Abstract
The impact of intrinsic aging upon human peripheral blood T-cell subsets remains incompletely quantified and understood. This impact must be distinguished from the influence of latent persistent microorganisms, particularly cytomegalovirus (CMV), which has been associated with age-related changes in the T cell pool. In a cross-sectional cohort of 152 CMV-negative individuals, aged 21–101 years, we found that aging correlated strictly to an absolute loss of naïve CD8, but not CD4, T cells, but, contrary to many reports, did not lead to an increase in memory T cell numbers. The loss of naïve CD8 T cells was not altered by CMV in 239 subjects (range 21–96 years) but the decline in CD4+ naïve cells showed significance in CMV+ individuals. These individuals also exhibited an absolute increase in the effector/effector memory CD4+ and CD8+ cells with age. That increase was seen mainly, if not exclusively, in older subjects with elevated anti-CMV Ab titers, suggesting that efficacy of viral control over time may determine the magnitude of CMV impact upon T cell memory, and perhaps upon immune defense. These findings provide important new insights into the age-related changes in the peripheral blood pool of older adults, demonstrating that aging and CMV exert both distinct and joint influence upon blood T cell homeostasis in humans.
Keywords: Aging, T cell, homeostasis, CMV
Introduction
Advanced age is associated with increased risk of sickness and death from infection. Infections by microorganisms that were not previously encountered or infections by previously encountered organisms which have the capacity for antigenic variation (e.g. influenza virus), cause disproportionate morbidity and mortality in older adults(1, 2). Moreover, those over 65 exhibit diminished response to vaccination(3) and are up to 1,000 fold more likely to die from vaccine-preventable infection than children(4). The key underlying cause for this increase in susceptibility to infections is believed to be the age-related decline of immune function – immune senescence.
Utilizing a short lived laboratory species (mus musculus), we and others have shown that factors such as thymic involution, decreased production of naïve T cells, reduction in T-cell responsiveness, decline in TCR repertoire diversity, and dysregulation of the memory cell pool responding to persistent latent infections – “memory inflation” - all contribute to immunosenesce [rev. in(5–7) and references therein). Moreover, the loss of naïve T cells, accumulation of effector memory cells and TCR repertoire constriction were shown to predict poor vaccine responses in aged non-human primates (8). Yet, factors leading to each of these disturbances and their relative contributions to immune senescence remain incompletely understood.
Human CMV is a persistent herpes virus present in 60–90% of the adult population, and is normally asymptomatic in immunocompetent hosts (rev. in(9, 10)). Several studies have demonstrated that persistent infection with CMV is associated with changes in subset distribution, phenotype and, potentially, on the function of T cells in aging individuals(10, 11). While numerous hypotheses and studies were conducted to delineate the influence of CMV upon T cell homeostasis and function with aging (rev. in (6, 10, 11), and references therein), the relationship between this virus and human physiological aging remains incompletely understood, particularly as related to the absolute cellular changes induced by the virus and by age itself.
In this study, we sought to specifically delineate the effect of aging from that of CMV infection upon relative and absolute changes in blood T cell subsets using a cohort of 391 adults aged 21–101 years, separated into two subgroups based upon serological cytomegalovirus (CMV) status. Our results highlight distinct and separate, but also interacting, roles of aging and of CMV in modulating the circulating blood T cell subsets, and provide the framework for evaluation of both the impact of aging and CMV upon susceptibility to infection and for designing potential predictive biomarkers and immunomodulatory interventions to measure and increase vaccine response efficacy in older adults.
MATERIALS and METHODS
Human Subjects, Cell Preparation and CMV Elisa
This study was approved by the Institutional Review Boards at the University of Arizona and the Oregon Health & Science University. The cohort included of 391 healthy community-dwelling individuals, 21–101 years of age, 152 CMV− (84 females, 68 males) and 239 CMV+ (157 females and 82 males), recruited in Oregon, Arizona and Texas. Table 1 illustrates our cohort details, presenting exact numbers by age bin, entire age range, age mean, age median, gender and CMV status, all stratified by geographic region where subjects were drawn. The cohort included 6 individuals from Texas (samples shipped in heparin overnight and then processed in Oregon). The remainder of the samples were closely split between Oregon (194) and Arizona (191), and were both processed fresh at the respective sites and also cryopreserved for future analysis. Confirmatory analysis of a smaller cohort using frozen samples (supplementary data) was conducted in Arizona. All individuals were recruited over the period of 6 years. Cell processing, CMV ELISA and FCM phenotyping were performed by two technicians, in a highly standardized and reproducible manner with regard to cell viability, standard curve ELISA generation and FCM standards. Exclusion criteria included known immunosuppressive pathology, stroke, cancer or use of steroids within the last 5yr.
Table 1.
Cohort Demographics
| CMV+ (n) | CMV− (n) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geographic Location | Number of Subjects (n) | Age Span (yrs) | Average Age (yrs) | Age STD (yrs) | Median Age (yrs) | Total Females (n) | Total Males (n) | Females (n) | Males (n) | Total (n) | Females (n) | Males (n) | Total (n) |
| Oregon | 194 | 21–101 | 53 | 22 | 53 | 123 | 71 | 69 | 32 | 101 | 54 | 39 | 93 |
| Arizona | 191 | 21–93 | 57 | 16 | 60 | 115 | 76 | 86 | 48 | 134 | 29 | 28 | 57 |
| Texas | 6 | 45–71 | 58 | 10 | 61 | 3 | 3 | 2 | 2 | 4 | 1 | 1 | 2 |
| Total Cohort | 391 | 21–101 | 56 | 60 | 241 | 150 | 157 | 82 | 239 | 84 | 68 | 152 | |
| <40yrs | 41–49yrs | 50–64yrs | 65+yrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Geographic Location | CMV+ (n) | CMV− (n) | Total (n) | CMV+ (n) | CMV− (n) | Total (n) | CMV+ (n) | CMV− (n) | Total (n) | |
| Oregon | 30 | 44 | 74 | 4 | 35 | 30 | 65 | 35 | 16 | 51 |
| Arizona | 24 | 15 | 39 | 8 | 55 | 22 | 77 | 50 | 17 | 67 |
| Texas | 0 | 0 | 0 | 2 | 2 | 1 | 3 | 1 | 0 | 1 |
| Total Cohort | 54 | 59 | 113 | 14 | 92 | 53 | 145 | 86 | 33 | 119 |
| Ethnicity | Race | |||||||
|---|---|---|---|---|---|---|---|---|
| Geographic Location | Hispanic | Non Hispanic | Caucasion | African American | Asian | More than One Race | Other | Native American |
| Oregon | 12 | 182 | 180 | 3 | 3 | 3 | 3 | 2 |
| Arizona | 64 | 127 | 171 | 8 | 1 | 5 | 4 | 2 |
| Texas | 0 | 6 | 6 | |||||
| Total Cohort | 76 | 315 | 357 | 11 | 4 | 8 | 7 | 4 |
Blood was drawn into heparinized Vacutainer-CPT tubes (BD, Sunnyvale, CA, Cat 362753) and processed per manufacturers recommendations to isolate peripheral blood mononuclear cells (PBMC) and plasma; Li-EDTA tubes were used to determine complete blood counts. Some PBMC were utilized immediately for FCM; the rest were viably cryopreserved and stored for further analysis. The presence of antibodies against CMV was determined by ELISA, as previously described(12). CMV titer was determined utilizing a standard curve from a confirmed clinical positive control as the reference with a negative control cut-off of 1:30. Additionally a clinically verified negative CMV control was run with every plate. All sub-cohorts were tested with the identical assay titration (including the identical positive and negative reference controls and a small group of 80 samples (40 CMV+ and 40 CMV−) were independently verified by a national reference laboratory (ARUP Laboratories, Utah).
