Abstract
Background
Chronic viral hepatitis is a potentially important determinant of healthcare utilization among persons living with HIV (PLWH). We describe hospitalization rates and reasons for hospitalization among PLWH stratified by co-infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV).
Methods
Laboratory, demographic, and hospitalization data were obtained for all patients receiving longitudinal HIV care during 2010 at 9 geographically diverse sites. Hepatitis serostatus was assessed by hepatitis B surface antigen and/or hepatitis C antibody. ICD-9 codes were used to assign hospitalizations into diagnostic categories. Negative binomial regression was used to assess factors associated with all-cause and diagnostic category-specific hospitalizations.
Results
A total of 2,793 hospitalizations were observed among 12,819 patients. Of these patients, 49.3% had HIV mono-infection, 4.1% HIV/HBV, 15.4% HIV/HCV, 2.5% HIV/HBV/HCV and 28.7% unknown hepatitis serostatus. Compared to HIV mono-infection, risk of all-cause hospitalization was increased with HIV/HBV (adjusted incidence rate ratio (aIRR) 1.55 [1.17–2.06]), HIV/HCV (1.45 [1.21–1.74]) and HIV/HBV/HCV (1.52 [1.04–2.22]). Risk of hospitalization for non-AIDS-defining infection was also higher among patients with HIV/HBV (2.07 [1.38–3.11]), HIV/HCV (1.81 [1.36–2.40]) and HIV/HBV/HCV (1.96 [1.11–3.46]). HIV/HBV was associated with hospitalization for gastrointestinal/liver disease (2.55 [1.30–5.01]). HIV/HCV was associated with hospitalization for psychiatric illness (1.89 [1.11–3.26]).
Conclusions
HBV and HCV co-infection are associated with increased risk of all-cause hospitalization and hospitalization for non-AIDS-defining infections, as compared to HIV mono-infection. Policy-makers and third-party payers should be aware of the heightened risk of hospitalization associated with co-infection when allocating healthcare resources and considering models of healthcare delivery.
Keywords: HIV, hepatitis B, hepatitis C, hospitalizations, healthcare utilization
BACKGROUND
Chronic viral hepatitis is common among persons living with HIV (PLWH). In the United States, Europe, and Australia, approximately 4.8–9.0% of PLWH are also chronically infected with hepatitis B virus (HBV), 20–33% are chronically infected with hepatitis C virus (HCV) and 0.5–4.0% are chronically infected with both1–5. Patients with HIV/HBV co-infection experience faster progression to cirrhosis, more hepatocellular carcinoma and higher risk of liver-related mortality than patients with either infection alone5–8. Similarly, liver disease progression and its complications are more common in HIV/HCV co-infected patients than in HIV mono-infected patients2,8,9. Viral hepatitis, particularly HCV, has also been associated with extrahepatic complications that can include renal disease, cardiovascular disease, diabetes, autoimmunity, metabolic bone disease and neurocognitive decline10–16.
In the era of potent and widely available antiretroviral therapy, hospitalization rates have become an important outcome measure and an important healthcare cost among PLWH17–19. Comparing rates and reasons for hospitalizations among PLWH with and without hepatitis coinfection will be important to clinicians and policy-makers trying to understand the healthcare needs of these populations. Differences across these populations could suggest areas of unique clinical need and may influence the allocation of healthcare resources and the construction of healthcare delivery models.
The purpose of this study was to characterize the impact of hepatitis co-infection on inpatient healthcare utilization among HIV-infected patients in a multi-site, multi-state consortium of HIV care sites.
METHODS
Site Selection and Data Collection
The HIV Research Network (HIVRN) is a consortium of 17 sites providing longitudinal adult and pediatric HIV care in 11 U.S. cities. Sites abstract comprehensive demographic, laboratory, and treatment data from clinical records, then de-identify and submit them to a data coordinating center where they are reviewed and combined into a uniform database20. In 2010, nine of the participating sites submitted details of hospital admissions for adult patients (3 Northeast, 3 West, 2 South, and 1 Midwest). Seven of these sites have academic affiliations and 2 are community-based. Inclusion in this retrospective cohort study was restricted to patients who enrolled in care before July 1, 2010, and were in active care during 2010. Active care was defined as having at least one outpatient HIV provider visit and one CD4 cell count during the calendar year. Institutional review boards at each site and at the data coordinating center at Johns Hopkins University approved the collection and use of these data for analysis and publication.
Definitions of Variables
Hepatitis serostatus was assessed by detection of hepatitis B surface antigen (HBsAg) and/or hepatitis C antibody (anti-HCV) at any time prior to or during 2010. At each site, patients are screened for chronic HBV and chronic HCV at the discretion of their providers. If a patient had multiple serologies performed over time, a single positive test was considered sufficient to categorize the patient as positive for that assay. HBV DNA and HCV RNA levels were not available. Patients were assigned to one of five hepatitis serostatus categories. Patients with negative results for both hepatitis serologies were categorized as HIV mono-infected. Patients with a positive HBsAg and negative anti-HCV were categorized as HIV/HBV co-infected. Patients with a negative HBsAg and positive anti-HCV were categorized as HIV/HCV coinfected. Patients with positive results for both hepatitis serologies were categorized as HIV/HBV/HCV tri-infected. Patients without known results from one or both tests were categorized as unknown hepatitis serostatus.
Age was assessed on July 1, 2010 and divided into 4 categories: 18–34, 35–49, 50–64 and 65 or more years. Race/ethnicity was categorized based on self-report as White, Black, Hispanic or other/unknown. HIV transmission risk factor was classified as one of four mutually exclusive categories: injection drug use (IDU), men who have sex with men (MSM), heterosexual transmission, or other/unknown. Patients who reported IDU in addition to any other risk factor were categorized as IDU. Men who reported sex with both men and women were categorized as MSM.
The CD4 T-cell count and HIV-1 RNA values used in this analysis were the first available measurements in 2010. CD4 count was categorized as ≤50, 51–200, 201–500 or >500 cells/mm3. HIV-1 RNA was categorized as <400 or ≥400 copies/mL. Antiretroviral therapy (ART) was defined as the concurrent use of three or more antiretroviral medications from at least two classes at any time during calendar year 2010. Insurance status was categorized as Medicaid, Medicare, Private, Ryan White/Uninsured or missing. Patients with dual eligibility for Medicaid and Medicare were included in the Medicare category.
