Abstract
Background
Survival after out-of-hospital cardiac arrest (OHCA) remains poor. Acute coronary obstruction is a major cause of OHCA. We hypothesize that early coronary reperfusion will improve 24h-survival and neurological outcomes.
Methods
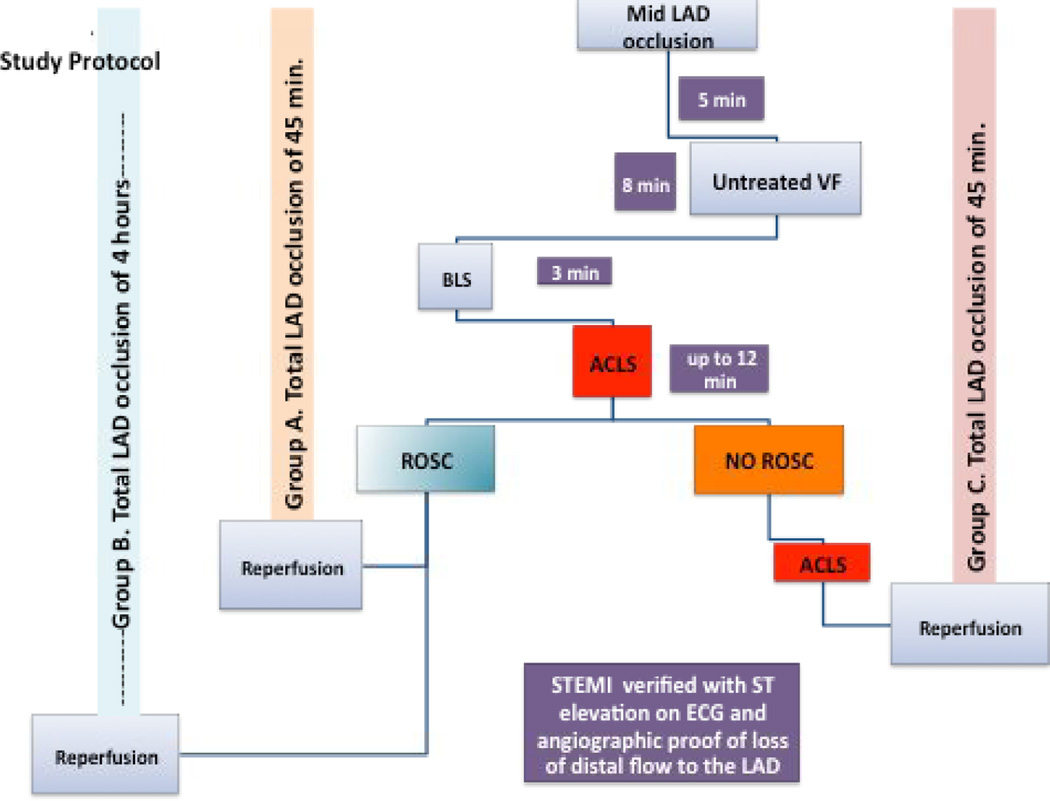
Total occlusion of the mid LAD was induced by balloon inflation in 27 pigs. After 5 minutes, VF was induced and left untreated for 8 minutes. If return of spontaneous circulation (ROSC) was achieved within 15 minutes (21/27 animals) of cardiopulmonary resuscitation (CPR), animals were randomized to a total of either 45 minutes (group A) or 4 hours (group B) of LAD occlusion. Animals without ROSC after 15 minutes of CPR were classified as refractory VF (group C). In those pigs, CPR was continued up to 45 minutes of total LAD occlusion at which point reperfusion was achieved. CPR was continued until ROSC or another 10 minutes of CPR had been performed. Primary endpoints for groups A and B were 24-hour survival and cerebral performance category (CPC). Primary endpoint for group C was ROSC before or after reperfusion.
Results
Early compared to late reperfusion improved survival (10/11 versus 4/10, p= 0.02), mean CPC (1.4±0.7 versus 2.5±0.6, p= 0.017), LVEF (43±13 versus 32±9%, p=0.01), troponin I (37±28 versus 99±12, p=0,005) and CK-MB (11 ±4 versus 20.1±5, p=0.031) at 24-hr after ROSC. ROSC was achieved in 4/6 animals only after reperfusion in group C.
Conclusions
Early reperfusion after ischemic cardiac arrest improved 24h survival rate and neurological function. In animals with refractory VF, reperfusion was necessary to achieve ROSC.
