Abstract
Objective: To deliver cells deep into injectable calcium phosphate cement (CPC) through alginate-chitosan (AC) microcapsules and investigate the biological behavior of the cells released from microcapsules into the CPC. Methods: Mouse osteoblastic MC3T3-E1 cells were embedded in alginate and AC microcapsules using an electrostatic droplet generator. The two types of cell-encapsulating microcapsules were then mixed with a CPC paste. MC3T3-E1 cell viability was investigated using a Wst-8 kit, and osteogenic differentiation was demonstrated by an alkaline phosphatase (ALP) activity assay. Cell attachment in CPC was observed by an environment scanning electron microscopy. Results: Both alginate and AC microcapsules were able to release the encapsulated MC3T3-E1 cells when mixed with CPC paste. The released cells attached to the setting CPC scaffolds, survived, differentiated, and formed mineralized nodules. Cells grew in the pores concomitantly created by the AC microcapsules in situ within the CPC. At Day 21, cellular ALP activity in the AC group was approximately four times that at Day 7 and exceeded that of the alginate microcapsule group (P<0.05). Pores formed by the AC microcapsules had a diameter of several hundred microns and were spherical compared with those formed by alginate microcapsules. Conclusions: AC microcapsule is a promising carrier to release seeding cells deep into an injectable CPC scaffold for bone engineering.
Keywords: Injectable scaffold, Calcium phosphate cement, Osteoblast, Microencapsulation, Cell release, Chitosan
1. Introduction
The need for bone repair arising from congenital malformations, trauma, tumor resections, and skeletal diseases has increased in recent years (Laurencin et al., 1999; Ambrosio et al., 2001; Lavik and Langer, 2004; Salgado et al., 2004; Mao et al., 2006; Kretlow et al., 2009). However, both autogenous and allogeneic grafts have their own drawbacks (Laurencin et al., 1999; Salgado et al., 2004). Tissue engineering approaches provide promising alternatives and extensive efforts have been made to improve the properties and performance of bone substitute biomaterials such as hydroxyapatite, other calcium phosphate bioceramics, and calcium phosphate cements (CPCs). Among these biomaterials, preformed scaffolds have excellent biological and mechanical properties but their use usually requires machining the graft or reaming of the surgical site to achieve a precise fit in the bone cavity. Injectable polymeric carriers are advantageous when used for seeding cells or injected in minimally-invasive surgeries but they are too weak for load-bearing applications (Kretlow et al., 2009). Compared with currently available injectable carriers such as hydrogel scaffolds, solidified CPC has two significant advantages. First, it can be converted to hydroxyapatite, the major inorganic component of human bone. Second, it possesses a certain degree of strength ((5.64±0.24) MPa), which is important to define the shape of the regenerated tissue (Qiao et al., 2013). CPCs are subsequently osteointegrated into the bone structure after implantation in bone defects and can be molded or injected during surgery (Habibovic and Barralet, 2011). However, the degradable time of CPC seems to be too slow to match the formation of new bone (Shindo et al., 1993; Friedman et al., 1998; Chow, 2000).
To improve the effectiveness of CPCs, osteoblasts and stem cells embedded within alginate beads have been seeded into CPC paste and alginate hydrogel has been shown to protect the implanted cells from the mixing and injection forces, as well as from the CPC setting reaction (Simon et al., 2004; Weir et al., 2006; Weir and Xu, 2010; Xu et al., 2010; Zhao et al., 2010a; 2010b; 2011). However, the implanted cells could not be released from the alginate beads or attached onto the CPC. Partially oxidized alginate microbeads were developed to deliver stem cells into CPC paste (Zhou and Xu, 2011) and the released cells differentiated into osteogenic lineage and synthesized bone minerals on CPC (Chen et al., 2012; Zhou et al., 2012). However, this type of microbead was only either partially embedded into the CPC surface or placed onto a flattened CPC surface and completely covered with another layer of CPC paste, rather than being completely mixed with the CPC.
Chitosan, a biodegradable cationic natural copolymer of glucosamine and mucoadhesive polysaccharide, promotes the physiological bone formation and healing processes, and a CPC-chitosan scaffold has been shown to support mesenchymal stem cell attachment and proliferation (Moreau and Xu, 2009).
Based on these previous results, the aim of the present study was to develop a new type of cell-encapsulating microcapsule, alginate-chitosan (AC) microcapsule, that could release the cells throughout the CPC scaffold and concomitantly create macropores of several hundred micrometers in diameter in situ for cell migration and proliferation after being mixed with CPC, and to investigate the attachment, proliferation, and osteogenic differentiation of the released cells in the CPC.
2. Materials and methods
2.1. β-TCP/CPC powder and liquid
The mixture of CPC powder consisted of different molar amounts of α-tricalcium phosphate (α-TCP; α-Ca3(PO4)2), monocalcium phosphate (MCPA; Ca(H2PO4)2), and calcium carbonate (CC; CaCO3), which were ball-milled in ethanol for 48 h, dried at 80 °C, and sieved to obtain a homogenous powder mixture. The β-TCP/CPC powder was then obtained by adding β-TCP into CPC. The mass fraction of β-TCP was 50%. A solution of 0.6 mol/L Na2HPO4/NaH2PO4 was used as the liquid component. Before use, the combined β-TCP/CPC powder and liquid was sealed and sterilized by 60Co γ-radiation with 25 kGy and stored at 4 °C. For use in this experiment, a powder to liquid ratio of 1 g/ml was used. β-TCP/CPC powder and liquid were kindly provided by Beijing Key Lab of Fine Ceramics, Institute of Nuclear and New Energy Technology, Tsinghua University, China.