Outcomes
The primary outcome of this study was all-cause hospitalization in 2010. We also investigated cause-specific hospitalization rates using 18 diagnostic categories, including non-AIDS-defining infection, cardiovascular, gastrointestinal/liver and AIDS-defining illness (see Table, Supplemental Digital Content 1, for complete list). Using a previously published algorithm, several steps were taken to assign each hospitalization to a single diagnostic category18,21. First, the primary diagnostic code for the hospitalization was assigned using the first-listed ICD-9 code that did not refer to HIV (042, V08, 795.71, V01.79), chronic HBV (070.22, 070.23, 070.32, 070.33), chronic HCV (070.44, 070.54, 070.70, 070.71), or oral candidiasis (112.0). These codes represent comorbidities that are frequently recorded for billing purposes but are not, by themselves, sufficient to justify hospitalization. Second, Clinical Classifications Software (CCS) developed by the Agency for Healthcare Research and Quality was used to assign the primary ICD-9 code into one of 18 “first-level” CCS categories 22. Finally, we modified the CCS diagnostic categories in three ways: we reassigned infections from organ system categories to the infection category (for example pneumonia was reassigned from pulmonary to infection); we combined congenital, perinatal, and unclassified (together < 1% of admissions) into a single category; and we created an AIDS-defining illness (ADI) category according to the 1993 Centers for Disease Control and Prevention criteria 23. After the ADI category was created, we renamed the remaining infection category “non-AIDS-defining infection” and the remaining malignancy category “non-AIDS-defining cancer.”
Within each diagnostic category, ICD-9 codes were used to identify the most frequently occurring individual diagnoses. Highly similar ICD-9 codes were grouped (Appendix Table). The most common individual diagnoses were tallied and reported as percentages of admissions within the corresponding diagnostic category.
Data Analysis
Hospitalization rates were calculated as total number of admissions divided by the number of years of patient follow-up and multiplied by 100 to obtain rates per 100 person-years (PY). Patients who enrolled in care or died during the observation period contributed less than one year of follow-up, so a variable person-time denominator was used in rate calculations.
Preliminary exploration of the hospitalization count data revealed that the variance was not equal to the mean of the distribution, making negative binomial regression a more robust analytic method than Poisson regression. Unadjusted negative binomial regression was therefore used to estimate incidence rate ratios for all-cause and diagnostic category-specific hospitalization rates associated with hepatitis serostatus and other predefined clinical and demographic variables.
Adjusted negative binomial models compared incidence rates for all-cause hospitalization and diagnostic category-specific hospitalizations between each of the hepatitis serostatus groups (including the unknown group), controlling for age, race, sex, HIV risk factor, CD4, HIV RNA, ART use, and insurance. Adjusted models also included categorical indicators for each clinical care site to control for site-specific variability in healthcare utilization (results suppressed).
A sensitivity analysis was performed in which patients with one positive hepatitis serology and one missing hepatitis serology were re-categorized from the unknown hepatitis status group into either the HIV/HBV or HIV/HCV group.
A two-sided type I error of 5% was considered statistically significant. All analyses were performed using Stata 12.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Demographic and clinical characteristics of the study population are presented in Table 1. Of the 12,819 patients included in this analysis, 49.4% had HIV monoinfection, 4.1% HIV/HBV co-infection, 15.4% HIV/HCV co-infection, 2.5% HIV/HBV/HCV tri-infection and 28.7% had unknown hepatitis serostatus. IDU was reported in 17.4% of patients overall with higher percentages in the HIV/HCV (59.4%) and HIV/HBV/HCV (32.7%) groups. MSM comprised 39.4% of patients overall with higher percentages in the HIV/HBV (56.6%) and HIV-monoinfected (47.5%) groups. MSM was relatively less common as a sole HIV risk factor in the HIV/HCV (15.7%) and HIV/HBV/HCV (26.1%) groups. Median CD4 counts and percentages of patients with HIV RNA <400 copies/mL were similar across all the hepatitis serostatus groups. There were 117 deaths and 885 new enrollments in care during the study period, resulting in less than one year of observation time for these individuals. Median follow-up of these patients was 230 days among patients in the HIV/HBV group and 245 days in all other hepatitis serostatus groups.