Flow cytometry (FCM)
Freshly isolated PBMC (1–5×106) were stained with T-cell markers (in various combinations): αCD3 APC-Cy7 (BD 557832), αCD4 PerCP-Cy5.5 (eBioscience), αCD8β-ECD (Beckman Coulter 6607123), αCD95-APC (Invitrogen MHCD9050), αCD28-Biotin (eBioscience 13-0289), and SA-Q-dot 525 (Invitrogen Q10141MP). Additional surface markers (CD14-PE-Cy7, CD11a-FITC, CD62L-PE, CD16-Pacific Blue) were included in this panel in order to evaluate these and other cell types (NK, Monocyte/Mf, etc.). Unfortunately, the consistency of CD62L staining proved to be insufficiently robust to use it in cohort analysis.
Cryopreserved PBMC (1×106/sample) were stained with αCD4-A700 (eBioscience 56-0048-82), αCD8β-ECD, αCD95-APC and αCD28-PerCP-Cy5.5, in select experiments CD3-A700, CD4-APCe780, CD45RA – A450, CCR7- PE, CD95-PECy7 from eBioscience, BD and BioLegend as well as the LIVE/DEAD® Fixable Dead Cell Stain – YFR dye (L-34959, Invitrogen) were also used. FCM analysis was performed with the 4-laser BD LSR II or BD Fortessa (exclusively used for analysis of cryopreserved specimens, which were all processed and analyzed in Arizona regardless of the site of their recruitment) instruments, using DiVa acquisition (BDIS, Mountain View, CA) and the FlowJo analysis software (TreeStar, Inc., Ashland, OR). All data were collected using either the same or similar 4-laser configuration, as follows 405; 488, 532 (LSR II in both OR and AZ) or 561 (Fortessa, AZ); and 640 nm from the same manufacturers, set to optimized standard voltages and calibrated using Rainbow beads (BD Biosciences) on a daily basis to achieve consistency.Maximal tolerated daily variation was <3% between the coefficients of variation measured by day-to-day rainbow bead controls.
Absolute CD4 and CD8 T cell numbers were calculated by multiplying % by the total lymphocyte number determined from the whole blood counts using the Coulter Counter (Beckman-Coulter, Hialeah, FL). Subset definitions and gating strategies are outlined in the text and/or the figure legends. The numbers of a defined subset (definitions specified in the text; e.g. naïve (N): CD28intCD95lo; central memory, or CM:CD28hiCD95hi; and effector/effector memory, or EM:CD28lo or −CD95hi) for CD4 and CD8 were obtained by multiplying the numbers of CD4 or CD8 by the of the respective memory subsets.
Statistical Analysis
The absolute number (Count) and proportions of CD4 and CD8 T cells (and their respective subsets) were modeled as a function of age and CMV status using a multiple regression model. Interaction between age and CMV status was also added as a covariate to test the statistical hypothesis that the slope of regression line for CMV− and CMV+ cohorts are the same. Detailed description of the multiple regression model, more precisely a multiple regression with indicator variable (13), is given by
where Count is the measured response variable; β0, is a regression coefficient associated with intercept; β1, β2, β3 are regression coefficients associated with independent variables of Age, CMV status, and interaction between age and CMV status, respectively; and e is the model error. The model error was added because the statistical/mathematical model does not fully represent the actual relationship between the dependent variable (T cell subset count) and the independent variables (age, CMV status). The model error (e) is defined by the deviations of each actual observation from a model regression line, it is assumed to be a random variable normally distributed (Gaussian distribution) with mean 0 and constant variance σ2. The ICMV is an indicator variable, and assignment of this indicator variable was ‘0’ to CMV− subject and ‘1’ to CMV+ subject. As a result, for the CMV−,
And for the CMV+,
This indicator variable provides a merit of statistical hypothesis testing on not only the difference in intercept or starting subset counts (statistical hypothesis of β2=0) between CMV− and CMV+ but also the difference in slope or rate of change (statistical hypothesis of β3=0) between CMV− and CMV+. Prior to applying regression models, normality assumption was evaluated using Shapiro-Wilk test, and logarithmic transformation was applied if normality assumption was not held. To evaluate variation of CMV− and CMV+ cohort by age group, we categorized age into three age groups (age < 40, 50 ≤ age < 65, and age ≥ 65) and then performed pairwise Levene’s tests for the equal variance followed by Bonferoni correction for the multiple comparisons. Two-way Analysis of Variance (ANOVA) followed by pair-wise comparisons was used to compare the T-cell counts between age groups and CMV titer. There are three categories of CMV titer (CMV−, CMV low, CMV high) and these were determined by the median CMV titer value 349, a titer above which was assigned CMV high, and below which was assigned CMV low where as a titer value of 0 was assigned CMV(−). Prior to applying two-way ANOVA, normality and equal variance assumptions were evaluated using Shapiro-Wilk test and Levene’s test, respectively. Due to the unequal variance, CD8 counts were logarithmically transformed with the base 10 (log10). Bonferroni multiple comparisons correction was used to control overall levels of type I error. Correlation and Bland Altman plot was used to evaluate agreement and disagreement with any systematic bias between CD95loCD28hi and CCR7+CD45RA+ measurement. A simulation study was performed to understand cohort size effect/small sample behavior. In that simulation, we randomly select subsample size of 80 and 120 from whole cohort (n = 391), repeated regression analysis to see if the subset analysis results same conclusion. We repeated the simulation100 times, and reported false positive rate and false negative rate as measures of sensitivity for cohort size effect (Table 2). Statistical significance was determined at the significance level of 0.05. These analyses were conducted using the Statistical Analysis System (SAS) version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Table 2.