Keywords: Early coronary revascularization, cerebral performance category (CPC) score, 24h survival rate, return of spontaneous circulation (ROSC)
Introduction
Sudden out-of-hospital cardiac arrest (OHCA) remains a serious public health problem associated with very poor hospital survival rates. Steady but small progress has been made over the last 50 years and survival rates from cardiopulmonary resuscitation (CPR) have remained relatively stable over the past half century.1 In the best emergency medical systems in the world overall survival does not exceed 15% for all presenting rhythms. Under optimal conditions patients presenting with a shockable rhythm have >50% chance of survival with good neurological outcome.2,3 Although less than 30% of patients presenting with out of hospital cardiac arrest have a shockable rhythm, this subset represents 90% of the patients that leave the hospital with good neurological function.4,5,6
Acute coronary obstruction is a major cause of cardiac arrest.7 A large proportion of patients that present with shockable rhythms such as ventricular fibrillation and tachycardia (VF/VT) have significant coronary artery disease.8,9,10 Although early primary percutaneous intervention and revascularization has been associated with improved odds for survival in observational studies, there are no randomized data in humans to support the practice, both for STEMI or NSTEMI patients.7
We hypothesize that early revascularization will improve post-resuscitation hemodynamic stability and lead to improved 24-hour survival with good neurological function. We further hypothesize that in refractory ventricular fibrillation due to an occluded coronary artery the only way to achieve return to spontaneous circulation is to successfully perform coronary revascularization and establish reperfusion. In order to answer those two questions, we utilized a highly reproducible left anterior descending coronary artery myocardial infarction porcine model of cardiac arrest and resuscitation.11
MATERIALS AND METHODS
All studies were performed by a qualified, experienced research team in 27 Yorkshire female farm pigs weighing 36 ± 4 kg. The protocol was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation. All animals received treatment and care in compliance with the 1996 Guide for the Care and Use of Laboratory Animals by the National Research Council in accordance with the United States Department of Agriculture Animal Welfare Act, Public Health Service Policy, and the American Association for Accreditation of Laboratory Animal Care.
Preparatory phase
The anesthesia, surgical preparation, data monitoring, and recording procedures used in this study have been described previously.12 Briefly, we employed aseptic surgical conditions, using initial sedation with intramuscular ketamine (7 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) followed by inhaled isoflurane at a dose of 0.8–1.2%. Pigs were intubated with a size 7.0 endotracheal tube. The animal’s bladder temperature was maintained at 37.5 ± 0.5°C with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN). Central aortic blood pressure was recorded continuously with a micromanometer-tipped (Mikro-Tip Transducer, Millar Instruments, Houston, TX) catheter placed at the beginning of the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. All animals received an intravenous heparin bolus (100 units/kg) and 500 units of heparin every hour until surgical repair was completed. An ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, NY) was placed to the right internal carotid artery to record blood flow (mL/min). The animals were then ventilated with room air, using a volume-control ventilator (Narcomed, Telford, PA), with a tidal volume of 10 mL/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mmHg and PaO2 of 80 mmHg (blood oxygen saturation >95%), as measured from arterial blood (Gem 3000, Instrumentation Laboratory, Lexington, MA) to adjust the ventilator as needed. Surface electrocardiographic tracings were continuously recorded. All data were recorded with a digital recording system (BIOPAC MP 150, BIOPAC Systems, CA). End-tidal CO2 (ETCO2), tidal volume, minute ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (CO2SMO Novametrix Medical Systems, Wallingford, CT).
Measurements and recording
Thoracic aortic pressure, right atrial pressure, ETCO2, and carotid blood flow (CBF) were continuously recorded. Coronary perfusion pressure during CPR was calculated from the mean arithmetic difference between right atrial pressure and aortic pressure during the decompression phase. Carotid artery blood flow was reported in mL/sec.
Coronary artery occlusion methodology 11
Under fluoroscopic guidance, a 6F short AL 0.75 guide catheter was used to engage the left main coronary artery. Using a nonhydrophilic 0.014-in guidewire, we advanced an over-the-wire 3.0 to 3.5 x 12-mm balloon into the left anterior descending coronary artery (LAD) and placed it after the first diagonal regardless of the size of the vessel or distal territory. Continuous limb and 2 precordial ECG tracings were recorded. The average time from the guide catheter engagement of the left main to balloon inflation was 6+1.5 minutes and not different between groups.
Experimental protocol
After the surgical preparation was complete, oxygen saturation on room air was >95% and ETCO2 was stable between 35 and 42 mmHg for 5 minutes. Total LAD occlusion, as described above, was induced via balloon inflation. After 5 minutes, ventricular fibrillation (VF) was induced by delivering intracardiac current via a temporary pacing wire (St. Jude Medical, Minnetonka, MN) and was left untreated for 8 minutes. The ventilator was disconnected from the endotracheal tube. All but one animal had electrical induction of VF. One animal in group A had spontaneous VF at minute 4 of LAD occlusion.