Table 1.
Study Population Characteristics Stratified by Hepatitis Serostatus
| Characteristic |
Overall n=12,819 |
HIV Mono-infected n=6,317 (49.3%) |
HIV/HBV Co-infected n=532 (4.2%) |
HIV/HCV Co-infected n=1,969 (15.4%) |
HIV/HBV/HCV Tri-infected n=318 (2.5%) |
Unknown Serostatus n=3,683 (28.7%) |
|---|---|---|---|---|---|---|
| Total hospitalizations in 2010 [No.] | 2,793 | 1,160 | 155 | 762 | 82 | 634 |
| Age on July 1, 2010 [years] | ||||||
| Median (IQR) | 47 (40–53) | 45 (37–51) | 46 (41–51) | 50 (45–56) | 48 (42–53) | 47 (40–54) |
| 18 – 34 [No. (%)] | 1,908 (14.9) | 1,244 (19.7) | 56 (10.5) | 93 (4.7) | 25 (7.9) | 490 (13.3) |
| 35 – 49 | 6,112 (47.7) | 3,147 (49.8) | 304 (57.1) | 785 (39.9) | 158 (49.7) | 1,718 (46.6) |
| 50 – 64 | 4,348 (33.9) | 1,708 (27.0) | 152 (28.6) | 1,032 (52.4) | 127 (39.9) | 1,329 (36.1) |
| ≥ 65 | 451 (3.5) | 218 (3.4) | 20 (3.8) | 59 (3.0) | 8 (2.5) | 146 (4.0) |
| Gender [No. (%)] | ||||||
| Male | 9,196 (71.7) | 4.540 (71.9) | 443 (83.3) | 1,387 (70.4) | 229 (72.0) | 2,597 (70.5) |
| Female | 3,623 (28.3) | 1,777 (28.1) | 89 (16.7) | 582 (29.6) | 89 (28.0) | 1,086 (29.5) |
| Race/Ethnicity [No. (%)] | ||||||
| White | 3,420 (26.7) | 1,860 (29.4) | 201 (37.8) | 429 (21.8) | 86 (27.0) | 844 (22.9) |
| Black | 6,305 (49.2) | 2,819 (44.6) | 249 (46.8) | 1,119 (56.8) | 176 (55.4) | 1,942 (52.7) |
| Hispanic | 2,661 (20.8) | 1,432 (22.7) | 65 (12.2) | 384 (19.5) | 54 (17.0) | 726 (19.7) |
| Other/unknown | 433 (3.4) | 206 (3.3) | 17 (3.2) | 37 (1.9) | 2 (0.63) | 171 (4.6) |
| HIV risk factor* [No. (%)] | ||||||
| MSM | 5,044 (39.4) | 2,998 (47.5) | 301 (56.6) | 309 (15.7) | 83 (26.1) | 1,353 (36.7) |
| Heterosexual | 4,824 (37.6) | 2,656 (42.0) | 181 (34.0) | 424 (21.5) | 123 (38.7) | 1,440 (39.1) |
| IDU | 2,225 (17.4) | 313 (5.0) | 23 (4.3) | 1,169 (59.4) | 104 (32.7) | 616 (16.7) |
| Other/unknown | 726 (5.7) | 350 (5.5) | 27 (5.1) | 67 (3.4) | 8 (2.5) | 274 (7.4) |
| First CD4 count in 2010 (cells/mm3) | ||||||
| Median (IQR) | 446 (268–645) | 454 (278–648) | 407 (231–622) | 400 (233–607) | 405 (220–642) | 464 (289–673) |
| ≤ 50 [No. (%)] | 515 (4.0) | 254 (4.0) | 31 (5.8) | 81 (4.1) | 13 (4.1) | 136 (3.7) |
| 51–200 | 1,657 (12.9) | 749 (11.9) | 79 (14.8) | 328 (16.7) | 59 (18.6) | 442 (12.0) |
| 201–500 | 5,223 (40.7) | 2,594 (41.1) | 216 (40.6) | 841 (42.7) | 118 (37.1) | 1,454 (39.5) |
| >500 | 5,424 (42.3) | 2,720 (43.1) | 206 (38.7) | 719 (36.5) | 128 (40.2) | 1,651 (44.8) |
| First HIV-1 RNA in 2010 (copies/mL) [No. (%)] |
||||||
| < 400 | 8,637 (67.4) | 4,190 (66.3) | 369 (69.4) | 1,299 (66.0) | 219 (68.9) | 2,560 (69.5) |
| ≥ 400 | 3,827 (29.8) | 1,949 (30.8) | 153 (28.8) | 616 (31.3) | 94 (29.6) | 1,015 (27.6) |
| Unknown | 355 (2.8) | 178 (2.8) | 10 (1.9) | 54 (2.7) | 5 (1.6) | 108 (2.9) |
| ART** [No. (%)] | ||||||
| Yes | 11,171 (87.1) | 5,541 (87.7) | 490 (92.1) | 1,725 (87.6) | 296 (93.1) | 3,119 (84.7) |
| No | 1,182 (9.2) | 588 (9.3) | 32 (6.0) | 206 (10.5) | 22 (6.9) | 334 (9.1) |
| Unknown | 466 (3.6) | 188 (3.0) | 10 (1.9) | 38 (1.9) | 0 (0) | 230 (6.2) |
| Insurance [No. (%)] | ||||||
| Medicaid | 4,212 (32.9) | 1,923 (30.4) | 166 (31.2) | 852 (43.3) | 95 (29.9) | 1,176 (31.9) |
| Medicare/dual eligible | 2,655 (20.7) | 1,217 (19.3) | 117 (22.0) | 427 (21.7) | 89 (28.0) | 805 (21.9) |
| Private | 2,818 (22.0) | 1,367 (21.6) | 109 (20.5) | 356 (18.1) | 32 (10.1) | 954 (25.9) |
| Ryan White/uninsured | 2,537 (19.8) | 1,497 (23.7) | 122 (22.9) | 287 (14.6) | 79 (24.8) | 552 (15.0) |
| Unknown | 597 (4.7) | 313 (5.0) | 18 (3.4) | 47 (2.4) | 23 (7.2) | 196 (5.3) |
IQR interquartile range; MSM men who have sex with men; IDU injection drug use; ART antiretroviral therapy
HIV risk factors were considered mutually exclusive; subjects who reported IDU in addition to any other risk factor were categorized as IDU, men who reported sex with men and women were categorized as MSM
ART was defined as concurrent use of 3 or more antiretroviral medications from at least 2 classes at any time during calendar year 2010
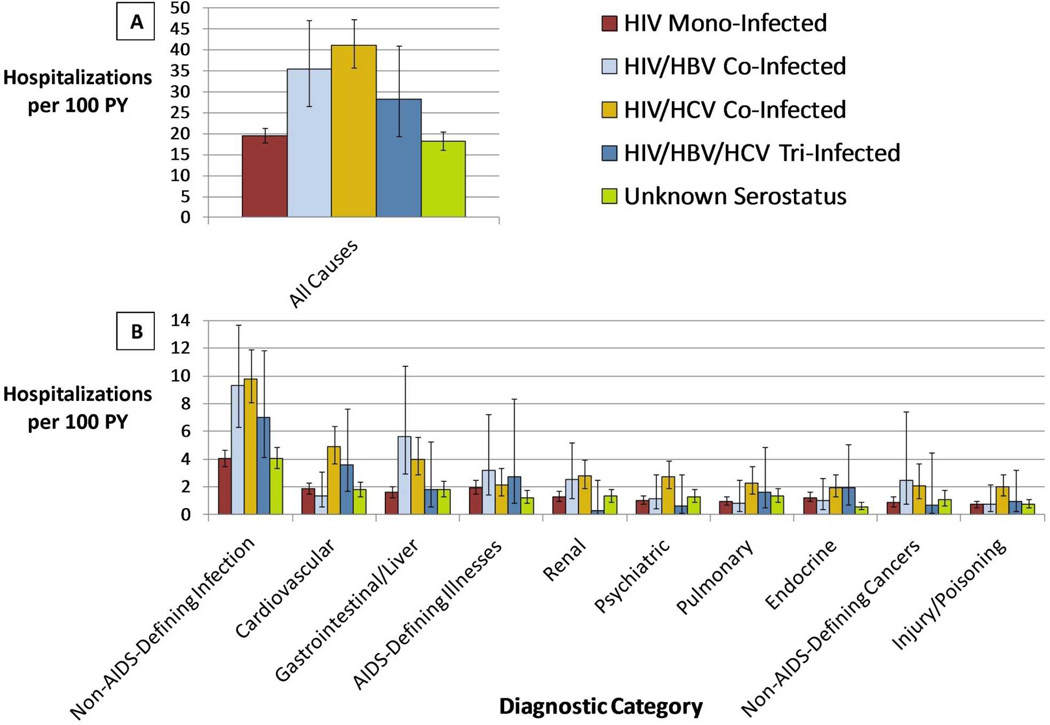
There were 2,793 hospitalizations in total. Unadjusted all-cause hospitalization rates stratified by hepatitis serostatus are presented in Figure 1A. Rates were highest among HIV/HCV co-infected patients (41.1 hospitalizations per 100 PY [95% CI 35.7–47.2]), followed by HIV/HBV co-infected (35.4/100 PY [26.6–47.0]), then HIV/HBV/HCV tri-infected (28.2/100 PY [19.4–40.9]). All-cause hospitalization rates were similar among HIV mono-infected patients (19.5/100 PY [17.9–21.3]) and patients with unknown hepatitis serostatus (18.2/100 PY [16.2–20.5]).