Impact of CMV Status on:
| Sample Size (Number of Subjects) | ||
|---|---|---|
| N=80 | N=120 | |
| CD8 Naïve: CMV + vs. CMV− (% False Positives) | 13% | 6% |
| CD8 Effector Memory: CMV+ vs. CMV− (%False Negatives) | 74% | 52% |
RESULTS
Question and study cohort
It has been long noted that the representation of circulating T cell populations changes with aging [e.g.(14–17), rev. in(18)]. However, such changes: (i) were rarely, if ever, corroborated in large and diverse human populations around the world; (ii) were commonly described only in relative (percentages), rather than absolute (cell number) terms. Therefore, it remains unclear whether the changes in a given T cell subset were directly caused by numerical increases or decreases in that particular T cell subset, or indirectly, so that lower representation of a given subset (e.g. the naïve cells) could have been due not to an absolute loss of that subset, but to an absolute increase in another cell subset (e.g. one of the memory cell subsets). Most importantly, (iii) the potentially confounding impact of CMV was not properly accounted for, and the impact of this virus relative to the impact of aging itself remains unclear. Indeed, only three studies so far evaluated changes in cell numbers with aging and CMV infection (19–21), using different markers to define cell subsets, and reaching somewhat disparate conclusions. To conclusively assess the above issues, we recruited a large healthy community-dwelling cohort of CMV− (152 individuals, age range 21–101) subjects from three very different regions of North America (the US states of Arizona, Oregon and Texas) and compared them in a cross-sectional manner to 239 CMV+ subjects in the same age and geographical range group.
Changes in proportional representation and absolute numbers of CD8 and CD4 T subsets with aging in CMV− subjects
We first analyzed relative representation of different T cell populations, using CD28 and CD95 as the main discrimination markers. We elected these markers due to stable and unwavering expression of CD95 on memory cells, and the informative delineation between effector and central memory cells based on CD28 downregulation (22, 23). We had other markers present in the panel, to evaluate additional cell subsets; of T cell markers, we also initially employed CD45RA and CD11a, the addition of which to the CD28 and CD95 demarcation did not show improved resolution over the use of CD28 and CD95 alone. We therefore focused the initial analysis on these two markers. Consistent with several other reports (14, 18, 24) and references therein) confirmed that in the absence of CMV infection, the percentages of total CD8 and CD8 naïve (defined as CD28int95lo) cells significantly decline with age, with a concomitant significant increase in the percentage of both EM (CD28low95hi) and CM(CD28hi95hi) CD8 subsets (Figure 1 A–D - red closed circles). By contrast, representation of total CD4 cells increased significantly (p ≤ 0.0001). Within the CD4 pool, naïve and EM CD4 T cells did not exhibit statistically significant age-related changes (p-values =0.1196 and 0.5864, respectively, supplementary Table ST1); CM CD4 cells exhibited a mild relative increase with aging (p-value = 0.0315, Fig. 1, A–D - green open circles). Analysis of frozen and thawed samples from the same subjects (Supplemental Fig. 1) corroborated these conclusions, establishing that the thawing process did not significantly affect representation of different T cell subsets.
Figure 1. Age-related proportional alterations in CD8 but not CD4 T cell subsets in CMV− subjects.
Fresh PBMC were phenotyped by multi-color FCM (see methods). Events (≥ 3×105) were gated on lymphocytes using forward and side scatter. T cells were defined as CD3+ CD4+ or CD3+CD8β+ and within each, N, CM and EM T cells were defined as CD28int95low, CD28hi95hi and CD28low95hi, respectively. Data shows changes in relative representation of total (A), naïve (B), CM (C) and EM (D) CD8 (gray closed circles) and CD4 (black open circles) cells with aging. Data originate from n = 152 individuals, ranging in age from 21–101. Each dot represents an individual. The lines drawn in the figure and p values are based upon the regression parameters as detailed in the methods and illustrated within supplemental Table, part B (See Table ST1).
More insightful results were obtained when we examined the absolute numbers of cell subsets (Fig. 2). Overall numbers of CD4 T cells remained broadly variable but did not show consistent trends depending on age. Numbers of total CD8 T cells, by contrast, declined with age at a rate of nearly 1.7% CD8 cells/μl/year (Fig. 2A). This was almost entirely due to an absolute loss of naïve CD8 T cells, which in the CMV− cohort correlated directly to the age of the subject, falling from an estimated average of 200 (199.8) cells/μl at 21 y to an average of 24 (24.3) cells/μl at 80 y of age (Fig. 2B). Importantly, the increase in representation of CD8 EM and CM in CMV− subjects (see Fig. 1D) with age was only relative, representing a combination of absolute loss of the naïve CD8 T cells, and the relatively constant or only minimally declining numbers of CD8 CM and EM cells with aging (mean levels ≤100 cells/μl for CM and EM cells showed little change across ages, Fig 2. C and D, respectively).
Figure 2. Absolute numbers of CD8 T cells and CD8 naïve cells decline with age in CMV− subjects.
Absolute cell numbers for each subset were obtained from fresh whole blood counts and from FCM analysis of fresh PBMC, as detailed in Methods. Subject cohort, staining, gating, phenotypic definition and statistical treatment were as in Fig. 1. Absolute numbers of total (A), naïve (B), CM (C) and EM(D) CD8 (gray closed circles) and CD4 (black open circles) cells are plotted against age. The lines drawn in the figure and p values are based upon the regression parameters as detailed in the methods and illustrated within supplemental Table, part A (ST1).
The absolute numbers of the CD4 CM and CD4 EM (Fig 2. C, D) cells also did not show changes in individuals of different age, and as expected, numbers of CD4 EM cells remained particularly low in these CMV (−) subjects. However, unlike with the naïve CD8 T cells, the absolute numbers of the CD4 naïve T cells also did not show alterations in individuals of different ages (Fig. 2B), and high levels of naïve CD4 T cells were maintained in the old, as well as in the adult, subjects. Again, these results obtained on fresh PBMC were closely mirrored by data from cryopreserved PBMC samples (Fig. S1). All regression calculations and extended data are provided in supplemental table (Table ST1).
Age-related T cell pool changes and CMV infection
We next analyzed 239 CMV+ subjects and found similar patterns of cellular representation across ages, with declining percentages of naïve and increased percentages of memory cell subsets (not shown). Importantly, CMV+ individuals showed an absolute increase in the EM CD4+ and CD8+ cells, with an estimated average of 67 (67.2) EM CD8+ cells/μl of blood at 21 y, and 110 (109.65) EM CD8+ cells/μl at 80 y in CMV+ subjects, compared to 38 (38.3) EM CD8+ cells/μl at 21 and 24 (24.15) EM CD8+ cells/μl at 80y for CMV− subjects. Although we clearly do not know the time of infection in our subjects, the differences between CD8+ EM cells in CMV+ and CMV− subjects (67 vs 38 cells/μl) were evident already at 21 y of age, suggesting that CMV causes inflation of the EM compartment promptly post infection even in young subjects, consistent with the published literature (20, 25–27). The EM CD4+ T cells were almost twice as numerous in 80y old in the presence of CMV compared to 21y old (15 cells/μl at 21 and 28 cells/μl at 80y, Fig. 3D), consistent with previous observations by Chidrawar et al. (20).