2.2. MC3T3-E1 cell culture and microencapsulation
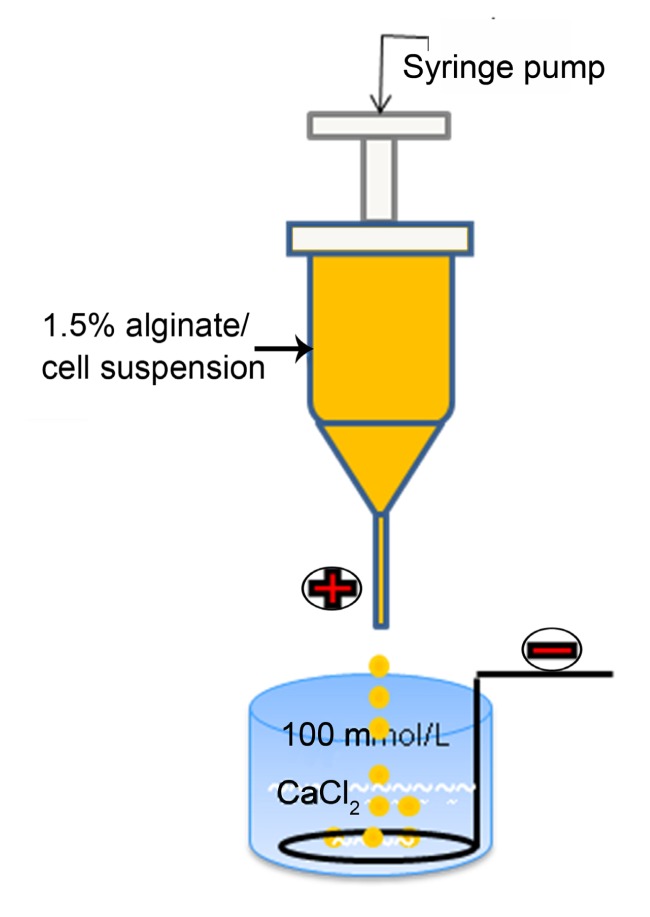
MC3T3-E1 cells (Cell Resource Center, IBMS, CAMS/PUMC, Beijing, China) were cultured in α-modified Eagle’s medium (α-MEM; Cell Resource Center) supplemented with 10% fetal bovine serum (FBS; Gibco, Auckland, NZ) and 1% penicillin/streptomycin (M&C Gene Technology, Beijing, China) at 37 °C in a fully humidified atmosphere with 5% CO2. The osteogenic medium consisted of culture medium plus 10 nmol/L dexamethasone, 10 mmol/L β-glycerophosphate, and 0.05 mmol/L ascorbic acid (Sigma, Beijing, China) (Taira et al., 2003). At 90% confluence, cells were harvested, centrifuged, and resuspended in a 1.5% (w/w) sterile-filtered sodium alginate solution (400 kDa, 100 mPa·s; Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China). Cell concentration was titrated to a density of 2.5×106 cells/ml alginate solution. The suspension was transferred into a 5-ml syringe connected to a syringe-driven pump and extruded into a 100 mmol/L sterile calcium chloride solution at an appropriate flow rate. The drops were incubated in the sterile calcium chloride for at least 15 min to obtain cell-encapsulating calcium alginate microcapsules (A-cell microcapsules), as schematically shown in Fig. 1.
Fig. 1.
Schematic diagram of the microcapsule generator
2.3. MC3T3-E1 cell viability after microencapsulation
Chitosan has osteoconductive properties (Moreau and Xu, 2009; Muzzarelli, 2011) and cell-encapsulating AC microcapsules (AC-cell microcapsules) were prepared just before mixing with the CPC paste. As a preliminary investigation, MC3T3-E1 cells were cultured in A-cell microcapsules in a culture medium to investigate the cell viability after microencapsulation. The medium was changed every 3 d. A Wst-8 kit (Dojindo, Beijing, China) was used for this assay at Days 1, 4, 7, 14, and 21 after encapsulation. At each time point, 100 μl of A-cell microcapsules were placed at the bottom of one well of a 24-well plate and washed with 1 ml of Tyrode’s HEPES buffer (140 mmol/L NaCl, 0.34 mmol/L Na2HPO4, 2.9 mmol/L KCl, 10 mmol/L HEPES, 12 mmol/L NaHCO3, 5 mmol/L glucose; pH 7.4) (Zhao et al., 2011). Then, 500 μl of Tyrode’s HEPES buffer and 50 μl of Wst-8 solution were added to the well (n=4). After 6 h incubation at 37 °C, 200 μl of each reaction mixture was transferred into a 96-well plate and the absorbance at 450 nm was measured with a microplate reader (Bio-Tek Instruments ELX800). Microcapsules without cells were used as blank controls (n=3). A live/dead assay was performed at Days 1, 4, 7, 14, and 21. The cell culture medium was removed and the A-cell microcapsules were removed from the flasks and washed with Tyrode’s HEPES buffer. Then, 100 μl A-cell microcapsules, 300 μl Tyrode’s HEPES buffer, and 200 μl phosphate buffered saline (PBS) containing 0.002 mmol/L calcein-AM (acetomethoxy derivate of calcein) and 0.004 mmol/L propidium iodide (PI) (Dojindo) were added to each specimen (n=6). After 15 min incubation at 37 °C, the cells were observed by epifluorescent microscopy (Nikon Eclipse TE-2000US).
2.4. CPC-cell construct preparation
A-cell microcapsules were washed with sterilized saline (155 mmol/L NaCl) thrice, immersed in a 0.5% (w/w) chitosan liquid (degree of deacetylation (DD)>90%, molecular weight (MW)=57 000; Dalian Institute of Chemical Physics, China), stirred with a glass rod continuously for 5 min, and washed with sterilized saline thrice to obtain AC-cell microcapsules (Xie et al., 2010). AC-cell microcapsules were then mixed with fresh CPC paste (n=6), which we referred to as the “CPC-AC-cell” group. For each sample, 0.2 g β-TCP/CPC powder was added to one well of a 24-well plate and mixed with 0.2 ml of liquid to form a paste sufficient to cover the bottom completely; the paste volume was approximately 200 μl. Then, 200 μl of AC-cell microcapsules were thoroughly mixed with fresh CPC paste. The ratio of the volume of microcapsules to the total volume of CPC plus microcapsules was approximately 50% (Simon et al., 2004; Deville et al., 2006; Weir et al., 2006; Zhao et al., 2010a; 2010b). Following incubation for 15 min (initial setting time, I-ST) (van den Vreken et al., 2010), 1.5 ml of fresh medium was added into each well until the construct was completely submerged.
Because previous studies indicated that the CPC induced pH and ionic changes of the culture medium during the incubation period (Taira et al., 2003), the culture medium was changed after the first 12 h co-incubation. For the first 2 d, the medium was changed once a day. From Day 3 on, it was changed every 3 d. A-cell microcapsules mixed with fresh CPC paste (n=6) were referred to as the “CPC-A-cell” group.
