Abstract
Objectives
Cirrhosis and hepatic encephalopathy (HE) can adversely affect survival, but their effect on socioeconomic and emotional burden on the family is not clear. The aim was to study the emotional and socioeconomic burden of cirrhosis and HE on patients and informal caregivers.
Methods
A cross-sectional study in two transplant centers (Veterans and University) of cirrhotic patients and their informal caregivers was performed. Demographics for patient/caregivers, model-for-end-stage liver disease (MELD) score, and cirrhosis complications were recorded. Patients underwent a cognitive battery, sociodemographic, and financial questionnaires. Caregivers were given the perceived caregiver burden (PCB; maximum = 155) and Zarit Burden Interview (ZBI)-Short Form (maximum = 48) and questionnaires for depression, anxiety, and social support.
Results
A total of 104 cirrhotics (70% men, 44% previous HE, median MELD 12, 49% veterans) and their caregivers (66% women, 77% married, relationship duration 32 ± 14 years) were included. Cirrhosis severely impacted the family unit with respect to work (only 56% employed), finances, and adherence. Those with previous HE had worse unemployment (87.5 vs. 19%, P = 0.0001) and financial status (85 vs. 61%, P = 0.019) and posed a higher caregiver burden; PCB (75 vs. 65, P = 0.019) and ZBI (16 vs. 11, P = 0.015) compared with others. Cognitive performance and MELD score were significantly correlated with employment and caregiver burden. Veterans and non-veterans were equally affected. On regression, depression score, MELD, and cognitive tests predicted both PCB and ZBI score.
Conclusions
Previous HE and cognitive dysfunction are associated with worse employment, financial status, and caregiver burden. Cirrhosis-related expenses impact the family unit's daily functioning and medical adherence. A multidisciplinary approach to address this burden is required.
Introduction
Chronic liver disease and cirrhosis are one of the leading causes of morbidity and mortality in the United States (1,2). While the impact of cirrhosis on the health-care system has been studied, the burden of cirrhosis on the family unit consisting of patients and their caregivers from a medical, emotional, and socioeconomic perspective needs to be investigated further. This is important because the adherence and transplant eligibility depend on the ability of the family to absorb the challenges of this medical condition. The financial burden specifically needs study because it can further add to the cost of health care and often goes unrecognized. Hepatic encephalopathy (HE), a condition associated with waxing and waning mental status, can be difficult to manage and can result in persistent cognitive deficits similar to Alzheimer's disease (3–5). The importance of burden on the families in Alzheimer's disease has been well evaluated and specific strategies to educate and counsel family members have been elaborated (6–10).
The current study was undertaken with the objective to study the emotional and financial burden of cirrhosis on the patients and informal caregivers. Our a priori hypothesis was the cirrhotic patients with previous HE would place a significantly higher burden than those without HE on their caregivers and the family finances.
Methods
Consecutive patients with cirrhosis and their informal caregivers who fit the inclusion and exclusion criteria were recruited from clinics at Virginia Commonwealth University Medical Center and McGuire VA Medical Center from November 2009 to October 2010, for a one-time study visit after written informed consent. Subjects were recruited from both transplant and nontransplant clinics at both institutions. Patients and caregivers were interviewed separately. The protocol was approved by the Institutional Review Boards at both sites.
Only patients with documented cirrhosis who were able to give informed consent, whose mini-mental status exam was > 25 and with a caregiver were included. We did not include patients with unresolved HE (defined as mini-mental status < 25) at the time of the study, as we needed to get an accurate personal perception of their situation. Patients with hepatocellular cancer, those who were on disability before the diagnosis of cirrhosis and those with other significant end-stage organ diseases (congestive heart failure, chronic obstructive pulmonary disease requiring oxygen, on dialysis, non-HE psychiatric issues requiring hospitalization, those on anti-psychotic and anti-seizure medications, and a current active cancer diagnosis) were excluded (11). We excluded all cancer, including hepatocellular cancer to improve our understanding of the non-cancer-related burden in the context of cirrhosis complications. We required caregivers to know the patients for at least 3 years.
Patient data
Demographics, comorbidities felt to impact burden (diabetes, depression, anxiety, chronic pain), medications used, cirrhosis details including etiology/duration, complications, and duration of first complication (HE, variceal bleeding, ascites, spontaneous bacterial peritonitis, hepato-renal, and hepato-pulmonary syndromes), HE treatment (lactulose and/or rifaximin; those on rifaximin were considered severe HE), and their current model-for-end-stage liver disease (MELD) score were collected (12,13). The patients then underwent the following:
Cognitive test battery consisting of seven tests: number connection-A/B (NCT-A/B), digit symbol (DST), serial dotting (SDT), line tracing errors (LTT errors) and time (LTT time), Block Design (BDT), and the inhibitory control (ICT; lures and targets) was administered as part of a recommended battery (14–17). A high score on NCT-A/B, ICT lures, SDT, LTT errors and time indicate worse cognition, whereas a low score on BDT, DST, and ICT targets indicates poor performance. Before initiating these measures, alertness and orientation were evaluated using the mini-mental status exam and Clinical Hepatic Encephalopathy Staging Scale (18).