Figure 3. Inflation of Effector Memory CD8 and CD4 cells in CMV+ subjects with age.
Methodology, analysis and panels are identical to Figure 2, except that n = 239 individuals ranging in age from 21– 96, and seropositive for CMV were analyzed. The lines drawn in the figure and p values are based upon the regression parameters as detailed in the methods and illustrated within supplemental Table (ST1).
CMV infection did not alter the rate of naïve CD8+ T cell loss seen with aging alone. CMV+ subjects had an estimated average of 238 (238.1) naive CD8+ cells/μl of blood at 21 and 21 (21.1) naive CD8+ cells/μl at 80y (Fig. 3B). This was not significantly different from the CMV− group (200 naive CD8+ cells/μl of blood at 21y, and 24 naive CD8+ cells/μl at 80y, Fig. 2), and the slopes between the two regression lines were not significantly different (p=0.14). The same results held with frozen PBMC (Fig. S1).
Other herpesviruses and homeostatic T cell changes with aging
It could be argued that CMV is not unique in its ability to drive the above changes in the T cell pool and that other herpesviruses, or for that matter other persistent infections, could lead to similar changes. We examined the potential impact of two other herpesviruses upon our conclusions, Herpes Simplex type 1 (HSV-1) and the Epstein-Barr Virus (EBV). In our cohort, we could see no effect of HSV upon naïve, CM or EM subset abundance (Fig. S2), even though CMV+ subjects had a much greater (3.4-fold) probability of being HSV+. We could not formally evaluate the impact of EBV, because 93.5% of our cohort was EBV+ and we therefore had no cohort power to address this question. However, we can conclude that it was unlikely that EBV played any role in the results obtained, because EBV+, CMV− subjects exhibited much lower EM numbers with aging (and no accumulation of this subset) compared to EBV+ CMV+ subjects. Therefore, we conclude that the other two herpesviruses surveyed do not impact the EM pool abundance to the same level (if at all) as CMV.
CD4:CD8 ratio, aging and CMV infection
The CD4:CD8 ratio was proposed to be one of the components of the Immune Risk Profile (IRP) in the original studies by Wikby, Pawelec and colleagues (20, 25) and the inversion of that ratio correlated with an increased risk of mortality in older adults exhibiting the inversion. Our studies were not longitudinal, and did not follow individual mortality. However, on a cohort level, we found this ratio to be affected by age, although in an unexpected manner (Fig. S3). Namely, in CMV (−) individuals, the CD4:CD8 ratios did not decrease with age. This is in contrast to reports which show an age-associated decline in the CD4:CD8 ratio (26) but is consistent with other studies finding no such changes with aging (27–29). In fact, we saw an increase in the CD4:CD8 ratio with age, driven primarily by the loss of (naïve) CD8 T cells. Examination of the CMV (+) subjects also revealed no significant decrease in the CD4:CD8 ratios with age (Fig. S3A), although it appeared that more individuals over the age of 65 exhibited lower CD4:8 ratios. Analysis of frozen/thawed PBMC confirmed the above findings (Fig. S3B). We are currently in the process of longitudinally following these individuals.
Analysis of naïve CD4 T cell numbers suggested that CMV infection may precipitate a mild loss of these cells with aging (p=0.028); moreover, absolute numbers of CD4 T cells appeared to be lower in CMV-infected individuals compared to CMV-negative subjects at all ages (even in young individuals), but the results did not reach statistical significance due to enormous variability. The issue of relationship between naïve CD4 T cells and CMV will require further study in longitudinal cohorts.
T cell subset definition, cohort size, CMV and aging
The above results posed a question as to why other authors (19–21) may have not observed some of the results described above, most notably the loss of naïve T cells irrespective of CMV and the increase in EM but not CM cell pool which grew more pronounced with age. Some of the reasons were rather obvious, e.g. Looney et al. have used a single marker, CD28, which was the key obstacle in properly delineating CM and N cells from one another (19). Therefore, in searching for possible underlying causes for discrepant conclusions, we considered both the cohort size effects and the differences in the phenotypic definition of T cell subsets.
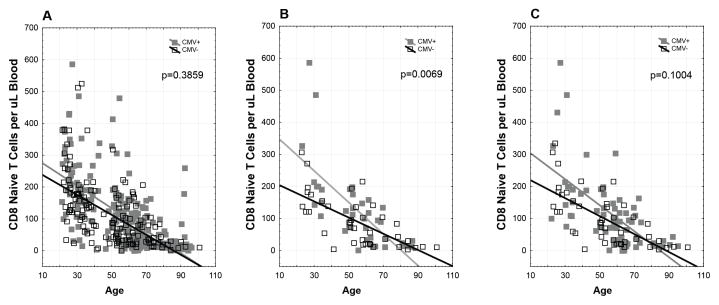
Given that our cohort was 3–10x larger than those used in prior reports, we next explored the effect of cohort size. We first compared how the cohort size impacts on the conclusions that naïve CD8+ T cells decline with aging independently of CMV (one of the points of difference between our conclusions and those of other three studies). We reduced the cohort size to test how this may affect the conclusions and evaluated the correlation between naïve T cell numbers and age. Fig. 4A shows that in the entire cohort (391 subjects) naïve T cells decline with age regardless of CMV status (no difference between the slopes in CMV− and CMV+ subjects). However, when we randomly selected 40 + 40 subjects (not weighting for variability, but approximating the cohort size used in past reports and spanning the entire age spectrum (20, 21), statistical analysis suggested that CMV increased the naïve CD8 T cell loss (Fig. 4B); that effect was lost when we increased cohort size in a weighted fashion to account for the increased variability of the naïve T cell pools we observed in younger subjects [increasing the number of adults and CMV+ subjects to bring the sample size to 120, with N=73 CMV+ and 47 CMV−] (Fig. 4C). We next conducted a simulation study to understand cohort size effect/small sample behavior in a more systematic manner. We randomly select subsample size of 80 individuals (40 CMV+ and 40 CMV+) from the whole cohort (n = 391), and repeated regression analysis to see if the subset analysis can reproduce the result from the whole cohort analysis. We repeated this 100 times (Table 2). As stated above, using the whole cohort (n=391), we observed no significant difference in slope between CMV+ and CMV− individuals (see Figs 2B and 3B and the text above), and have concluded that CMV does not palpably impact the numerical loss of naïve CD8 T cells, which occurs as a consequence of aging. With 100 randomly selected subset of 80 individuals each, 13 out of 100 subsample sets resulted in a statistically significant difference in CD8 N cell regression slopes between CMV+ and CMV− groups. Therefore, the false positive rate was 13% with the subsample size of 80 (Table 2, top). However, the false positive rate decreased to 6% when we increased the subsample size to 120 (using weighed addition of CMV− and CMV+ individuals to match the group variability). Results were even more striking when evaluating the CD8 EM counts (Table 2, bottom). Analysis of the entire cohort of 152 CMV− and 239 CMV+ individuals showed statistically different regression slopes (Figs. 2D and 3D) between the CMV− and CMV+ groups, confirming inflation of the EM compartment in the presence of CMV. However, 74 out of 100 subsample sets of 80 individuals showed no such statistical significance, presenting a false negative rate of a whopping 74% (equivalent to a mere 26% power to detect differences with this cohort size). This false negative rate was reduced to a lower, but still utterly unacceptably high false negative rate (52%) when another 40 subjects were added. This powerfully illustrates the dangers of undersampling, and leads us to conclude that the most likely explanation for the disparate conclusions in the literature lies in undersampling/underpowering of the cohorts in question by other studies.