2.5. Microencapsulated MC3T3-E1 cell release after mixing with CPC paste
β-TCP/CPC powder (0.2 g) was put into a sterile plastic ring (11 mm in diameter, 4 mm in height) in a well of a 12-well plate and 0.2 ml of liquid (0.6 mol/L Na2HPO4/NaH2PO4 solution) was added to form a paste. Immediately, 0.2 ml of A-cell microcapsules were seeded onto the paste and they remained in place for 15 min. The ring was then removed and 2.0 ml of culture medium was added into the well, completely submerging the newly setting CPC disc. The culture medium was changed after 12 h. A live/dead assay was performed at Days 1, 7, and 14 to observe the released cells on CPC discs. The cell culture medium was removed from each well and cells were washed with PBS thrice, then stained with 2.0 ml of PBS containing 0.002 mmol/L calcein-AM for 15 min at 37 °C. Next, the dye solution was removed, 2.0 ml of PBS containing 0.002 mmol/L PI was added to each specimen, and the plate was stained for another 5 min. The specimens were then washed with PBS and observed by a confocal laser scanning microscopy (Leica TCS SP5). The XYZ scanning model was selected because the surface of the CPC was not very smooth. We selected 50 μm from the uppermost surface down as the observation range and images were taken every 10 μm as predetermined. Live cells were stained green, dead cells red. Released cells attached onto the bottom of the 12-well plate were also observed using an inverted phase contrast microscope (n=6).
2.6. Viability of released MC3T3-E1 cells
Cell viability of the released cells in CPC scaffolds was analyzed at Days 1, 7, and 14 of the culture (n=4). The Wst-8 kit was used for this assay. Every specimen was washed with PBS and 500 μl of culture medium, and then 50 μl of Wst-8 solution was added to each well. After 6 h incubation at 37 °C, 200 μl of each reaction mixture was transferred to a 96-well plate and the absorbance at 450 nm was measured with a microplate reader (Xu et al., 2007; Klammert et al., 2009; van den Vreken et al., 2010). A-cell microcapsules grown in culture medium were used as controls (n=4).
2.7. Osteogenic differentiation of released MC3T3-E1 cells
Osteogenic differentiation of released MC3T3-E1 cells in the CPC scaffolds was analyzed after 7, 14, and 21 d of culture in an osteogenic medium (n=6). At each time point, the osteogenic medium was removed and every sample in the plate was washed with cold PBS. Then 0.25 ml of cell lysate (50 mmol/L Tris, 0.98 mmol/L MgCl2·6H2O, 0.5% (v/v) Triton X-100; pH=7.6) was added to each well and the discs were carefully broken into small pieces. The samples were then processed through three freeze-thaw cycles (−20 and 37 °C, 30 min each) to rupture the cell membrane and extract the proteins (de Ruijter et al., 2001). The disc and 0.25 ml of cell lysate were placed in a 1.5-ml centrifuge tube and centrifuged at 14 000 r/min for 10 min. The upper liquid (150 μl) was then collected and used for the following experiment. A p-nitrophenyl phosphate (pNPP) liquid substrate system (Nanjing Jiancheng Bioengineering Institute, China) was used to analyze alkaline phosphatase (ALP) activity in accordance with the manufacturer’s protocol. Total protein content was determined by the bicinchoninic acid (BCA) method in aliquots of the same samples using the Pierce protein assay kit (Rockford, IL, USA), read at 562 nm, and calculated according to a series of albumin (bovine serum albumin) standards. Finally, ALP levels were normalized to total protein content. Empty microcapsules (without cells) mixed with CPC paste were used as the negative control (n=3).
2.8. Cell attachment evaluated by environment scanning electron microscopy
Attachment of MC3T3-E1 cells to the CPC scaffolds was investigated after 1, 7, and 14 d of culture. An environment scanning electron microscopy (ESEM) was performed at each time point to observe attachment of the released cells and the macropores formed in the scaffold. At each time point, MC3T3-E1 cells cultured in CPC following delivery by one of the two constructs were rinsed with PBS thrice, fixed with 2.5% (w/w) glutaraldehyde for 12 h, and examined with ESEM (FEI Quanta 200 FEG, low vacuum model).
2.9. Staining of mineralized nodules formed by released MC3T3-E1 cells
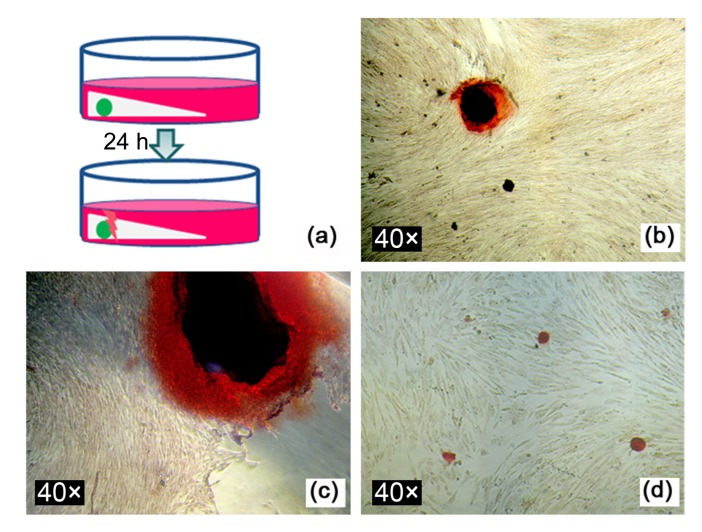
To enable convenient collection of released cells, cell-encapsulating alginate and AC capsules of 2 mm in diameter were fabricated and seeded into CPC paste. The constructs were retained in position for 15 min and 2.0 ml of fresh medium was added into each well until the constructs were completely submerged. The culture medium was changed after the first 12 h. After a further 12 h, the constructs were transferred into new wells and carefully broken away from the capsule, as schematically illustrated in Fig. 10a. Next, 2.0 ml of fresh osteogenic medium was added to each well and the pore formed by the capsule in situ was gently washed with medium to re-suspend and collect the released cells. The CPC disc was then removed because its mineral composition would interfere with the staining of mineralization by the cells. At Day 21 of the culture, the medium was removed, and alizarin red staining was performed to observe the formation of mineralized nodules. MC3T3-E1 cells directly seeded into wells of a culture plate were used as controls.
Fig. 10.
Staining of mineralized nodules produced by released MC3T3-E1 cells
(a) Schematic diagram of the process of collecting released MC3T3-E1 cells; (b) Mineralized nodules formed by cells released from alginate capsules at Day 21 in osteogenic medium; (c) Mineralized nodules formed by cells released from AC capsules at Day 21 in osteogenic medium; (d) Mineralized nodules formed by MC3T3-E1 cells seeded directly into culture wells
2.10. Statistical analysis
One-way analysis of variance (ANOVA) and two-way ANOVA were performed to detect significant effects of the experimental variables. Tukey’s multiple comparison tests were used at a P value of 0.05.