MacArthur foundation sociodemographic questionnaire: inquires about family income, debts accrued and net worth with/without debts and the time that the family could continue their standard of living if debts were due (http://www.macses.ucsf.edu/research/socialenviron/sociodemographic.php).
Specific financial questions: Since the diagnosis of cirrhosis, patients were asked about their employment. The medical adherence questions pertained to the past 12 months. This questionnaire also asked about the specific sacrifices that the patients and their families had to make over the past 3 years due to their medical expenses only because of their cirrhosis treatment (Supplementary Material online).
Caregiver
They were asked about their relationship, comorbid conditions, and medications and then were administered:
Perceived Caregiver Burden (PCB) Scale: This is a 31-item questionnaire validated in Alzheimer's disease which has 5 domains: (i) impact on finances, (ii) impact on schedule, (iii) sense of abandonment, (iv) impact on health, and (v) sense of entrapment. A higher score reflects a higher burden (10).
Zarit Burden Interview (ZBI)-short form: this is a shorter 12-question burden instrument with a total score of 48 (indicates a higher burden) (19–21).
Beck Depression Inventory: a 21-item validated questionnaire assessing depression (22).
Beck Anxiety Inventory: is a validated 21-item anxiety questionnaire (23).
Interpersonal Support Evaluation List—Short Form (ISEL-SF): a 16-question instrument, is a validated questionnaire of social support for the caregiver in which respondents mark “probably true” or “probably false” to each of the questions. A higher score is worse (24).
Statistical analysis
Descriptive analysis was performed for all variables. Comparisons were made between patients with/without previous HE with respect to all patient and caregiver variables. Continuous variables for which the normality assumption was valid were analyzed using a one-way analysis of variance or t-test; if the normality assumption was not valid the variables were analyzed using the Kruskal–Wallis test. Discrete variables were analyzed using a χ2 test. Stepwise multiple linear regression was performed with Zarit and PCB as outcomes separately. The variables included in this model were based on previous assessments of burden in similar situations using the same questionnaires. These variables were the patient age, duration of cirrhosis, MELD score, alcoholic cirrhosis, duration of relationship, spouse or not, family income for the last 12 months, chronic pain medication use, depression, anxiety, and ISEL-SF scores. For the cognitive testing, the model was analyzed using two different methods: in one previous HE was used and in the other individual cognitive tests were used. This was done to understand the role of cognitive testing as a continuum as proposed recently. Data are presented as mean (standard deviation) unless otherwise specified.
Results
We approached 142 cirrhotic patients; 13 refused participation, 15 did not have any caregiver, and 10 had a caregiver who knew them for < 3 years. Therefore, we included 104 patients and caregivers. This included 73 (70%) men with a mean education of 13 (7) years. The predominant etiology of cirrhosis was chronic hepatitis C (44%), followed by non-alcoholic steato-hepatitis (23%), alcohol with hepatitis C (11%), alcohol alone (7%), and others (15%). Fifty-one (49%) were veterans, while the rest had private insurance. In all, 45% of patients were recruited at the time of liver transplant evaluation. The majority (83%) were Caucasians, while 12% were African-Americans and 5% were Hispanics. The mean duration of cirrhosis diagnosis before the study was 8 ± 4 years. The mean interval between the study and the first diagnosis of HE that required therapy was 23 ± 14 months in those with previous HE. Diabetes was present in 35% of patients, chronic pain requiring narcotics (11%), or nonsteroidal anti-inflammatory drugs (8%) at least twice a week in 15%, anxiety requiring treatment in 10%, and depression needing therapy in 23% of patients.
The median MELD score was 12 (range: 6–20) and 44% had previous HE (all were on lactulose while 23% had severe previous HE; both rifaximin and lactulose). In all, 15% had previous variceal bleeding, 6% spontaneous bacterial peritonitis, 6% hepato-renal syndrome, and 1% hepato-pulmonary syndrome. The mean age of caregivers was 51 (14) years and 66% were women. The mean relationship duration was 32 (14) years and 77% were married partners (remaining: unmarried partners (12%), children (6%), or close friends (5%)). Eighty-two percent shared living space for a mean of 21 (16) years. The majority of caregivers were not on any medications but 17% were on anti-depressants, 12% on nonsteroidal anti-inflammatory drugs, and 1% on narcotics.
Cognitive testing
Although the mental status determined by mini-mental status exam (27 (3)) and Clinical Hepatic Encephalopathy Staging Scale score (median 1) were normal, overall cognition was severely affected in all domains. The mean score on number connection (NCT)-A was 43 (18) s, NCT-B: 122 (68) s, digit symbol (DST): 47 (16), block design (BDT): 22 (13), line tracing (LTT) time: 109 (44) s, LTT errors: 37 (34), serial dotting (SDT): 74 (31) s, ICT lures: 15 (9) and ICT targets%: 91 (11).
Employment
After cirrhosis diagnosis, 57% of patients thought they were worse off regarding work (defined as decreasing hours and income or losing their job). In all, 63% felt that their financial status was significantly worse after the diagnosis of cirrhosis. Only 56% of patients had worked since being diagnosed with cirrhosis and of these 53% had to decrease their hours. Eighty-four percent thought continuing to work was very important and 57% thought their work was a welcome source of support. The people who were employed were equally white- or blue-collar workers. A significantly higher percentage of blue-collar workers thought that their financial status was worse compared with white-collar workers (45 vs. 0%, P = 0.008). Of the 44% of people who were not currently working (all had stopped working after their cirrhosis diagnosis), 71% thought it was important to continue working and wished they were able to work.