Active compression decompression (ACD) CPR was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described.13 An impedance threshold device (ITD-16) (ResQPOD, Advanced Circulatory Systems, Inc, Minneapolis, MN) was used to provide ACD+ITD CPR.4
During CPR, uninterrupted chest compressions were performed at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter were provided. Asynchronous positive-pressure ventilations were delivered with room air (FiO2 of 0.21) with a manual resuscitator bag. The tidal volume was maintained at ~10mL/kg and the respiratory rate was 10 breaths/min.
Following 8 minutes of untreated VF and 3 minutes of CPR (simulating basic life support), epinephrine was administered in a 0.5 mg (~15µg/kg) bolus at minute 3 of CPR. Cardioversion shocks were delivered with 200J every 3 minutes and advanced cardiac life support (ACLS) was continued until either return of spontaneous circulation (ROSC) was achieved or 15 minutes of CPR were performed unsuccessfully.
If ROSC was achieved within 15 minutes, the animals were randomized to either 45 minutes (group A) or 4 hours (group B) of total mid-LAD occlusion before reperfusion. In both groups, the primary end-points were 24-hour left ventricular ejection fraction (LVEF) and survival with good neurological function. We chose 45 minutes of occlusion since in our porcine model that period of time gives on average about 50% of infarction size to area at risk. The infarction size expressed as a percentage of the area at risk is not related to the site of occlusion in this model.14 For longer duration of occlusion (e.g. >90 minutes) the ratio of infarction to area at risk approaches >70% and reperfusion offers no or minimal benefit.11 We chose this time in order to simulate early access (<4 hours human equivalent) to the catheterization laboratory for patients with ST elevation myocardial infarction when benefits from revascularization are clinically observed.15,16,17
If ROSC was not achieved within 15 minutes, pigs were included in the refractory VF (group C) and ACLS was continued up to a total of 45 minutes of LAD occlusion. Defibrillation and epinephrine as well as amiodarone were given per AHA 2010 guidelines.18,19 Amiodarone (20 mg) was administered bolus intravenously after the third unsuccessful shock cycle. if VF was refractory another bolus was given within the next 10 minutes. If after 45 minutes of LAD occlusion ROSC was not obtained, reperfusion was achieved via LAD balloon deflation and removal (simulating primary coronary intervention with on-going CPR) and efforts continued until ROSC was achieved or another 10 minutes of CPR were performed. If animals were successfully resuscitated they were observed for one hour and then were sacrificed. The end-point in these animals was successful ROSC (Figure 1).
Figure 1.
Timeline of experimental protocol. ACLS: advanced cardiac life support; BLS: basic life Support; ECG: electrocardiogram; LAD: left anterior descending; ROSC: return of spontaneous circulation; STEMI: ST elevation myocardial infarction; VF: ventricular fibrillation.
Post-ROSC Care
After ROSC was achieved, animals were connected to the mechanical ventilator. Supplemental oxygen was added only if arterial saturation was lower than 90% and to the minimum amount required to achieve saturations >92%. Animals were observed under general anesthesia with isoflurane until hemodynamically stable. Hemodynamic stability was defined as a mean aortic pressure >55 mmHg without pharmacologic support for 10 minutes and normalization of ETCO2 and acidosis. Epinephrine post ROSC was given as small boluses of 0.1 to 0.2 mg when needed. No dose was given for 20 minutes prior to the echocardiographic evaluation. If hypotension was present, a bolus was given after the echo was performed. No drips were given. If pH was lower than 7.2, 50–100 mEq of NaHCO3 were given intravenously. This was repeated as needed for significant acidosis. At that point, vascular repair of the internal jugular and the left common femoral artery were then performed. Arterial blood gases were obtained at baseline, at 5 mins of CPR, and every 30 mins following ROSC. Animals were kept normothermic for the duration of the protocol and up until their return to the runs.
Survivors were given intramuscular analgesic injections of nonsteroidal anti-inflammatory medication as previously described and had free access to water and food.12 There was no other post-ROSC medical care provided after the vascular repair. Animals were returned to their runs and were observed every 2 hrs for the first 6 hrs for signs of distress or accelerated deterioration of their function. If animals met predetermined criteria or if the veterinarian judged that they were in severe distress they were euthanized per Institutional Animal Care and Use Committee protocol.
Neurological assessment
Twenty-four hours after ROSC, a certified veterinarian, blinded to the intervention, assessed the pigs’ neurologic function based on a cerebral performance category (CPC) scoring system modified for pigs. The veterinarian used clinical signs, such as response to opening the cage door, response to noxious stimuli if unresponsive, response to trying to lift the pig, whether the animal could stand, move all four limbs, walk, eat, urinate, defecate, and respond appropriately to the presence of a person walking into the cage. The following scoring system was used: 1 normal; 2 slightly disabled; 3 severely disabled but conscious; 4 vegetative state; a 5 was given to animals that died in the lab due to unachievable ROSC or died in the cage following ROSC.12 Except the veterinarian, post-resuscitation care was not blinded since the same team performed CPR and provided post-ROSC care.
