Abstract
This study used functional magnetic resonance imaging to investigate brain activation during preparatory and regulatory control while participants (N = 24) were instructed either to simply view or decrease their emotional response to, pleasant, neutral or unpleasant pictures. A main effect of emotional valence on brain activity was found in the right precentral gyrus, with greater activation during positive than negative emotion regulation. A main effect of regulation phase was evident in the bilateral anterior prefrontal cortex (PFC), precuneus, posterior cingulate cortex, right putamen and temporal and occipital lobes, with greater activity in these regions during preparatory than regulatory control. A valence X regulation interaction was evident in regions of ventromedial PFC and anterior cingulate cortex, reflecting greater activation while regulating negative than positive emotion, but only during active emotion regulation (not preparation). Conjunction analyses revealed common brain regions involved in differing types of emotion regulation including selected areas of left lateral PFC, inferior parietal lobe, temporal lobe, right cerebellum and bilateral dorsomedial PFC. The right lateral PFC was additionally activated during the modulation of both positive and negative valence. Findings demonstrate significant modulation of brain activity during both preparation for, and active regulation of positive and negative emotional states.
Keywords: cognitive control, positive emotion, negative emotion, fMRI, brain
INTRODUCTION
Cognitive control of emotion refers to the process by which individuals voluntarily regulate their emotional states (Gross, 1998). The inability to regulate emotional responses has been identified as a critical contributor to inappropriate social behaviors (Karrass et al., 2006) as well as various mental disorders, including impulsive aggression (Davidson et al., 2000) and depression (Johnstone et al., 2007). Previous research has demonstrated that efforts to consciously control emotional states such as erotic arousal (Beauregard et al., 2001), sadness (Levesque et al., 2003), fear (Hariri et al., 2003) and negative affect more broadly (Ochsner et al., 2002, 2004; Phan et al., 2005) produce significant effects on neural systems. In general, such studies have reported increased activity in the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) as well as reduced activity in the amygdala during cognitive control. For example, increased activity in the lateral and medial PFC was found during the downregulation of negative emotion in conjunction with attenuated report of negative affect (Ochsner et al., 2002). Another study examining the voluntary suppression of sadness found that the right orbitofrontal cortex (OFC) and lateral PFC were activated (Levesque et al., 2003). Additionally, increased prefrontal and ACC activation as well as reduced amygdala activity were observed in a study examining the regulation of responses to fearful stimuli (Hariri et al., 2003) and in other studies during the downregulation of negative affect (Phan et al., 2005; Urry et al., 2006). In sum, studies to date have demonstrated consistent effects of emotion regulation on activity in underlying neural systems.
Although prior work has been successful in identifying changes in brain activity associated with emotion regulation, it has not fully addressed the regulatory process, being limited in two major aspects. First, studies to date have not examined brain activation effects associated with preparatory control for emotion regulation. Preparation prior to upcoming emotional events can be viewed as an adaptive coping mechanism that facilitates planned action, and some available data provide evidence of distinct neural systems associated with preparatory control (MacDonald et al., 2000; Braver et al., 2009). Thus, a need exists for further investigation of preparatory effects in the context of emotional regulation.
Second, previous studies have generally included only a single category of affective stimuli (most typically negative), so that direct comparisons of neuronal activity associated with the regulation of positive versus negative emotion are limited. Thus far, only one study has compared positive and negative emotions within a task, detecting bilateral PFC activation during regulation of both positive and negative emotion, while showing selective activation in ventromedial PFC (VmPFC) and ACC regions during the downregulation of negative as compared with positive emotion (Kim and Hamann, 2007). These findings highlight the need for further research directed at elucidating the brain systems underlying regulation of positive and negative emotion. In particular, the control of positive emotion is crucial in understanding problem behaviors, as difficulty regulating positive affect or appetitive reactivity is often associated with pathological conditions such as mania (Elliott et al., 2004) or addictive disorders (Koob and Le Moal, 2001). There is also evidence that distinct but interacting neural systems are involved in processing of positive as compared with negative emotional stimuli (Ahern and Schwartz, 1985; Davidson and Irwin, 1999; Carver, 2001) and in affect-related individual differences (Davidson, 1998). Thus, further studies using both positive and negative affective stimuli within the same processing task are needed to advance understanding of brain systems underlying emotion regulation.
With these considerations in mind, this study was designed to investigate brain systems involved in the regulation of positive and negative emotion using functional magnetic resonance imaging (fMRI). The study extended prior work by examining activity in differing brain regions during preparatory and regulatory phases of an emotion regulation task in which participants were instructed either to simply view, or to decrease emotional reactions to, positive and negative affective stimuli. As our emotion regulation procedure, we utilized cognitive reappraisal as documented in previous studies (Ochsner et al., 2002, 2004), and for preparatory control, we utilized a variant of a naturalistic prospective-memory procedure entailing active remembering and preparing to implement an intended action in an upcoming event (Simons et al., 2006).
Based on prior research findings (MacDonald et al., 2000), we hypothesized that the left lateral PFC, especially dorsolateral PFC (DLPFC), would be selectively activated during the preparatory phase in which attentional control would be expected to predominate. In addition, during the emotion regulation phase, we expected to find bilateral activations in subdivisions of PFC (ventromedial, orbitofrontal and dorsolateral) and in ACC, regions known to be involved in on-line emotion regulation (Ochsner et al., 2002, 2004; Kim and Hamann, 2007) and response conflict monitoring (Botvinick et al., 1999). We also hypothesized that distinct brain regions, such as the VmPFC and ACC (Kim and Hamann, 2007), would be differentially involved in the regulation of positive versus negative emotion. Regarding modulatory effects of regulation on subcortical regions, we expected, on the basis of prior research (Beauregard et al., 2001; Ochsner et al., 2002, 2004; Levesque et al., 2003), to observe reduced activity in the amygdala. In addition, we examined the ventral striatum (VS) in view of evidence for its involvement in positive emotion (Everitt et al., 2000; Knutson et al., 2001), although previous studies have generally not reported changes in this region (for an exception, see Phan et al., 2005).
MATERIALS AND METHODS
Participants
Participants were 24 right-handed females (age: M = 19.58 years, s.d. = 1.21 years) recruited from undergraduate psychology classes and from an advertisement in the student newspaper. Female subjects were recruited exclusively for this study to circumvent moderating effects of gender on responses to emotional stimuli (Cahill et al., 2001; Seo et al., 2011). All individuals included in the study had normal or corrected-to-normal vision, and were free of hearing impairments or major psychiatric disorders. The study procedures were approved by the Institutional Review Board at the University of Minnesota, and participants provided informed written consent prior to initiation of testing. Compensation for participation consisted either of course credit or a cash payment of $52.