Financial and socioeconomic status
The median patient's individual income before tax for the past 12 months was between $16,000 and 24,999, whereas the combined family income was between $35,000 and 49,999. If patients lost all sources of income they could maintain their current standard of living for 3–6 months. After liquidation of all resources, the median finances available would be between $10,000 and 19,999 which would be reduced to $500–4,999 if current debt was subtracted from it.
Effect of cirrhosis on daily activities and medical adherence of the family
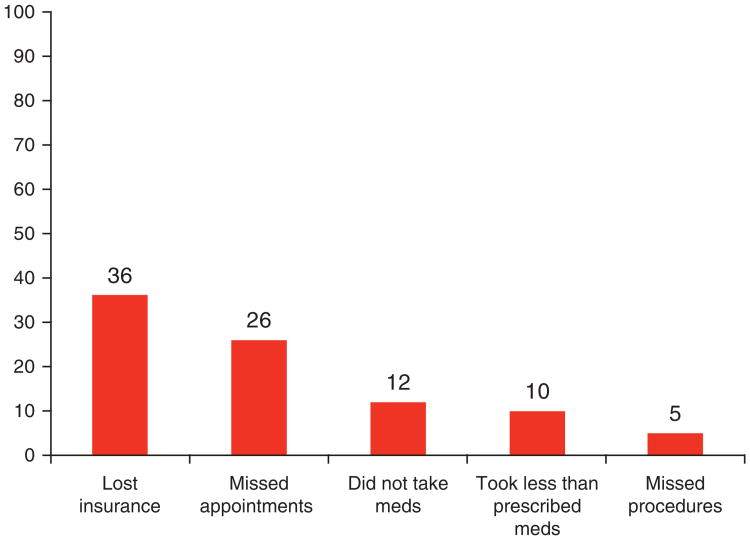
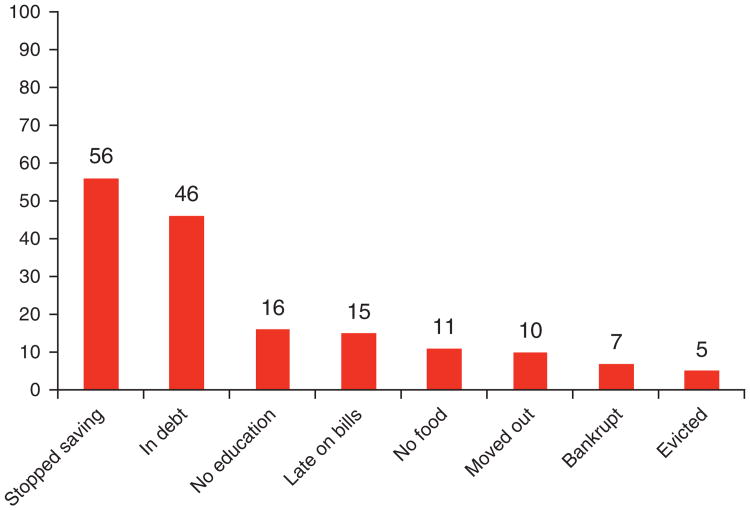
The effect of the financial burden on medical adherence after the diagnosis of cirrhosis compared with before they were diagnosed with cirrhosis is displayed in Figure 1. The specific limitations in the activities of the family were for the last 3 years due to cirrhosis-related medical expenses compared with what they were 3 years previous are shown in Figure 2. Cirrhosis and related expenses severely impacted each sphere of daily life for the family unit and this also had a negative impact on patient adherence.
Figure 1.
The impact of cirrhosis-related medical expenses on adherence with medical appointments, medications, and procedures for the past 12 months. The percentage of patients who responded yes is shown in the bar graphs. Meds, medication.
Figure 2.
The impact of cirrhosis-related medical expenses on daily activities of the affected family within the last 3 years. No education: a family member's education suffered; late on bills: late on utility bills, rent, and car payments; no food: had to skip food; moved out: needed to move to a cheaper place; bankrupt: had to declare personal bankruptcy. The percentage of patients who responded yes is shown in the bar graphs.
Impact of cirrhosis on caregivers
The mean PCB score was 71 (21) (median 65, range 31 – 134), while the mean ZBI score was 13.5 (8.6) (median 12, range 0–39); both scores were highly correlated (r = 0.67, P = 0.0001). The mean scores on the five domains of the PCB were: finances: 10.0 (3.6), abandonment: 14.3 (5.6), schedule: 14.0 (7.2), personal health: 16.7 (4.0), and entrapment: 15.1 (7.4).
Burden severity was significantly higher for spouses compared with other caregivers with respect to Zarit (16 vs. 11, P = 0.012) and PCB scores (75 vs. 66, P = 0.05). Within the PCB, a spouse had a significantly higher disruption of schedule (P = 0.05), personal health (P = 0.002), and entrapment (P = 0.004), but not financial (P = 0.21) nor abandonment (P = 0.16). There was no significant difference between caregivers of those with alcoholic cirrhosis compared with others with respect to PCB (P = 0.562) or Zarit Burden (P = 0.94).