Echocardiographic evaluation of left ventricular function
A transthoracic echocardiogram was obtained on all survivors 1, 4, and 24 hours post ROSC. Images were obtained from the right parasternal window, which provides similar views as the long and short parasternal windows in humans.20 Ejection fraction was assessed using Simpson’s method of volumetric analysis by an independent clinical echocardiographer blinded to the treatments.21 Before echocardiographic evaluation, any inotropic support was stopped for at least 20 mins and, if needed, was restarted immediately after the echocardiographic evaluation.
Statistical analysis
Values were expressed as mean ± standard deviation. Baseline data, hemodynamics and blood gases during CPR and post resuscitation between groups A and B were compared using a t-test for normally distributed continuous variables, and the Wilcoxon rank sum test for continuous variables that were not normally distributed. Lilliefors test of normality was used to determine if continuous variables were normally distributed or not. Survival outcomes were analyzed with Fisher exact test. Results were considered to be statistically significant if p < 0.05.
Results
Baseline and intra CPR hemodynamics and blood gases were not different between groups. Duration of CPR during first ROSC was similar in the two groups: 7.5±3 versus 8.1±2.6 minutes for groups A and B, respectively. Hemodynamic differences with aortic systolic and diastolic pressure were seen between groups at minute 30 post ROSC due to the higher need of epinephrine due to recurrent CPR efforts in group B. (Table 1) Twenty one out of 27 animals were successfully resuscitated within 15 minutes.
Table 1.
Comparison of ABGs and hemodynamics in groups A and B before, 5 and 30 minutes after ROSC.
| ABGs and Hemodynamics | Early reperfusion | Delayed Reperfusion | p value | |
|---|---|---|---|---|
| pH | Baseline | 7.43±0.04 | 7.44±0.04 | p=0.42 |
| 5 min after ROSC | 7.24±0.09 | 7.28±0.09 | p=0.34 | |
| 30 min after ROSC | 7.29±0.07 | 7.27±0.05 | p=0.44 | |
| pCO2 (mmHg) | Baseline | 42±5 | 40±5 | p=0.49 |
| 5 min after ROSC | 53±19 | 50±13 | p=0.69 | |
| 30 min after ROSC | 44±7 | 45±4 | p=0.76 | |
| pO2 (mmHg) | Baseline | 206.3 ± 42 | 261.0 ± 52 | p=0.43 |
| 5 min after ROSC | 153 ± 33 | 152.2 ± 38 | p=0.98 | |
| 30 min after ROSC | 142.1 ± 26 | 161.4 ± 27 | p=0.62 | |
| Lactate (mmol/l) | Baseline | 1.2±0.71 | 0.8±0.3 | p=0.08 |
| 5 min after ROSC | 7.1±2.1 | 6.1±1.4 | p=0.24 | |
| 30 min after ROSC | 6.8±1.8 | 6.8±1.6 | p=0.99 | |
| HCO3- (mEq/l) | Baseline | 28±1.7 | 27.4±1.7 | p=0.56 |
| 5 min after ROSC | 21.7±4 | 22±5 | p=0.92 | |
| 30 min after ROSC | 21±2 | 20.6±2 | p=0.73 | |
| Base Deficit (mEq/l) | Baseline | 3.6±1.4 | 3.3±1.4 | p=0.65 |
| 5 min after ROSC | −5.7±4 | −5.6±5 | p=0.93 | |
| 30 min after ROSC | −5.6±3 | −6.3±3 | p=0.64 | |
| SBP (mmHg) | Baseline | 94.6 ± 3 | 100.4 ± 6 | p=0.3 |
| 2–4th min of CPR (mean) | 72±12 | 68±14 | p=0.3 | |
| 5 min after ROSC | 108 ± 10 | 101± 4 | p=0.52 | |
| 30 min after ROSC | 71 ± 2 | 91 ± 6 | p=0.009 | |
| DBP (mmHg) | Baseline | 53± 3 | 67± 4 | p=0.02 |
| 2–4th min of CPR (mean) | 32±8.6 | 29± 11 | p=0.32 | |
| 5 min after ROSC | 75± 8 | 66 ± 4 | p=0.37 | |
| 30 min after ROSC | 48± 2 | 65± 4 | p=0.003 | |
| CPP (mmHg) | Baseline | 48± 2 | 57 ± 6 | p=0.2 |
| 2–4th min of CPR (mean) | 28± 7 | 26± 9 | P=0.4 | |
| 5 min after ROSC | 78 ± 10 | 61 ± 6 | p=0.20 | |
| 30 min after ROSC | 48± 2 | 61.18 ± 4 | p=0.02 | |
| ETCO2 (mmHg) | Baseline | 37±1.6 | 36±1.5 | p=0.4 |
| 2–4th min of CPR (mean) | 22± 8 | 23± 6.5 | p=0.5 | |
| 5 min after ROSC | 43± 2.8 | 45± 2.7 | p=0.42 | |
| 30 min after ROSC | 36± 2.1 | 36± 2 | p=0.8 | |
| Carotid Blood flow (ml/min) | Baseline | 152± 30 | 174± 18 | p=0.5 |
| 2–4th min of CPR (mean) | 45±31 | 58±17 | p=0.2 | |
| 5 min after ROSC | 198±32 | 203±20 | p=0.9 | |
| 30 min after ROSC | 179±29 | 156±7 | p=0.48 | |
Legend: ABGs and hemodynamics in groups with early and delayed reperfusion are shown at baseline, 5 and 30 minutes after ROSC. All values are expressed as mean ± SD.
