Abstract
Mismanagement of the composting process can result in emissions of CH4, N2O, and NH3, which have caused severe environmental problems. This study was aimed at determining whether CH4, N2O, and NH3 emissions from composting are affected by bulking agents during rapid composting of pig manure from the Chinese Ganqinfen system. Three bulking agents, corn stalks, spent mushroom compost, and sawdust, were used in composting with pig manure in 60 L reactors with forced aeration for more than a month. Gas emissions were measured continuously, and detailed gas emission patterns were obtained. Concentrations of NH3 and N2O from the composting pig manure mixed with corn stalks or sawdust were higher than those from the spent mushroom compost treatment, especially the sawdust treatment, which had the highest total nitrogen loss among the three runs. Most of the nitrogen was lost in the form of NH3, which accounts for 11.16% to 35.69% of the initial nitrogen. One-way analysis of variance for NH3 emission showed no significant differences between the corn stalk and sawdust treatments, but a significant difference was noted between the spent mushroom compost and sawdust treatments. The introduction of sawdust reduced CH4 emission more than the corn stalks and spent mushroom compost. However, there were no significant differences among the three runs for total carbon loss. All treatments were matured after 30 d.
Keywords: Organic waste, Composting, Bulking agent, Gaseous emission, Pig manure
1. Introduction
China’s agricultural industry has been intensified significantly since the early 1980s. However, intensive agricultural activities generate large amounts and different types of organic waste (Dias et al., 2010). Therefore, the pressure to properly handle the organic waste has been mounting in China. Composting, one of the most effective technologies for treating organic waste, can reduce the mass and volume of the waste, destroy the pathogens and weed seeds, and provide organic fertilizers as valuable end-products for agriculture. However, harmful gases emitted from mismanagement of the composting process have caused severe environmental problems (He et al., 2000; Fukumoto et al., 2003; Szanto et al., 2007). Ammonia (NH3), among others, can cause acidification and nutrient loss, which is also one of the principal malodorous compounds produced in composting. Nitrous oxide (N2O) and methane (CH4) are both greenhouse gases, which effect global warming potentials over a 100-year time horizon of 23 times for CH4 and 296 times for N2O per mole of gas compared to CO2 (Jiang et al., 2011; Shen et al., 2011).
The emissions result from physical and biological reactions (El Kader et al., 2007). Other than CO2, ammonia gas is the main compound found in exhaust gases when organic wastes are composted (Beck-Friis et al., 2001). Temperature, pH, and initial ammonium content are the most important parameters affecting the amount of nitrogen emitted as ammonia (Pagans et al., 2006). The pattern of ammonia emissions is greatly dependent on the process temperature (Pagans et al., 2006). Maintaining an alkaline pH during the thermophilic phase of composting may lead to substantial losses of gaseous ammonia (Jarvis et al., 2009). Furthermore, the rate of intermittent aeration is an important factor influencing gas emissions during composting, and the emission of ammonia was reduced with a decrease in the aeration rate (Shen et al., 2011). A lower C/N ratio is preferable to increasing the NH3 emissions, and the moisture content can affect the ammonia emission during composting, but not significantly (Jiang et al., 2011). N2O released from nitrification or denitrification can be the end-products of either ammonium oxidation or incomplete denitrification (Beck-Friis et al., 2000). N2O emission was a function of the compost age, pile depth, temperature, and water filled pore space (Czepiel et al., 1996). The static system showed that higher N2O emissions were due to the prevalence of anaerobic regions in combination with a high ammonia content and the absence of readily available carbon (Szanto et al., 2007). In addition, the chemodenitrification of NO2 − to N2O plays an important role in the early N2O emission (Hao et al., 2001). CH4 is produced through biodegradation of the organic fraction of waste under anaerobic conditions (He et al., 2000), which is found to have a high rate in the composting heap center during the thermophilic phase (Sommer and Moller, 2000). A high aeration rate can reduce the amount of CH4 emitted during composting (Jiang et al., 2011; Shen et al., 2011). Meanwhile, there is a possibility of changing the gas emission rates simply by changing the scale of the compost pile (Fukumoto et al., 2003). Traditional composting can take several months, but land shortage coupled with a large volume of animal manure in China requires more effective treatment (Liu et al., 2011). Using composting technologies such as forced aeration and windrow turning can result in a shorter composting time (Szanto et al., 2007). Therefore, we selected forced aeration that can accelerate the composting process and is widely used in China (Jiang et al., 2011).
Organic manure cannot be composted alone due to the high moisture content and low C/N ratio, especially pig manure. This is the case even for its mechanically separated solid fraction (Imbeah, 1998). Therefore, the addition of a bulking agent is necessary to compost the separated solid fraction of pig manure (Nolan et al., 2011). A bulking agent is the material that adjusts the C/N ratio, provides optimum free air space (FAS), and regulates the water content of the waste to be composted (Gea et al., 2007; Kato and Miura, 2008; Iqbal et al., 2010; Jolanun and Towprayoon, 2010; Doublet et al., 2011), which are usually fibrous with carbonaceous material (Iqbal et al., 2010). The bulking agent represents a large proportion of the composting mixture as a source of carbon and energy for micro-organisms (Adhikari et al., 2009). The composting processes are affected by the type of bulking agent used, its particle size, and the proportion of bulking agent in the composting mixture (Atkinson et al., 1996; Gea et al., 2007; Chang and Chen, 2010). The addition of biodegradable C fractions will balance the initial C/N ratio of the mixture and reduce the N losses during the composting process (Dias et al., 2010). The type of bulking agent has a significant effect on N and C losses caused by volatilization (Barrington et al., 2002), and the bulking agents with a high content in lingocellulose have a lower microbial degradability and lead to higher nitrogen losses (Martin et al., 1993). In other words, a higher bulking agent content in the composting mixture increases the water absorption capacity and the composting rate, reduces the composting and acidification time, and lowers the pH value when food waste is composted with sawdust as the bulking agent (Chang and Chen, 2010). Numerous bulking agents, including sawdust, rice husk, and rice bran, were used extensively in Asian waste composting studies (Chang and Chen, 2010) for the composting of animal manure and other wet feedstocks. Although most of these studies used several parameters for evaluating the composting performance, only a few studies have been conducted on the effect of bulking agent characteristics on NH3, CH4, and N2O emissions during rapid composting.
In light of the above considerations, the objective of this investigation was to evaluate the influence of bulking agents (corn stalks, spent mushroom compost, and sawdust) on NH3, CH4, and N2O emissions for composting with pig manure using rapid composting technology.
