Abstract
The term anhedonia has long been used in the psychiatric literature to describe reward-processing dysfunction in psychopathology, especially depression and schizophrenia. Although anhedonia literally describes a lack of pleasurable experiences in everyday life, recent advances in both the basic science and clinical literatures indicate that reward deficits in these disorders are much broader than hedonic responses. In this article, we summarize some of the recent theoretical and empirical advances in understanding deficits in reward processing and their neurobehavioral mechanisms, with a particular focus on the neural underpinnings of motivation and effort-based decision making. We also highlight the potential of translational neuroscience to enhance diagnostic clarity by defining clinical symptoms in terms of underlying pathophysiology.
Keywords: anhedonia, depression, schizophrenia, dopamine, motivation, nosology
Imagine it is Friday, and you have plans to meet up with a small group of friends for what would normally be an enjoyable evening. However, tonight it feels like the gathering will be more draining than pleasurable, and you would much prefer to stay in by yourself. In fact, over the last few weeks, you have been less interested in most social activities as well as most of your hobbies. It is not that you have had less time or fewer opportunities, but you were unmotivated to pursue them; and even when you did, you ended up feeling bored and checking your watch the whole time. Distressed by this, you might wonder whether there was a name for what you were feeling, at which point you would likely discover that you were experiencing what clinical psychologists and psychiatrists call anhedonia, which is a common symptom in patients with major depression or schizophrenia
The term anhedonia is generally defined as a significant reduction in the capacity to experience pleasure. It is important to note that this definition implies a causal explanation for your hypothetical bout of listlessness: You are feeling less motivated and interested in various activities because you are enjoying them less. This makes some intuitive sense; after all, does not everyone reduce their motivation for something if they begin to enjoy it less? Perhaps, but a wealth of data suggests that it is possible to be less motivated for a reward without enjoying it any less or to continue chasing something long after the pleasure of its attainment has faded away. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994) tries to address this issue by defining anhedonia in the context of depression as a lack of interest or pleasure. Although this definition is more inclusive, it treats motivation and hedonic aspects of anhedonia as being equivalent. Yet, such equivalence has found little support in empirical research (Berridge & Robinson, 2003; Salamone, Correa, Farrar, & Mingote, 2007). In this review, we describe (a) how the multifaceted nature of reward processing raises important conceptual questions regarding the ontology of anhedonia and (b) how translational neuroscience may help improve clinical diagnosis and treatment of disorders with reward-processing abnormalities.
Anhedonia and Study of Reward Processing
Though often discussed as a singular entity, reward processing is not a unitary construct. Behavioral scientists have identified a number of or subdomains within the reward-processing construct, including incentive salience, motivation, cost–benefit decision making, hedonic responses, and action–outcome association updating, to name but a few (Berridge & Robinson, 2003; Kahneman, Wakker, & Sarin, 1997; Rangel, Camerer, & Montague, 2008). Psychiatric disorders often involve alterations in many of these subdomains, whereas clinical assessments have either exclusively focused on hedonic capacity or have treated all aspects of reward processing as functionally equivalent.
The emphasis on hedonic deficits as opposed to other reward processes reflects both historical precedence and the frequency of anhedonic complaints in patient populations. Patients with mood or thought disorders reliably endorse diminished enjoyment when assessed by self-report and clinical interview (Watson & Naragon-Gainey, 2009). However, quantifying enjoyment and isolating it from other aspects of reward processing is a tricky business (Havermans, 2011), as past decisions about what activities to partake in may influence these reports (Ariely & Norton, 2008; Brehm, 1956).
The complexity increases when subjects are asked to rate hypothetical or future rewards, commonly referred to as affective forecasting. A large empirical literature documents people’s startlingly poor ability to accurately estimate how much they will enjoy an anticipated reward (Wilson & Gilbert, 2005). Difficulty in affective forecasting may provide the appearance of deficits in consummatory reward experience, despite normal hedonic response to rewards when they are received. For example, when asked to rate their enjoyment of pleasurable stimuli in the moment, patients with schizophrenia show no differences from controls (Barch & Dowd, 2010; Strauss & Gold, 2012). In patients with depression, a similar lack of group differences is observed for certain types of stimuli, such as sweet tastes (for a broader discussion, see Treadway & Zald, 2011). However, patients with both depression and schizophrenia report significantly reduced enjoyment when asked to rate prospective, retrospective, or hypothetical experiences, as is common in symptom inventories (Strauss & Gold, 2012; Watson & Naragon-Gainey, 2009). Taken together, these studies suggest that although diminished enjoyment may play a role in these disorders, standard clinical assessment methodologies may obfuscate the presence of multiple reward-processing deficits in clinical populations.