3. Results
3.1. A-cell and AC-cell microcapsules
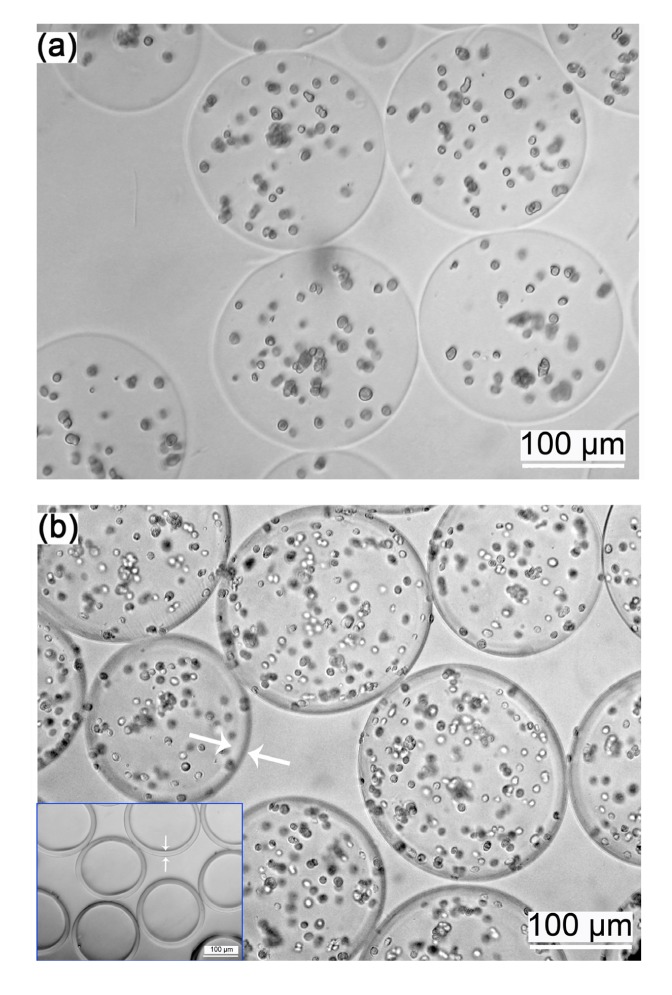
Fig. 1 shows the schematic representation of the electrostatic droplet generator used to produce the microcapsules. A-cell microcapsules (Fig. 2a) and AC-cell microcapsules (Fig. 2b) were spherical and the cells appeared as small dots. The AC polyelectrolyte complex membrane was clearly visible (Fig. 2b arrows and inset figure). AC microcapsules without cells (Fig. 2b inset figure) showed the typical structure of core-shell microcapsules.
Fig. 2.
Images of A-cell (a) and AC-cell (b) microcapsules
The cells appeared as small dots and in the AC-cell microcapsules the AC polyelectrolyte complex membrane was clearly visible (white arrows); Inset figure in (b) shows AC-microcapsules without cells, showing the typical structures of the core-shell microcapsules
3.2. MC3T3-E1 cell viability after microencapsulation
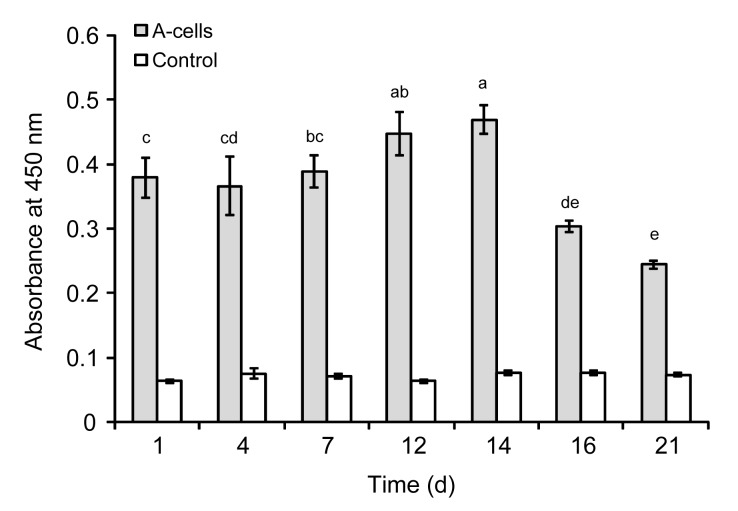
Cell viability after microencapsulation (mean±standard deviation (SD); n=4) was measured using the Wst-8 assay (Fig. 3). From Days 1 to 7, the absorbance at 450 nm (arbitrary units) was similar in each group with no significant differences. At Days 12 and 14, viability was observed to have increased, with values of 0.447±0.034 and 0.469±0.022, respectively, whereas at Day 21, the value fell to 0.244±0.006. The difference in viability between the microencapsulated cells and the control group was significant (P<0.05).
Fig. 3.
Wst-8 viability assay of microencapsulated A-cells in vitro
The viability of cells was quantified by measuring the dehydrogenase activity (arbitrary unit). Each value is mean±SD (n=4). Cell viability increased at Days 12 and 14. Dissimilar letters in the plot indicate that values are significantly different (P<0.05, Tukey’s multiple comparison test, family confidence coefficient 0.95)
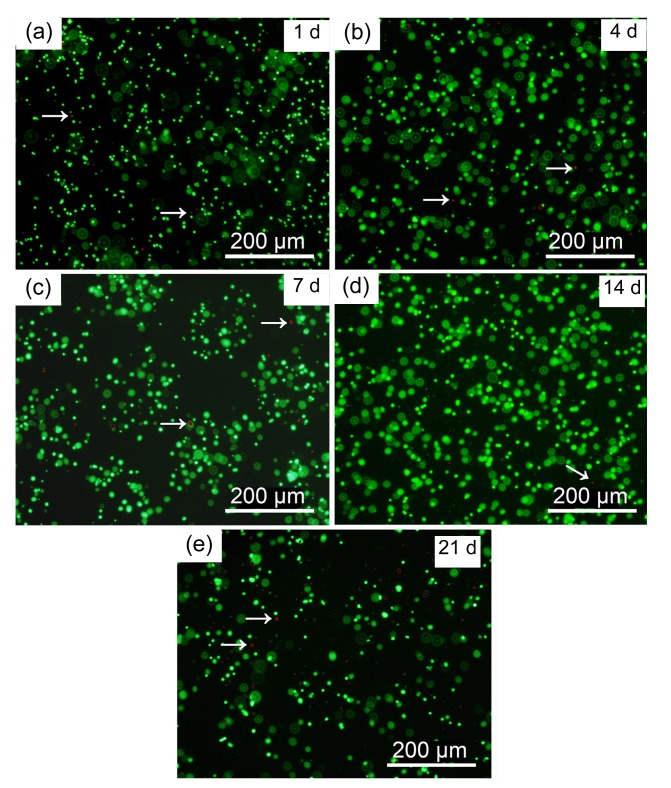
Fluorescence microscopy was used to observe live/dead stained microencapsulated MC3T3-E1 cells cultured from Days 1 to 21 (Fig. 4). Live cells appeared as small green dots in the hydrogel while dead cells appeared as red dots (arrows). Very few dead cells were evident and no cell release from the alginate microcapsules was observed during the entire culture period.