2. Materials and methods
2.1. Composting materials and experimental design
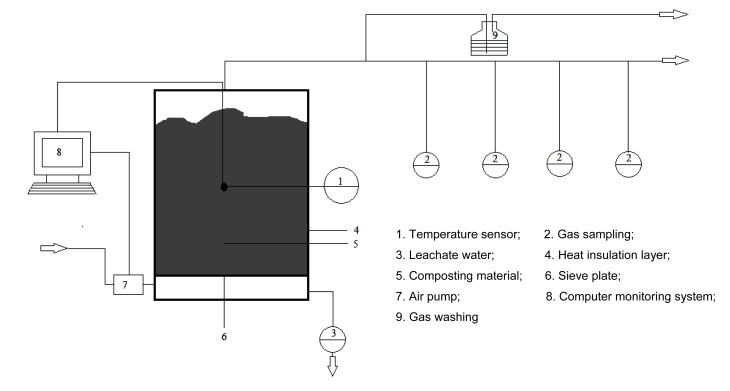
Three composting experiments were performed in insulated fermenters (Fig. 1) using the same pig manure mixed with three different bulking agents: corn stalks, spent mushroom compost, and sawdust. The pig manure was collected from the ZHOU Chun-sheng Pig Farm (Shujiatuo Town, Haidian District, Beijing, China), where the Ganqingfen system was used for manure collection (there was a separate collection of the manure from the concrete floor before flushing the urine with water). Corn stalks, taken from Shangzhuang Station, China Agricultural University, were cut into 1–5 cm fragments. Spent mushroom compost, the solid residue generated at the end of edible fungus cultivation, was collected from Fangshan County, Beijing, China. The sawdust was obtained from fresh wood cuts from local sawmills. The mixing ratio was adjusted according to the same C/N ratio (18) of the three mixtures, and the initial moisture content was adjusted to around 65%. All mixtures were elaborated to identify the influence of each bulking material. Table 1 presents the physicochemical properties of the raw materials.
Fig. 1.
Sketch map of the closed composting vessel
Table 1.
Physicochemical properties of the organic materials used for the composting mixtures
| Material | pH | Moisture (%) | TOC (g/kg) | Total N (g/kg) | C/N |
| Pig manure | 8.70 (0.22) | 75.0 (0.48) | 350.0 (3.23) | 27.0 (0.73) | 12.9 (0.24) |
| Corn stalk | 7.50 (0.08) | 9.3 (0.06) | 419.0 (2.98) | 9.9 (0.25) | 4.2 (0.18) |
| Sawdust | 7.20 (0.09) | 20.6 (0.12) | 454.0 (1.35) | 3.0 (0.09) | 151.3 (0.89) |
| Spent mushroom compost | 6.51 (0.42) | 46.9 (0.32) | 351.0 (3.97) | 16.0 (0.30) | 21.9 (0.49) |
TOC: total organic carbon. Values are expressed as mean of triplicates (dry weight basis), values in parentheses are standard error
The volume of composting reactors is about 60 L (inner diameter, 0.36 m; height, 0.6 m; composting materials were added to a height of 0.5 m from the bottom of the reactor), with a temperature sensor connected to a computer and controlled by a C-LGX program designed by our laboratory, so that the aeration can be automatically controlled by time or temperature. The air flow under continuous aeration was 0.16 m3/h. Composting was performed throughout one month with turning on Days 5, 7, 14, and 22. Water was added to keep the moisture content at 55%–65% in the reactor during turning, and solid samples were taken. All samples were weighed before being introduced, as was each turning, and the mixtures and reactors were eventually sampled and re-introduced into the reactors, which were weighed again. The mass losses related to sampling and turning were taken into account in the overall mass balance of composting. The leachate was not measured or collected for analysis during the process. The design of the experiment is shown in Table 2.
Table 2.
Design of experiment
| Group | Pig manure (kg) | Corn stalk (kg) | Spent mushroom compost (kg) | Sawdust (kg) |
| CS | 23.70 | 3.95 | ||
| SMC | 13.57 | 15.08 | ||
| SWD | 25.00 | 5.00 |
2.2. Compost sampling and analytical methods
Solid samples of the compost were collected four times during the experimental period (on Days 0, 5, 22, and 30) by mixing six subsamples taken from different locations in the reactor. The samples were divided into two parts, one part being analyzed immediately, and the other air-dried before being passed through a 1-mm sieve. Ammonia gas released during composting was captured in a boric acid wash bottle (sampling lasted 30 min) and was titrated using sulfuric acid (H2SO4, 0.1 mol/L), while O2 was monitored using an oxygen sensor (DH6511A, China). Gas samples were taken from the gas sampling port and analyzed for CO2 using a gas chromatograph (GC-9900, China). N2O and CH4 were analyzed using a gas chromatograph (3420A, Beifen, China). N2O was examined using an electron capture detector at the following temperatures: detector 280 °C, injector 120 °C, and column 80 °C, while CH4 was examined using a flame ionization detector at the following temperatures: detector 200 °C, injector 120 °C, and column 80 °C.
The total nitrogen (N) was analyzed using the Kjeldahl method (Kimberly and Roberts, 1905). Samples were analyzed for organic carbon by oxidation with potassium dichromate (K2CrO7) (Yeomans and Bremner, 1988). Results are expressed in terms of dry matter. The moisture contents of different samples were determined based on weight loss at 105 °C and evaluating the constant weight at the end. Inorganic nitrogen (NH4 +-N, NO3 −-N) in the samples was extracted with 2 mol/L KCl at a 1:10 soil:water (w/v) ratio, and then analyzed using an auto analyzer (Auto Analyzer 3, Seal, Germany). To analyze the pH, electrical conductivity (EC), and germination index (GI), a 20-g fresh sample was extracted with 200 ml distilled water by stirring for 1 h and then centrifuged at 4000 r/min. The pH was then measured using a pH meter, while EC was measured using a DDS-12A conductivity meter.
Seed germination was used to assess the phytotoxicity (Sellami et al., 2008): 20 Lepidium sativum seeds were evenly distributed on filter paper in Petri dishes (diameter 10 cm) and moistened with 5 ml compost extract. A total of 5 ml of distilled water replaced the extract as a control, and then, the dishes for each sample were incubated at 25 °C for 3 d. The number of germinating seeds and root length were measured at every treatment. GI was calculated as GI (%)=(Seed germination of compost sample×root length of compost sample)×100%÷(seed germination of control×root length of control).
2.3. Statistical analysis
SAS 9.1 for Windows was used for all statistical analyses. One-way analysis of variance (ANOVA) was performed and shown for chemical parameters to compare the variations of different composting treatments. Data are reported as the mean and standard error of three replicates.