Experimental stimuli
The task stimuli consisted of 150 positive (60), neutral (30), negative (60) pictures selected from the International Affective Picture System (IAPS; Lang et al., 1999). Positive and negative picture sets were matched for arousal (Ms = 5.70 and 5.86, respectively, versus 3.09 for neutral pictures; s.d. = 0.75, 0.90 and 0.68) based on IAPS rating norms (Lang et al., 1999), as a whole and across instructional conditions (view, decrease). Mean normative ratings of valence (pleasantness) for positive, negative and neutral picture categories were 7.52, 2.47 and 4.91, respectively (s.d. = 0.66, 0.74 and 0.33). The pictures of the same valence type were also counterbalanced across instruction conditions (decrease, view). Picture stimuli were presented in 6 blocks of 25 trials. To more effectively isolate brain activity associated with preparation for regulation of positive versus negative emotion, positive and negative picture stimuli were presented in separate blocks (3 positive blocks and 3 negative blocks). Neutral pictures were also included in blocks of each type, but only within the view condition. The order of positive blocks and negative blocks was counterbalanced across participants, such that half started with a positive block and the other half started with a negative block. Participants were not cued at the beginning of each block and thus were unaware of the valence of upcoming blocks. For each of five conditions (positive–decrease, positive–view, negative–decrease, negative–view and neutral–view), 30 pictures were presented. Each picture was presented only once and the order of picture presentation was randomized within blocks and across participants.
Task
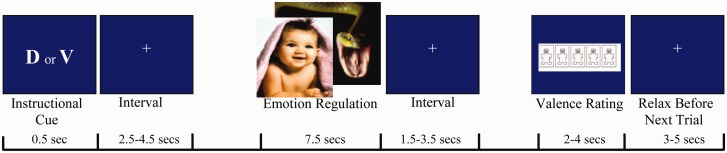
Each task trial consisted of four stimulus events (Figure 1). An event-related design was used that included a “time-jitter” technique to effectively differentiate blood-oxygen-level dependent (BOLD) activity and to separate events of interest within a trial (Dale, 1999). First, an instructional cue (V for view, or D for decrease) appeared for 500 ms, followed by a jittered inter-stimulus interval that varied from 2.5 to 4.5 s (mean = 3.5 s). Participants were instructed to actively prepare to regulate emotion when a “D” cue was presented and to simply view the picture without making an effort when a “V” cue was presented. Specifically, participants were asked to prepare to reappraise their emotional responses to pictures during an upcoming presentation period, by rehearsing the preparatory cue (signaling the downregulation by reappraisal) during decrease trials. Second, a picture appeared for 7.5 s, at which point participants implemented the prior instructional cue, followed by a variable interval ranging from 1.5 to 3.5 s (mean = 2.5 s). In “decrease” trials, participants were instructed to decrease their emotional states using a reappraisal strategy, which was rehearsed before the scanning session. The reappraisal strategy entailed reinterpreting the actions, feelings and outcomes of the people or scenarios depicted in the emotional pictures to attenuate emotional reactions evoked by the pictures (Gross, 1998; Ochsner et al., 2002, 2004). Following this, an affective valence rating scale (self-assessment manikin; Lang, 1980) appeared, consisting of numbered options ranging from 1 (very negative) through 9 (very positive). The participant pressed buttons to register a rating of valence (2–4 secs, depending on individual speed). At the end of the trial, a fixation cross appeared for the duration of a jittered inter-trial interval (mean 4 s, range 3–5 s), during which participants relaxed until the onset of the next trial.
Fig. 1.
Schematic time course of a single trial. Participants were instructed to view or to decrease their emotional states in response to a series of pictures using a reappraisal strategy. D, decrease; V, view.
Procedure
Three to four days prior to scanning, participants received a 1.5 h training session on reappraisal strategies and preparatory control for the emotion regulation task. The training procedure was standardized across participants. Participants completed a block of 25 practice trials using stimuli different from those employed in the main experiment. The MRI session took place at the University of Minnesota's Center for Magnetic Resonance Research, using a 3.0 Tesla Siemens Trio scanner. The scan session lasted approximately 1.5 h, with 1 h devoted to the fMRI task and 30 min for set-up and structural MRI scanning. While in the scanner, participants used a mirror mounted above the head coil apparatus to view images projected onto a screen.
Data acquisition
Standard T1-weighted anatomical image volumes were first acquired using a 3D Magnetization Prepared Rapid Gradient Echo (MP-RAGE) sequence (1 mm isotropic voxels). Functional images were then collected using an 8-channel phased array coil. The functional data were acquired using an Echo-Planar Imaging pulse sequence (36 slices, slice thickness = 2 mm with a 1.5-mm gap; field of view = 22.4 × 22.4 cm, matrix size = 64 × 64 for a nominal resolution of 3.5 mm; TE = 28 ms; TR = 2 s; flip angle = 80 degrees). Slice orientation was oblique, roughly aligned with a line connecting the base of the cerebellum with the base of the frontal lobe. This orientation was used to obtain optimal signal-to-noise ratio in the OFC (Deichmann et al., 2003) and amygdala (Chen et al., 2003).
Data analysis
Affective ratings were analyzed using a two-way repeated measures analysis of variance (ANOVA), in which picture valence (positive, negative) and instructional condition (decrease, view) were included as within-subject factors. Follow-up t-tests were used to clarify the basis of significant effects. A one-way repeated measure ANOVA was used to evaluate effects for picture valence (positive, neutral and negative) within the view condition.
Preprocessing of fMRI data was conducted using the FSL suite from the Oxford FMRIB software library. The data were motion corrected using MCFLIRT (Jenkinson et al., 2002). Functional images were spatially smoothed using a 5 mm Gaussian kernel and temporally high-pass filtered. General linear model analyses were employed to examine the imaging data, and each event was modeled using a double-gamma function. High-resolution anatomical images were spatially normalized and registered to the Montreal Neurological Institute (MNI)-152 template using FLIRT. For group analysis, a 2 × 2 repeated measures ANOVA was conducted on individual beta weights (decrease–view) to evaluate main effects of emotional valence (positive, negative) and regulation phase (preparation, regulation) and their interaction. As a follow-up to this, simple effect analyses (t tests) were conducted to clarify the sources of effects that emerged as significant. In addition, conjunction analyses were performed to identify brain regions commonly involved in levels within each main effect (valence, regulation phase), collapsing over the levels of the other factor. To correct for multiple comparisons, the Family Wise Error rate (FWE) correction was implemented such that a FWE cluster corrected, significance threshold of Z > 2.3 with P < 0.05 (Worsley et al., 1992; Friston et al., 1994) was applied across all analyses within FSL. Significant activations identified with cluster corrections are displayed in figures as a color overlay on the Colin27 Brain T1-weighted anatomy in MNI space (Holmes et al., 1998). To examine the effects of emotion regulation on activity in subcortical regions, two brain regions corresponding to specific regions of interest (ROIs) were defined a priori based on prior research (Ochsner et al., 2002; Levesque et al., 2003; Phan et al., 2005), and we additionally examined activity in ROI regions based on small volume corrections; these regions included amygdala, and VS as defined by Harvard–Oxford atlases.