How Translational Neuroscience Can Help
Translational neuroscience is organized around the principle of leveraging preclinical models of brain and behavior to guide clinical neuroscience investigations into the pathophysiology of neurological and psychiatric disorders. The problem of accurately assessing abnormalities, such as the reward deficits described in the previous section, is not uncommon to medicine; a single medical symptom can frequently result from multiple pathologies (equifinality). Consider the example of fever, which can arise following either a viral or bacterial infection, among other causes. The mere presence of a fever does not aid in differential diagnosis, much like a subject’s report of reduced enjoyment does not necessarily differentiate between impaired motivation and decreased hedonic capacity. However, because the pathophysiology of viral and bacterial infections is well understood, tests can be performed to isolate the cause of infection in each case and to determine the best treatment. A similar scenario may one day be possible for reported decreases in pleasure, in which specific biological tests will help determine whether these experiences result from one or more reward-processing deficits. Though we lack this ability at present, translational research approaches are already aiding in the identification of distinct behavioral and neural markers of reward processing, particular those underlying motivational deficits in psychopathology.
This work began by drawing on animal models suggesting that the mesolimbic dopamine (DA) system may be selectively involved in reward motivation but not hedonic response. The mesolimbic DA system encompasses a specific subpopulation of DA neurons that innervate the ventral striatum, a key region involved in the processing of reward-relevant information (Haber & Knutson, 2010). Early evidence for the role of mesolimbic DA in motivation was provided by effort-based, decision-making tasks in rodents. In these paradigms, animals must choose whether to consume freely available but less desirable food rewards (low effort) or to exert physical effort in exchange for more palatable food rewards (high effort). Healthy rats exhibit a strong preference for the high effort option, whereas attenuation or blockade of DA—especially in the ventral striatum—results in a behavioral shift toward low effort options (Cousins & Salamone, 1994; Salamone et al., 2007). Critically, DA blockade does not reduce overall consumption, highlighting a selective role in willingness to work rather than appetitive drive. Moreover, potentiation of DA produces the opposite effects, resulting in an increased willingness to work for preferred rewards (Bardgett, Depenbrock, Downs, Points, & Green, 2009). In contrast to this strong evidence for DA in motivation, attenuation or even complete absence of DA appears to have little effect on measures of hedonic response, including sucrose preference and hedonic facial reactions (for a review, see Berridge & Kringelbach, 2008).
To translate these findings to clinical research, several groups have begun to explore the relationship between DA and motivation by adapting effort-based, decision-making paradigms to humans. One such task developed by our group is the Effort-Expenditure for Rewards Task (EEfRT, pronounced “effort”; Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009). During this task, subjects perform a series of trials in which they are asked to choose between completing a high effort task and a low effort task to obtain varying levels of monetary rewards.