3. Results and discussion
3.1. General description of the composting progress
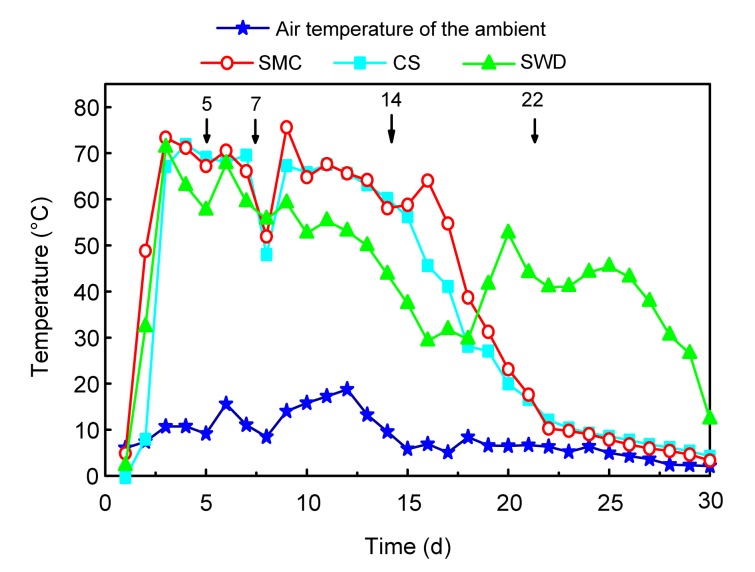
The changes in the compost temperature for all runs are shown in Fig. 2. The temperatures of all the runs increased rapidly after the first day; the peak temperatures (>55 °C) of the CS and SWD runs were obtained by Day 3, but that for the SMC run was obtained by Day 9, which shows that the temperature increase during composting is due to the heat produced by aerobic degradation of the organic matter (Szanto et al., 2007). The interruptions in the temperature curve by Day 7 due to a turning and a rapid recovery of thermophilic conditions were observed on the following day. Compost temperatures in the CS and SMC runs showed a downward trend after Day 17, and further turning did not result in a rapid temperature increase. However, the temperature in the SWD run increased rapidly after the fourth turning and then decreased gradually. The nature of bulking agents influences the time needed to reach similar organic matter (OM) stability and the biochemical evolution of OM (Doublet et al., 2011). Sawdust had the higher content of lingocellulose materials and low available carbon sources for microorganism than corn stalk and spent mushroom compost, and therefore, temperatures in the SWD run were lower than others in the first 17 d. However, after that, turning activities made the composting materials more homogeneous especially the easily degradation lignocelluloses of sawdust, which made the activities of microbes increase after Day 17 in the SMC run. However, after the fourth turning, the easily degradation lignocellulose was exhausted. The temperature performance in all the runs fulfilled the Chinese National Standard (GB 7959-87: to maintain a temperature above 50–55 °C for 5–7 d) for sanitation in the composting application into the soil in China. The ambient temperature, which fluctuated during the experiment, was averaged at about 9 °C.
Fig. 2.
Temperature changes during composting by different treatments
Arrows show the turning days
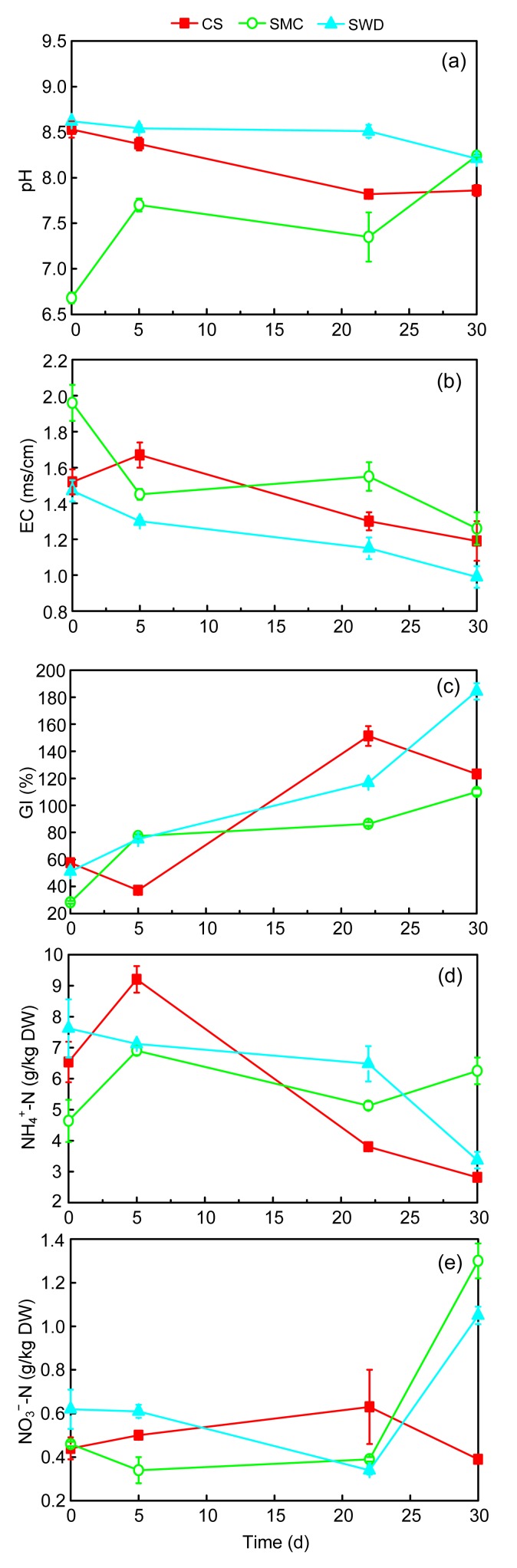
The changes in the absolute contents of total organic carbon (TOC) and total nitrogen in composting different runs are shown in Table 3. The initial TOC and TN absolute contents were influenced by the characteristics of the bulking agent used in the composting. TOC content varied from 1.41 to 3.51 kg, with the highest TOC value found in the SMC run (3.51 kg). Most of the degradation occurred after Day 5. The extractable nitrogen (NH4 +-N and NO3 −-N) contents are shown in Fig. 3. NH4 +-N increased in the first 5 d in the CS and SMC runs (Fig. 3d), possible because organic N changed to NH4 +-N via ammonification (Ren et al., 2010), and then decreased later on, which caused by NH3 emission and nitrification (Jiang et al., 2011). Compared with NH4 +-N, NO3 −-N was always at a very low level (<1.3 g/kg DW) in the process (Fig. 3e).
Table 3.