RESULTS
Affective valence ratings
For the two-way repeated-measures ANOVA, significant effects were found for both picture valence [F(1,23) = 73.62, P < 0.001] and instruction [F(1,23) = 30.8, P < 0.001] (Figure 2). Additionally a Valence × Instruction interaction was evident [F(1,23) = 120.35, P < 0.001]. For negative picture stimuli, participants showed reduced ratings of unpleasantness for the decrease condition compared with the view condition [t(23) = 9.82, P < 0.001]. For positive picture stimuli, diminished ratings of pleasantness were evident for the decrease condition compared with the view condition [t(23) = −11.13, P < 0.001]. Additionally, the magnitude of the instructional effect (decrease–view) on affective ratings was greater for positive than for negative pictures [t(23) = 5.55, P < 0.001], suggesting more effective regulation of positive emotion than negative emotion. Additionally, a one-way repeated measures ANOVA comparing valence ratings during the viewing of positive, neutral and negative pictures yielded a significant effect [F(1,23) = 270.55, P < 0.001], with follow-up tests indicating enhanced and diminished ratings of valence, respectively, for positive and negative pictures in relation to neutral [t (23) = 12.85 and −16.6, P < 0.001].
Fig. 2.
Mean self-reports of affect ratings for positive, neutral and negative emotion. On the affective rating scale, 9 was the most positive and 1 the most negative. Relative to the view condition, pleasantness during the decrease condition was significantly reduced for positive emotion and unpleasantness was diminished for negative emotion, suggesting successful modulation of both positive and negative emotion. The magnitude of positive valence modulation (decrease–view) was significantly greater than negative valence modulation (decrease–view). ***P < 0.001.
Brain imaging results
Repeated measures ANOVA
The results of the 2 × 2 repeated measures ANOVA conducted on decrease–view (Figure 3 and Table 1) indicated meaningful modulatory effects in the form of significant main effects of valence (positive, negative) and regulation phase (preparation, regulation), along with an interaction effect (P < 0.05, whole-brain FWE corrected). The main effect of valence was significant in the right precentral gyrus, with simple effects analysis revealing greater activity in this region during positive than negative regulation (P < 0.05, whole-brain FWE corrected). The main effect of regulation phase was evident in the bilateral anterior PFC (BA 10), left DLPFC (BA 9/46), precuneus, posterior cingulate cortex, right putamen and selected areas of left superior/middle temporal gyrus (TG), occipital lobe and cerebellum. Simple effects analysis revealed greater activity in these regions during the preparatory phase compared with the regulatory phase, and none of these regions were found to be more active during the regulatory than the preparatory phase. The two-way interaction effect was also significant for the VmPFC and ACC regions, with simple effects analysis indicating significantly greater activity in the VmPFC/ACC during negative than positive emotion regulation, during the active regulation phase but not the preparatory phase.
Fig. 3.
Whole brain voxel-based fMRI images showing valence (positive, negative) X regulation phase (preparation, regulation) main effects and an interaction (left panel; P < 0.05, whole-brain FWE corrected) and corresponding simple effects (right panel; P < 0.05, whole-brain FWE corrected). (A) A main effect of valence was evident in right precentral gyrus, with greater activity during positive than negative emotion regulation. (B) A main effect of regulation phase was shown in the bilateral anterior PFC, left DLPFC, right putamen and thalamus, precuneus, superior/middle temporal and occipital lobes, with greater activity in these regions during preparatory than regulatory control. (C) A valence X regulation phase interaction was shown in the VmPFC and ACC with greater activity during negative than positive emotion regulation, but only in the regulatory phase (not preparation). L, left; R, right. Coordinates are given in MNI space.
Table 1.
Significant brain activation showing main effects and interaction (decrease–view)
| Regions | Lat | BA | 2 × 2 ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | Coordinates |
Volume (mm3) | Z | Coordinates |
Volume (mm3) | |||||||
| X | Y | Z | X | Y | Z | |||||||
| Simple effects |
||||||||||||
| Main effect (valence) | Positive > negative | |||||||||||
| Precentral gyrus | R | 4, 6 | 4.97 | 36 | −22 | 44 | 4904 | 3 | 35 | −23 | 53 | 3 227 |
| Main effect (regulation phase) | Preparatory > regulatory | |||||||||||
| Lateral anterior PFC/DLPFC | L | 10, 9, 46 | 3.51 | −31 | 48 | 16 | 14 776 | 3 | −29 | 45 | 17 | 14 421 |
| Lateral anterior PFC | R | 10 | 3 | 31 | 50 | 16 | 5163 | 2.9 | 32 | 49 | 13 | 3219 |
| Putamen/thalamus | R | — | 3.1 | 23 | −7 | 8 | 7951 | 2.93 | 26 | −10 | 4 | 7364 |
| Superior/middle TG | L | 21, 22 | 3 | −58 | −21 | −1 | 7769 | 2.85 | −52 | −23 | 5 | 7811 |
| Posterior cingulate cortex/MFG | L, R | 6, 23, 24, 31 | 3.04 | 9 | −23 | 53 | 29 932 | 2.9 | 9 | −25 | 47 | 29 300 |
| Precuneus/occipital lobe/cerebellum | L, R | 7, 17, 18 | 3.56 | −2 | −72 | −2 | 94 942 | 3.05 | −1 | −72 | −5 | 82 175 |
| Interaction | Negative > positive (regulation phase) | |||||||||||
| VmPFC/ACC | L, R | 10, 11, 32 | 3 | 8 | 54 | −2 | 4248 | 2.8 | −1 | 45 | −4 | 6449 |
P < 0.05, whole-brain FWE corrected. Lat, Laterality; L, left; R, right; BA, Brodmann's area. MNI coordinates were used. MFG, middle frontal gyrus.