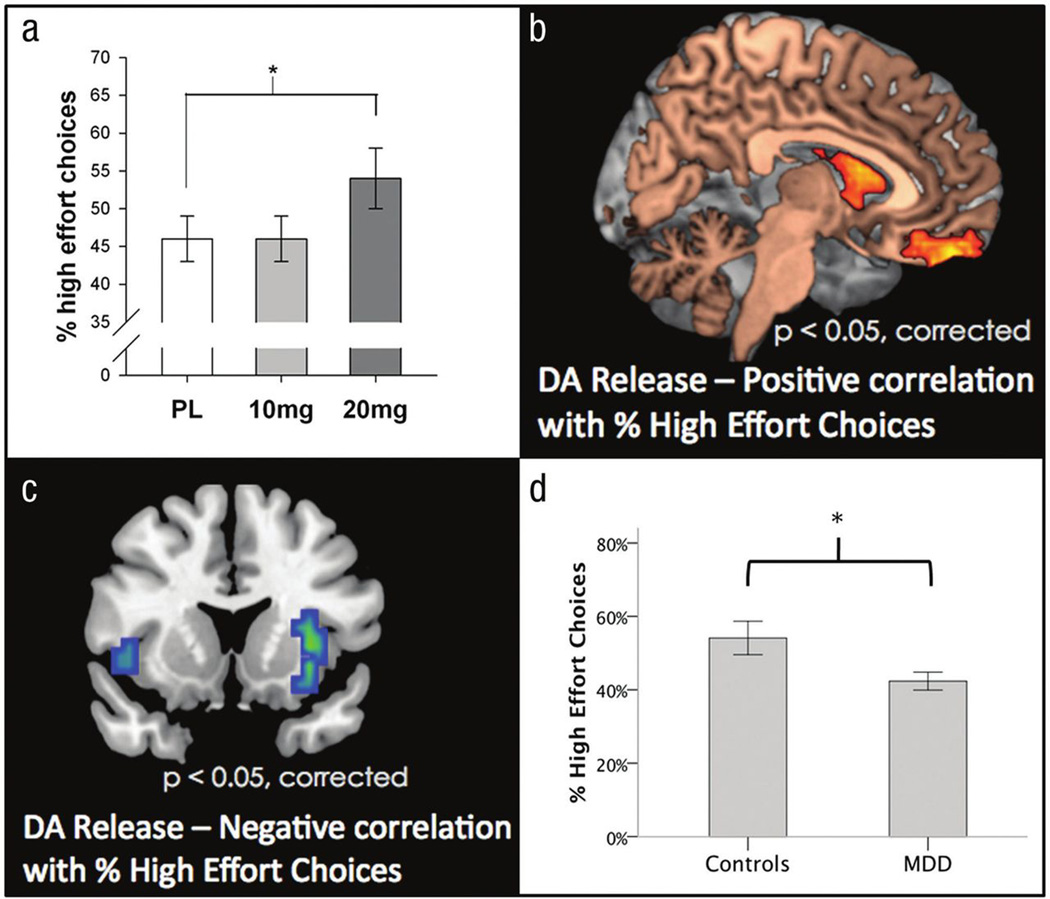
Using the EEfRT and related tasks, recent studies have begun to map out the role of mesolimbic DA circuitry in normal and abnormal reward motivation. Mirroring the effects of DA potentiation in rats, we found that administration of the DA agonist d-amphetamine produced a dose-dependent increase in the willingness to work for rewards as assessed by the EEfRT (Wardle, Treadway, Mayo, Zald, & de Wit, 2011; see Figure 1A). These effects were strongest during trials for which probability of reward receipt was low, suggesting that DA may be involved in helping animals overcome probabilistic discounting as well as effort related response costs. Similar effects of DA enhancement with the DA precursor L-Dopa have been observed on measures of reward anticipation and an optimism bias (Sharot, Guitart-Masip, Korn, Chowdhury, & Dolan, 2012; Sharot, Shiner, Brown, Fan, & Dolan, 2009)—two constructs that are closely related to motivation.
Figure.
Associations among dopamine (DA), depression, and the Effort-Expenditure for Rewards Task. (A) Amphetamine increases the percentage of high effort choices (Wardle, Treadway, Mayo, Zald, & de Wit, 2011). PL = Placebo. Error bars represent the standard deviation. *p < .05. (B) Positron emission tomography imaging shows that individual differences in the percentage of high effort choices are positively associated with the magnitude of amphetamine-induced DA release in striatum and are (C) negatively associated with DA release in the insula (Treadway, Buckholtz, et al., 2012). (D) Patients with major depressive disorder (MDD) chose fewer high effort choices than matched controls (Treadway, Bossaller, Shelton, & Zald, 2012). Error bars represent the standard error of the mean. *p < .05.
To further elucidate the role of DA function as a predictor of individual differences in motivation, we used DA receptor positron emission tomography imaging to test associations between amphetamine-induced DA release (a probe of DA system reactivity) and willingness to work for rewards during the EEfRT (Treadway, Buckholtz, et al., 2012). Here, we found that the magnitude of DA release in the striatum positively predicted the proportion of high effort choices that subjects made during low probability trials (see Figure 1B). Localization to this region is consistent with preclinical findings (Cousins & Salamone, 1994; Salamone et al., 2007) as well as human functional neuroimaging studies (Croxson, Walton, O’Reilly, Behrens, & Rushworth, 2009; Kurniawan et al., 2010; Schmidt, Lebreton, Clery-Melin, Daunizeau, & Pessiglione, 2012). Intriguingly, in our study we also found a negative relationship between the percentage of high effort choices and DA release in the insula (see Figure 1C). Although insula DA function has not been examined in rodent models of effort-based decision making, one prior functional magnetic resonance imaging study showed increased insula activation when subjects chose not to expend effort (Prevost, Pessiglione, Metereau, Clery-Melin, & Dreher, 2010). Although further investigation is necessary, these data suggest that the insula and striatum may play somewhat antagonistic roles in determining whether an individual is willing to overcome effort costs.