Social support measured through the mean score on the ISEL-SF was 8 (2). Among the specific questions, < 50% of caregivers answered “true” to questions asking if they knew any person who could give them financial advice (21%) or objective feedback (25%), who could take them to the doctor if they were sick (12%), or do chores for them if they were not able to (18%).
Depression and anxiety in caregivers
The mean depression score was 10.4 (8.5); 18% of caregivers had mild, 5% had moderate, and 5% had severe depression. The mean anxiety score was 8.9 (10.0); 61% did not have anxiety, 22% had mild, 12% had moderate/severe, whereas 5% had severe anxiety. Both emotional inventory scores were correlated (r = 0.76, P = 0.0001).
The depression score was highly correlated with PCB (r = 0.47, P = 0.0001) and Zarit score (r = 0.61, P = 0.0001). The anxiety score was also significantly correlated with PCB (0.43, P = 0.0001) and Zarit score (r = 0.58, P = 0.0001). Both anxiety and depression scores were significantly correlated with all PCB domains apart from sense of abandonment. The depression score was significantly correlated with the amount of social support, i.e., ISEL-SF (r = 0.28, P = 0.006) but not the anxiety score.
Neither score was correlated with MELD, those with previous HE or other complications, cognition, or income. There was also no significant difference between caregivers of patients with alcoholic cirrhosis compared with those without with respect to depression (9.6 vs. 10.6, P = 0.65) or anxiety scores (9.9 vs. 8.9, P = 0.75).
Effect of previous HE
Previous HE adversely affected several aspects of financial status and burden (Table 1). Previous HE on impacted yearly family income (HE ($35,000–49,999) vs. without HE ($50,000–74,999), P = 0.17), cash after asset liquidation (HE ($5,000–9,999) vs. without HE ($20,000–49,999), P = 0.44), and cash minus debt (HE ($500) vs. without HE ($5,000–9,999), P = 0.25) but it did not reach statistical significance.
Table 1. Differences between patients with and without previous HE.
| Variable | Patients without previous HE (n = 58) |
Patients with previous HE (n = 46) |
P value |
|---|---|---|---|
| Age (years) | 58.6 (6.3) | 57.1 (7.0) | 0.272 |
| MELD score | 10.7 (5.0) | 15.5 (5.7) | 0.00001 |
| Diabetes mellitus | 35% | 37% | 0.84 |
| On chronic pain therapy (including narcotics and NSAIDs) | 16% | 15% | 0.83 |
| Depression requiring therapy | 32% | 14% | 0.08 |
| Anxiety requiring therapy | 10% | 10% | 1.0 |
| Currently working | 81% | 12.5% | 0.001 |
| Need to decrease hours | 39% | 71% | 0.017 |
| Worse off regarding job | 47% | 74% | 0.009 |
| Worse off regarding financial status | 61% | 85% | 0.019 |
| Median duration they would continue to live if all income stopped | 1–2 months | 7–12 months | 0.001 |
| Median longest period free of work | 21 days | 365 days | 0.035 |
| Debt from cirrhosis | 36% | 54% | 0.06 |
| Burden on caregivers | |||
| Zarit burden interview | 11.5 (8.4) | 16 (9) | 0.016 |
| Total perceived caregiver burden | 65 (21.8) | 75.4 (19.2) | 0.015 |
| Impact on finances | 9.3 (3.3) | 10.6 (4.1) | 0.112 |
| Sense of abandonment | 14.6 (7.2) | 13.8 (3.3) | 0.45 |
| Impact on schedule | 11.9 (7.0) | 16.1 (6.2) | 0.005 |
| Impact on personal health | 15.6 (4.1) | 17.8 (3.7) | 0.006 |
| Sense of entrapment | 13.4 (6.5) | 17.3 (8.3) | 0.016 |
MELD, model-for-end-stage liver disease; NSAID, nonsteroidal anti-inflammatory drug.
Bold values signify P-values that are significant at < 0.05.
Patients with previous HE were a significantly higher burden on their caregivers compared with those without (Table 1). Zarit (19 vs. 12, P = 0.005) and PCB (85 vs. 68, P = 0.008) were significantly higher in caregivers of those with severe previous HE compared with those with previous HE controlled on lactulose and those without previous HE.
As expected cognitive testing was significantly worse in patients with previous HE for most of the tests compared with those without HE; NCT-A (38 vs. 46, P = 0.016), NCT-B (100 vs. 144, P = 0.004), DST (54 vs. 41, P = 0.0001), LTT time (99 vs. 119, P = 0.019), SDT (64 vs. 86, P = 0.002), BDT (26 vs. 20, P = 0.024), ICT lures (13 vs. 17, P = 0.023), and ICT targets (94 vs. 87%, P = 0.014). LTT errors were similar (34 vs. 39, P = 0.45).
Other cirrhosis complications and comorbidities
There was no significant difference in the total Zarit score between the caregivers of patients with/without previous spontaneous bacterial peritonitis (13 vs. 11, P = 0.48), variceal bleeding (17 vs. 13, P = 0.16), hepato-renal syndrome (13 vs. 12, P = 0.92), or hepato-pulmonary syndrome (13 vs. 12.5, P = 0.88). Similarly there was no difference in PCB score between the caregivers of those with/without previous spontaneous bacterial peritonitis (82 vs. 69, P = 0.39), variceal bleeding (69 vs. 70, P = 0.94), hepato-pulmonary syndrome (70 vs. 66, P = 0.44), or hepato-renal syndrome (77 vs. 69, P = 0.53).