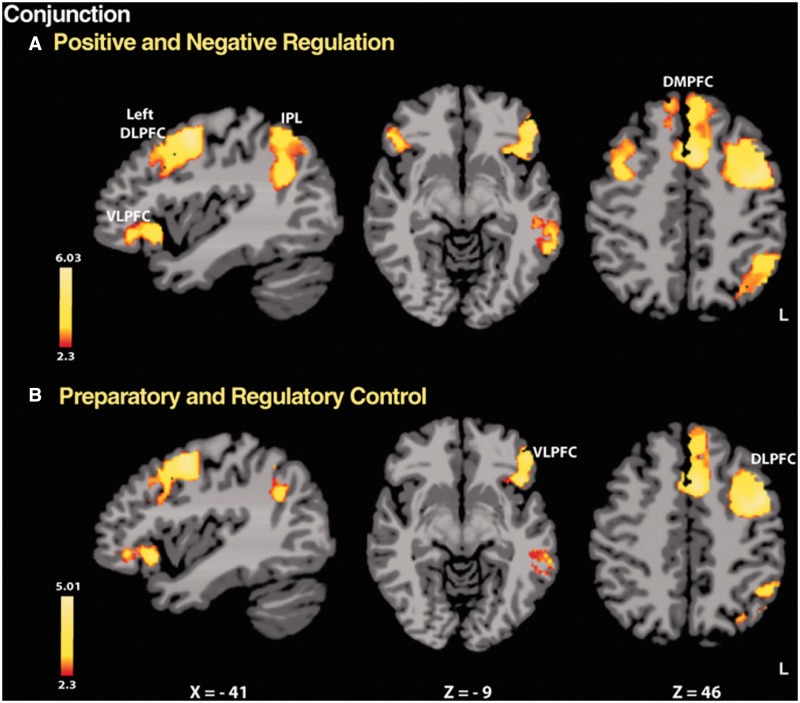
Conjunction analyses
The results of conjunction analyses indicated that selected areas of the PFC, temporal and parietal lobes were commonly involved in different types of regulation (P < 0.05, whole-brain FWE corrected; Figure 4 and Table 2). The analyses were conducted on the levels of each main effect (valence, regulation phase), averaging over the levels of the other factor. Brain regions that showed common activation during the regulation of both positive and negative emotion included the bilateral PFC (ventrolateral and dorsolateral), dorsomedial PFC, right cerebellum and the left side of the superior/middle temporal and inferior parietal lobes. Further, during both preparatory and regulatory controls, left-lateralized PFC (ventrolateral and dorsolateral), superior/middle temporal and inferior parietal lobes, along with bilateral dorsomedial PFC and right cerebellum, were commonly activated.
Fig. 4.
Results of conjunction analyses for two main effects. (A) valence (positive and negative) and (B) regulation phase (preparation and regulation), collapsing across levels of the other factor (P < 0.05, whole-brain FWE corrected). During differing types of control, selected areas of the lateral PFC, dorsomedial PFC and left superior/middle temporal and inferior parietal lobes were commonly activated. Specifically, (A) during the regulation of both positive and negative emotion, bilateral PFC activity was displayed, whereas (B) during both preparatory and regulatory control, left lateral PFC activity was found. DMPFC, dorsomedial PFC; IPL, inferior parietal lobe; L, left. Coordinates are given in MNI space.
Table 2.
Results of conjunction analyses (decrease–view)
| Regions | Lat | BA | Positive and negative regulation |
Preparatory and regulatory control |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | Coordinates |
Volume (mm3) | Z | Coordinates |
Volume (mm3) | |||||||
| X | Y | Z | X | Y | Z | |||||||
| Lateral PFC (ventro/dorsolateral) | L | 47, 44/45, 6/8/9 | 3.66 | −41 | 16 | 27 | 31 209 | 3.36 | −41 | 15 | 27 | 24 355 |
| R | 47, 45, 6/8/9 | 3 | 43 | 17 | 37 | 4499 | — | — | — | — | ||
| Dorsomedial PFC/middle CG | L, R | 6/8/9, 32 | 3.6 | −4 | 24 | 49 | 29 544 | 3.37 | −4 | 22 | 50 | 21 680 |
| Temporo-parietal regions | L | 21/22, 39/40 | 3.38 | −51 | −50 | 20 | 18 185 | 3.17 | −54 | −48 | 15 | 10 898 |
| Cerebellum | R | — | 3.24 | 26 | −72 | −35 | 8569 | 3.21 | 26 | −71 | −34 | 5397 |
P < 0.05, whole-brain FWE corrected. MNI coordinates were used. Lat, laterality; L, left; R, right; BA, Brodmann's area; CG, cingulate gyrus. Temporo-parietal regions include superior/middle TG and inferior parietal lobe.
Modulation of activity in subcortical ROI
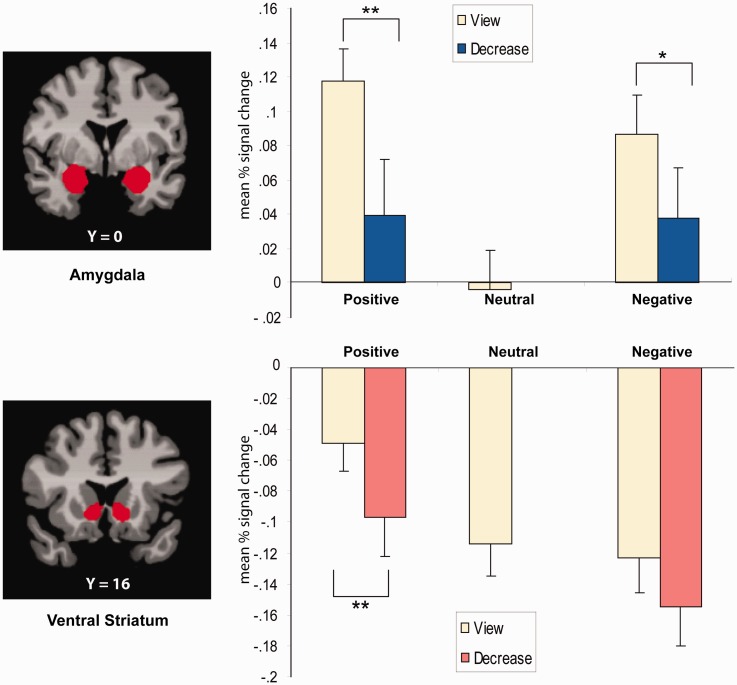
To examine activity in specific a priori subcortical regions (amygdala and VS) in relation to regulatory control, two-way repeated measures ANOVAs were performed in which valence (positive/negative) and instruction condition (decrease/view) as included as within subject factors (Figure 5). Laterality (left, right) was not included in this analysis, as preliminary tests revealed no significant hemispheric effects for either the amygdala or VS. For the amygdala, a significant main effect was found [F(1,23) = 10.7, P < 0.01] indicating a general decrease in amygdala activity during the regulation of emotion [t(23) = −3.27, P < 0.01] as compared with the view condition. The magnitude of this regulatory effect was similar for positive and negative picture stimuli [t (23) = −2.98 and −2.29, respectively, P < 0.05]. No main effect of valence or two-way interaction effect (instruction × valence) was found.
Fig. 5.
Means and standard errors of mean for signal changes of the amygdala and VS during view and decrease conditions for positive, neutral and negative trials. During positive emotion regulation, the activity in the amygdala and VS was diminished compared with the view condition. During negative emotion regulation, amygdala activity was reduced relative to the view condition. Brain images show the amygdala and VS of the Harvard–Oxford structural atlas from which mean signal changes were obtained. MNI coordinates were used. *P < 0.05, **P < 0.01.