Human studies also support the absence of DAergic effects on hedonic responsiveness reported in preclinical studies (Berridge & Kringelbach, 2008). This may seem surprising, as many DA-acting drugs, such as amphetamine and cocaine, are associated with subjective reports of euphoria. However, when these drugs are taken in conjunction with an opioid antagonist, euphoria is significantly diminished (Jayaram-Lindstrom, Wennberg, Hurd, & Franck, 2004). In contrast, subtler augmentations of DA, such as L-Dopa administration, show no effect on hedonic responses to rewarding stimuli (Liggins, Pihl, Benkelfat, & Leyton, 2012; Sharot et al., 2012, 2009), despite clear effects on anticipatory reward processing (Sharot et al., 2012, 2009). In the opposite direction, transient reduction of DA with an amino-acid depletion protocol resulted in less willingness to work for rewards during a progressive ratio task (Venugopalan et al., 2011) and increased subjective experiences of boredom and apathy, as well as reduced willingness to overcome probabilistic costs during a gambling task (McLean, Rubinsztein, Robbins, & Sahakian, 2004).
Taken together, clinical findings regarding the role of DA in human motivation parallel preclinical findings. Building upon this translational parity, clinical researchers have recently employed effort-based, decision-making paradigms in psychiatric populations. Using the EEfRT, we found that patients with depression were less willing to expend effort for rewards as compared with controls, which is consistent with a hypothesis that depression is associated with a core motivational deficit (Treadway, Bossaller, et al., 2012; see Figure 1D). It is worth noting that this deficit was most pronounced during trials in which rewards were larger or more certain, and normally would cause a greater willingness to expend more effort. Using a related effort-expenditure task, Gold et al. (2012) also observed a similar pattern of results in a sample of subjects with schizophrenia. The failure of reward incentives to modulate effort-expenditure was also reported in a separate depression study, in which patients with major depressive disorder (MDD) both exerted less force than controls (raw force only; not normalized per subject) and failed to modulate their effort expenditure on the basis of monetary incentives (Clery-Melin et al., 2011). Sherdell, Waugh, and Gotlib (2011), who examined the willingness of patients with MDD to expend effort to view humorous cartoons, also found evidence for reduced sensitivity to reward-predicting cues, but they did not observe any differences in effort-expenditure.
It is notable that the most consistent finding across these studies is a failure to modulate effort-production as a function of reward information. This deficit was present across studies with and without overall reductions in effort-expenditure and is consistent with findings from prior studies of cue-evoked reward anticipation and reward learning in populations with MDD or schizophrenia (Juckel et al., 2006; Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008; Smoski et al., 2009). Taken together, these findings suggest a consistent deficit across experimental paradigms and diagnostic categories in which reward-relevant information does not result in the optimal recruitment of neural systems involved in motivation, learning, and salience. Although further studies will be required to clarify the best interpretation of these data, they cannot be explained by a simple deficit in “enjoying” the reward.
Clinical Implications.
Though it will be some time before we are able to use neural assessments to differentially diagnosis reward-processing pathologies, the value of translational neuroscience is already evident. Using translational approaches, the field is identifying specific motivational circuits that may have transdiagnostic implications for reward-processing deficits in multiple disorders. For example, in addition to depression and schizophrenia, motivational deficits are frequently observed in chronic fatigue syndrome, Parkinson’s disease, as well as in response to proinflammatory medical treatments (Dantzer, Meagher, & Cleeland, 2012). Of note, these latter manifestations of motivational impairment are often discussed in terms of “fatigue” or “low energy” rather than reductions in pleasure and motivation, despite potentially overlapping biological mechanisms (Capuron et al., 2012). This suggests the possibility of a shared pathophysiology, which may have been masked by differences in terminology.
Another implication of this research is the need for caution when the term anhedonia is used. Previously, we have recommended the adoption of the term motivational anhedonia to distinguish between motivational and consummatory deficits (Treadway & Zald, 2011), whereas others have suggested abandoning the term anhedonia all together (Strauss & Gold, 2012). What is clear is that precision in the description of different types of reward-processing deficits will be necessary if clinical practice is to take full advantage of advances in the understanding of the neurobiology of reward processing.