Chemical analysis of the organic wastes at different composting time
| Composting time (d) | TOC (kg) | Total N (g) | C/N |
| CS pig manure: corn stalk | |||
| 0 | 2.89±0.03a | 160.62±0.12c | 18.0±0.32 |
| 5 | 2.65±0.02a | 157.27±0.10b | 16.8±0.14 |
| 22 | 1.71±0.01b | 132.24±0.13a | 12.9±0.18 |
| 30 | 1.53±0.06c | 129.10±0.08a | 11.9±0.17 |
| SMC pig manure: spent mushroom compost | |||
| 0 | 3.51±0.07a | 194.74±0.07d | 18.0±0.26 |
| 5 | 3.01±0.09b | 188.79±0.15c | 15.9±0.32 |
| 22 | 1.95±0.01c | 178.47±0.11b | 10.9±0.10 |
| 30 | 1.69±0.04d | 173.18±1.02a | 9.8±0.16 |
| SWD: pig manure: sawdust | |||
| 0 | 3.10±0.06a | 172.93±0.97d | 17.9±0.08 |
| 5 | 2.84±0.02b | 167.68±1.04c | 16.9±0.12 |
| 22 | 1.83±0.04c | 130.99±0.86b | 14.0±0.15 |
| 30 | 1.41±0.01d | 110.70±1.05a | 12.7±0.43 |
Values are expressed as mean±standard error of triplicates (dry weight basis). Means with different subscripts in the same column for the same trial were significantly different (P<0.05)
Fig. 3.
Changes of pH (a), EC (b), GI (c), NH4 +-N (d), and NO3 −-N (e) during different composting treatments
The parameters pH and EC were also very important for the evolution of the composting process. The pH of the three runs was maintained at 6.68–8.62 during the composting process (Fig. 3a). A noticeable decrease in the pH of the CS and SWD runs was observed in the degradation progress, which could have resulted from organic acids produced by incomplete oxidation of the organic matter (Liu et al., 2011). Further, the large quantities of CO2 that are released during the composting process might be responsible for the decrease in pH (Huang et al., 2004). After composting, the SMC and SWD runs had a higher pH value compared to the CS run, and the pH analysis of all runs indicated alkaline conditions.
EC and GI were used to test the salinity and phytotoxicity of the compost (Shen et al., 2011). EC values of the CS, SMC, and SWD runs from 1.52, 1.96, and 1.47 ms/cm decreased to 1.19, 1.26, and 0.99 ms/cm, respectively, during the composting process (Fig. 3b), lower than 3.00 ms/cm, which was identified as the limit for safe growth of plants (García et al., 1991). At the end of the experiment, the GI of all treatments was higher than 80%, and consequently the mixtures were mature (Fig. 3c).
3.2. Influence of bulking agents on kinetics of O2 and CO2 concentrations and emissions of N2O and CH4
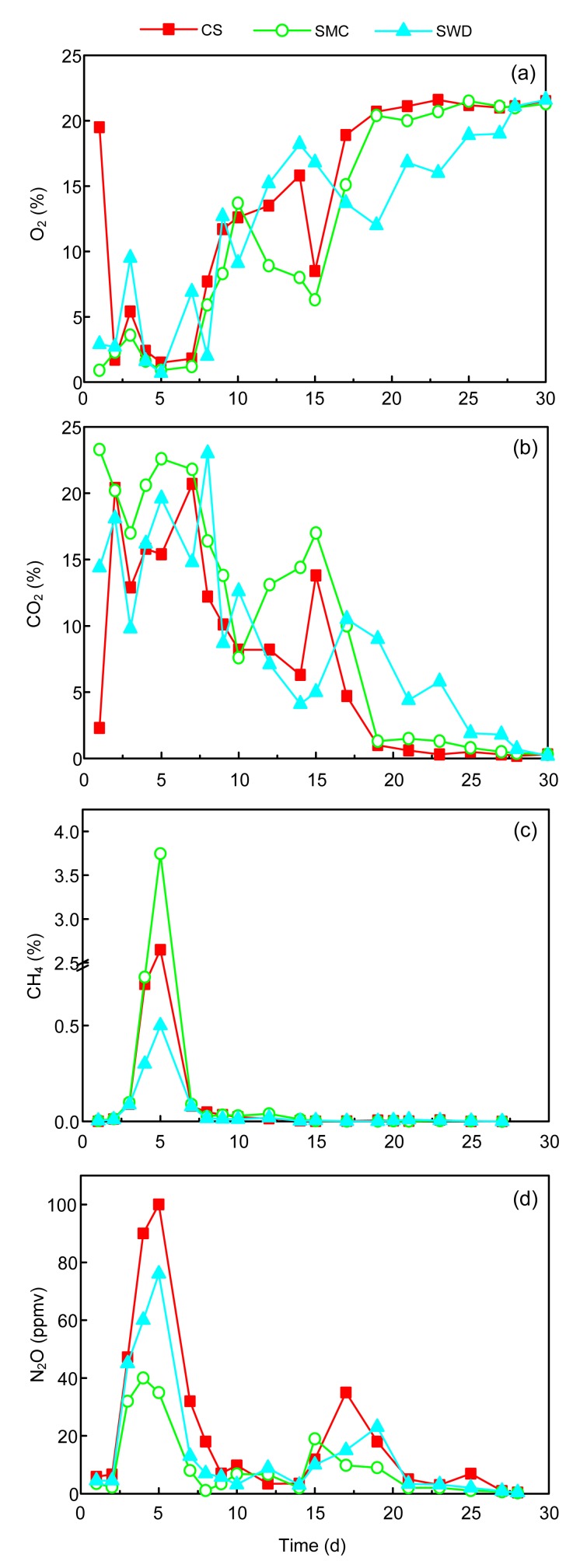
The O2 and CO2 concentrations in the outlet of the composting reactors are shown in Figs. 4a and 4b, respectively. The decrease in O2 was accompanied by an increase in CO2. One-way ANOVA for CO2 concentrations showed no significant differences among the three runs (F=1.07, P=0.3493). For the three runs, oxygen concentration decreased rapidly to 1.7%, 2.3%, and 2.7% by Day 2 in the CS, SMC, and SWD runs, respectively. Oxygen concentration in the CS and SMC runs was maintained at a low level before the second turning, while it increased gradually after the third turning. The low O2 level inside the composting reactors indicated that these systems had more anaerobic regions, which were confirmed by its high CH4 content. The result was similar to the finding of Szanto et al. (2007). However, the pattern of O2 concentrations in the SWD run was higher than those in the CS and SMC runs during the first 17 d. It reached 18.2% by Day 14 and then decreased until the fourth turning. One-way ANOVA showed no significant differences among the three runs (F=0.48, P=0.6234).