Statistically significant difference with p < 0,05.
CPP: central pulmonary pressure; DBP: diastolic blood pressure; ETCO2: end-tidal CO2; SBP: systolic blood pressure.
ECG findings post ROSC
Immediately after ROSC, eight of the 21 (38%) animals demonstrated ST elevation in the precordial lead (V3-V4 territory) of the surface ECG and 13 /21 (62%) did not. (Figure 2) Animals that did not demonstrate ST elevation after ROSC, had non-specific ST-T wave changes and ST depression in combination with wide QRS complexes. ST elevation was present again in all animals after 15±8 minutes post ROSC.
Figure 2.
Examples of the post resuscitation ECG showing presence (a) and absence of ST elevation in a precordial lead (V3 equivalent) (b) accompanied by their coronary angiograms showing proximal LAD occlusion and Left Circumflex with patent flow. The coronary wires are also visible at 7 o’clock.
Early versus late revascularization (Groups A and B)
24 hour survival
Early reperfusion (group A) significantly improved 24 hour survival rate compared to delayed reperfusion after completion of the myocardial infarction (Group B) (10/11 versus 4/10, p= 0.02)
24-hour neurological function
Early reperfusion (group A) significantly improved mean CPC score at 24 hours post resuscitation compared to delayed reperfusion (Group B) (1.4 ±0.7 versus 2.5± 0.6, p= 0.017)
Post resuscitation stability
Early revascularization (group A) led to significantly fewer episodes of recurrent VF/VT and CPR recurrence. Total epinephrine and amiodarone doses were similar in both groups for achievement of ROSC. (Table 2)
Table 2.
Stability parameters in group A and B.
| Stability parameters | Early Reperfusion |
Delayed Reperfusion |
p-value |
|---|---|---|---|
| Defibrillation Shocks until Reperfusion | 8.25±4 | 19.7±12 | 0.02 |
| Times of CPR needed after ROSC | 2.85±1.2 | 5.3±2.5 | 0.04 |
| Epinephrine total dose to ROSC (mg) | 0.8±0.45 | 1.1±0.6 | 0.4 |
| Post ROSC Epinephrine dose( mg) | 0.3±0.2 | 0.8±0.6 | 0.02 |
| Amiodarone total (mg) | 25±9 | 30±11.5 | 0.33 |
| Total 0.9N (L) infused | 2.23±0.7 | 2.44±0.63 | 0.44 |
Legend: Comparison of haemodynamic and electrical stability parameters between group with early and delayed reperfusion. All values are expressed as mean ± SD.
Statistically significant difference with p < 0,05.
CPR: cardiopulmonary resuscitation; ROSC: return of spontaneous circulation.
Cardiac function and biomarkers of injury
Echocardiographic evaluation at 1 hour, 4 hours and 24 hours revealed that left ventricular ejection fraction was similar at 1 and 4 hours with a mean EF for groups A and B of 32±8% and 37±12% versus 35±11% and 38±9 respectively, p>0.5). There was a significant difference at 24 hours with improvement in EF for groups A compared to group B (43±13 versus 32±9% respectively, p=0.01). All animals had anterior, apical and septal hypo/akinesis.
Cardiac troponin I and CK-MB were not different between groups A and B at 4 hours but at 24 hours survivors from group B had significantly higher levels of biomarkers. Liver enzymes and kidney function were not significantly different at 4 hours and 24 hours between groups. (Table 3)
Table 3.