Figure 1. Unadjusted Hospitalization Rates by Diagnostic Category.
Unadjusted rates of all-cause (panel A) and diagnostic category-specific (panel B) hospitalization. Rates are standardized as hospitalizations per 100 PY of AU7 follow-up. Unadjusted negative binomial regression was performed to construct 95% confidence intervals.
Analyses of factors associated with all-cause hospitalization are presented in Table 2. Decreasing CD4 count was the strongest predictor of all-cause hospitalization with an adjusted incidence rate ratio (aIRR) of 8.14 (95% CI 6.27–10.58) for persons with CD4 <50 cells/mm3, compared to CD4 >500 cells/mm3. Risk of hospitalization increased in those with HIV/HBV (aIRR 1.55 [1.17–2.06]), HIV/HCV (aIRR 1.45 [1.21–1.74]) and HIV/HBV/HCV (aIRR 1.52 [1.04–2.22]) compared to HIV mono-infection. Other factors independently associated with hospitalization included age, gender, HIV transmission risk factor, HIV-1 RNA, and insurance.
Table 2.
Univariate and Multivariate Analyses of Risk Factors for All-Cause Hospitalization
| Characteristic | IRR (95% CI) | Adjusted IRR (95% CI) |
|---|---|---|
| Hepatitis co-infection status | ||
| HIV mono-infection | 1.0 (Ref) | 1.0 (Ref) |
| HIV/HBV co-infection | 1.81 (1.35–2.44) | 1.55 (1.17–2.06) |
| HIV/HCV co-infection | 2.10 (1.78–2.48) | 1.45 (1.21–1.74) |
| HIV/HBV/HCV tri-infection | 1.44 (0.99–2.12) | 1.52 (1.04–2.22) |
| Unknown serostatus | 0.93 (0.81–1.08) | 1.06 (0.90–1.24) |
| Age (years) | ||
| 18–34 | 1.0 (Ref) | 1.0 (Ref) |
| 35–49 | 1.08 (0.89–1.30) | 0.93 (0.77–1.12) |
| 50–64 | 1.40 (1.16–1.71) | 1.21 (0.99–1.48) |
| ≥65 | 1.93 (1.36–2.74) | 1.92 (1.37–2.68) |
| Gender | ||
| Male | 1.0 (Ref) | 1.0 (Ref) |
| Female | 1.36 (1.19–1.55) | 1.41 (1.22–1.64) |
| Race | ||
| White | 1.0 (Ref) | 1.0 (Ref) |
| Black | 1.33 (1.14–1.54) | 1.06 (0.90–1.26) |
| Hispanic | 1.19 (0.99–1.43) | 0.92 (0.76–1.12) |
| Other/Unknown | 0.58 (0.38–0.88) | 0.57 (0.38–0.87) |
| HIV transmission risk factor* | ||
| MSM | 1.0 (Ref) | 1.0 (Ref) |
| Heterosexual | 1.30 (1.13–1.50) | 0.99 (0.83–1.17) |
| IDU | 2.29 (1.94–2.70) | 1.37 (1.13–1.66) |
| Other/Unknown | 1.70 (1.30–2.22) | 1.41 (1.08–1.84) |
| First CD4 count in 2010(cells/mm3) | ||
| >500 | 1.0 (Ref) | 1.0 (Ref) |
| 201–500 | 1.72 (1.50–1.97) | 1.67 (1.45–1.92) |
| 51–200 | 3.85 (3.24–4.58) | 3.57 (2.99–4.28) |
| ≤50 | 8.39 (6.51–10.81) | 8.14 (6.27–10.58) |
| First HIV-1 RNA in 2010 (copies/ml) | ||
| <400 | 1.0 (Ref) | 1.0 (Ref) |
| ≥400 | 2.04 (1.80–2.31) | 1.26 (1.10–1.44) |
| ART** | ||
| Yes | 1.0 (Ref) | 1.0 (Ref) |
| No | 0.89 (0.72–1.11) | 1.04 (0.84–1.30) |
| Unknown | 1.52 (1.12–2.07) | 1.31 (0.86–1.99) |
| Insurance | ||
| Medicaid | 1.0 (Ref) | 1.0 (Ref) |
| Medicare/Dual eligible | 0.87 (0.74–1.02) | 0.99 (0.84–1.17) |
| Private | 0.48 (0.40–0.56) | 0.55 (0.46–0.66) |
| Ryan White/Uninsured | 0.38 (0.32–0.46) | 0.51 (0.42–0.62) |
| Unknown/Missing | 0.41 (0.29–0.56) | 0.62 (0.44–0.87) |
IRR incidence rate ratio; CI confidence interval; MSM men who have sex with men; IDU injection drug use; HIV human immunodeficiency virus; HBV hepatitis B virus; HCV hepatitis C virus Incidence rate ratios and 95% confidence intervals were calculated using negative binomial regression. The adjusted model included the listed characteristics as well as an indicator variable for clinical care site. IRRs in bold are statistically significant (p≤0.05).
HIV risk factors were considered mutually exclusive; subjects who reported IDU in addition to any other risk factor were categorized as IDU, men who reported sex with men and women were categorized as MSM
ART was defined as concurrent use of 3 or more antiretroviral medications from at least 2 classes at any time during calendar year 2010
In unadjusted analyses, non-AIDS-defining infections accounted for significantly more hospitalizations per 100 person-years in each of the hepatitis co-infected groups than in the HIV mono-infected group (Figure 1B). Gastrointestinal/liver-related hospitalizations were more common in the HIV/HBV (5.6 per 100 PY [2.9–10.7]) and HIV/HCV (4.0 per 100 PY [2.9–5.6]) groups than in the HIV mono-infected group (1.6 per 100 PY [1.3–2.0]). Compared to HIV mono-infected patients, patients with HIV/HCV had significantly higher unadjusted hospitalization rates in the cardiovascular, renal, psychiatric, pulmonary, and injury/poisoning categories (p<0.001).