Neither diabetes (Zarit 15 vs. 12, P = 0.2, PCB: 72 vs. 69, P = 0.42) nor chronic pain (Zarit 14 vs. 13, P = 0.8, PCB 73 vs. 69, P = 0.48) significantly affected burden. Similarly patients' depression (Zarit 14 vs. 13, P = 0.34, PCB:66 vs. 70, P = 0.34) and anxiety (Zarit 10 vs. 13, P = 0.12, PCB 64 vs. 70, P = 0.43) did not significantly influence caregiver burden.
Differences between veterans and non-veterans
Veterans included in the study had a higher MELD score (15 vs. 12, P = 0.008) and alcoholic cirrhosis (28 vs. 8%, P = 0.008). As expected, the majority of veterans were men (96%) compared with non-veterans (45%, P = 0.0001). Age (57 vs. 56 years, P = 0.76) and years of education (13 vs. 13, P = 0.9) were similar. There was a significantly higher prevalence of severe previous HE in veterans (P = 0.001) and a trend toward higher previous HE overall (51 vs. 36%, P = 0.15) compared with non-veterans. Despite the higher MELD score, alcoholic contribution of cirrhosis and trend toward higher prevalence of patients with previous HE, there was no statistically significant difference in the income and socioeconomic status between veterans and non-veterans. In fact, the percentage of patients who had to decrease medications was significantly lower in veterans (2 vs. 22%, P = 0.01) and a higher percentage of patients had their prescriptions completely covered (54 vs. 11%, P = 0.0001). There was no significant difference in Zarit (14 vs. 13, P = 0.67) and a trend toward higher PCB (75 vs. 67, P = 0.06) in caregivers of veterans.
Liver disease severity and financial status/burden
MELD score was significantly correlated with total Zarit (r = 0.30, P = 0.003) and PCB (r = 0.335, P = 0.001), as well as most individual domains of the PCB (finances, r = 0.21, P = 0.05; abandonment, r = 0.01, P = 0.35; schedule, r = 0.35, P = 0.001; personal health, r = 0.266, P = 0.008, and entrapment, r = 0.246, P = 0.017). MELD score in those employed patients was significantly lower than others (15 vs. 12, P = 0.03), but it, however, was not correlated with income variables.
Those evaluated for liver transplant compared with others
As expected, those evaluated for liver transplant had a higher MELD score (15 vs. 11, P = 0.01) and, as MELD was correlated with burden, posed a higher burden as evidenced by both PCB (78 vs. 63, P = 0.0001) and total Zarit (16 vs. 12, P = 0.026) compared with those who were not evaluated for transplant.
Cognitive profile and caregiver burden and financial status
The cognitive profile showed striking correlations with total Zarit and PCB score indicating an intimate relationship between patients' cognition and caregiver burden (Table 2). Employed patients had a significantly better cognitive performance compared with unemployed patients with respect to key cognitive tests (NCT-A: 39 vs. 49, P = 0.007, DST: 51 vs. 43, P = 0.024, BDT: 26 vs. 19, P = 0.006, and ICT lures: 13 vs. 17, P = 0.027).
Table 2. Correlation of patients' cognitive test performance with burden on caregivers.
| Perceived caregiver burden | Zarit burden interview | |||
|---|---|---|---|---|
|
|
|
|||
| R | P value | R | P value | |
| Higher score is worse | ||||
|
| ||||
| NCT-A (s) | 0.24 | 0.02 | 0.13 | 0.22 |
|
| ||||
| NCT-B (s) | 0.35 | 0.001 | 0.2 | 0.06 |
|
| ||||
| LTT time (s) | 0.38 | 0.0001 | 0.27 | 0.009 |
|
| ||||
| LTT errors (number) | 0.03 | 0.81 | 0.1 | 0.48 |
|
| ||||
| SDT (s) | 0.33 | 0.01 | 0.30 | 0.003 |
|
| ||||
| ICT lures (number responded) | 0.3 | 0.01 | 0.21 | 0.04 |
|
| ||||
| Lower score is worse | ||||
|
| ||||
| DST (raw score) | −0.41 | 0.0001 | −0.29 | 0.005 |
|
| ||||
| BDT (raw score) | −0.24 | 0.023 | −0.07 | 0.53 |
|
| ||||
| ICT targets (%) | −0.04 | 0.70 | 0.03 | 0.79 |
BDT, block design; DST, digit symbol; ICT, inhibitory control test; LTTe, line tracing errors; LTT, line tracing time; NCT-A/B, number connection-A/B; SDT, serial dotting.
This table displays significant correlations of several cognitive tests taken by the patients with burden placed on caregivers. The bold values indicate the statistically significant correlations (P < 0.05) of individual cognitive tests. A high score on NCT-A/B, ICT lures, SDT, LTT errors, and time indicate worse cognition, whereas a low score on BDT, DST, and ICT Targets indicates poor performance. A high total PCB and Zarit score demonstrate a high burden on caregivers.