Biomarkers in groups with early and delayed reperfusion at 4 and 24 hours after ROSC.
| Biomarkers | Early Reperfusion |
Delayed Reperfusion |
p-value |
|---|---|---|---|
| Troponin I (4-H) | 46±20 | 74±32 | 0.09 |
| Troponin I (24-H) | 37±28 | 99±12 | 0.005 |
| CK-MB (4-H) | 44±37 | 62±68 | 0.57 |
| CK-MB (24-H) | 11±4 | 20.1±5 | 0.031 |
| Creatinine (4-H) | 1.19±0.5 | 1.11±0.26 | 0.73 |
| Creatinine (24-H) | 1.08±0.27 | 1.1±0.27 | 0.6 |
| AST (4-H) | 196±125 | 375±302 | 0.21 |
| AST (24-H) | 303±179 | 273±153 | 0.78 |
| ALT (4-H) | 53±16 | 57±23 | 0.78 |
| ALT (24-H) | 99.3±36 | 103±25 | 0.85 |
Legend: Myocardial necrosis, liver and kidney biomarkers in groups with early and delayed reperfusion at 4 and 24 hours after ROSC. All values are expressed as mean ± SD.
Bold fonts: Statistically significant difference with p < 0,05.
ALT: alanine transaminase; AST; aspartate transaminase; CK-MB; creatine kinase-MB.
Refractory VF (Group C)
In group C, animals received CPR for a total of 30 minutes and at that time point, LAD occlusion had been present for a total of ~45 min. Per protocol the balloon was deflated and coronary flow was reestablished and verified with coronary angiography to eliminate the possibility of dissection or no flow. All 6 animals had TIMI 3 flow with reperfusion while performing CPR. After reperfusion 4/6 animals had successful return of circulation and survived one hour. Before reperfusion the animals received an average of 22±8 DC shocks unsuccessfully. After reperfusion the animals that were successfully resuscitated had an average 3±2 DC shocks. Echocardiographic evaluation at one hour post ROSC showed moderate to severe LV dysfunction with a mean ejection fraction of 33±8% and anterior, apical, and septal hypo/akinesis. The two animals that were not resuscitated developed pulseless electrical activity after reperfusion and systolic function did not recover.
Discussion
In this animal model of ischemic cardiac arrest an early reperfusion strategy significantly improved survival rates at 24 hours with good neurological function compared to a delayed approach of reperfusion allowing for completion of the myocardial infarction. Early reperfusion resulted in fewer re-fibrillations and fewer episodes where CPR was needed again.
Second, the finding of better cardiac function after early reperfusion, as reflected by ejection fraction and cardiac biomarkers of injury at 24 hours, is consistent with previous experience.22,23 Our study shows that even in the setting of cardiac arrest, whenever a reversible cause of cardiac arrest can be identified and treated, outcomes may improve.
Furthermore, we found that a more aggressive approach during refractory VF due to ischemia could potentially improve ROSC rates. Only after reperfusion was achieved, (after a total of 45 minutes of LAD occlusion) ROSC was achieved with a few shocks in 4/6 animals. This model of cardiac arrest represents a patient population that currently faces 100% mortality even if they are witnessed and receive bystander CPR since the majority of CPR efforts are stopped in the field within 30 minutes. In the absence of randomized clinical trial data, our study provides evidence that early mobilization of patients with witnessed refractory VF should be considered. Those patients should be considered for immediate access to the cardiac catheterization laboratories with on going CPR.
Acute coronary artery occlusion is the cause of cardiac arrest in 50–70% of post VF/VT arrest survivors 7,24 and coronary artery thrombi or atherosclerotic plaque fissuring have been identified in 95% of patients who die from sudden cardiac death due to a presumed cardiac etiology.25 Our study findings of better outcomes with prompt coronary reperfusion give credence to the notion that rapid access to the catheterization laboratory with the intention to perform PCI may offer a clinical benefit to OHCA survivors presenting with a shockable rhythm.
It is widely accepted based on registry data that survival rates in OHCA patients with ROSC improve with early coronary angiography and percutaneous coronary intervention (PCI).7,24 Nevertheless, to our knowledge, no randomized studies exist on the use of acute emergency coronary angiography and PCI following OHCA. Evidence in favor of immediate angiogram and PCI amongst survivors of OHCA is limited to single-center, non-randomized, observational studies, that have shown improved patient outcomes compared to historical controls. Recent AHA/ACC and ERC guidelines recommend immediate coronary angiography with PCI if indicated for patients successfully resuscitated after OHCA with presumed cardiac cause. The guidelines state: “it may be reasonable to include cardiac catheterization in a standardized post-cardiac-arrest protocol as part of an overall strategy to improve neurologically intact survival in this patient group”.26
It appears that the post resuscitation period is critical for brain recovery and episodes of hypotension, hypoxia and metabolic derangement are poorly tolerated and lead to worse outcomes.27–31 Early reperfusion resulted in less hemodynamic and electrical instability after ROSC and better survival, improved neurological outcomes.