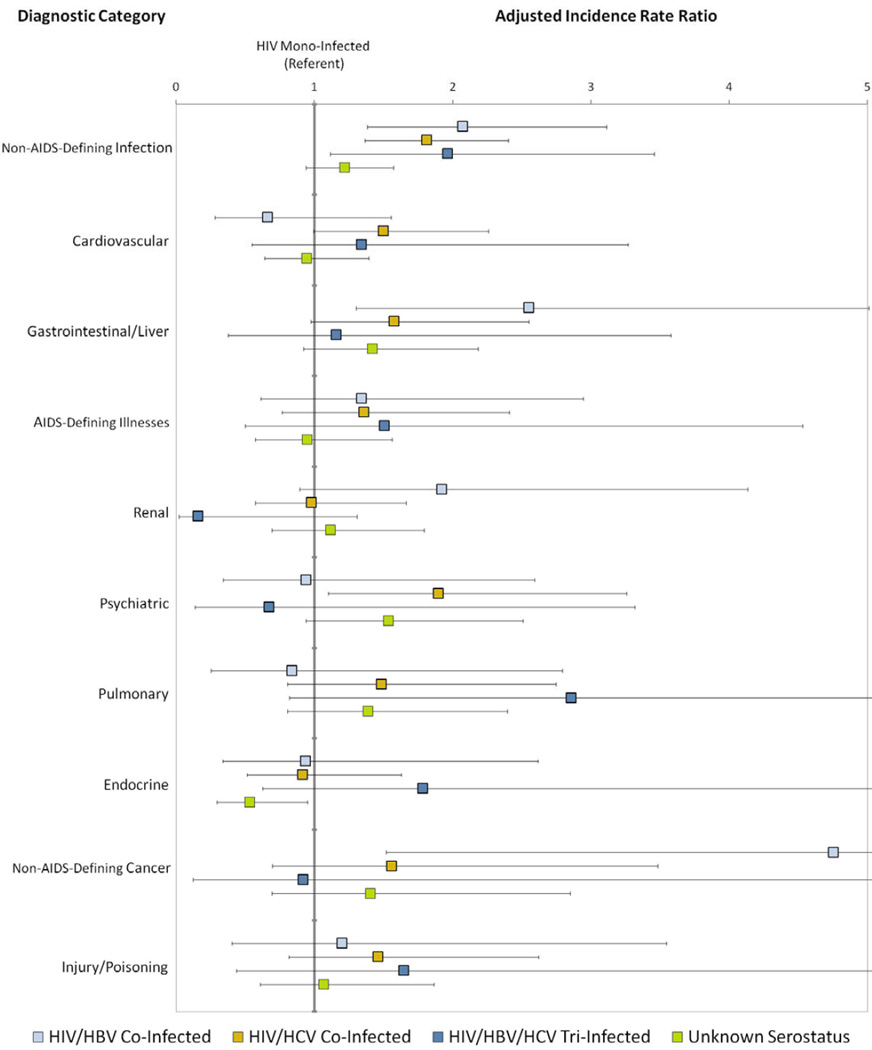
Adjusted relative rates of hospitalization for the ten most common diagnostic categories are presented in Figure 2. Compared to HIV mono-infection, the relative rate of hospitalization for non-AIDS-defining infection was higher among patients with HIV/HBV (aIRR 2.07 [1.38–3.11]), HIV/HCV (aIRR 1.81 [1.36–2.40]) and HIV/HBV/HCV (aIRR 1.96 [1.11–3.46]). The relationship between hepatitis co-infection and hospitalization for gastrointestinal/liver disease was attenuated in multivariate analysis, with only HIV/HBV remaining independently associated with risk of hospitalization (aIRR 2.55 [1.30–5.01]). Patients with HIV/HCV had higher risk of hospitalization for psychiatric illness (aIRR 1.89 [1.11–3.26]) and patients with HIV/HBV had higher risk of hospitalization for non-AIDS-defining cancers (aIRR 4.75 [1.52–14.88]) than the HIV mono-infected reference group.
Figure 2. Adjusted Incidence Rate Ratio of Hospitalization by Diagnostic Category.
Adjusted incidence rate ratios and 95% confidence intervals were calculated using negative binomial regression and are interpreted as the relative rate of admissions compared to the reference group (HIV mono-infection) after controlling for age, gender, race, HIV risk factor, CD4 count, HIV-1 RNA, ART, insurance and clinical care site.
Table 3 lists the most common diagnostic categories and individual diagnoses within these categories. Among non-AIDS-defining infections, bacterial pneumonia was the most common diagnosis overall and among most hepatitis serostatus groups. Complications of cirrhosis (including admissions for cirrhosis, hepatic encephalopathy, portal hypertension and ascites) were the most common reason for GI/liver admissions overall, although the proportion of admissions did not differ significantly from the proportions for pancreatitis or diarrhea. Complications of cirrhosis accounted for only 4.12% of GI/liver admissions in the HIV monoinfected group. Among AIDS-defining illnesses, Pneumocystis jiroveci was the most common diagnosis overall and among most hepatitis serostatus groups.
Table 3.
Most Common Individual Diagnoses Within Diagnostic Categories
|
Diagnostic Category Common Diagnoses |
Overall n=2,793 (%) |
HIV Mono-infected n=1,160 (%) |
HIV/HBV Co-infected n=155 (%) |
HIV/HCV Co-infected n=762 (%) |
HIV/HBV/HCV Tri-infected n=82 (%) |
Unknown Serostatus n=634 (%) |
|---|---|---|---|---|---|---|
| Non-AIDS-Defining Infection | 637 (22.8) | 245 (21.1) | 44 (28.4) | 183 (24.0) | 22 (26.8) | 143 (22.6) |
| Bacterial pneumonia | 130 (20.4) | 46 (18.8) | 7 (15.9) | 47 (25.7) | 3 (13.6) | 27 (18.9) |
| Sepsis/bacteremia | 92 (14.4) | 38 (15.5) | 9 (20.4) | 24 (13.1) | 5 (22.7) | 16 (11.2) |
| Cellulitis | 88 (13.8) | 26 (10.6) | 6 (13.6) | 33 (18.0) | 1 (4.55) | 22 (15.4) |
| Cardiovascular | 290 (10.4) | 114 (9.83) | 7 (4.52) | 94 (12.3) | 11 (13.4) | 64 (10.1) |
| Chest pain | 57 (19.7) | 17 (14.9) | 1 (14.3) | 28 (29.8) | 1 (9.09) | 10 (15.6) |
| Heart failure | 54 (18.6) | 29 (25.4) | 0 (0) | 9 (9.57) | 8 (72.73) | 8 (12.5) |
| CAD/MI | 43 (14.8) | 13 (11.4) | 1 (14.3) | 14 (14.9) | 0 (0) | 15 (23.4) |
| Gastrointestinal/Liver | 265 (9.49) | 97 (8.36) | 22 (14.2) | 77 (10.1) | 5 (6.10) | 64 (10.1) |
| Complication of cirrhosis* | 45 (17.0) | 4 (4.12) | 10 (45.4) | 18 (23.4) | 0 (0) | 13 (20.3) |
| Pancreatitis | 41 (15.5) | 10 (10.3) | 0 (0) | 20 (26.0) | 1 (20.0) | 10 (15.6) |
| Diarrhea | 40 (15.1) | 23 (23.7) | 2 (9.09) | 7 (9.09) | 1 (20.0) | 7 (10.9) |
| AIDS-Defining Illness | 217 (7.77) | 113 (9.74) | 15 (9.68) | 41 (5.38) | 7 (8.54) | 41 (6.47) |
| Pneumocystis jiroveci | 59 (27.2) | 36 (31.9) | 3 (20.0) | 10 (24.4) | 0 (0) | 10 (24.4) |
| Cryptococcus | 33 (15.2) | 22 (19.5) | 1 (6.67) | 9 (22.0) | 0 (0) | 1 (2.44) |
| Recurrent bacterial pneumonia | 21 (9.68) | 10 (8.85) | 2 (13.3) | 7 (17.1) | 0 (0) | 2 (4.88) |
| Renal | 191 (6.84) | 78 (6.72) | 12 (7.74) | 53 (6.96) | 1 (1.22) | 47 (7.41) |
| Acute renal failure | 125 (65.4) | 49 (62.8) | 8 (66.7) | 36 (67.9) | 0 (0) | 32 (68.1) |
| Hypertension with chronic kidney disease |