Fig. 4.
Fluorescent images of live/dead staining of A-cell microcapsules
Cultured A-cell microcapsules at Days 1 (a), 4 (b), 7 (c), 14 (d), and 21 (e). Green dots: live cells; Red dots (arrows): dead cells (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
3.3. MC3T3-E1 cell release into the CPC scaffold
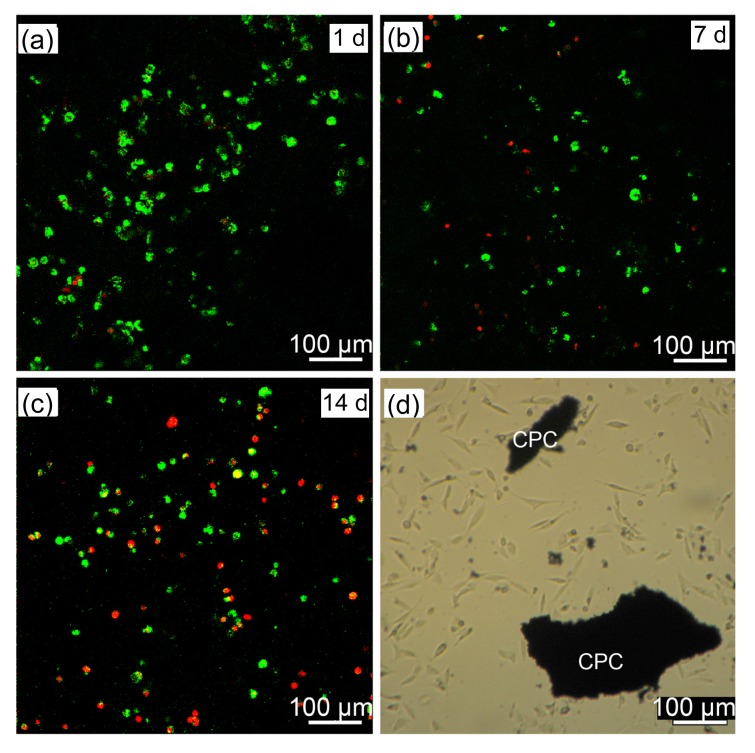
Released MC3T3-E1 cells attached to CPC are shown in Figs. 5a–5c, in which live cells are stained green and dead cells red. At Days 1 and 7, there were more live cells than dead cells, while at Day 14, many dead cells could be seen. Fig. 5d shows released cells attached to the bottom of a 12-well plate at Day 1. The cells exhibited a spindle-shaped or polygonal morphology and developed cytoplasmic extensions.
Fig. 5.
MC3T3-E1 cell release when mixed with CPC paste
Released cells growing on CPC at Days 1 (a), 7 (b), and 14 (c). (d) Released cells attached to the bottom of a 12-well plate at Day 1. Green dots: live cells; Red dots: dead cells (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
3.4. Viability of released MC3T3-E1 cells in the CPC scaffold
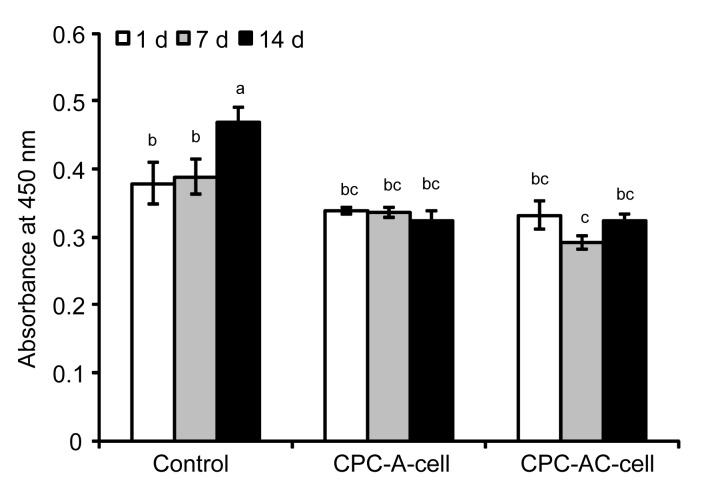
The viability of the surviving MC3T3-E1 cells released into CPC is illustrated in Fig. 6. The viability of cells encapsulated in alginate or AC microcapsules seeded into CPC changed only minimally compared to that of the controls. Longer incubation time had no influence on cell proliferation throughout the course of the study.
Fig. 6.
Cell viability of released MC3T3-E1 cells in CPC
Results are displayed as mean±SD (n=4). Dissimilar letters in the plot indicate that values are significantly different (P<0.05, Tukey’s multiple comparison test, family confidence coefficient 0.95)
3.5. Osteogenic differentiation of released MC3T3-E1 cells
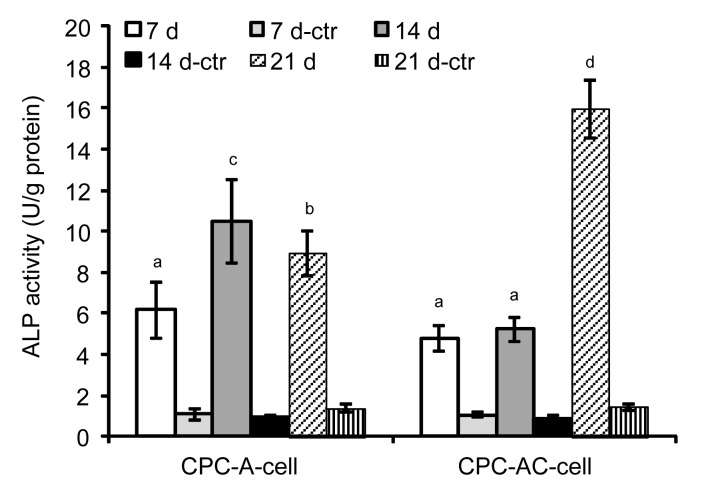
The ALP activity of released MC3T3-E1 cells is shown in Fig. 7. ALP activity of the CPC-A-cell construct increased at Day 14, but then showed minimal change until Day 21. In the CPC-AC-cell construct, we observed a continuous increase of ALP over the course of 21 d and the datum for Day 21, which was about four times that at Day 7, remained significantly higher compared to the data of other time points in the CPC-AC-cell group as well as the data of different time points in the CPC-A-cell group (P<0.05). The difference in ALP between the experimental group and its control group was statistically significant (P<0.05).