Although there is absence of randomized clinical trial data supporting the concept that early revascularization improves the neurologic status of the OHCA survivors from VF/VT, recently published data show high rates (80–92%) 4,5,32 of full neurological recovery in this patient population. On the other hand, other investigators concluded that the more aggressive approach to post-resuscitation care does not improve neurologic outcome.33 Our data, support the notion that early identification of a reversible cause and early reperfusion of an occluded artery leads to better cardiac and cerebral outcomes due to improvement of post resuscitation hemodynamics and possibly of the humoral substrate.
The present study has several limitations. First, since it is an animal study, extrapolation to humans is reasonable, but not proven. The differences in the arrhythmogenicity and in collateral flow between pigs and humans can alter the temporal window of opportunity for revascularization, which in humans can be extended out in time compared to our model. It appears only logical though that there should be no reason to postpone coronary angiography and possible angioplasty if the probability of coronary occlusion is high. Besides, according to our results, which are in accordance with the existing human data,7 the early post resuscitation ECG has low negative prognostic value as far as the exclusion of a myocardial infarction as the cause of a cardiac arrest. Second, neurological outcomes were measured only 24 hours later and we cannot exclude the potential for later neurological improvement in the late reperfusion group. That possibility could not alter the difference in overall survival between groups observed in our study nonetheless. Third, all animals had an acute myocardial infarction before VF induction. A significant proportion of patients (30–40%) that present with shockable rhythms do not have coronary obstruction as the main reason for their arrest and it is possible that coronary angiography could be harmful for this population, although clinical observational studies do not support this position.7,32,34 Finally, we cannot comment of the neurological and survival status of the refractory VF group since we only observed them for one hour. It is highly probable that those animals would have suffered heavy neurological insults due to the prolonged CPR efforts.
Conclusions
Early reperfusion therapy improves twenty-four hour survival rates and neurological status in a porcine model of VF cardiac arrest due to acute myocardial infarction and resuscitation. In animals with refractory VF, immediate coronary artery reperfusion appears to be the only option leading to successful ROSC.
Acknowledgments
The Cardiovascular Division at the University of Minnesota funded the study.
Abbreviations
- ACD
active compression decompression
- ACLS
advanced cardiac life support
- CK-MB
creatine kinase-MB
- CPC
cerebral performance category
- CPR
cardiopulmonary resuscitation
- ITD
impendence threshold device
- OHCA
out of hospital cardiac arrest
- ROSC
return of spontaneous circulation
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
There are no conflicts from any of the authors in relation to this manuscript.
References
- 1.Eisenberg MS, Horwood BT, Cummins RO, Reynolds-Haertle R, Hearne TR. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990;19:179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Aufderheide TP, Eigel B, et al. American Heart Association Emergency Cardiovascular Care Committee; Council on Arteriosclerosis, Thrombosis, and Vascular Biology; Council on Cardiopulmonary, Critical Care, Perioperative, and Resuscitation; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Advocacy Committee Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 3.Aufderheide TP, Yannopoulos D, Lick CJ, et al. Implemeting the 2005 American Heart Association Guidelines improves outcomes after out-of-hospital cardiac arrest. Heart Rhythm. 2010;7:1357–1362. doi: 10.1016/j.hrthm.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aufderheide TP, Nichol G, Rea TD, et al. Resuscitation Outcomes Consortium (ROC) Investigators. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med. 2011;365:798–806. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kragholm K, Skovmoeller M, Christensen AL, Fonager K, Tilsted HH, Kirkeggard H, DE Haas I, Rasmussen BS. Employment status 1 year after out-of-hospital cardiac arrest in comatose patients treated with therapeutic hypothermia. Acta Anaesthesiol Scand. 2013 Aug;57(7):936–943. doi: 10.1111/aas.12142. [DOI] [PubMed] [Google Scholar]
- 7.Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 8.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CA, Yarzebski J, Goldberg RJ, Lessard D, Gore JM, Dalen JE. Changes over time in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: perspectives from the Worcester Heart Attack Study. Am Heart J. 2000;139:1014–1021. doi: 10.1067/mhj.2000.106160. [DOI] [PubMed] [Google Scholar]