Fig. 4.
Changes of concentrations of O2 (a), CO2 (b), CH4 (c), and N2O (d) in different composting treatments
ppmv: parts per million by volume
CH4 content from all runs was high in the first several days with low O2 concentration, but it was at a very low level, and there were no significant differences among the three runs during the rest of the composting time (Fig. 4c). The emission patterns of CH4 in this study resembled those reported by other works (Osada et al., 1997; Fukumoto et al., 2003; Shen et al., 2011). The maximum value of CH4 concentration appeared on the fifth day, reaching 2.65%, 3.75%, and 0.5% for the CS, SMC, and SWD runs, respectively. The high CH4 emission demonstrated the involvement of anoxic/anaerobic micro-organisms (He et al., 2000). The higher CH4 value of the SMC run should be explained in two aspects. First, CH4 was produced through degradation of soluble lipids, carbohydrates, organic acids, and proteins (Fukumoto et al., 2003), which are limited to strict anaerobic conditions (Jiang et al., 2011). Thereby, the higher value of CH4 concentrations in the SMC run by Day 5 was probably due to the large amount of organic acids inferring from the pH value of the SMC run. Second, the characteristics of the bulking agent in the starting mixture may be related to the amount of released CH4. The spent mushroom compost used in the experiment contains particles with diameters of some centimeters, in which oxygen cannot infiltrate into the center. One-way ANOVA showed no significant differences among the three runs (F=0.52, P=0.5961).
The N2O emissions composting in all the runs started and increased until the first turning (Fig. 4d). The nitrification process would not have occurred because autotrophic nitrifier activity ceases above 40 °C, and thus N2O would not have been generated from this step (Sanchez-Monedero et al., 2001; Fukumoto et al., 2003). Therefore, the emission of N2O in the early stages of composting was probably due to N2O produced in the manure before the pile was made (El Kader et al., 2007). On the contrary, Angnes et al. (2013) showed that nitrification was not inhibited by high temperature. The highest N2O emissions of all runs were observed in the first week, similar to previous studies (He et al., 2000; El Kader et al., 2007; Shen et al., 2011). There were two other high emission periods around the first and third turnings, which confirmed previous observations (Fukumoto et al., 2003; El Kader et al., 2007; Jiang et al., 2011). They attributed this to the fact that the turning could enable the transportation of NO2 −/NO3 − from the aerobic portion (the surface layer of the pile) to the anoxic portion (the center of the pile), resulting in a homogeneous product. However, it may be assumed that denitrification caused the highest N2O emission as a result of the low available carbon levels, which was supported by the decrease of compost temperature and low CO2 emission, especially the third turning. The same result was observed by He et al. (2000), which reported that N2O emission began to increase shortly after the depletion of available organic carbon. For the CS, SMC, and SWD runs, the maximum concentration of N2O reached 100, 35, and 76 parts per million by volume (ppmv), respectively, on the fifth day. The higher N2O concentration of the CS and SWD runs could be explained by the fact that the N2O emission in the early stage of the composting resulted from the preparation of pig manure. Therefore, the amount of pig manure added in order to maintain the C/N ratio for the experiment could result in this phenomenon, in such an order as CS>SWD>SMC. The same trend has been found in the study of He et al. (2000), who observed that N2O emission was linearly correlated with NO2 − content in waste in the manure-applied treatments, while no significant correlation was observed in non-manure treatments. One-way ANOVA showed no significant differences among the three runs (F=1.31, P=0.2772).
3.3. Influence of bulking agents on emission of NH3
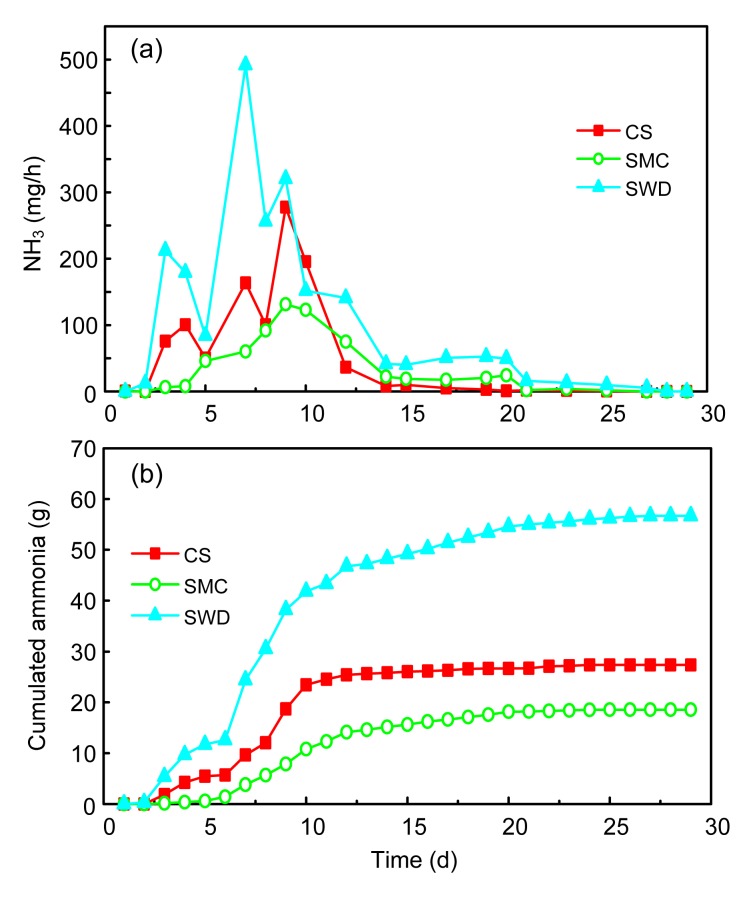
NH3 concentration in the outlets for all the runs was between 0 and 492 mg/h (Fig. 5a), and the emission patterns of ammonia showed a similar trend for the CS, SMC, and SWD runs (Fig. 5b). In general, ammonia emission surged with the rising temperature during the first 4 d. The first peak of the NH3 emission came on the fourth day and then decreased from Days 4 to 5 as the temperature dropped. The subsequent increase came after the first turning. The emission patterns were similar to that in the previous studies (Ren et al., 2010; Jiang et al., 2011; Shen et al., 2011). A possible explanation for the ammonia emission pattern is that, at the initial stage of composting, degradation of large amounts of easily biodegradable organic compounds with high nitrogen content provokes a release of ammonia gas that is exponentially dependent on temperature, as is expected for free soluble ammonia (Pagans et al., 2006). NH3 concentration had two other peaks on Days 7 and 10, which were sustained as the high temperature accelerated the emission of gaseous components inside the compost pile due to intensive microbiological activity (Fukumoto et al., 2003). NH3 emission at a relatively high level was observed during the composting process except on Day 10 in the SWD run. The maximum concentrations of NH3 reached 277.63, 131.39, and 491.57 mg/h for the CS, SMC, and SWD runs, respectively. Ammonia emissions are expected to depend strongly on the temperature and substrate pH (Komilis and Ham, 2006), due to the fact that ammonia emissions could begin to appear at thermophilic temperatures (>45 °C) (Sommer and Moller, 2000), and based upon the data of the pH, NH4 +-N, and the temperature in three runs, the NH4 +-N potential is less negative when the NH4 +-N content is higher. Therefore, the higher pH value could possibly result in higher ammonia emissions in this study, especially in the SWD run, which had the highest pH value that is influenced by the characteristic of the sawdust. NH3 emissions ceased in all the runs 28 d after composting began. One-way ANOVA showed no significant differences between the CS and SWD treatments, but a significant difference between the SMC and SWD treatments.