Regression with burden scales as outcomes
The variables associated with Zarit interview were MELD score (P = 0.0001), depression (P = 0.034), and ICT lures (P = 0.006) along with anxiety score (P = 0.019) total ISEL-SF (P = 0.06) and patient age (P = 0.07). When previous HE was used instead of cognitive tests, the variable predicting Zarit were similar; MELD score (P = 0.0001), previous HE (P = 0.021), alcoholic etiology (P = 0.003), depression (P = 0.08), anxiety (P = 0.021), and years of relationship (P = 0.001).
The variables that predicted total PCB score were based on two cognitive tests: LTT time (P = 0.002) and ICT lures (P = 0.05), MELD score (P = 0.002), income for the past 12 months (P = 0.01), and depression score (P = 0.0001). When previous HE was used, the variables were MELD score (P = 0.0001), being married to the patient (P = 0.026), previous HE (P = 0.05), and anxiety score (P = 0.07).
Discussion
The current study shows that cirrhosis places a significant financial, socioeconomic, and personal burden on patients and their caregivers. The effect of cirrhosis-related medical expenses was felt by the affected family in all aspects of daily living and several sacrifices needed to be made by the family unit to continue with the medical therapy in cirrhosis. The severity of liver disease was associated with all financial, caregiver burden and socioeconomic parameters. Patients with previous HE were a significantly higher burden on their caregivers, with specific effects on schedule, sense of entrapment, and personal health. Previous HE also negatively affected the employment capability and financial status of patients. Of interest, there was a direct correlation between key cognitive tests and burden on caregivers as well as a worse cognitive status in those who were not able to work.
Burden of disease on family members and caregivers is an important issue to consider when evaluating the impact of the disease. Uniformly, it is shown that caregivers are placed under significant amounts of strain that can manifest financially and can even increase mortality in the caregivers (8,25–28). Previous experience with Zarit-SF has shown that the included study caregivers are at similar stress levels compared with caregivers of patients with Alzheimer' s disease (score 15±10) and advanced cancer (score 12±8.5), but at a lower level compared with caregivers with acute brain injury (score: 21.7±10.1) (21). Also consistent with Alzheimer's disease (7,29) the burden was most intense if the caregiver was the spouse (30–32). Similar to previous studies in dementia and spinal cord injury, our study found a relatively scant social support in caregivers, which was in turn correlated with depression in these individuals (33,34). Both burden questionnaires, in turn were related to depression and social support, which replicate findings in other chronic diseases (26–28). Our study also confirmed findings noted in other diseases, such as mild cognitive impairment in Alzheimer's disease, major depression, Parkinson's disease, and stroke, that the degree of caregiver burden is highly correlated with the patient's cognitive impairment (35–38). Therefore, the burden seen in caregivers of cirrhotic patients, especially the higher burden in previous HE, mirrors that seen in several chronic neurological diseases in its severity and pattern.
In our study, a strong relationship between the spectrum of neuro-cognitive impairment in cirrhosis (SONIC) and caregiver burden was observed, both when individual cognitive tests or previous HE were used (4). The cognitive functioning also predicted whether they were working or not (39,40). As SONIC and HE result in persistent cognitive deficits, which have been associated with employment and driving difficulties, the extra burden placed on caregivers of patients with previous HE is expected (2,5,41,42). As proposed in the SONIC model, in which each test is a continuous cognitive measure, performance on LTT time, and ICT lures were predictive of the burden. This is probably because LTT time and ICT lures evaluate frontal lobe function and visuo-spatial ability, which are required to perform daily instrumental activities (4). Interestingly, impairment in similar tests has been associated with caregiver stress in other diseases (35,36). Even with the categorical approach using previous HE instead of cognitive tests, previous HE was still a significant contributor to caregiver burden. The MELD score was also highly predictive of burden and a degree of collinearity with previous HE is to be expected. However, the multi-variable analysis clearly showed that cognitive performance or previous HE independently affected scores on caregiver burden.
The study also showed the significant financial hurdles posed by cirrhosis, which impact all aspects of daily living, including being able to afford food and shelter. This all-pervasive financial difficulty also affects medical adherence. The patients were specifically asked to only respond to the questions pertaining to their cirrhosis diagnosis and related medical expenses. As these are personal perceptions of the patients, they are susceptible to attribution bias and that other comorbid conditions could also affect these responses. Regardless of this, patients and family units seemed to be severely impacted financially. This is important because financial issues, as a contributor to medical non-adherence, are not routinely investigated by clinicians (43). Also almost half of this group was evaluated for transplant, indicating a selectively better patient population. Therefore, the presence of these issues even in these patients implies that nontransplant candidates may have worse financial challenges. The dynamic of limited financial resources in the setting of poor social support (low ISEL score) predisposes to treatment non-adherence, not due to patient or caregiver issues, but rather out of financial necessity (43).
The downward spiral of financial insecurity in the setting of a life-threatening disease resulting in non-adherence is likely to make the patients poor candidates for therapy or transplant evaluation (44). The humanitarian and financial costs to society can be mitigated by a multidisciplinary effort, one that includes psychological and social work input, while focusing on the family system. This has been performed in other diseases in the form of a caregiver focused family psychoeducation intervention (45). In cirrhosis, specific goals would include inquiring about financial issues, increasing knowledge regarding the physical and cognitive concomitants (e.g., SONIC) of the disorder, and teaching both the patient and their caregivers healthy ways of coping with the emotional and financial dimensions of burden, as well as providing mental health resources and support groups.
