SUMMARY
Peripheral blood myelomonocytic cells are important for cytomegalovirus dissemination to distal organs such as salivary glands where persistent replication and shedding dictates transmission patterns. We find that this process is markedly enhanced by the murine cytomegalovirus (MCMV)-encoded CC chemokine, MCK2, which promotes recruitment of CX3CR1hi patrolling monocytes to initial infection sites in the mouse. There, these cells become infected and traffic via the bloodstream to distal sites. In contrast, inflammatory monocytes, the other major myelomonocytic subset, remain virus negative. CX3CR1 deficiency prevents patrolling monocyte migration on the vascular endothelium and interrupts MCMV dissemination to the salivary glands independent of antiviral NK and T cell immune control. In this manner, CX3CR1hi patrolling monocytes serve as immune-privileged vehicles to transport MCMV via the bloodstream to distal organs. MCMV commandeers patrolling monocytes to mediate systemic infection and seed a persistent reservoir essential for horizontal transmission.
Keywords: CMV, MCK2, dissemination, herpesvirus, latency
INTRODUCTION
Human cytomegalovirus (HCMV) is an opportunistic betaherpesvirus of medical significance in settings of compromised immune systems. HCMV has a tropism for epithelial, endothelial, myeloid, neuronal and fibroblast cell types in patients and tissue culture settings (Revello and Gerna, 2010); however, pathogenesis in immunocompetent individuals remains to be established. Different peripheral blood leukocyte (PBL) subsets have been implicated in viremia (Revello et al., 1998) and latency (Reeves and Sinclair, 2008; Slobedman and Mocarski, 1999a) in both immune compromised and competent subjects. Non-permissive monocytes likely serve as latent reservoirs that support dissemination by maturing into permissive macrophages or dendritic cells that reactivate virus (Hertel et al., 2003; Reeves et al., 2005; Sinclair, 2008b). Overall, a definitive understanding of the cell type that disseminates virus or contributes to the latent reservoir remains to be elucidated.
Murine CMV (MCMV) has revealed key principles of the virus–host interaction involved in immune control, pathogenesis and latency (Daley-Bauer and Mocarski, 2012; Reddehase et al., 2008; Scalzo et al., 2007). Virus exposure results in systemic infection. Dissemination from initial infection sites occurs via a leukocyte-associated viremia, seeding distal organs such as salivary glands – a site of persistent infection that is key to transmission (Collins et al., 1994; Stoddart et al., 1994). Host innate and adaptive immune mechanisms collaborate to control infection, setting the stage for lifelong latency (Pollock et al., 1997; Reddehase et al., 2008). Studies showing MCMV seeding visceral organs directly following systemic inoculation (Hsu et al., 2009; Sacher et al., 2008), add to earlier observations that systemic infection relies on bone marrow (BM)-derived myelomonocytic cells that disseminate virus via the blood (Bale and O'Neil, 1989; Cheung and Lang, 1977; Noda et al., 2006; Stoddart et al., 1994). Further, macrophages are a major latent reservoir (Pollock et al., 1997), analogous to humans (Revello et al., 1998) but difficult to track in naturally infected immunocompetent adults. Regardless of immune status, cell-free virus is not produced in mice (Roback et al., 2006) or humans (Gilbert et al., 1989; Yeager et al., 1981). A blood mononuclear phagocyte transports MCMV (Stoddart et al., 1994), potentially acquiring virus from vascular endothelial cells at initial sites (Sacher et al., 2008). A model consistent with current concepts suggests local infection spreads once vascular endothelial cells pass the virus to monocytes as they traverse the vasculature (Mocarski et al., 2006) to promote acute systemic infection. Once resolved, latency may be preserved in BM-derived, tissue-resident macrophages or endothelial cells (Pollock et al., 1997; Reddehase et al., 2008). Monocytes circulate in the blood and give rise to macrophages (Auffray et al., 2007; Yona et al., 2013), underscoring a need to clarify their role during CMV pathogenesis.
Two functionally distinct monocyte subsets give rise to macrophages and dendritic cells in humans and mice (Auffray et al., 2009). Inflammatory and patrolling monocytes (IMs and PMs, respectively) are distinguished based on migratory and proinflammatory differences (Robbins and Swirski, 2010). Both subsets have been implicated in CMV pathogenesis (Daley-Bauer and Mocarski, 2012; Daley-Bauer et al., 2012). In mice, BM-resident IMs become dramatically mobilized to accumulate at infection sites (Serbina et al., 2008), whereas PMs circulate continuously in blood in close apposition to the vasculature, poised to contribute to early inflammatory responses (Auffray et al., 2007). Human CD14+ IMs and CD16+ PMs (Cros et al., 2010) parallel murine CX3CR1intLy6Chi IMs and CX3CR1hiLy6C− PMs (Geissmann et al., 2003). Murine PMs arise from both IM-dependent (Sunderkotter et al., 2004; Varol et al., 2007; Yona et al., 2013) and IM-independent precursors (Hanna et al., 2011). Nucleic acid-mediated inflammatory cues trigger PMs to adhere to the vasculature without extravasating into inflamed tissues (Carlin et al., 2013); a pattern of contact and migration likely to facilitate MCMV dissemination. The established value of heterozygous Cx3cr1gfp/+ and homozygous Cx3cr1gfp/gfp mice to identify PMs (Geissmann et al., 2003) and dissect their function (Auffray et al., 2009) presents an opportunity to determine the contribution of CX3CR1hi PMs in MCMV pathogenesis.