Figure 4. Cohort size considerations.
PBMC from the entire cohort (n=391) (239 CMV+, grey closed squares; 152 CMV−, black open squares, A.) or its subsets of 80 (40 CMV+ and 40 CMV− donors, B) or 120 (80 + another 40 weighed; total 73 CMV+ and 47 CMV−, C) individuals were analyzed for the possible impact of CMV upon the decline of naive T cells plotted against age. Note the changes in significance (p value by regression analysis) as a consequence of cohort size.
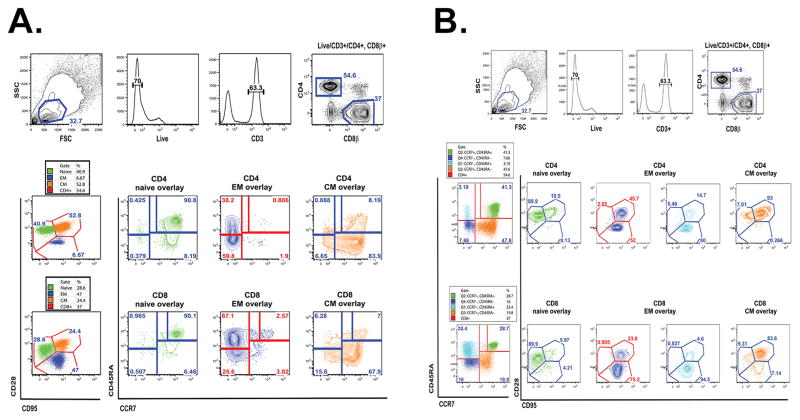
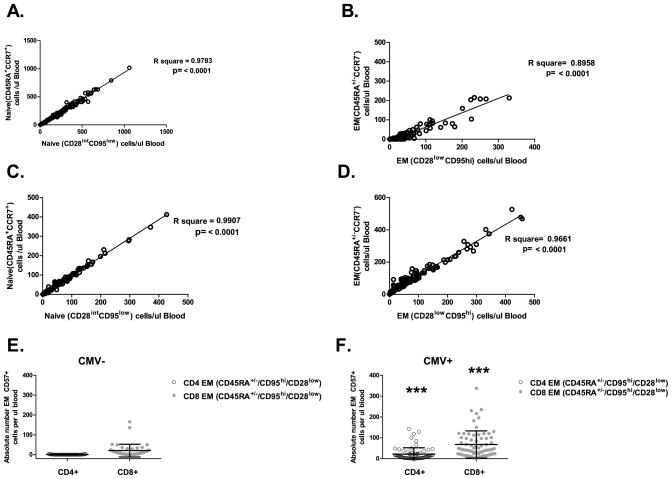
To insure that our conclusions were not biased by inappropriately chosen differentiation markers, we reanalyzed our data using other recommended markers (rev. in (28, 29). Figs. 5 & 6 shows superimposed analysis of naïve T cell subsets in CMV− subjects defined by CD28int95lo (phenotype definition 1) or CCR7hi CD45RAhi (phenotype definition 2) phenotypes for different subsets of subjects chosen from our cohort using a weighed method. Figure 5 provides gating strategies and superimposition of one N, CM, EM definition (Fig. 5A, primary definition CD28/95; Fig. 5B, primary definition CD45RA/CCR7) onto the other for both CD4 and CD8 T cells in a representative subject. Figure 6 then analyzed to what extent these definitions correspond to one another in a larger cohort of subjects for both CD4 and CD8 cells, by comparing concordance between N (Fig. 6A, C) and EM (Fig. 6 B, D) numbers. This was done separately for naïve T cells defined by our original (CD28int95lo) N definition (A and B for CD4 and CD8 T cells respectively) and another accepted phenotypical definition of N cells (CCR7hiCD45RAhi) cells; and also for EM cells defined by our original (CD28lo95hi) EM definition (C and D for CD4 and CD8 T cells respectively) and another accepted phenotypical definition of EM cells [conventional (CCR7−CD45RAlo) + CD45RA-reexpressing (TEMRA) CCR7−CD45RAhi]. In each case, we saw an outstanding correlation between them for both CD4 and CD8 T cells (Fig. 6A–D).
Figure 5. Multi-color FCM gating strategy (A, B).
Representative flow cytometric analysis of one donor demonstrating gating to identify T cell subset populations (naïve (N), central memory (CM), and effector memory(EM)) and their respective overlaps between phenotypic gating strategy (CD28/CD95 vs. CD45ra/CCR7). Color coding as follows: green = naïve cells, orange = central memory cells, blue (dark and lt. blue) = effector memory and terminal effector memory respectively.
Figure 6. Impact of phenotyping marker selection.
Multiple surface marker definitions confirm fluctuations of N and EM T cells with age and CMV, respectively. Frozen PBMC from a weighted subset of our cohort, consisting of a total of 116 individuals, comprised of CMV− (n=45) and CMV+(n=71) donors were thawed, rested overnight and phenotyped by multi-color FCM as in methods.≥ 3 × 105 events were analyzed, gated via the lymphocyte forward/size scatter gate, then on live cells, then with the main T cell subsets defined as CD3+CD4+ or CD3+CD8β+ and the naïve cells defined as CD28int95lo or CD45RAhiCCR7hi. Correlation between absolute numbers for Naive, left panels A (CD4), C (CD8) and Effector Memory (right panel B (CD4), D(CD8) cells defined as CD28int95low (phenotype 1, closed circles) and CD45RA+CCR7+ (phenotype 2, open circles) for the naïve and CD28hi95hi (phenotype 1, closed circles) and CD45RA+ or - CCR7− (phenotype 2, open circles) for the effector memory is shown. p values of R2 from regression concordance are all highly significant. E, F = Corroboration of EM changes using CD57. The same cohort was analyzed by gating on CD3+CD4+ (open circles) or CD3+CD8+ (gray closed circles) cells to enumerate CD95+, CD45RA+or- CD28− CD57+ EM cell. The analysis shows that this cell type accumulates solely in the presence of CMV infection (F) but not its absence (E).