Fig. 5.
Emission of NH3 in different composting treatments
Changes of the NH3 concentration (a) and cumulated ammonia (b)
3.4. Carbon and nitrogen balance during composting
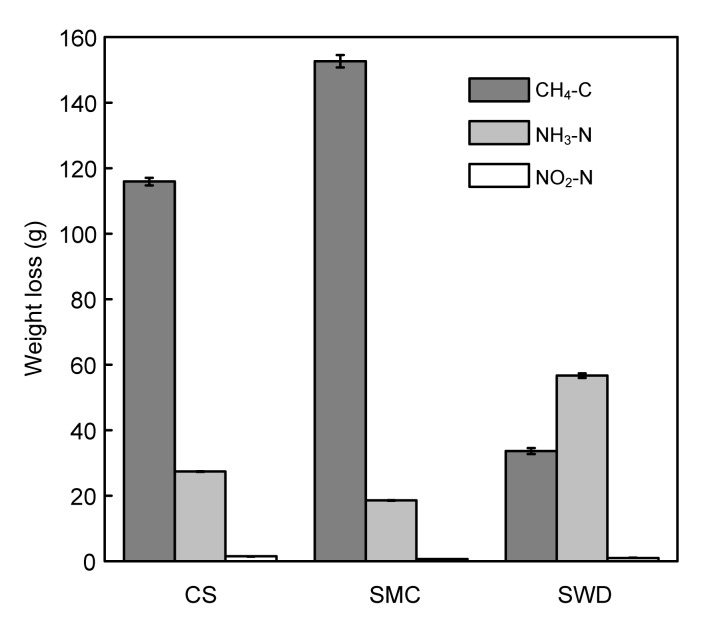
The loss of carbon in the study was over 40% during the composting process (Table 4). For the SWD run, the TOC loss reached 54.40%, of which only 1.99% was emitted in the form of CH4, and it reached 8.55% and 8.40% in the CS and SMC runs, respectively (Fig. 6). The introduction of sawdust induced a higher biodegradability of the initial mixture than corn stalk and spent mushroom compost. Woody materials have a high content of ligin, which is extremely resistant to chemical and enzymatic degradation (Nolan et al., 2011). Michel et al. (2004) and Hao et al. (2004) found a lower decomposition of the compost substrate when using bulking materials rich in lignin (woodchip and sawdust) compared to composting with straw, which had a lower lignin content. In this study, the higher degradability of the sawdust may have been associated with its larger surface area-to-mass ratio, and also with the fact that the small bulking agent particles create a real porous structure and a homogeneous porous size distribution inside the material that acts as an efficient oxygen diffuser (Gea et al., 2007). However, an experiment with pig manure alone or pig manure with an inert bulking agent was not performed in this study, so it was not possible to evaluate the degradability of the bulking agents alone. Szanto et al. (2007) found that CH4 emissions are due to complete anaerobic degradation of organic matter to CH4 in the turned system lower than that in the static system when composting straw-rich pig manure. Fukumoto et al. (2003) showed that a large-scale compost pile increased the emission rate of gaseous components, especially N2O and CH4. The bulking agent influenced the C dynamics during composting, which was related to their own degradability, more specifically, the nature and recalcitrance of their holocellulosic fractions (Doublet et al., 2011), so that they could influence the biodegradability of organic matter during the composting process. However, CH4 production was not only related to the content of carbohydrates (Fukumoto et al., 2003), but more importantly to the physicochemical characteristics of the composting mixture (e.g., temperature, strict anaerobic condition, and compost structure) (Szanto et al., 2007; Beck-Friis et al., 2000; Jiang et al., 2011). This was probably because sawdust used as the bulking agent in the study had a higher degradation rate of TOC whereas a lower emission rate of CH4-C for the composting of pig manure.
Table 4.
Carbon and nitrogen balance during composting
| Group | TOC |
TN |
||||||||
| W initial (kg) | W final (kg) | Lossa (%) | CO2-C (kg) | CH4-C (g) | W initial (kg) | W final (kg) | Lossa (%) | NH3-N (g) | N2O-N (g) | |
| CS | 2.89 (0.12) | 1.53 (0.07) | 46.97 | 1.13 (0.03) | 115.91 (0.13) | 160.37 (0.12) | 129.22 (0.07) | 19.42 | 27.36 (0.08) | 1.48 (0.02) |
| SMC | 3.51 (0.11) | 1.69 (0.06) | 51.77 | 1.54 (0.05) | 152.61 (0.10) | 194.84 (0.18) | 173.11 (0.06) | 11.16 | 18.56 (0.09) | 0.68 (0.008) |
| SWD | 3.10 (0.13) | 1.41 (0.08) | 54.40 | 1.51 (0.07) | 33.61 (0.09) | 172.39 (0.09) | 110.86 (0.08) | 35.69 | 56.67 (0.10) | 1.06 (0.009) |
Nutrient loss is calculated as (W initial−W final)×100%/W initial
TOC and TN mentioned above are the absolute contents. Values are expressed as the mean (standard error) of triplicates on the dry weight basis
Fig. 6.