Fig. 7.
ALP activity of released MC3T3-E1 cells in CPC
Results are displayed as mean±SD (n=6). Dissimilar letters in the plot indicate that values are significantly different (P<0.05, Tukey’s multiple comparison test, family confidence coefficient 0.95). ctr: control
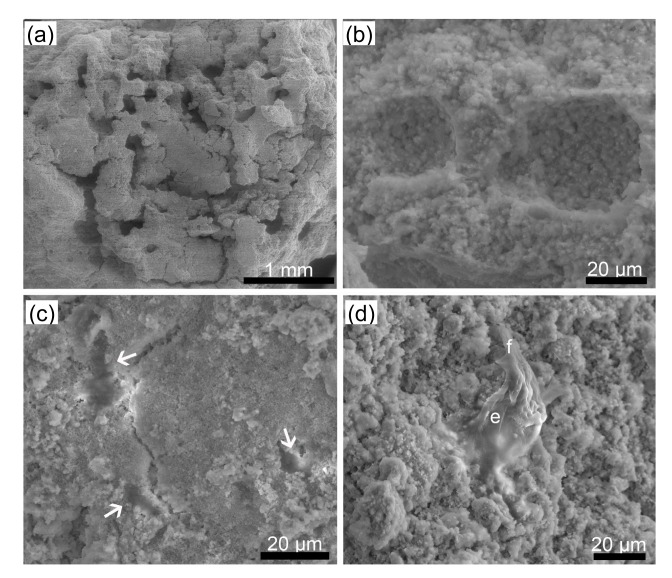
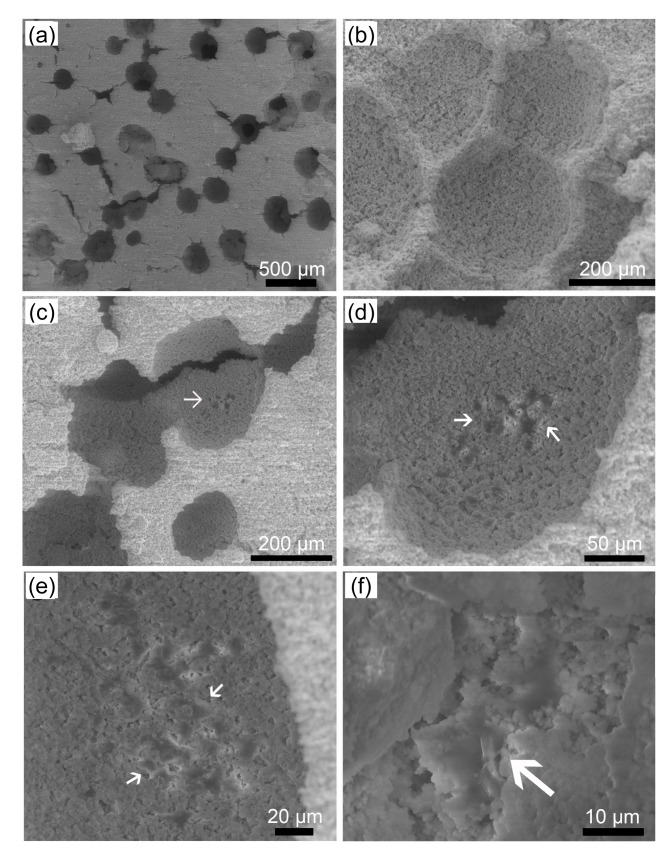
3.6. Cell attachment investigated by ESEM
The surfaces of the two types of constructs and the released cells that attached onto the CPC were observed using ESEM and the images are shown in Figs. 8 and 9. Images of the surface and of a transverse section of the CPC setting discs of the CPC-A-cell construct are shown in Figs. 8a and 8b. The released cells (Fig. 8c arrows) attached onto the CPC and developed short cytoplasmic extensions anchoring them onto the CPC (Fig. 8d). Images of the surface and of a transverse section of the CPC setting discs of the CPC-AC-cell construct are shown in Figs. 9a and 9b. Figs. 9c–9f show that cells (white arrows) remained in the pores of the construct, as shown under different magnifications, and developed short cytoplasmic extensions that anchored them onto the CPC (Fig. 9f). More pores were created by the AC microcapsules in situ than by the alginate microcapsules.
Fig. 8.
ESEM images of CPC-A-cell construct and released MC3T3-E1 cells
(a) The surface of the CPC-A-cell construct; (b) Transverse section of the CPC-A-cell construct; (c, d) Released MC3T3-E1 cells (arrows) under different magnifications. e: MC3T3-E1 cell; f: short cytoplasmic extension
Fig. 9.
ESEM images of CPC-AC-cell construct and released MC3T3-E1 cells
(a) The surface of the CPC-AC-cell construct; (b) Transverse section of the CPC-AC-cell construct; (c, d, e, f) Released MC3T3-E1 cells (arrows) under different magnifications
3.7. Alizarin red staining of mineralization by released MC3T3-E1 cells
Alizarin red staining showed that the MC3T3-E1 cells released from both alginate and AC capsules were able to form mineralized nodules by Day 21 (Figs. 10b and 10c). Fig. 10d shows mineralized nodules formed by MC3T3-E1 cells directly seeded into a culture plate.
4. Discussion
CPC, initially reported by Brown and Chow (1983), has become widely used due to its biocompatibility, osteoconductivity, self-setting properties, and injectability. It generally consists of a powder phase and a liquid phase. Powder phases of CPCs are mixed with the liquid phase, usually double-distilled water or various concentrations of NaxH3−xPO4, at a moderate powder to liquid (P/L) ratio, to form CPC paste that can self-set within a few minutes (Miyamoto et al., 1995).