8 (4.19) | 5 (6.41) | 0 (0) | 2 (3.77) | 0 (0) | 1 (2.13) |
| Urinary calculus | 8 (4.19) | 3 (3.85) | 0 (0) | 1 (1.89) | 1 (100) | 3 (6.38) |
| Psychiatric | 169 (6.05) | 63 (5.43) | 6 (3.87) | 52 (6.82) | 2 (2.44) | 46 (7.26) |
| Depression | 44 (26.0) | 15 (23.8) | 2 (33.3) | 21 (40.4) | 1 (50.0) | 5 (10.9) |
| Drug abuse/withdrawal | 40 (23.7) | 13 (20.6) | 0 (0) | 12 (23.1) | 1 (50.0) | 14 (30.4) |
| Psychosis/schizophrenia | 34 (20.1) | 15 (23.8) | 1 (16.7) | 6 (11.5) | 0 (0) | 12 (26.1) |
| Pulmonary | 157 (5.62) | 59 (5.09) | 4 (2.58) | 42 (5.51) | 5 (6.10) | 47 (7.41) |
| Asthma/COPD | 71 (45.2) | 25 (42.4) | 0 (0) | 21 (50.0) | 0 (0) | 25 (53.2) |
| Acute respiratory failure | 31 (19.8) | 10 (17.0) | 1 (25.0) | 9 (21.4) | 1 (20.0) | 10 (21.3) |
| Pleural effusion | 8 (5.10) | 6 (10.2) | 0 (0) | 1 (2.38) | 0 (0) | 1 (2.13) |
| Endocrine | 145 (5.19) | 76 (6.55) | 5 (3.23) | 37 (4.86) | 6 (7.32) | 21 (3.31) |
| Electrolyte abnormalities | 62 (42.8) | 32 (42.1) | 2 (40.0) | 14 (37.8) | 2 (33.3) | 12 (57.1) |
| Diabetes | 40 (27.6) | 16 (21.0) | 3 (60.0) | 14 (37.8) | 2 (33.3) | 5 (23.8) |
| Cachexia | 9 (6.21) | 6 (7.89) | 0 (0) | 2 (5.41) | 1 (16.7) | 0 (0) |
| Non-AIDS-Defining Cancer | 140 (5.01) | 53 (4.57) | 11 (7.10) | 38 (4.99) | 2 (2.44) | 36 (5.68) |
| Lymphoma | 41 (29.3) | 25 (47.2) | 1 (9.09) | 9 (23.7) | 0 (0) | 6 (16.7) |
| Liver cancer | 10 (7.14) | 0 (0) | 0 (0) | 7 (18.4) | 1 (50.0) | 2 (5.56) |
| Lung cancer | 6 (4.29) | 2 (3.77) | 0 (0) | 3 (7.89) | 0 (0) | 1 (2.78) |
| Injury/Poisoning | 118 (4.22) | 45 (3.88) | 4 (2.58) | 39 (5.12) | 3 (3.66) | 27 (4.26) |
| Device/procedure complications | 35 (29.7) | 17 (37.8) | 1 (25.0) | 12 (30.8) | 0 (0) | 5 (18.5) |
| Poisoning | 32 (27.1) | 7 (15.6) | 1 (25.0) | 15 (38.5) | 1 (33.3) | 8 (29.6) |
| Fracture | 24 (20.3) | 9 (20.0) | 1 (25.0) | 6 (15.4) | 2 (66.7) | 6 (22.2) |
Diagnostic categories and individual diagnoses are listed in order of frequency in the overall study population. The number of hospitalizations during 2010 that were associated with each diagnostic category and individual diagnosis are reported. The percentage listed for each diagnostic category represents the percentage of all hospitalizations associated with that diagnostic category. The percentage listed for each individual diagnosis represents the percentage of hospitalizations within that diagnostic category. See Appendix Table for ICD-9 codes used to identify each diagnosis.
CAD coronary artery disease; MI myocardial infarction; COPD chronic obstructive pulmonary disease
Complication of cirrhosis includes admissions for cirrhosis, hepatic encephalopathy, portal hypertension, and ascites
In our sensitivity analysis, 67 participants from the unknown hepatitis serostatus group were recategorized as HIV/HBV co-infected and 291 as HIV/HCV co-infected on the basis of one positive serology and an unknown second hepatitis serology. Multivariable models using this definition of hepatitis serostatus yielded similar results to our original analysis. Compared to HIV mono-infection, the relative rate of all-cause hospitalization was again increased in those with HIV/HBV (IRR 1.50 [1.15–1.97]), HIV/HCV (IRR 1.38 [1.16–1.65]) and HIV/HBV/HCV (IRR 1.52 [1.04–2.22]). Inferences about the relationship between hepatitis serostatus and risk of diagnostic category-specific hospitalizations were also unchanged (data not shown).
DISCUSSION
This study is the first to demonstrate, in a contemporary cohort of PLWH, that hospitalization rates are higher among patients with HBV and/or HCV co-infection. This finding is consistent with prior studies demonstrating high rates of morbidity and mortality in co-infected populations2,5–13,16. Since hospitalizations are a significant driver of healthcare costs among PLWH, the higher frequency of hospitalization in hepatitis co-infected populations results in increased healthcare costs for these populations19. Policy-makers should be aware of the financial implications of co-infection among PLWH as they allocate scarce healthcare resources and establish capitated costs for accountable care organizations.