We believe the current study is the largest study to date analyzing the impact of cirrhosis on the emotional, cognitive, and financial burden on the family unit. This study utilized more than one burden measure and evaluated this construct in a multi-dimensional manner. The caregiver data are likely to be reliable because of the stable relationship and low rates of mood disorders or comorbidities in that group. The study found equivalence in the financial and socioeconomic parameters between veterans and non-veterans despite a worse MELD score in the veterans. This is likely due to the insurance and medication coverage provided by the veterans hospitals compared with the private sector.
The cross-sectional nature of this study is a limitation, as is also the large proportion of male patients with female caregivers. Although the duration of cirrhosis and previous HE was accounted for, the interpretation of the data may be limited by varying durations in this cross-sectional design. Almost half of our population was being considered for transplant. This automatically selects out patients with stable caregivers and good adherence behavior, which could have affected the results. Although those with significant end-organ diseases were excluded and there was no significant difference in comorbidities between those with and without HE, it is possible that they could confound the results. Ideally, it would be best to examine the relationship between burden, comorbidities, and illness variables in a prospective longitudinal manner. All patients seen in our study had existing health insurance; therefore, the real financial burden that results in patients losing insurance was not captured. Also there were relatively few patients with alcoholic cirrhosis and non-HE complications in our study population (28). Another potential limitation of our study concerns a potential geographical selection bias that may limit its generalizability to other regions or countries with different health systems.
In conclusion, cirrhosis places a tremendous socioeconomic and emotional burden on patients and their caregivers. Cognitive function in cirrhosis is linked to employment and socioeconomic status, as well as the burden of cirrhosis on caregivers. The financial impact of cirrhosis and related medical expenses on the family unit severely impacts their activities of daily living and their adherence with therapy. The study results underline the need for a multi-disciplinary approach with psychology, social work, and medical professionals to examine these areas in all patients and develop strategies for defining and overcoming this immense burden.
Supplementary Material
Study Highlights.
What is Current Knowledge
Hepatic encephalopathy (HE) is a significant complication of cirrhosis in terms of effect on patients' functioning.
Patients with HE are cognitively impaired and have poor quality of life.
The financial and emotional burden on cirrhosis and HE on caregivers and the whole family unit has not been clearly elucidated.
What is New Here
Cirrhosis has a severe negative financial impact on the daily lives of patients and their caregivers.
Financial and employment issues have an adverse effect on medical compliance.
Cirrhotic patients with previous HE are a significantly higher burden on their caregivers and this is proportional to their cognitive dysfunction.
Acknowledgments
Financial support: This study was partly supported by the American College of Gastroenterology Junior Faculty Development Award, NCCAM, NIH Grant UO1 AT004428 (awarded to JSB) and the McGuire Research Institute. L.R.T. was supported by NIH grant UL1RR031990.
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/ajg
Portions of this study were presented as an oral presentation in the Annual Liver Meeting in Boston 2010.
Conflict of Interest: Guarantor of the article: Jasmohan S. Bajaj, MD, MS.
Potential competing interests: None.
Specific author contributions: Jasmohan S. Bajaj was involved in all aspects of the paper; James B. Wade, Douglas P. Gibson, Douglas M. Heuman, and Arun J. Sanyal were involved in study concept and design and critical revision of the manuscript; Debulon E. Bell, Melanie B. White, and Nicole Noble were responsible for research recruitment and coordination; Velimir Luketic, R. Todd Stravitz, Richard K. Sterling, Puneet Puri, Michael Fuchs, HoChong Gilles, and Katherine Morton were involved in recruitment and data acquisition; Leroy R. Thacker was involved in the statistical analysis.