Loss of CH4-C, NH3-N, and NO2-N for different treatments during composting
Values are the mean±standard error of triplicates
Considering carbon loss, the SWD run also showed a higher N loss (35.69%) than the CS (19.42%) and SMC (11.16%) runs (Table 4). During composting, the comparative carbon losses were larger than that of nitrogen. However, the small composting reactors and no replicated treatments of this experiment may limit the validity of the carbon and nitrogen balances. Nitrogen loss in the form of NH3-N reached 87.85%, 85.38%, and 92.1% for the CS, SMC, and SWD runs (Fig. 6), respectively, which was in accordance with other previous works (Ren et al., 2010; Shen et al., 2011). Some management strategies reducing NH3 emission such as regulating the C:N ratio in the substrate, controlling temperature, controlling pH through chemical additives and controlling the microbial populations during the composting process, have been studied (Velasco-Velasco et al., 2011). In this study, the use of corn stalks might have a positive effect on reducing N losses. Losses of nitrogen compounds in leachate were not accounted for, since they were negligible. The ammonia emission could be related to the effect of free air space, and it also depends on the carbon available for micro-organisms (El Kader et al., 2007). Velasco-Velasco et al. (2011) reported that the addition of C readily available to microorganisms in the pre-composting process can significantly reduce NH3 emission by up to 50%, but adding cellulose such as paper and straw did not show any significant differences on ammonia emission (Liang et al., 2006). In this study, the N2O-N emissions were varied from 1.72% to 4.76% of the total nitrogen loss, and reached 4.76%, 3.12%, and 1.72% for the CS, SMC, and SWD runs, respectively (Fig. 6). These results were similar to those of Fukumoto et al. (2003) and Jiang et al. (2011). The bulking agent could increase the N availability by enhancing the N organization and limiting N losses by volatilization during composting (Doublet et al., 2011), and the loss of N by volatilization was affected by the loss of C during the composting process rather than by the moisture content or aeration regime (Barrington et al., 2002), and also by the initial C/N ratio of the composting mixtures and the presence of recalcitrant carbon fractions such as lignin (Goyal et al., 2005). However, N2O produced by both nitrifiers and denitrifiers was optimized under microaerobic conditions (Czepiel et al., 1996). Szanto et al. (2007) believed that N2O emissions became prevalent when readily available carbon was depleted, and it was also closely related to the forms and amount of the nitrogen (He et al., 2000). Therefore, in the study, it could be seen that the emission pattern of NH3 was the same as the pattern of nitrogen loss but the N2O-N emission had a different trend, in an order of corn stalks>sawdust>spent mushroom compost.
4. Conclusions
The effect of bulking agents (corn stalks, spent mushroom compost, and sawdust) on CH4, N2O, and NH3 emissions was investigated in this study. The introduction of corn stalks is preferable to increasing the NO2 emission. The introduction of spent mushroom compost induced a higher CH4 emission by reducing the air spaces and increasing the large amount of organic acids inferring from the pH value, whereas the emissions of N2O and NH3 reduced. The concentration of NH3 from composting pig manure with sawdust was higher than that mixed with corn stalk or spent mushroom compost. The nature of bulking agents mixed with pig manure before composting had only a small influence on the loss of total organic carbon, whereas it significantly influenced the loss of total nitrogen.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 41275161 and 41075110), the National Science & Technology Pillar Program (Nos. 2012BAD14B01 and 2012BAD14 B03), the Sino-German Cooperation Project of Recycling of Organic Residues from Agricultural and Municipal Residues in China (No. BMBF FKZ 0330847), and the Chinese Universities Scientific Fund
Compliance with ethics guidelines: Xiang-ping SUN, Peng LU, Tao JIANG, Frank SCHUCHARDT, and Guo-xue LI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Adhikari BK, Barrington S, Martinez J, et al. Effectiveness of three bulking agents for food waste composting. Waste Manage. 2009;29(1):197–203. doi: 10.1016/j.wasman.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Angnes G, Nicoloso RS, da Silva MLB, et al. Correlating denitrifying catabolic genes with N2O and N2 emissions from swine slurry composting. Bioresource Technol. 2013;140:368–375. doi: 10.1016/j.biortech.2013.04.112. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson CF, Jones DD, Gauthier JJ. Biodegradabilities and microbial activities during composting of oxidation ditch sludge. Compost Sci Utiliz. 1996;4(1):84–96. doi: 10.1080/1065657X.1996.10701821. [DOI] [Google Scholar]
- 4.Barrington S, Choinière D, Trigui M, et al. Effect of carbon source on compost nitrogen and carbon losses. Bioresource Technol. 2002;83(3):189–194. doi: 10.1016/S0960-8524(01)00229-2. [DOI] [PubMed] [Google Scholar]
- 5.Beck-Friis B, Pell M, Sonesson U, et al. Formation and emission of N2O and CH4 from compost heaps of organic household waster. Environ Monit Assessm. 2000;62(3):317–331. doi: 10.1023/A:1006245227491. [DOI] [Google Scholar]
- 6.Beck-Friis B, Smars S, Jonsson H, et al. Gaseous emissions of carbon dioxide, ammonia and nitrous oxide from organic household waste in a compost reactor under different temperature regimes. J Agric Eng Res. 2001;78(4):423–430. doi: 10.1006/jaer.2000.0662. [DOI] [Google Scholar]
- 7.Chang JI, Chen YJ. Effects of bulking agents on food waste composting. Bioresource Technol. 2010;101(15):5917–5924. doi: 10.1016/j.biortech.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Czepiel P, Douglas E, Harriss R, et al. Measurements of N2O from composted organic wastes. Environ Sci Technol. 1996;30(8):2519–2525. doi: 10.1021/es950841j. [DOI] [Google Scholar]
- 9.Dias BO, Silva CA, Higashikawa FS, et al. Use of biochar as bulking agent for the composting of poultry manure: effect on organic matter degradation and humification. Bioresource Technol. 2010;101(4):1239–1246. doi: 10.1016/j.biortech.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Doublet J, Francou C, Poitrenaud M, et al. Influence of bulking agents on organic matter evolution during sewage sludge composting; consequences on compost organic matter stability and N availability. Bioresource Technol. 2011;102(2):1298–1307. doi: 10.1016/j.biortech.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 11.El Kader NA, Robin P, Paillat JM, et al. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresource Technol. 2007;98(14):2619–2628. doi: 10.1016/j.biortech.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto Y, Osada T, Hanajima D, et al. Patterns and quantities of NH3, N2O and CH4 emissions during swine manure composting without forced aeration—effect of compost pile scale. Bioresource Technol. 2003;89(2):109–114. doi: 10.1016/S0960-8524(03)00060-9. [DOI] [PubMed] [Google Scholar]