Microencapsulation of living cells has become widely used in recent years. In this study, A-cell and AC-cell microcapsules were prepared using an electrostatic droplet generator. There were no significant differences in cell viability between the encapsulated cells and the controls (Xie and Wang, 2007). The diameter of the AC-cell microcapsules was approximately 200 μm (Fig. 2), so that influx of nutrients and outflux of biological metabolites produced by the encapsulated cells could occur easily due to the small size and high surface area of the microcapsules compared with the larger beads (Zhou and Xu, 2011). However, encapsulating cells inside the alginate gel caused contact inhibition between cells (Markusen et al., 2006). As a result, cells showed no obvious proliferation when cultured in the medium (Figs. 3 and 4).
Alginate, a natural polysaccharide, is biocompatible and can form a three-dimensional polymer network with water as the predominant dispersal medium (Drury and Mooney, 2003). It has been used for cell delivery because it can be cross-linked under mild conditions by the addition of divalent cations (Gombotz and Wee, 1998). However, it is inherently non-degradable in mammals (Lee and Mooney, 2012). Biodegradation of materials can be characterized by changes in their mechanical and physicochemical properties (disintegration and dissolution) (Ding, 2007). Ionically cross-linked alginate gels can be dissolved by release of the divalent ions due to exchange reactions with monovalent cations such as Na+ (Lee and Mooney, 2012). Taqieddin and Amiji (2004) found that PO4 3− was able to extract the calcium ions from AC microcapsules and convert the solid calcium alginate core into a liquid sodium alginate core. In this study, we used β-TCP/CPC as the powder and 0.6 mol/L Na2HPO4/NaH2PO4 solution as the liquid. Since there were many PO4 3− ions in the freshly-made CPC paste, the core of the AC microcapsules dissolved in the setting CPC paste to release the cells. Alginate microcapsules used in this study were dissolved once mixed with CPC paste, releasing the encapsulated MC3T3-E1 cells (Fig. 5).
MC3T3-E1 cells released within the CPC scaffolds showed no proliferation but survived and differentiated (Figs. 6 and 7). Cell proliferation was compromised, which might have resulted from cell differentiation in osteogenic medium or from inadequate cell density of the surviving cells. Moreover, the influx of nutrients and the outflux of biological metabolites produced by these cells presented difficulties in a static culture system. However, the results of the in vitro study demonstrated that β-TCP/CPC had no pronounced negative effect on the viability of MC3T3-E1 cells. This finding confirmed the study of van den Vreken et al. (2010) that showed that MC3T3-E1 cells grew well but failed to proliferate on CPC. ALP activity of MC3T3-E1 cells in the CPC-A-cell construct peaked at Day 14. In contrast, that of the CPC-AC-cell construct peaked at Day 21, when it was approximately four times that at Day 7 and exceeded that of the CPC-A-cell group. This confirmed the previously reported observation that chitosan has an osteoconductive effect (Moreau and Xu, 2009; Muzzarelli, 2011). It was also found that by Days 14 and 21, the MC3T3-E1 cells had entered the third stage of maturation, demonstrated by increased ALP activities and the formation of mineralized nodules (Hong et al., 2010). In this study, released cells growing for more than 14 d on CPC and demonstrating increased ALP activities were considered as mature osteoblasts although the time of peak ALP activity differed between the two groups. The released cells were able to form mineralized nodules at Day 21 (Figs. 10b and 10c).
CPC appears to be an unsuitable substrate for attachment of cells released from microcapsules in vitro compared with some biofunctionalized substrates (Chen et al., 2012; Zhou et al., 2012). Cells attaching onto CPC (Figs. 5a–5c, 8 and 9) did not exhibit spindle-shaped or polygonal morphology or develop long cytoplasmic extensions, unlike the morphology in a two-dimensional culture system or on the very smooth surface of CPC discs (Moreau et al., 2009; van den Vreken et al., 2010). The cytoplasmic extensions are regions of the cell plasma membrane and are favorable for movement of the migrating cells along a substratum (Moreau et al., 2009). This may be due to the unsuitable surface of CPC which, independent of the calcium and phosphate ion concentration changes in the medium, may have an effect on cell behavior (Habibovic and Barralet, 2011). Consequently it is evident that the performance of CPC used in this study is also in need of improvement. Many cells dispersed at the bottom of the pores formed by AC-cell microcapsules in situ (Figs. 9c–9e) showed higher ALP activity than that of the CPC-A-cell group even after extended incubation time. Furthermore, pores formed by AC-cell microcapsules (Figs. 9a and 9b) were larger and more spherical than those formed by A-cell microcapsules (Figs. 8a and 8b). This was because an AC polyelectrolyte complex membrane formed around the outside of the microcapsules and the AC-cell microcapsules could therefore retain their spherical shape in CPC paste while A-cell microcapsules were directly exposed to CPC paste and began to dissolve once mixed with CPC. This observation implies that a CPC-AC-cell construct would be more suitable for injection than a CPC-A-cell one, forming a porous scaffold after setting. Further study is also needed to determine whether the proliferative ability of the cells could be improved and to investigate the mechanical properties of the CPC-AC-cell construct both in vitro and in vivo. The ultimate goal is to develop an injectable bioactive bone-substitute scaffold for moderate stress-bearing bone tissue engineering.