For the VS region, the analysis revealed significant main effects of instruction [F(1,23) = 10.73, P < 0.01] and valence [F(1,23) = 27.75, P < 0.001], but no instruction × valence interaction. Ventral striatal activity was significantly greater during positive than negative picture trials [t(23) = 4.5, P < 0.001]. Further, ventral striatal activity was decreased significantly during the regulation of emotion [t(23) = −3.28, P < 0.01] as compared with the view condition. This regulatory effect was evident for both positive [t(23) = −2.97, P < 0.01] and negative [t(23) = −2.0, P = 0.058] pictures.
To compare activity in the amygdala and VS regions during the viewing of positive, neutral and negative pictures, one-way repeated measures ANOVAs were conducted (Figure 5). For the amygdala, significant main effects were found [F(1,23) = 16.63, P < 0.001], with greater activity observed during viewing of both positive [t(23) = 5.74, P < 0.001] and negative [t(23) = 4.94, P < 0.001] pictures relative to neutral pictures. No difference in amygdala activation was found between positive and negative pictures. A significant effect was also evident for the VS [F(1,23) = 16.37, P < 0.001], with activity in this region significantly greater during viewing of positive pictures relative to both neutral [t(23) = 5.41, P < 0.001] and negative pictures [t(23) = 4.51, P < 0.001]. Neutral and negative pictures did not differ in terms of activity in the VS.
Correlational analysis
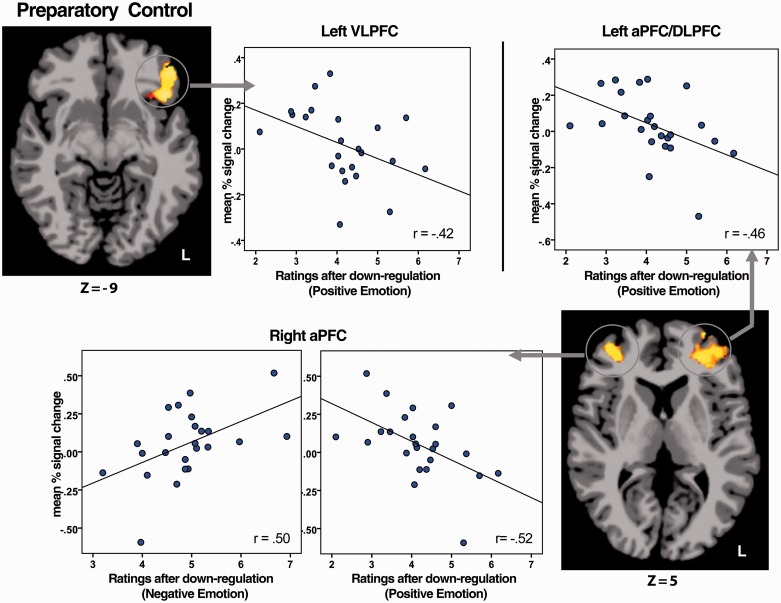
To understand the relationship between functionally defined ROIs and modulation of perceived emotional experience, mean signal changes in brain regions showing task-related activations (Figures 3 and 4 and Tables 1 and 2) were independently correlated with modulated affective ratings. Results indicated that activity during preparatory control in the bilateral anterior PFC (aPFC) (Figure 3B) and left ventrolateral PFC (VLPFC) (Figure 4B) showed significant correlations with reduced affective ratings (Figure 6). There was no significant correlation between brain activity during the regulatory phase and modulation of ratings. As the association with ratings was found for the preparatory control phase in the main effect and the conjunction map, the results of correlations focused on mean signal changes for brain regions collapsed across the levels of the valence factor.
Fig. 6.
Associations between brain activity during preparatory control and modulation of affective ratings (1 = most negative; 9 = most positive). During preparatory control, increased activity in the left VLPFC (r = −0.42, P < 0.05) and left anterior PFC/DLPFC (r = −0.46, P < 0.05) was associated with reduced ratings of pleasantness following downregulation of positive emotion. Increased right anterior PFC activity was also significantly associated with reduced pleasantness of positive pictures (r = −0.52, P = 0.01) and with reduced unpleasantness of negative pictures (r = 0.50, P = 0.013) following downregulation. No outliers were found in these associations. L, left. Coordinates are given in MNI space.
During preparatory control, increased right aPFC (BA 10) was significant associated with reduced pleasantness in relation to positive stimuli (r = −0.52, P = 0.01) and reduced unpleasantness in relation to negative stimuli (r = 0.50, P = 0.013). Increased left aPFC/DLPFC (BA 9/10/46) and left VLPFC activity (BA 47) were also associated with reduced pleasantness ratings for positive stimuli (r = −0.46 and −0.42, respectively, P < 0.05) and trend-level reductions in unpleasantness ratings for negative stimuli (r = 0.40 and 0.39, respectively, P = 0.056 and 0.058). No outliers were found in these associations. To examine whether the predictive strength of brain activity in these regions differed for positive versus negative ratings, the difference in absolute r values for the two was tested using Steiger’s Z test. No significant difference was found in the r for right aPFC (Z = 0.21, P = n.s.), left aPFC/DLPFC (Z = 0.60, P = n.s.) or left VLPFC (Z = 0.3, P = n.s.), indicating a comparable level of modulation for positive and negative ratings. There were also no correlations between task-related brain activity and responses of the amygdala and VS.
DISCUSSION
This study used fMRI to investigate brain systems involved in preparatory and regulatory control over positive and negative emotion. The results demonstrate differential activity in specific brain regions during preparatory as compared with active emotion regulation phases, as well as differential activity between the regulation of positive and negative emotional states. Emotion modulatory effects were also evidenced by reduced activation in the amygdala and VS along with reduced subjective ratings of pleasantness and unpleasantness for positive and negative pictures, respectively. These findings help to elucidate mechanisms underlying preparatory and regulatory control of emotion by showing common and differential brain regions associated with these processes.
First, brain regions commonly involved in different types of control (preparatory and regulatory; positive and negative) were found, including the left-lateralized PFC, inferior parietal and superior/middle temporal lobes, dorsomedial PFC and right cerebellum. The concomitant involvement of these regions suggests potentially shared neural networks during different types of cognitive control entailing reappraisal. Specifically, increased activity in the left lateral PFC (ventro- and dorsolateral) was evident for different types of regulation. Emotion regulation requires implementation of regulatory instructions to modulate emotional response, which may primarily involve modulatory activity exerted in the left DLPFC and VLPFC, as indicated by prior neuroimaging studies of cognitive reappraisal (Ochsner et al., 2002, 2004; Kim and Hamann, 2007). Specifically, the DLPFC is known to mediate cognitive control via goal representation and guidance of planned behavioral actions (Miller and Cohen, 2001). In particular, previous studies have reported that the left DLPFC plays a crucial role in cognitive control during both preparation (MacDonald et al. 2000; Braver et al., 2009) and online emotion regulation (Ochsner et al., 2002), suggesting a central role of this region in differing phases of cognitive control.