- 13.García C, Hernández T, Costa F. Study on water extract of sewage-sludge composts. Soil Sci Plant Nutr. 1991;37(3):399–408. doi: 10.1080/00380768.1991.10415052. [DOI] [Google Scholar]
- 14.Gea T, Barrena R, Artola A, et al. Optimal bulking agent particle size and usage for heat retention and disinfection in domestic wastewater sludge composting. Waste Manage. 2007;27(9):1108–1116. doi: 10.1016/j.wasman.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Goyal S, Dhull SK, Kapoor KK. Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresource Technol. 2005;96(14):1584–1591. doi: 10.1016/j.biortech.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Hao XY, Chang C, Larney FJ, et al. Greenhouse gas emissions during cattle feedlot manure composting. J Environ Qual. 2001;30(2):376–386. doi: 10.2134/jeq2001.302376x. [DOI] [PubMed] [Google Scholar]
- 17.Hao XY, Chang C, Larney FJ. Carbon, nitrogen balances and greenhouse gas emission during cattle feedlot manure composting. J Environ Qual. 2004;33(1):37–44. doi: 10.2134/jeq2004.3700. [DOI] [PubMed] [Google Scholar]
- 18.He YW, Inamori Y, Mizuochi M, et al. Measurements of N2O and CH4 from the aerated composting of food waste. Sci Total Environ. 2000;254(1):65–74. doi: 10.1016/S0048-9697(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 19.Huang GF, Wong JWC, Wu QT, et al. Effect of C/N on composting of pig manure with sawdust. Waste Manage. 2004;24(8):805–813. doi: 10.1016/j.wasman.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Imbeah M. Composting piggery waste: a review. Bioresource Technol. 1998;63(3):197–203. doi: 10.1016/S0960-8524(97)00165-X. [DOI] [Google Scholar]
- 21.Iqbal MK, Shafiq T, Ahmed K. Characterization of bulking agents and its effects on physical properties of compost. Bioresource Technol. 2010;101(6):1913–1919. doi: 10.1016/j.biortech.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis Å, Sundberg C, Milenkovski S, et al. Activity and composition of ammonia oxidizing bacterial communities and emission dynamics of NH3 and N2O in a compost reactor treating organic household waste. J Appl Microbiol. 2009;106(5):1502–1511. doi: 10.1111/j.1365-2672.2008.04111.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Schuchardt F, Li G, et al. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J Envir Sci-China. 2011;23(10):1754–1760. doi: 10.1016/S1001-0742(10)60591-8. [DOI] [PubMed] [Google Scholar]
- 24.Jolanun B, Towprayoon S. Novel bulking agent from clay residue for food waste composting. Bioresource Technol. 2010;101(12):4484–4490. doi: 10.1016/j.biortech.2010.01.116. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Miura N. Effect of matured compost as a bulking and inoculating agent on the microbial community and maturity of cattle manure compost. Bioresource Technol. 2008;99(9):3372–3380. doi: 10.1016/j.biortech.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Kimberly AE, Roberts MG. A method for the direct determination of organic nitrogen by the kjeldahl process. Public Health Pap Rep. 1905;31(2):109–122. doi: 10.1016/S0043-1354(00)00222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komilis D, Ham R. Carbon dioxide and ammonia emissions during composting of mixed paper, yard waste and food waste. Waste Manage. 2006;26(1):62–70. doi: 10.1016/j.wasman.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Leonard JJ, Feddes JJR, et al. Influence of carbon and buffer amendment on ammonia volatilization in composting. Bioresource Technol. 2006;97(5):748–761. doi: 10.1016/j.biortech.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Zhang R, Wu H, et al. Changes in biochemical and microbiological parameters during the period of rapid composting of dairy manure with rice chaff. Bioresource Technol. 2011;102(19):9040–9049. doi: 10.1016/j.biortech.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 30.Martin AM, Evans J, Porter D, et al. Comparative effects of peat and sawdust employed as bulking agents in composting. Bioresource Technol. 1993;44(1):65–69. doi: 10.1016/0960-8524(93)90210-3. [DOI] [Google Scholar]
- 31.Michel FC, Jr, Pecchia JA, Rigot J, et al. Mass and nutrient losses during the composting of dairy manure amended with sawdust or straw. Compost Sci Utiliz. 2004;12(4):323–334. doi: 10.1080/1065657X.2004.10702201. [DOI] [Google Scholar]
- 32.Nolan T, Troy SM, Healy MG, et al. Characterization of compost produced from separated pig manure and a variety of bulking agents at low initial C/N ratios. Bioresource Technol. 2011;102(14):7131–7138. doi: 10.1016/j.biortech.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 33.Osada T, Kuroda K, Yonaga M. VOERMANS, JAM &. MGJ Iternational Symposium on Ammonia and Odour Control from Animal Production Facilities. the Netherlands: Vinkeloord; 1997. N2O, CH4 and NH3 emissions from composting of swine waste; pp. 373–380. [Google Scholar]
- 34.Pagans E, Barrena R, Font X, et al. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere. 2006;62(9):1534–1542. doi: 10.1016/j.chemosphere.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Ren L, Schuchardt F, Shen Y, et al. Impact of struvite crystallization on nitrogen losses during composting of pig manure and cornstalk. Waste Manage. 2010;30(5):885–892. doi: 10.1016/j.wasman.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Monedero MA, Roig A, Paredes C, et al. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresource Technol. 2001;78(3):301–308. doi: 10.1016/S0960-8524(01)00031-1. [DOI] [PubMed] [Google Scholar]
- 37.Sellami F, Hachicha S, Chtourou M, et al. Maturity assessment of composted olive mill wastes using UV spectra and humification parameters. Bioresource Technol. 2008;99(15):6900–6907. doi: 10.1016/j.biortech.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, Ren L, Li G, et al. Influence of aeration on CH4, N2O and NH3 emissions during aerobic composting of a chicken manure and high C/N waste mixture. Waste Manage. 2011;31(1):33–38. doi: 10.1016/j.wasman.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Sommer SG, Moller HB. Emission of greenhouse gases during composting of deep litter from pig production—effect of straw content. J Agric Sci. 2000;134(3):327–335. doi: 10.1017/S0021859699007625. [DOI] [Google Scholar]
- 40.Szanto GL, Hamelers HVM, Rulkens W, et al. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresource Technol. 2007;98(14):2659–2670. doi: 10.1016/j.biortech.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Velasco-Velasco J, Parkinson R, Kuri V. Ammonia emissions during vermicomposting of sheep manure. Bioresource Technol. 2011;102(23):10959–10964. doi: 10.1016/j.biortech.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 42.Yeomans JC, Bremner JM. A rapid and precise method for routine detemination of organic carbon in soil. Commun Soil Sci Plant Anal. 1988;19(13):1467–1476. doi: 10.1080/00103628809368027. [DOI] [Google Scholar]