5. Conclusions
In this study, a new type of MC3T3-E1 cell-encapsulating AC microcapsule of several hundred microns in diameter was randomly mixed with a CPC paste for the first time. The encapsulated MC3T3-E1 cells were released from the microcapsules and successfully attached, survived, and differentiated within the CPC scaffold. The AC microcapsules were more favorable for implantation of MC3T3-E1 cells, allowing them to differentiate while concomitantly creating macropores in situ. This is a promising way to achieve seeding of cells deep into an injectable CPC scaffold to improve its effectiveness and function.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30772447) and the Talent Introduction Project of Peking University Health Science Center (No. bmu2009139), China
Compliance with ethics guidelines: Peng-yan QIAO, Fang-fang LI, Li-min DONG, Tao XU, and Qiu-fei XIE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ambrosio AM, Sahota JS, Khan Y, et al. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res. 2001;58(3):295–301. doi: 10.1002/1097-4636(2001)58:3<295::AID-JBM1020>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Brown W, Chow LC. A new calcium-phosphate setting cement. J Dent Res. 1983;62(SI):672. [Google Scholar]
- 3.Chen W, Zhou H, Weir MD, et al. Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012;8(6):2297–2306. doi: 10.1016/j.actbio.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow LC. Calcium phosphate cements: chemistry, properties, and applications. In: Boston MA, editor. Mineralization in Natural and Synthetic Biomaterials. USA: Warrendale, Materials Research Society; 2000. pp. 27–37. [Google Scholar]
- 5.de Ruijter JE, Brugge PJ, Dieudonne SC, et al. Analysis of integrin expression in U2OS cells cultured on various calcium phosphate ceramic substrates. Tissue Eng. 2001;7(3):279–289. doi: 10.1089/10763270152044143. [DOI] [PubMed] [Google Scholar]
- 6.Deville S, Saiz E, Tomsia AP. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials. 2006;27(32):5480–5489. doi: 10.1016/j.biomaterials.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Ding SJ. Biodegradation behavior of chitosan/calcium phosphate composites. J Non-Cryst Solids. 2007;353(24-25):2367–2373. doi: 10.1016/j.jnoncrysol.2007.04.020. [DOI] [Google Scholar]
- 8.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/S0142-9612α03)00340-5. [DOI] [PubMed] [Google Scholar]
- 9.Friedman CD, Costantino PD, Takagi S, et al. BoneSource™ hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res. 1998;43(4):428–432. doi: 10.1002/(SICI)1097-4636(199824)43:4<428::AID-JBM10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–285. doi: 10.1016/S0169-409X497)00124-5. [DOI] [PubMed] [Google Scholar]
- 11.Habibovic P, Barralet JE. Bioinorganics and biomaterials: bone repair. Acta Biomater. 2011;7(8):3013–3026. doi: 10.1016/j.actbio.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Hong D, Chen HX, Yu HQ, et al. Morphological and proteomic analysis of early stage of osteoblast differentiation in osteoblastic progenitor cells. Exp Cell Res. 2010;316(14):2291–2300. doi: 10.1016/j.yexcr.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klammert U, Reuther T, Jahn C, et al. Cytocompatibility of brushite and monetite cell culture scaffolds made by three-dimensional powder printing. Acta Biomater. 2009;5(2):727–734. doi: 10.1016/j.actbio.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Kretlow JD, Young S, Klouda L, et al. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21(32-33):3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurencin CT, Ambrosio AM, Borden MD, et al. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1(1):19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Lavik E, Langer R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65(1):1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 17.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85(11):966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markusen JF, Mason C, Hull DA, et al. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12(4):821–830. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto Y, Ishikawa K, Fukao H, et al. In vivo setting behaviour of fast-setting calcium phosphate cement. Biomaterials. 1995;16(11):855–860. doi: 10.1016/0142-9612(95)94147-D. [DOI] [PubMed] [Google Scholar]
- 21.Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials. 2009;30(14):2675–2682. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau JL, Weir MD, Xu HH. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J Biomed Mater Res A. 2009;91(2):605–613. doi: 10.1002/jbm.a.32248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzzarelli RA. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohyd Polym. 2011;83(4):1433–1445. doi: 10.1016/j.carbpol.2010.10.044. [DOI] [Google Scholar]
- 24.Qiao P, Wang J, Xie Q, et al. Injectable calcium phosphate-alginate-chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo . Mat Sci Eng C Mater. 2013;33(8):4633–4639. doi: 10.1016/j.msec.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 26.Shindo ML, Costantino PD, Friedman CD, et al. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119(2):185–190. doi: 10.1001/archotol.1993.01880140069012. [DOI] [PubMed] [Google Scholar]
- 27.Simon CJ, Guthrie WF, Wang FW. Cell seeding into calcium phosphate cement. J Biomed Mater Res A. 2004;68(4):628–639. doi: 10.1002/jbm.a.20008. [DOI] [PubMed] [Google Scholar]
- 28.Taira M, Nakao H, Takahashi J, et al. Effects of two vitamins, two growth factors and dexamethasone on the proliferation of rat bone marrow stromal cells and osteoblastic MC3T3-E1 cells. J Oral Rehabil. 2003;30(7):697–701. doi: 10.1046/j.1365-2842.2003.01118.x. [DOI] [PubMed] [Google Scholar]
- 29.Taqieddin E, Amiji M. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials. 2004;25(10):1937–1945. doi: 10.1016/j.biomaterials.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 30.van den Vreken NM, Pieters IY, Declercq HA, et al. Characterization of calcium phosphate cements modified by addition of amorphous calcium phosphate. Acta Biomater. 2010;6(2):617–625. doi: 10.1016/j.actbio.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Weir MD, Xu HHK. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomater. 2010;6(10):4118–4126. doi: 10.1016/j.actbio.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir MD, Xu HH, Simon CJ. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res A. 2006;77(3):487–496. doi: 10.1002/jbm.a.30626. [DOI] [PubMed] [Google Scholar]
- 33.Xie HG, Li XX, Lv GJ, et al. Effect of surface wettability and charge on protein adsorption onto implantable alginate-chitosan-alginate microcapsule surfaces. J Biomed Mater Res A. 2010;92(4):1357–1365. doi: 10.1002/jbm.a.32437. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Wang CH. Electrospray in the dripping mode for cell microencapsulation. J Colloid Interface Sci. 2007;312(2):247–255. doi: 10.1016/j.jcis.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Xu HH, Carey LE, Simon CJ, et al. Premixed calcium phosphate cements: synthesis, physical properties, and cell cytotoxicity. Dent Mater. 2007;23(4):433–441. doi: 10.1016/j.dental.2006.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HH, Zhao L, Weir MD. Stem cell-calcium phosphate constructs for bone engineering. J Dent Res. 2010;89(12):1482–1488. doi: 10.1177/0022034510384623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L, Weir MD, Xu HH. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials. 2010;31(14):3848–3857. doi: 10.1016/j.biomaterials.2010.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31(25):6502–6510. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L, Tang M, Weir MD, et al. Osteogenic media and rhBMP-2-induced differentiation of umbilical cord mesenchymal stem cells encapsulated in alginate microbeads and integrated in an injectable calcium phosphate-chitosan fibrous scaffold. Tissue Eng Part A. 2011;17(7-8):969–979. doi: 10.1089/ten.tea.2010.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32(30):7503–7513. doi: 10.1016/j.biomaterials.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Chen W, Weir MD, et al. Biofunctionalized calcium phosphate cement to enhance the attachment and osteodifferentiation of stem cells released from fast-degradable alginate-fibrin microbeads. Tissue Eng Part A. 2012;18(15-16):1583–1595. doi: 10.1089/ten.tea.2011.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]