The VLPFC is involved in the implementation of intended goals and interacts with the medial PFC/OFC to modulate emotional responses in limbic regions (Sakagami and Pan, 2007). Moreover, in the current data, increased left VLPFC activity during preparatory control was associated with reduced affective ratings, suggesting a relationship between the left VLPFC and perceived experience of emotional modulation. This observed association may reflect the contribution of the VLPFC to implementation of controlled behaviors, by serving as a bridge between cognition and emotion (Gray et al., 2002; Sakagami and Pan, 2007). Notably, the left VLPFC demonstrates a specific association with cognitive control through language-based, semantic processing (Badre and Wagner, 2007), providing a plausible explanation for the left-specific VLPFC activation during preparatory and regulatory control phases in our task.
In addition, enhanced left-lateralized activity was noted in the inferior parietal lobe and superior/middle TG. The left inferior parietal lobe is known to play a role in inhibitory control (Bellgrove et al., 2004) and mood regulation (Koven et al., 2010). The TG and Broca’s area—regions involved in language and semantic processing as well as storytelling (Bartha et al., 2003; Steinvorth et al., 2005)—were also activated. During the preparatory phase, participants were instructed to rehearse the instructional cue to maintain focused control, an activity expected to recruit language-processing regions. During the active emotion regulation phase, participants were instructed to utilize reappraisal strategies, which may also entail semantic-linguistic processing involving the TG and Broca’s area. These findings are in turn consistent with those of previous studies utilizing reappraisal strategies (Ochsner et al., 2002, 2004; Kim and Hamann, 2007). More specifically, left-lateralized TG activation has been demonstrated during the processing of verbalizable materials, whereas the right side is implicated more in the processing of visual-spatial information (Golby et al., 2001; Weintrob et al., 2002).
The dorsomedial PFC has been associated with higher cognitive function related to mental operations during verbal processing (Hanakawa et al., 2002), impression formation (Mitchell et al., 2004), monitoring and adjustment of action plans (Nachev et al., 2005), and the generation of response strategies (Matsuzaka et al., 2012). Accordingly, increased dorsomedial PFC activity can be interpreted as reflecting higher cognitive operations while exerting regulatory efforts. The role of the cerebellum in cognitive and emotional processing has also been noted. For example, Krienen and Buckner (2009) concluded that cerebellar activity typically occurs contralaterally to affiliated cortical activity during emotion and cognition processing (Krienen and Buckner, 2009). This pattern could account for the occurrence of right cerebellar activity in our task. This result also coincides with work showing left PFC activity accompanied by right cerebellar activity during a cognitive task involving semantic processing (Roskies et al., 2001). In sum, our finding of concurrent activity in left-lateralized PFC, temporal and inferior parietal lobes, and right cerebellum under different types of regulatory demands indicates that these regions may operate together to orchestrate higher order executive function and implement regulation of emotional processes.
In addition to the brain regions discussed above, right lateral PFC activity was also evident during the regulation of both positive and negative emotional valence, displaying bilateral activation. Bilateral PFC activation has also been reported in previous studies investigating the downregulation of positive and negative emotion (Kim and Hamann, 2007), as well as in a study that focused on negative emotion only (Ochsner et al., 2004). Other work has shown that emotion (both positive and negative) plays a role in orienting attention through elicitation of arousal (Lang, 2010), suggesting that the right PFC may be related to modulation of the arousal component of emotion. In support of this, Dichter and Belger (2008) found the right lateral PFC to be selectively activated during the regulation of highly arousing emotional pictures regardless of valence. Previous studies have also demonstrated increased right PFC activation during the inhibition of affective response to both pleasant and unpleasant stimuli including erotic images (Beauregard et al., 2001), sadness inductions (Levesque et al., 2003) and fearful stimuli (Hariri et al., 2003), indicating a role of the right lateral PFC in modulation of both positive and negative emotion.
This study also indicated valence-specific brain regions of emotion regulation. When trials involving regulation of positive emotion were compared with those involving negative emotion, the right precentral gyrus was preferentially activated. Positive or reward stimuli are known to elicit appetitive motivation and action (Lang, 2010) and activate limbic and motor regions (Roesch and Olson, 2003). The premotor cortex is involved in the modulation of motivation and reward (Roesch and Olson, 2003; Roesch and Olson, 2004) and goal-directed action control (Christensen et al., 2007; Pastor-Bernier et al., 2012). In particular, the right precentral gyrus has been associated with modulation of sustained attention (Cabeza and Nyberg, 2000), self-related awareness (Theoret et al., 2004) and greater use of cognitive reappraisal (Leung and Lee, 2012). Based on this, it can be inferred that participants in the current study were more engaged in modulating affect-driven attention through reappraisal during positive stimulus blocks.
In addition, distinct brain regions (VmPFC/ACC) differentiating the regulation of negative from positive emotion were found, but only during the active emotion regulation phase, suggesting that online processing of emotional stimuli is necessary to differentially activate these regions. This result is consistent with previous work demonstrating greater involvement of the medial division of anterior PFC (aPFC; BA 10) in processing of externally presented stimuli (Burgess et al., 2005; Gilbert et al., 2005). The VmPFC also has dense anatomical connections with the amygdala and exerts inhibitory influence on this region (Cavada et al., 2000; Fisher et al., 2009). It follows that the observed modulation in the VmPFC/ACC may be associated with regulation of limbic activation triggered by external stimuli, especially with negative content. The role of the VmPFC/ACC in regulating negative emotion is also consistent with previous work (Kim and Hamann, 2007) reporting greater VmPFC/ACC activation during the downregulation of negative than positive emotion. This finding is also consistent with other data indicating a role of the VmPFC in the regulation of negative emotion (Urry et al., 2006; Goldin et al., 2008). The ACC has also been associated with negative feedback (Ruchsow et al., 2002) and negative reinforcement learning (Brown and Braver, 2005). In addition, individuals who exhibit difficulty in regulating negative emotion evidence impairments in VmPFC/ACC function (Davidson et al., 2000). These results collectively indicate that the VmPFC/ACC activation specific to active attenuation of negative emotion may reflect online inhibitory control over stimuli with negative content.