Non-AIDS-defining infections accounted for about a quarter of all hospital admissions, and the relative risk of hospitalization for this reason was elevated among patients in all the hepatitis-infected categories. Consistent with prior studies, the most common non-AIDS-defining infection in our study was bacterial pneumonia24–26. Chronic viral hepatitis is known to be associated with dysregulation of hepatitis-specific immune responses, but further investigation is needed to explore potential mechanisms underlying an increased risk of bacterial infections27,28. Preventable infections such as influenza and pneumococcal pneumonia should be proactively addressed in PLWH with appropriate vaccinations to potentially reduce morbidity and hospitalizations29,30.
While complications of cirrhosis among PLWH with viral hepatitis co-infection deserve attention due to their seriousness and their associations with mortality, such hospitalizations accounted for only 2.8% (28 out of 999, Table 3) of all hospitalizations among the three viral hepatitis groups combined5–9. We did not perform formal diagnostic tests specifically on complications of cirrhosis because of small sample sizes. By way of comparison, however, they accounted for 0.3% (4 out of 1,160) of all hospitalizations among HIV mono-infected persons.
The increased risk for hospitalization due to psychiatric disease in the HIV/HCV co-infected group highlights the need for mental health care in this population. The most common diagnosis among psychiatric admissions was depression. Although drug use may play a role in hospitalizations related to depression, this finding is consistent with existing evidence that HIV/HCV co-infection is associated with higher prevalence and severity of neuropsychiatric disease than either infection alone16,31. Integrated mental health and HIV care programs have been shown to improve rates of HIV viral suppression, retention in care, substance abuse and psychiatric symptoms as well as decrease hospitalization costs32,33. Co-location of mental health services may offer particular benefit to the HIV/HCV co-infected population.
Interestingly, we did not observe significantly increased risk of hospitalization for renal, cardiovascular or endocrine diagnoses among HIV/HCV co-infected patients, despite evidence that morbidity and mortality related to such diagnoses are increased with HCV co-infection 10–13. Our study may not have included enough cardiovascular events to detect a statistically significant difference. Our adjusted relative risk estimates for renal and endocrine hospitalizations, on the other hand, suggested no trend towards increased hospitalizations for these diagnoses. One possible explanation for this discrepancy is that these complications are being successfully managed in the outpatient setting and, while present, are not contributing to excess hospitalizations.
Therapy directed against hepatitis B and/or hepatitis C has been shown to decrease progression to cirrhosis among co-infected PLWH34. Further investigation is needed to evaluate the effects of hepatitis therapy on all-cause and cause-specific hospitalization rates. Treatment of HBV is common among PLWH, and more than 75% of HIV/HBV co-infected patients in the HIVRN are prescribed agents with activity against both HIV and HBV (Moore RD, Personal Communication on 31 May 2013). Conversely, prior studies have reported treatment rates of only 20–40% for HCV in the routine clinical care of PLWH, with less than half of these persons achieving sustained virologic response35,36. With the development of more effective and better tolerated anti-HCV medications, increased utilization of anti-HCV therapy among co-infected patients is expected in the near future37,38. If hepatitis therapy decreases hospitalization rates, this could provide an economic counterbalance to the high cost of treating hepatitis, especially with the newest anti-HCV medications38,39.
A potential limitation of this study is the reliance on hepatitis C antibodies as evidence of hepatitis C co-infection. Unfortunately, HCV RNA data were not available to confirm chronic infection. The bias introduced by misclassifying patients who cleared HCV viremia as being chronically infected would likely make the HIV mono-infected and HIV/HCV co-infected groups appear more similar. Inferences made based on significant differences between these groups should therefore be robust despite the misclassification. Also, HIV co-infection decreases spontaneous clearance of HCV to fewer than 10% of cases, so it is expected that most patients in this analysis with positive anti-HCV were chronically infected with HCV40.
Use of ICD-9 codes to determine cause for hospitalization may be less accurate than physician chart review, although validation studies within individual institutions have suggested high concordance with chart review21. Hospitalizations occurring outside of each patient’s HIV care institution may not be completely captured, though efforts are made by all HIVRN sites to capture utilization data from neighboring hospitals. The inclusion of relatively few patients with HIV/HBV/HCV tri-infection in this study limits our power to draw conclusions about this unique patient population. The population of patients at HIVRN sites is not nationally representative and our findings may not be generalizable to populations served by smaller clinics, located in more rural settings, or cared for by providers with less HIV subspecialty experience.
This study demonstrates that chronic viral hepatitis is associated with increased risk of hospitalization and therefore increased healthcare costs among PLWH. Policy-makers and third-party payers should be aware of the heightened risk of hospitalization associated with co-infection when allocating healthcare resources and considering models of healthcare delivery. Our findings also underscore the importance of targeting patients who are co-infected with HIV and viral hepatitis with preventive measures such as routine vaccinations and integrated mental health services that may help to curb their increased risk of hospitalization. Further investigation is needed to evaluate the effects of therapy against hepatitis on hospitalization rates.
ACKNOWLEDGMENTS
HIVRN Participating Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children's Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Community Health Network, Rochester, New York (Roberto Corales, D.O.)
Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.)
Fenway Health, Boston, Massachusetts (Stephen Boswell, M.D.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., M.P.H., Richard Moore, M.D., M.H.S., Allison Agwu, M.D., Sc.M.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.)
Montefiore Medical Center, Bronx, New York (Lawrence Hanau, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.)
St. Jude's Children's Hospital and University of Tennessee, Memphis, Tennessee (Aditya Gaur, M.D.)
St. Luke's Roosevelt Hospital Center, New York, New York (Victoria Sharp, M.D., Stephen Arpadi, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
Wayne State University, Detroit, Michigan (Jonathan Cohn, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D., Faye Malitz, M.S.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., M.H.S., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., M.P.H., Cindy Voss, M.A.)
Source of Funding: This work was supported by the Agency for Healthcare Research and Quality (HHSA290201100007C), the Health Resources and Services Administration (HHHSH250201200008C), the National Institute of Allergy and Infectious Diseases (K23 AI084854), and the National Institute on Drug Abuse (R01 DA016078).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Disclaimer: The views expressed in this paper are those of the authors. No official endorsement by DHHS, the National Institutes of Health, or the Agency for Healthcare Research and Quality is intended or should be inferred.