To ensure that these results were further robustly validated, we have assessed whether the two phenotypic definitions similarly quantified N and EM cell abundance in each subject, using the Bland-Altman comparison of methods(30). The Bland-Altman analysis compares the agreement between two different assays or approaches, essentially testing whether the two different assays or methods are comparable across different magnitudes of data. This analysis (not shown) demonstrated that for CD8N cells, the two phenotypes were within ± 2SD of being superimposable. Concordance was high for other subsets as well, although for CD4N and CD4EM cells there was a tendency of phenotype 1 to score fewer cells than phenotype 2. FCM overlays (Fig. 5) demonstrated that this overestimation occurs because both EM and TEMRA subsets as defined by CD45RA and CCR7 (CCR7- fraction) contain subsets (5–25%) of CD28hi95hi cells that probably belong to memory populations that are more differentiated than conventional CM cells and likely represent transitional cells between CM and EM (4). Importantly, however, none of these relationships changed at either high or low abundance of either N or EM cells. Overall, this further suggested that the two phenotypic definitions were concordant over a large span of parametric values.
Finally, we have also used CD57 (22, 31) as an additional marker of highly differentiated EM cells, finding a very strong (p< 0.0001) significance that the CD57+ (gated via CD8 or CD4 and also CD45RA+or-, CD95hiCD28low population) EM cells only exist in the CMV+ CD4 and CD8 memory compartment but not in the CMV− CD4 and CD8 memory compartment (Fig. 6E, F). For example, mean ± SEM of CMV+ CD4+CD57+: EM cells was 21.44 ± 3.684 (N=69; Fig. 6F) as opposed to 0.5282 ± 0.1446 (N=45; Fig. 6E) in CMV− subjects.
Therefore, regardless of the precise marker sets used for definition of naïve or EM cells, aging and CMV resulted in specific, largely non-overlapping patterns of T cell pool changes, the former leading to loss of naïve cells and the latter to accumulation of EM cells.
Interaction of aging and CMV – a link to anti-CMV Ab titers
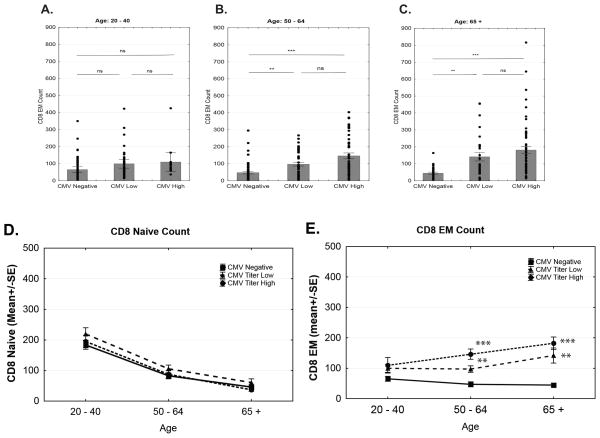
Bulk analysis of the data shown in Figures 1–3 did not reveal a clear interaction between CMV infection and aging with regard to T cell subset homeostasis. Activation status of CMV-specific T cells suggests that hCMV undergoes reactivation throughout the lifespan. However, subtle in vivo reactivation of CMV in humans has been exceptionally difficult to detect by virological methods in the absence of manifest immune suppression or additional comorbidity (32). Anti-CMV Ab titers have been used to describe the activity of the host immune system against hCMV(32), with high titers of anti-CMV Ab being taken as a correlate of the propensity of CMV to frequently reactivate and the inability of the immune system (perhaps CD8 T cells) to control such reactivation (32, 33). We sought to test whether Ab levels correlated with the age and abundance of T cell subsets in our subject cohort. To preserve statistical power, we divided all subjects into the upper and lower titer half, with the median titer being 349 (limit of detection=10; maximal titer 1731). We compared the absolute counts of T cell subsets in CMV− subjects belonging to the three age groups (21–40; 50–64; 65 and older) to the age-matched subjects with high (>349 titer) and low(<349) Ab titers for. We found (Fig. 7A) that CD8 EM T cell counts in younger adult group remained relatively flat regardless of the CMV titer. By contrast, in the two older groups, CD8 EM T cell counts were significantly higher in CMVAb+ individuals (Fig. 7, B, C). While there was a trend of higher inflation of EM CD8 T cells in the>65 group compared to those between 50–64 yr of age, that trend was not statistically significant. When analyzed across age groups, CD8 N T cell numbers were not influenced by anti-CMV Ab titers; they declined with age with the same slope for all three groups (Fig. 7D). By contrast, in the CMV Abhi individuals, the older groups exhibited more pronounced accumulation of CD8 EM cells compared to younger cohorts (Fig 7E). Overall, CD8 EM cells were the only CD8 subset that showed interaction between CMV and aging (Fig 7E). The analysis of CD4 subsets did not show significant changes with anti-CMV Ab titer (not shown).
Figure 7. CD8 EM expansion is dependent upon CMV Titer and Age.
Results shown in Figs. 2 & 3 were plotted against anti-CMV Ab titers by separating the cohort (n=391) into upper and lower anti-CMV Ab titer half (lower<349>upper); multiple regression model with age and CMV status as covariates was used to calculate significance. CD8 EM numbers are shown for anti-CMV titer hi, lo and CMV(−): A, for individuals 40y and younger; B, individuals 50–64yrs of age; and C, for individuals 65yrs of age and older. D. Slope of absolute naïve CD8 cells count over age groups. E. Slope of absolute EM T cell inflation over age groups. Data from CMV−, CMVAblo and CMVAbhi subjects are shown in solid line/squares, dashed line/triangels and dotted line/circles, respectively. Significance ** p=0.01 to p=0.001, ***p>0.001 was determined by two-way ANOVA followed by Bonferroni correction for multiple comparisons. NS= not significant.