- 10.Huikuri HV, Castellanos A, Myerburg R. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos D, Matsuura T, McKnite S, et al. No assisted ventilation cardiopulmonary resuscitation and 24-h neurological outcomes in a porcine model of cardiac arrest. Crit Care Med. 2010;38:254–260. doi: 10.1097/CCM.0b013e3181b42f6c. [DOI] [PubMed] [Google Scholar]
- 12.Yannopoulos D, Zviman M, Castro V, et al. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation. 2009;120:1426–1435. doi: 10.1161/CIRCULATIONAHA.109.848424. [DOI] [PubMed] [Google Scholar]
- 13.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89:684–693. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Lyons JK, Yeung AC, Ikeno F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv. 2008;71(1):100–107. doi: 10.1002/ccd.21329. [DOI] [PubMed] [Google Scholar]
- 15.Van de WF, Bax J, Betriu A, et al. ESC Committee for Practice Guidelines (CPG). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 16.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54(23):2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Gunnar RM, Passamani ER, Bourdillon PD, et al. Guidelines for the early management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee to Develop Guidelines for the Early Management of Patients with Acute Myocardial Infarction) J Am Coll Cardiol. 1990;16(2):249–292. doi: 10.1016/0735-1097(90)90575-a. [DOI] [PubMed] [Google Scholar]
- 18.Caron MF, Kluger J, White CM. Amiodarone in the new AHA guidelines for ventricular tachyarrhythmias. Ann Pharmacother. 2001;35:1248–1254. doi: 10.1345/aph.10407. [DOI] [PubMed] [Google Scholar]
- 19.Ong ME, Pellis T, Link MS. The use of antiarrhythmic drugs for adult cardiac arrest: a systematic review. Resuscitation. 2011;82:665–670. doi: 10.1016/j.resuscitation.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Marino BS, Yannopoulos D, Sigurdsson G, et al. Spontaneous breathing through an inspiratory impedance threshold device augments cardiac index and stroke volume index in a pediatric porcine model of hemorrhagic hypovolemia. Crit Care Med. 2004;32(9 Suppl):S398–S405. doi: 10.1097/01.ccm.0000139950.39972.68. [DOI] [PubMed] [Google Scholar]
- 21.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 22.Zeymer U, Bauer T, Gersh BJ, et al. Beneficial effect of reperfusion therapy beyond the preservation of left ventricular ejection function in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol. 2011;21:177–180. doi: 10.1016/j.ijcard.2009.06.034. 146. [DOI] [PubMed] [Google Scholar]
- 23.Hallén J, Jensen JK, Fagerland MW, Jaffe AS, Atar D. Cardiac troponin I for the prediction of functional recovery and left ventricular remodelling following primary percutaneous coronary intervention for ST-elevation myocardial infarction. Heart. 2010;96:1892–1897. doi: 10.1136/hrt.2009.190819. [DOI] [PubMed] [Google Scholar]
- 24.Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) Registry. Circ Cardiovasc Interv. 2010;3:200–207. doi: 10.1161/CIRCINTERVENTIONS.109.913665. [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S787–S817. doi: 10.1161/CIRCULATIONAHA.110.971028. [DOI] [PubMed] [Google Scholar]
- 27.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 28.Jeung KW, Min YI, Heo T. Rapidly induced selective cerebral hypothermia using a cold carotid arterial flush during cardiac arrest in a dog model. Resuscitation. 2008;77:235–241. doi: 10.1016/j.resuscitation.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Zandbergen EG, de Haan RJ, Reitsma JB, Hijdra A. Survival and recovery of consciousness in anoxic-ischemic coma after cardiopulmonary resuscitation. Intensive Care Med. 2003;29:1911–1915. doi: 10.1007/s00134-003-1951-4. [DOI] [PubMed] [Google Scholar]
- 30.Krep H, Böttiger BW, Bock C, et al. Time course of circulatory and metabolic recovery of cat brain after cardiac arrest assessed by perfusion- and diffusion-weighted imaging and MR-spectroscopy. Resuscitation. 2003;58:337–348. doi: 10.1016/s0300-9572(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 31.Hao-Hui C. Dehydration therapy and hypotension in post- resuscitation cerebral oedema, and application of intra-ocular pressure measurement--a review of resuscitation work, part I. Resuscitation. 1980;8:195–209. doi: 10.1016/0300-9572(80)90013-1. [DOI] [PubMed] [Google Scholar]
- 32.Dumas F, White L, Stubbs BA, Cariou A, Rea TD. Long-term prognosis following resuscitation from out of hospital cardiac arrest: role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60(1):21–27. doi: 10.1016/j.jacc.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Nanjayya VB, Nayyar V. Immediate coronary angiogram in comatose survivors of out-of-hospital cardiac arrest-An Australian study. Resuscitation. 2012;83:699–704. doi: 10.1016/j.resuscitation.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds JC, Callaway CW, El Khoudary SR, Moore CG, Alvarez RJ, Rittenberger JC. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med. 2009;24(3):179–186. doi: 10.1177/0885066609332725. [DOI] [PMC free article] [PubMed] [Google Scholar]