When preparatory control was compared with regulatory control, selective areas of prefrontal and parietal cortices were found to be differentially active, including in the bilateral aPFC (BA 10), precuneus, PCC, right putamen and superior/middle temporal and occipital lobes. Given that the task in the preparatory control phase entailed actively preparing to reappraise while rehearsing the cue signaling this intended action, several processes governed by differing neural networks were likely invoked, including top–down modulation of attention, self-conscious recall of an intended action, and sensory processing of the attended cue (Carlsson et al., 2000; Simons et al., 2006).
With regard to the finding of increased activity in the lateral division of the aPFC (BA 10) during preparatory than regulatory control, this region plays a key role in online maintenance of an intended action (Burgess et al., 2001). Multiple studies demonstrated a role for the lateral aPFC in prospective memory, an executive function requiring attentional control during cue presentation and maintenance of an intended action during a delay period (Burgess et al., 2001, 2003; West and Ross-Munroe, 2002; den Ouden et al., 2005; Simons et al., 2006). Thus, enhanced lateral aPFC activity during preparatory control could reflect increased attention modulation and goal-directed maintenance of an intended action (reappraisal) for an upcoming event. Consistent with this, activity in the bilateral aPFC along with left DLPFC during preparatory control was also significantly associated with reductions in affective ratings in the current study, suggesting an important executive role for lateral aPFC during preparation for modulation of emotional reactivity.
In addition, the precuneus and PCC, brain regions related to modulating consciousness and self-reflection (Kelley et al., 2002; Lou et al., 2004), were more active during preparatory control. The precuneus is involved in self-conscious mental processes (den Ouden et al., 2005; Cavanna and Trimble, 2006) and attentive control (Luo et al., 2004), suggesting greater conscious, attention-driven processing of internal goals during this phase. This is in turn consistent with previous studies showing concurrent activity in the lateral aPFC, PCC and precuneus during prospective memory process (Burgess et al., 2001; den Ouden et al., 2005). Further, activity in the temporal and occipital cortices was elicited, which can be interpreted as reflecting the modulation of anticipatory attention in cue-related processing (Corbetta and Shulman, 2002; Serences and Yantis, 2006). Increased activity of the putamen has likewise been demonstrated in other studies of anticipatory processing (Haruno and Kawato, 2006), with concomitant activity of the lateral aPFC and striatum specifically observed during goal-directed anticipation of emotional stimuli (Rea et al., 2011). From the perspective of anticipation and preparation as processes facilitating awareness of one’s intentions in relation to an upcoming event, it can be inferred that the lateral aPFC, PCC and precuneus were involved in goal-directed preparation and self-conscious awareness of intended action during the preparatory phase of the current study, in conjunction with activity in sensory processing regions reflecting cue processing and readiness to receive upcoming stimulus information.
Although preparatory control elicited enhanced activity in these differing regions, regulatory control did not elicit greater activity than the preparation phase, suggesting that participants were more easily engaged in intended regulation during the preparatory phase than in the subsequent regulation phase, where cognitive control was needed to overcome bottom-up emotional arousal. This may in turn relate to instances in everyday life where advance cognitive preparation is found to be easier than regulation during unexpected stressors or conflicts. However, this result should be interpreted with caution. Although view trials were subtracted from decrease trials during both phases, the nature of regulatory control can be considered only roughly comparable for the two phases—given that regulatory control entailed context-driven reappraisal whereas preparatory control entailed anticipation of an unknown context. Accordingly, further studies will be needed to clarify commonalities and differences in processing within these two phases.
Emotion modulatory effects were also evident in responses of the amygdala and VS. During the regulation of positive and negative emotion, activity in both these subcortical regions decreased in comparison with the view condition. Consistent with this result, prior studies have reported reductions in amygdala activity during the regulation of both positive (Beauregard et al., 2001) and negative emotion (Levesque et al., 2003; Ochsner et al., 2002; Schaefer et al., 2002). The VS, a structure known to play a major role in pleasure and reward (Everitt et al., 2000; Knutson et al., 2001), evidenced greater activation during viewing of positive pictures than either negative or neutral pictures. During active regulation of positive emotion, ventral striatal activity significantly decreased in comparison with the view condition. For active regulation of negative emotion, a parallel trend-level reduction was evident. Another previous study also demonstrated a similar pattern, reporting decreased activity in the amygdala and VS during the regulation of negative emotion (Phan et al., 2005).
For positive picture trials, during both viewing and regulation conditions, an overall signal decrease in structurally defined VS was observed relative to baseline. This pattern contrasts with prior findings of increased VS signal during viewing of positive stimuli (Sabatinelli et al., 2007). Decreased VS response on positive picture trials relative to baseline in the current study could be attributable to our selection of a structurally defined ROI that was quite large in relation to a functionally defined area, localized more to the medial part of the VS. Within this more localized VS area, we did find increased BOLD signals relative to baseline during positive picture trials. However, the selection of structurally defined ROIs was necessary to effectively compare VS activity across different conditions. In this context, it should be noted that the pattern of structurally defined VS activity observed in the current study across positive, neutral and negative viewing trials (Positive > Neutral/Negative) coincides with findings from prior work (Sabatinelli et al., 2007; Wittmann et al., 2008).
In summary, this study provides neural evidence underlying the effects of emotion modulation during preparation for as well as during active implementation of emotion regulation. The findings of this study have important implications for the mechanisms underlying conscious control of evoked emotional responses. Specifically, in line with clinical studies showing that cognitive preparation enhances the performance and the effects of psychotherapy in anxiety disordered patients (Harve et al., 2000; Nilsson et al., 2011), our findings suggest potential benefits of mental preparation as a therapeutic strategy. In addition, the current study demonstrated differential regulatory systems specific to positive and negative emotions. Specifically, the right precentral gyrus was recruited specifically in regulating positive emotion, whereas the VmPFC and rostral ACC appear more important for regulating negative emotion during only regulatory phase. Some limitations of the current study warrant mention. First, our study included only female participants to optimize power to detect effects (i.e. in the absence of moderation by gender) and to permit comparisons with prior studies that have included only women (Ochsner et al., 2002; Kim and Hamann, 2007). More specifically, sex differences in brain responses to emotional stimuli have been reported (Goldstein et al., 2010; Seo et al., 2011). Although studying female participants provides specific, valuable information about individuals of this gender, there are clearly limitations in generalizing these findings to the population as a whole. A further limitation is that subjective ratings were collected for the regulation phase but not the preparatory phase, which constrains direct interpretations of brain activity effects observed during the preparatory phase. In addition, an optimal design for the preparatory control (e.g. the duration of jittered intervals) and its effects on emotion regulation should be further explored in future studies to advance our understanding on the nature of the preparatory control. Despite these limitations, this study contributes to basic understanding of neural systems underlying preparatory and regulatory control of emotion. Along more practical lines, our findings may also contribute to understanding of the mechanisms by which cognitive-behavioral strategies work to alleviate emotional suffering related to situational stressors or dysfunctional reward-seeking and in facilitating recovery of prefrontal regulatory function. Future research should continue to focus on the complex relationship between cognition and emotional control, especially in the area of understanding brain mechanisms underlying emotion regulatory deficits of individuals with mental disorders.