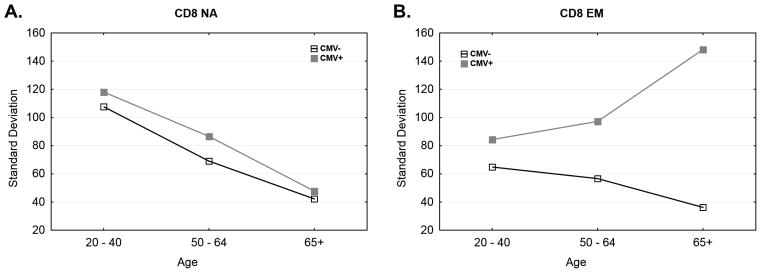
Corroboration of the above data was obtained when we analyzed variation in cell numbers by the state of infection and age. Variability of naïve T cell number tended to decreased with aging. Naïve T cell numbers appear to be more variable in younger subjects group compared to middle age and elderly group (Levene’s test p-values were 0.0112 and <0.0001 for comparisons between young adult and 50–64 y old, and young adult and 65 and older, respectively, Fig. 8). Furthermore there was significant difference in variability between 50–64 and >65y groups (p-value = 0.0017), with those over 65 showing the least variability. The variability pattern of N cells did not significantly differ between the CMV− and CMV+ cohorts (Fig. 8A, D, G Levene’s test p-value = 0.0677; reduction of variability between age groups also shown in Fig. 9A), consistent with our conclusion that CMV does not massively influence the numbers of naïve T cells. EM cell variability in younger subjects was also not sensitive to CMV status (Levene’s test p-value = 0.3529), despite the fact that even the younger adult CMV+ cohort exhibited significant increase in the absolute EM count (Bonferroni adjusted p-value = 0.0138). By contrast, focused analysis of CD8EM count variability in older age groups revealed striking differences between CMV− and CMV+ individuals with age. This was a consequence of several trends. First, while the variability was similar in younger and middle-aged individuals regardless of CMV infection (p=02199), the older CMV− subjects showed reduced EM cell variability; by contrast, the older CMV+ subjects exhibited remarkably higher variability compared to CMV− subjects (Levene’s test p-value = 0.0317 and 0.0049, for 50–64 and >65y age groups, respectively Fig. 8B, E, H).. Finally, the older groups exhibited more pronounced increase in absolute CD8EM count (Bonferroni adjusted p-value = 0.0001 for both the 50–64 and 65+ age groups). For comparison, the CMV+ younger adult group (<40) displayed 1.9 times higher CD8EM count than that of comparable CMV− group; whereas the CMV+ cohort in the old group (65+) displayed 3.9 times higher CD8EM count than that of CMV− cohort in the older group, and that difference was statistically significant (p-value = 0.0168). Therefore, CMV was associated with marked individual variations which was more pronounced specifically in older adults (Fig. 8E, H), and that is also illustrated in Figure 9B as a comparison between the three age groups. By contrast, age distribution variability was uniform between CMV+ and CMV− cohorts within each age group (Fig 8C, F, I), demonstrating that within-cohort age variability cannot explain the observed results.
Figure 8. Variance in Absolute Count is Significantly Increased in the Inflated Effector Memory Pool of CMV(+) Donors.
Absolute T cell inflation within age bins from CMV− (open squares) and CMV+ (closed squares) subjects. Plots show the standard deviation of absolute naïve (A, D, G) and EM (B, E, H) T cell counts. Overall N=391; CMV(+) = 239. CMV(−) =152, and as noted within axis of each panel. Panels C, F, and I illustrate that the CMV− and CMV+ cohorts are age matched within each age bin. Significance as noted within the panels.
Figure 9. Variance in Absolute Count of Naïve Pool Declines with Advancing Age Independent of CMV Status; Variance Increases Significantly in aged CMV+ donor subset.
Plots show the standard deviation of absolute naïve (A) and EM (B) T cell counts. N=391; CMV(+) = 239. CMV(−) =152. Absolute T cell inflation over age from CMV− (dark line, open squares) and CMV+ (gray line, closed squares) subjects.
DISCUSSION
In this study, we have investigated the role of aging in the absence or presence of CMV infection in modulating the balance of CD8 and CD4 T cell subsets in peripheral blood. While it has been known that aging is accompanied by changes in T cell subset balance, only a handful of studies have evaluated the impact of aging upon the absolute numbers of T lymphocyte subsets while controlling for the role of CMV (19–21, 34)
The study by Looney, Abraham and colleagues (19) examined old (>60y; mean age 72 for CMV− and 76 for CMV+) and young (<60; mean age 34.5 for CMV+ and 39.7 for CMV−) US subjects. Despite several shortcomings (treating age as a binary category - old or young; using 3-color FCM, with no precise distinction between naïve and CM subsets; assuming that all CD3 cells are either CD8 or CD4; using small numbers of CMV− subjects −22 old and 26 young, per above age groupings), this seminal study established the emergence of a CD28-CD8+ (and CD28-CD4+) cell phenotype as a strong correlate of CMV infection which was independent of aging, suggesting that memory inflation (35, 36) occurs in humans. Chidrawar et al.(20) analyzed T cell subsets from 122 healthy UK donors, aged 20–40 (8 CMV+; 18 CMV−) 40–60 (12 CMV+; 10 CMV−) and >60y (47 CMV+; 29 CMV−; oldest subjects >80y old) by 3-color FCM. These authors concluded that CMV led to an increase in CD8 memory pool, due to an increase in resting memory (CM) cells, a conclusion based on an incomplete and potentially misleading identification of CD45RA+ cells as CM. This study suggested that memory inflation occurs in both the CD8 and CD4 cells in the presence of CMV. The authors concluded that CMV reduces naïve CD8 T cells over and above the effects of aging, adding “40 years of age” to the effects seen in CMV− donors, but that conclusion was based upon relative, and not absolute, representation of naïve cells in 28 younger and 29 older CMV− subjects. As our studies show, limited cohort size is highly conducive to these types of errors (Fig. 4C). Litjens et al.(21) evaluated circulating T cell subsets in a Dutch cohort of young (20–40 y; 15 CMV− and 17 CMV+) and elderly (>60 y; 21 CMV− and 23 CMV+) subjects using 6-color FCM, with an appropriate discrimination of cell subsets but with limited power. They found no significant decline in total CD8 or CD4 pools, regardless of CMV infection; the increase in CD8 memory subsets with CMV was observed but was not significant except for the CD8 CM cells and total memory cells (EM + CM + EMRA); and a decline in naïve CD4 and CD8 cells was found significant in CD8 cells regardless of CMV and was significant for CD4 cells only in the presence of CMV. Finally, the Wikby et al. study (34) was focused upon a small cohort of nonagenerians. Neither of the above studies (19–21, 34) was powered to dissect, and three of them (19, 20, 34) did not explicitly delineate, the effects of aging in the absence of CMV infection, which was the primary goal of our work.
Our study used a large sample size of 152 CMV− subjects aged 21–101. We found that aging in the absence of the CMV infection led to both relative, and more importantly, absolute reduction in naïve CD8 cells, but, contrary to the prevailing views, did not lead to accumulation of CM or EM CD8 T cells, whose numbers remained stable or declined slowly (but not significantly within our statistical analysis) with aging. Therefore, physiological immune aging is accompanied by declining naïve and stable memory CD8 T cell pools. CD4 cells exhibited no significant relative or absolute changes in the naïve, CM or EM cell pools. Reasons for this difference remain unclear, although, CD8 T cells are known to undergo higher homeostatic turnover in non-human primates and humans compared to CD4 T cells (37, 38), and, when confronted with the same stimulus, proliferate faster and longer (rev. in (39). It is possible that such differences may lead to higher rates of apoptosis and/or conversion into true or virtual memory cells(40, 41), leading to depletion of the truly naïve cell pool. We also note that the loss of naïve CD8 T cells was not linear with age, an issue that will require additional investigation.