REFERENCES
- 1.Thomas DL, Leoutsakas D, Zabransky T, Kumar MS. Hepatitis C in HIV-infected individuals: cure and control, right now. Journal of the International AIDS Society. 2011;14:22. doi: 10.1186/1758-2652-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber R, Sabin C, Reiss P, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antiviral therapy. 2010;15(8):1077–1086. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 3.Sollima S, Caramma I, Menzaghi B, et al. Chronic coinfection with hepatitis B and hepatitis C viruses in an Italian population of HIV-infected patients. J Acquir Immune Defic Syndr. 2007 Apr 15;44(5):606–607. doi: 10.1097/QAI.0b013e318031d5b5. [DOI] [PubMed] [Google Scholar]
- 4.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Mar 15;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 5.Thio CL, Seaberg EC, Skolasky R, Jr., et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002 Dec 14;360(9349):1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 6.Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. Journal of hepatology. 2007 Oct;47(4):527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Salmon-Ceron D, Rosenthal E, Lewden C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalite 2005 study. Journal of hepatology. 2009 Apr;50(4):736–745. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006 Aug 14–28;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 9.Castellares C, Barreiro P, Martin-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. Journal of viral hepatitis. 2008 Mar;15(3):165–172. doi: 10.1111/j.1365-2893.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. The Journal of infectious diseases. 2012 Aug 15;206(4):469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 11.Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatology international. 2011 Jun 23; doi: 10.1007/s12072-011-9284-9. [DOI] [PubMed] [Google Scholar]
- 12.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV medicine. 2010 Aug;11(7):462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 13.Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World journal of gastroenterology : WJG. 2012 Apr 14;18(14):1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himoto T, Masaki T. Extrahepatic manifestations and autoantibodies in patients with hepatitis C virus infection. Clinical & developmental immunology. 2012;2012:871401. doi: 10.1155/2012/871401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiefke I, Fach A, Wiedmann M, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World journal of gastroenterology : WJG. 2005 Mar 28;11(12):1843–1847. doi: 10.3748/wjg.v11.i12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford DB, Evans SR, Yang Y, Gulick RM. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005 Oct;(19 Suppl 3):S64–S71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- 17.Crum-Cianflone NF, Grandits G, Echols S, et al. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use. J Acquir Immune Defic Syndr. 2010 Jul;54(3):248–257. doi: 10.1097/qai.0b013e3181c8ef22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry SA, Fleishman JA, Moore RD, Gebo KA. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. 2012 Apr 1;59(4):368–375. doi: 10.1097/QAI.0b013e318246b862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010 Nov 13;24(17):2705–2715. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hospital and outpatient health services utilization among HIV-infected patients in care in 1999. J Acquir Immune Defic Syndr. 2002 May 1;30(1):21–26. doi: 10.1097/00126334-200205010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Gebo KA, Diener-West M, Moore RD. Hospitalization rates differ by hepatitis C satus in an urban HIV cohort. J Acquir Immune Defic Syndr. 2003 Oct 1;34(2):165–173. doi: 10.1097/00126334-200310010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Clinical Classifications Software (CCS), 2013. Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2013. [computer program] [Google Scholar]
- 23.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 1992 Dec 18;41(RR-17):1–19. [PubMed] [Google Scholar]
- 24.Sogaard OS, Lohse N, Gerstoft J, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995–2007: a Danish population-based, nationwide cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Nov 15;47(10):1345–1353. doi: 10.1086/592692. [DOI] [PubMed] [Google Scholar]
- 25.Kohli R, Lo Y, Homel P, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006 Jul 1;43(1):90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 26.Mussini C, Galli L, Lepri AC, et al. Incidence, Timing, and Determinants of Bacterial Pneumonia Among HIV-Infected Patients: Data From the ICONA Foundation Cohort. J Acquir Immune Defic Syndr. 2013 Jul 1;63(3):339–345. doi: 10.1097/QAI.0b013e318295ab85. [DOI] [PubMed] [Google Scholar]
- 27.King E, Trabue C, Yin D, Yao ZQ, Moorman JP. Hepatitis C: the complications of immune dysfunction. Expert review of clinical immunology. 2007 Mar;3(2):145–157. doi: 10.1586/1744666X.3.2.145. [DOI] [PubMed] [Google Scholar]
- 28.Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. The Lancet infectious diseases. 2007 Dec;7(12):804–813. doi: 10.1016/S1473-3099(07)70289-X. [DOI] [PubMed] [Google Scholar]
- 29.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. The New England journal of medicine. 2010 Mar 4;362(9):812–822. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. The Journal of infectious diseases. 2012 Oct;206(8):1250–1259. doi: 10.1093/infdis/jis487. [DOI] [PubMed] [Google Scholar]
- 31.Hilsabeck RC, Castellon SA, Hinkin CH. Neuropsychological aspects of coinfection with HIV and hepatitis C virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 Jul 1;(41 Suppl 1):S38–S44. doi: 10.1086/429494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winiarski MG, Beckett E, Salcedo J. Outcomes of an inner-city HIV mental health programme integrated with primary care and emphasizing cultural responsiveness. AIDS care. 2005 Aug;17(6):747–756. doi: 10.1080/09540120412331336733. [DOI] [PubMed] [Google Scholar]
- 33.Weaver MR, Conover CJ, Proescholdbell RJ, et al. Cost-effectiveness analysis of integrated care for people with HIV, chronic mental illness and substance abuse disorders. The journal of mental health policy and economics. 2009 Mar;12(1):33–46. [PubMed] [Google Scholar]
- 34.Tuma P, Medrano J, Resino S, et al. Incidence of liver cirrhosis in HIV-infected patients with chronic hepatitis B or C in the era of highly active antiretroviral therapy. Antiviral therapy. 2010;15(6):881–886. doi: 10.3851/IMP1630. [DOI] [PubMed] [Google Scholar]
- 35.Reiberger T, Obermeier M, Payer BA, et al. Considerable under-treatment of chronic HCV infection in HIV patients despite acceptable sustained virological response rates in a real-life setting. Antiviral therapy. 2011;16(6):815–824. doi: 10.3851/IMP1831. [DOI] [PubMed] [Google Scholar]
- 36.Vellozzi C, Buchacz K, Baker R, et al. Treatment of hepatitis C virus (HCV) infection in patients coinfected with HIV in the HIV Outpatient Study (HOPS), 1999–2007. Journal of viral hepatitis. 2011 May;18(5):316–324. doi: 10.1111/j.1365-2893.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 37.Sulkowski M, Pol S, Mallolas J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. The Lancet infectious diseases. 2013 Jul;13(7):597–605. doi: 10.1016/S1473-3099(13)70149-X. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DL, Bartlett JG, Peters MG, Sherman KE, Sulkowski MS, Pham PA. Provisional guidance on the use of hepatitis C virus protease inhibitors for treatment of hepatitis C in HIV-infected persons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Apr;54(7):979–983. doi: 10.1093/cid/cir882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas DL. Curing hepatitis C with pills: a step toward global control. Lancet. 2010 Oct 30;376(9751):1441–1442. doi: 10.1016/S0140-6736(10)61497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA : the journal of the American Medical Association. 2000 Jul 26;284(4):450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]