Acknowledgments
The authors thank Cheryl M Lacadie, Elisabeth L Kallenberger and Michael Meldahl for their technical assistance for this study. This work was supported by the National Institutes of Health (R21-MH65137 to C.J.P., RC1-MH089727 to C.J.P., P50-MH 072850 (Project 4) to C.J.P., UL1-DE19586 to R.S. and R01-AA13892 to R.S.) and the Hathaway endowment at the University of Minnesota.
REFERENCES
- Ahern GL, Schwartz GE. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23(6):745–55. doi: 10.1016/0028-3932(85)90081-8. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bartha L, Brenneis C, Schocke M, et al. Medial temporal lobe activation during semantic language processing: fMRI findings in healthy left- and right-handers. Brain Research. Cognitive Brain Research. 2003;17(2):339–46. doi: 10.1016/s0926-6410(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–6. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7351–6. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–21. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–55. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–18. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, McLeod P, Phillips L, editors. Measuring the Mind: Speed, Control and Age. Oxford: Oxford University Press; 2005. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75(1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. Tickling expectations: neural processing in anticipation of a sensory stimulus. Journal of Cognitive Neuroscience. 2000;12(4):691–703. doi: 10.1162/089892900562318. [DOI] [PubMed] [Google Scholar]
- Carver CS. Affect and the functional bases of behavior: On the dimensional structure of affective experience. Personality and Social Psychology Review. 2001;5:345–356. [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10(3):220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen NK, Dickey CC, Yoo SS, Guttmann CR, Panych LP. Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: application to imaging of the amygdala. Neuroimage. 2003;19(3):817–25. doi: 10.1016/s1053-8119(03)00091-0. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Geertsen SS, Petersen TH, Paulson OB, Nielsen JB. Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nature Neuroscience. 2007;10(4):417–9. doi: 10.1038/nn1873. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. Affective style and affective disorders: perspectives from affective neuroscience. Cognition and Emotion. 1998;12(3):307–30. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289(5479):591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19(2 Pt 1):430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28(4):787–96. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Atypical modulation of cognitive control by arousal in autism. Psychiatry Research. 2008;164(3):185–97. doi: 10.1016/j.pscychresns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biological Psychiatry. 2004;55(12):1163–70. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. New York: Oxford University; 2000. pp. 353–90. [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cerebral Cortex. 2009;19(11):2499–507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:214–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. The European Journal of Neuroscience. 2005;21(5):1423–31. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, et al. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124(Pt 9):1841–54. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010;30(2):431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2(3):271–99. [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, et al. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereberal Cortex. 2002;12(11):1157–70. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology. 2006;95(2):948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Harve AG, Clark DM, Ehlers A, Rapee RM. Social anxiety and self-impression: cognitive preparation enhances the beneficial effects of video feedback following a stressful social task. Behaviour Research and Therapy. 2000;38(12):1183–92. doi: 10.1016/s0005-7967(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrass J, Walden TA, Conture EG, et al. Relation of emotional reactivity and regulation to childhood stuttering. Journal of Communication Disorders. 2006;39(6):402–23. doi: 10.1016/j.jcomdis.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koven NS, Roth RM, Garlinghouse MA, Flashman LA, Saykin AJ. Regional gray matter correlates of perceived emotional intelligence. Social Cognitive and Affective Neuroscience. 2010;6(5):582–90. doi: 10.1093/scan/nsq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereberal Cortex. 2009;19(10):2485–97. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in Mental Health Care Delivery Systems. Norwood, NJ: Ablex; 1980. pp. 119–l37. [Google Scholar]
- Lang PJ. Emotion and motivation: toward consensus definitions and a common research purpose. Emotional Reviews. 2010;2(3):229–33. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: Center for Research in Psychophysiology, University of Florida; 1999. International affective picture system (IAPS): technical manual and affective ratings. [Google Scholar]
- Leung M-K, Lee T. Minneapolis, MN: Organization for Human Brain mapping; 2012. Right precentral gyrus and cognitive reappraisal in Chinese middle-aged men. Poster 497. [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53(6):502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Niki K, Ding ZG, Luo YJ. Precuneus contributes to attentive control of finger movement. Acta Pharmacologica Sinica. 2004;25(5):637–43. [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Akiyama T, Tanji J, Mushiake H. Neuronal activity in the primate dorsomedial prefrontal cortex contributes to strategic selection of response tactics. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(12):4633–8. doi: 10.1073/pnas.1119971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. The Journal of Neuroscience. 2004;24(21):4912–7. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Current Biology. 2005;15(2):122–8. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson JE, Lundh LG, Faghihi S, Roth-Andersson G. The enhancement of beneficial effects following audio feedback by cognitive preparation in the treatment of social anxiety: a single-session experiment. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(4):497–503. doi: 10.1016/j.jbtep.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pastor-Bernier A, Tremblay E, Cisek P. Dorsal premotor cortex is involved in switching motor plans. Frontiers in Neuroengineering. 2012;5:5. doi: 10.3389/fneng.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Rea M, Kullmann S, Veit R, et al. Effects of aversive stimuli on prospective memory. An event-related fMRI study. PLoS One. 2011;6(10):e26290. doi: 10.1371/journal.pone.0026290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. Journal of Neurophysiology. 2003;90(3):1766–89. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–10. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13(6):829–43. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neuroscience Letters. 2002;325(3):203–6. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology. 2007;98(3):1374–9. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Current Opinion of Neurobiology. 2007;17(2):228–33. doi: 10.1016/j.conb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14(6):913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping. 2011;32(11):1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends in Cognitive Sciences. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44(8):1388–97. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43(4):479–96. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Theoret H, Kobayashi M, Merabet L, Wagner T, Tormos JM, Pascual-Leone A. Modulation of right motor cortex excitability without awareness following presentation of masked self-images. Brain Research. Cognitive Brain Research. 2004;20(1):54–7. doi: 10.1016/j.cogbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintrob DL, Saling MM, Berkovic SF, Berlangieri SU, Reutens DC. Verbal memory in left temporal lobe epilepsy: evidence for task-related localization. Annals of Neurology. 2002;51(4):442–7. doi: 10.1002/ana.10133. [DOI] [PubMed] [Google Scholar]
- West R, Ross-Munroe K. Neural correlates of the formation and realization of delayed intentions. Cognitive, Affective and Behavioral Neuroscience. 2002;2(2):162–73. doi: 10.3758/cabn.2.2.162. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schiltz K, Boehler CN, Duzel E. Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia. 2008;46(4):1000–8. doi: 10.1016/j.neuropsychologia.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow Metabolism. 1992;12(6):900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]