In sum, we have articulated the potential value of translational neuroscience in providing diagnostic clarity to the study of reward-related symptoms in psychopathology. Given recent advances in basic science and translational validation, we are now poised to leverage these approaches to better understand and even potentially reclassify these symptoms in terms of biologically derived constructs and measures.
Acknowledgments
Funding
The authors report no financial conflicts of interest. This work was supported by National Institute on Drug Abuse Grant R01 DA019670 (to David H. Zald), National Institute of Mental Health Grants R21 MH092751 (to David H. Zald) and F31 MH087015 (to Michael T. Treadway), and National Center for Advancing Translational Sciences Grant UL1 TR000445 (to the Vanderbilt Institute for Clinical and Translational Research).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: 1994. [Google Scholar]
- Ariely D, Norton MI. How actions create—Not just reveal—Preferences. Trends in Cognitive Sciences. 2008;12:13–16. doi: 10.1016/j.tics.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behavioral Neuroscience. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Brehm JW. Postdecision changes in the desirability of alternatives. Journal of Abnormal Psychology. 1956;52:384–389. doi: 10.1037/h0041006. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Miller AH. Dopaminergic mechanisms of reduced Basal Ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clery-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE. 2011;6:e23178. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacology Biochemistry and Behavior. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. Journal of Neuroscience. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature Reviews: Clinical Oncology. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.022. epub: Feb2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermans RC. “You say it’s liking, I say it’s wanting” On the difficulty of disentangling food reward in man. Appetite. 2011;57:286–294. doi: 10.1016/j.appet.2011.05.310. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. Journal of Clinical Psychopharmacology. 2004;24:665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Wakker PP, Sarin R. Back to Bentham? Explorations of experience utility. The Quarterly Journal of Economics. 1997;112:375–405. [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: The role of striatum in signaling physical effort of a chosen action. Journal of Neurophysiology. 2010;104:313–321. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins J, Pihl RO, Benkelfat C, Leyton M. The dopamine augmenter L-DOPA does not affect positive mood in healthy human volunteers. PLoS ONE. 2012;7:e28370. doi: 10.1371/journal.pone.0028370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean A, Rubinsztein JS, Robbins TW, Sahakian BJ. The effects of tyrosine depletion in normal healthy volunteers: Implications for unipolar depression. Psychopharmacology. 2004;171:286–297. doi: 10.1007/s00213-003-1586-8. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost C, Pessiglione M, Metereau E, Clery-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. Journal of Neuroscience. 2010;30:14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews: Neuroscience. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology. 2012;10:e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Guitart-Masip M, Korn CW, Chowdhury R, Dolan RJ. How dopamine enhances an optimism bias in humans. Current Biology. 2012;22:1477–1481. doi: 10.1016/j.cub.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Shiner T, Brown AC, Fan J, Dolan RJ. Dopamine enhances expectation of pleasure in humans. Current Biology. 2009;19:2077–2080. doi: 10.1016/j.cub.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology. 2011;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. American Journal of Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O’Hara C, O’Loughlin J, Benkelfat C, Fellows LK, Leyton M. Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology. 2011;36:2469–2476. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: Effects of d-amphetamine on human effort-based decision-making. Journal of Neuroscience. 2011;31:16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2009;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TD, Gilbert DT. Affective forecasting: Knowing what to want. Current Directions in Psychological Science. 2005;14:131–134. [Google Scholar]
Recommended Reading
- Barch DM, Dowd EC. 2010 (See References). A thorough and lucid review of reward processing deficits in schizophrenia.
- Salamone JD, Correa M, Farrar A, Mingote SM. 2007 doi: 10.1007/s00213-006-0668-9. (See References). Comprehensive theoretical review of dopamine’s role in motivated responding in preclinical studies.
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences, USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. An elegant demonstration of the respectively dissociable effects of dopamine and opioid systems on motivational and hedonic aspects of reward processing in rodents.
- Treadway MT, Zald DH. 2011 (See References). A detailed review of reward processing in deficits in major depressive disorder.
- Venugopalan VV, Casey KF, O’Hara C, O’Loughlin J, Benkelfat C, Fellows LK, Leyton M. 2011 doi: 10.1038/npp.2011.135. (See References). Translational evidence for role of dopamine on motivation in humans.