References
- 1.Kim WR, Brown RS, Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(Suppl 1):3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–5. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–21. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj JS, Schubert CM, Heuman DM, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–40. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gayomali C, Sutherland S, Finkelstein FO. The challenge for the caregiver of the patient with chronic kidney disease. Nephrol Dial Transplant. 2008;23:3749–51. doi: 10.1093/ndt/gfn577. [DOI] [PubMed] [Google Scholar]
- 7.Rankin ED, Haut MW, Keefover RW. Clinical assessment of family caregivers in dementia. Gerontologist. 1992;32:813–21. doi: 10.1093/geront/32.6.813. [DOI] [PubMed] [Google Scholar]
- 8.Sherwood PR, Given CW, Given BA, et al. Caregiver burden and depressive symptoms: analysis of common outcomes in caregivers of elderly patients. J Aging Health. 2005;17:125–47. doi: 10.1177/0898264304274179. [DOI] [PubMed] [Google Scholar]
- 9.Tong A, Sainsbury P, Craig JC. Support interventions for caregivers of people with chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2008;23:3960–5. doi: 10.1093/ndt/gfn415. [DOI] [PubMed] [Google Scholar]
- 10.Stommel M, Given CW, Given B. Depression as an overriding variable explaining caregiver burdens. J Aging Health. 1995;2:81–101. [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 13.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 14.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 15.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–73. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj JS, Hafeezullah M, Franco J, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. e1. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Saeian K, Verber MD, et al. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754–60. doi: 10.1111/j.1572-0241.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz M, Cordoba J, Doval E, et al. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther. 2007;26:859–67. doi: 10.1111/j.1365-2036.2007.03394.x. [DOI] [PubMed] [Google Scholar]
- 19.Bedard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–7. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 20.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–55. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 21.Higginson IJ, Gao W, Jackson D, et al. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63:535–42. doi: 10.1016/j.jclinepi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 23.Piotrowski C. The status of the Beck Anxiety Inventory in contemporary research. Psychol Rep. 1999;85:261–2. doi: 10.2466/pr0.1999.85.1.261. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S, Mermelstein R, Kamarck T, et al. Measuring the Functional Components of Social Support. Martinus Nijhoff Publishers; Dordrecht: 1985. [Google Scholar]
- 25.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–9. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 26.Cohen M, Katz D, Baruch Y. Stress among the family caregivers of liver transplant recipients. Prog Transplant. 2007;17:48–53. doi: 10.1177/152692480701700107. [DOI] [PubMed] [Google Scholar]
- 27.Bolkhir A, Loiselle MM, Evon DM, et al. Depression in primary caregivers of patients listed for liver or kidney transplantation. Prog Transplant. 2007;17:193–8. doi: 10.1177/152692480701700306. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki ET, Dos Santos R, Jr, Miyazaki MC, et al. Patients on the waiting list for liver transplantation: caregiver burden and stress. Liver Transpl. 2010;16:1164–8. doi: 10.1002/lt.22130. [DOI] [PubMed] [Google Scholar]
- 29.Pruchno RA, Resch NL. Husbands and wives as caregivers: antecedents of depression and burden. Gerontologist. 1989;29:159–65. doi: 10.1093/geront/29.2.159. [DOI] [PubMed] [Google Scholar]
- 30.Baumgarten M, Battista RN, Infante-Rivard C, et al. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol. 1992;45:61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- 31.Cohen D, Luchins D, Eisdofer C, et al. Caring for relatives with Alzheimer's disease: the mental health risks to spouses, adult children, and other family caregivers. Behavior, Health and Aging. 1990;1:171–82. [Google Scholar]
- 32.Barber CE, Pasley BK. Family care of Alzheimer's patients: the role of gender and generational relationship on caregiver outcomes. J Appl Gerontol. 1995;14:172–92. [Google Scholar]
- 33.Arango Lasprilla JC, Moreno A, Rogers H, et al. The effect of dementia patient's physical, cognitive, and emotional/behavioral problems on caregiver well-being: findings from a Spanish-speaking sample from Colombia, South America. Am J Alzheimers Dis Other Demen. 2009;24:384–95. doi: 10.1177/1533317509341465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arango-Lasprilla JC, Plaza SL, Drew A, et al. Family needs and psychosocial functioning of caregivers of individuals with spinal cord injury from Colombia, South America. NeuroRehabilitation. 2010;27:83–93. doi: 10.3233/NRE-2010-0583. [DOI] [PubMed] [Google Scholar]
- 35.Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson's disease. Brain Pathol. 2010;20:640–5. doi: 10.1111/j.1750-3639.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HM, Hou SY, Yeh YC, et al. Frontal function, disability and caregiver burden in elderly patients with major depressive disorder. Kaohsiung J Med Sci. 2011;26:548–54. doi: 10.1016/S1607-551X(10)70084-X. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher D, Ni Mhaolain A, Crosby L, et al. Dependence and caregiver burden in Alzheimer's disease and mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2011;26:110–4. doi: 10.1177/1533317510394649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigby H, Gubitz G, Eskes G, et al. Caring for stroke survivors: baseline and 1-year determinants of caregiver burden. Int J Stroke. 2009;4:152–8. doi: 10.1111/j.1747-4949.2009.00287.x. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World J Gastroenterol. 2008;14:3609–15. doi: 10.3748/wjg.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37–41. doi: 10.1023/a:1011610427843. [DOI] [PubMed] [Google Scholar]
- 41.DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl. 2009;15:121–3. doi: 10.1002/lt.21638. [DOI] [PubMed] [Google Scholar]
- 42.Bajaj JS, Hafeezullah M, Zadvornova Y, et al. The effect of fatigue on driving skills in patients with hepatic encephalopathy. Am J Gastroenterol. 2009;104:898–905. doi: 10.1038/ajg.2009.7. [DOI] [PubMed] [Google Scholar]
- 43.Stilley CS, DiMartini AF, de Vera ME, et al. Individual and environmental correlates and predictors of early adherence and outcomes after liver transplantation. Prog Transplant. 2010;20:58–66. doi: 10.7182/prtr.20.1.c903845857104k83. quiz 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiMartini A, Crone C, Fireman M, et al. Psychiatric aspects of organ transplantation in critical care. Crit Care Clin. 2008;24:949–81. x. doi: 10.1016/j.ccc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madigan K, Egan P, Brennan D, et al. A randomised controlled trial of carer-focussed multi-family group psychoeducation in bipolar disorder. Eur Psychiatry. 2011 Feb 18; doi: 10.1016/j.eurpsy.2010.12.008. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.