In CMV+ subjects, the loss of naïve CD8 cells was not exacerbated compared to CMV− subjects, suggesting that this virus does not grossly affect the abundance of circulating naïve CD8 T cells. We observed an absolute increase in EM (but not CM) pool in CMV+ subjects. Therefore, the main effect of CMV is to expand and increase the absolute size of the EM compartment, an effect that became more pronounced with aging, likely due to the massive EM response to periodically produced viral antigens. CD4 naïve cells were somewhat reduced, while the CD4 CM T cell subsets did not show numerical changes in the presence of CMV. However, the minor CD4 EM subset doubled in the presence of CMV infection.
We did not observe reduction or inversion of CD4:CD8 ratios in either CMV− or CMV+ subjects on a group level, and in fact we found that such ratios increase in CMV− subjects. This, however, does not address the value of the CD4:8 ratio as a biomarker of immune vitality or mortality risk(42).
The above results can be integrated into a comprehensive picture of age- and frequent persistent pathogen-mediated changes in the circulating T cell pool. Attrition of circulating naïve CD8 T cells was the most profound age-related change, with a tenfold reduction between 21 and 80 years of age. Yet, CMV infection does not further increase the loss of naïve CD8 cells. Similar conclusions were reached by the study of Vescovini et al. (in press) in a different cohort of CMV+ subjects, where the magnitude of the anti-CMV response correlated to the size of the EM pool, but not to age or to the loss of naïve cells. Correlation between CMV infection and impaired immune responsiveness has been reported in humans (43), but the ethical limits preclude direct investigation of the impact of these factors upon human immune defense. Mouse experiments with controlled CMV lifelong infection and direct testing of antimicrobial resistance recently (44–46) provided results are fully consistent with the conclusions of our study and with Vescovini et al. (submitted) - namely that aging leads to decline in naïve CD8 cells and reduced TCR diversity, whereas CMV does not impact naïve cells but induces memory inflation of EM cells independent of, but eventually synergistic with, aging. We found a sizable number of aged CMV+ donors who did not experience memory inflation. This finding suggests that CMV is necessary but not sufficient for memory inflation even after age 65. Experiments are in progress to address what other factors may be involved in precipitating memory inflation in the old age.
While we did not observe the same extent of profound numerical changes in the CD4 T cell pool with aging as found in CD8+ T cells, the relatively preserved pool shouldn’t be equated with intact CD4 function. Indeed, previously described functional defects in CD4 cells (47) likely impair immunity in their own right.
Limitations of our study include: (i) cross-sectional design, which lacks a longitudinal component; and (ii) implied assumption that time and course of CMV pathogen exposure were equal among subjects. Studies in progress now include longitudinal titers and assessments of CMV viral DNA in plasma, PBMC and urine. Overall, our findings, those by Vescovini et al. (in press), and the past literature (48–51) dictate that we move towards testing of predictive value of biomarkers defined in these studies. Specifically, vaccine responses should be studied prospectively with regards to the numbers, diversity and functional reactivity of different T cell subsets, with the goal to stratify human populations to improve vaccine efficacy.
Supplementary Material
Acknowledgments
We thank D. Wasserman; the Oregon Brain Aging Study (OBAS); the NIA-funded Oregon Alzheimer Disease Center (OADC), supported by P30 AG008017; and the Office of Research & Development, Clinical Sciences Research & Development Service, Department of Veterans Affairs, Portland VA Medical Center, for help with subject recruitment. We wish to acknowledge Dr S. Njoroge for subject recruitment and technical assistance, and the CTRC resource at OHSU and the Clinical and Translational Science Center (CATs) at the University of Arizona for facilitating blood draws. We are deeply thankful to all of our study participants without whose continued participation this study would not have been possible.
Abbreviations
- CM
central memory T cells
- CMV
cytomegalovirus
- EM
Effector memory T cells
- N
Naïve T cells
Footnotes
This study was supported by the NIH ADB Contract HHSN 272201100017C (NIH/NIAID N01-AI 00017) and the Bowman Trust (Endowed Professorship in Medical Research to J.N-Z).
References
- 1.Albright JF, Albright JW. Aging, Immunity and Infection. Humana Press; Totowa: 2003. [Google Scholar]
- 2.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 3.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner P, Pabbatireddy S. Vaccines for women age 50 and older. Emerg Infect Dis. 2004;10:1990–1995. doi: 10.3201/eid1011.040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchholz VR, Neuenhahn M, Busch DH. CD8+ T cell differentiation in the aging immune system: until the last clone standing. Curr Opin Immunol. 2011;23:549–554. doi: 10.1016/j.coi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengel H, Brune W, Koszinowski UH. Immune evasion by cytomegalovirus--survival strategies of a highly adapted opportunist. 1998;6:190–197. doi: 10.1016/s0966-842x(98)01255-4. [DOI] [PubMed] [Google Scholar]
- 10.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 13.Myers RM. Chapter 3.10 - Categorical or indicator variables (Regression models oand ANOVA models) In: Myers RM, editor. Classical and modern regression with applications. Duxbury Thomson Learning; Australia-Canada-Mexico-Singapoore-Spain-United Kingdom-United States: 1990. pp. 135–153. [Google Scholar]
- 14.Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Characterization of naive, memory and effector CD8+ T cells: effect of age. Exp Gerontol. 2004;39:545–550. doi: 10.1016/j.exger.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term Cytomegalovirus infection leads to significant changes in the composition of the CD8 T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, Vukmanovic-Stejic M, Yong K, Battistini L, Kern F, Soares MV, Akbar AN. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 20.Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litjens NH, de Wit EA, Betjes MG. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing. 2011;8:2. doi: 10.1186/1742-4933-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 25.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 26.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 27.Day EK, Carmichael AJ, ten Berge IJ, Waller EC, Sissons JG, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol. 2007;179:3203–3213. doi: 10.4049/jimmunol.179.5.3203. [DOI] [PubMed] [Google Scholar]
- 28.De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 29.Maecker HT, Lindstrom TM, Robinson WH, Utz PJ, Hale M, Boyd SD, Shen-Orr SS, Fathman CG. New tools for classification and monitoring of autoimmune diseases. Nat Rev Rheumatol. 2012;8:317–328. doi: 10.1038/nrrheum.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 31.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 32.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 34.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 35.Holtappels R, Podlech J, Geginat G, Steffens HP, Thomas D, Reddehase MJ. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 37.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 38.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 40.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 43.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 44.Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8:e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mekker A, V, Tchang S, Haeberli L, Oxenius A, Trkola A, Karrer U. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8:e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smithey MJ, Li G, Venturi V, Davenport MP, Nikolich-Zugich J. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J Immunol. 2012;189:5356–5366. doi: 10.4049/jimmunol.1201867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagnoni F, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiancy in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 49.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 50.Saurwein-Teissl M, Lung T, Marx F, Gschåsser C, Asch E, Blasko I, Parson W, Båck G, Schånitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of Antibody Production Following Immunization in Old Age: Association with CD8+CD28- T Cell Clonal Expansions and an Imbalance in the Production of Th1 and Th2 Cytokines1. Journal of Immunology. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.