CMV-encoded chemokines support dissemination by enhancing the inflammatory response (Daley-Bauer and Mocarski, 2012). HCMV UL146-encoded vCXCL1 (Penfold et al., 1999) and MCMV m131-m129-encoded MCK2 (MacDonald et al., 1999) recruit myeloid leukocytes. MCK2 expression promotes leukocyte recruitment, increasing viremia and promoting dissemination to ensure optimal systemic infection (Fleming et al., 1999; Saederup et al., 2001; Saederup et al., 1999). MCK2-enhanced viremia and dissemination proceed independent of adaptive immunity in immunocompromised an immunocompetent mice (Mocarski et al., 2006; Noda et al., 2006). Although IMs do not disseminate virus, they respond to MCK2 to modulate antiviral CD8 T cell responses (Daley-Bauer et al., 2012). Plasmacytoid dendritic cells also respond to MCK2 and modulate T cell control of virus (Wikstrom et al., 2013). Most recent evidence suggests MCK2 mediates infection of macrophages (Wagner et al., 2013). Thus, MCK2 may potentially control leukocyte migration, influence viral clearance and dictate susceptibility to infection.
We investigated the contribution of PMs to MCMV infection and show that the virus depends on MCK2 to regulate PM recruitment to inoculation sites. These monocytes become infected and traffic via the blood to disseminate virus to salivary glands and other tissues. CMV therefore employs the virus-encoded chemokine to hijack an early responding immune component as a key step in pathogenesis of infection.
RESULTS
CX3CR1 is necessary for viral dissemination following local inoculation
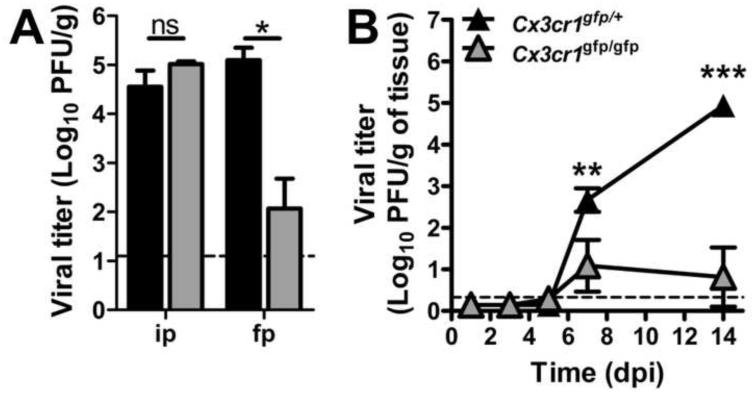
Optimal MCMV dissemination via the blood to the salivary glands relies on MCK2-dependent recruitment of a monocytic PBL independent of CCR2-mediated IM mobilization (Daley-Bauer et al., 2012; Noda et al., 2006; Stoddart et al., 1994). We sought to define CX3CR1-dependent PM control of viral dissemination during MCMV infection. Although CX3CR1 deficiency did not interrupt dissemination to the salivary glands following systemic, intraperitoneal (ip) inoculation, dissemination from a local footpad (FP) inoculation site was compromised (Figure 1A). Thus, direct delivery of cell-free virus to many organs (Hsu et al., 2009) overcomes a crucial CX3CR1-dependent step that is needed when inoculation mimics natural biting and grooming transmission patterns. Cx3cr1gfp/gfp mice showed ≥ 1000-fold lower viral titers compared to Cx3cr1gfp/+ controls. At 7 days post-infection (dpi), virus replicates in associated brown fat; whereas, virus replication is localized to salivary gland acinar epithelial cells at 14 dpi (Stoddart et al., 1994). Regardless of inoculation route, seeding of acinar epithelial cells depends on a host monocytic leukocyte (Noda et al., 2006; Saederup et al., 2001; Saederup et al., 1999). Viral titers increased dramatically from 7 to 14 dpi in Cx3cr1 gfp/+ but not Cx3cr1gfp/gfp mice (Figure 1B), revealing the crucial contribution of the CX3CL1-CX3CR1 axis in MCMV dissemination.
Figure 1.
CX3CR1 deficiency disrupts MCMV dissemination to the salivary glands. (A) Viral titers in Cx3cr1gfp/+ (black) and Cx3cr1gfp/gfp (gray) mice at 14 days following systemic (ip) or FP inoculation. (B) Viral titers in salivary glands of Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice at 1, 3, 5, 7 and 14 days following FP inoculation. Data points represent the mean viral titers ± SE for groups of five mice. Horizontal dashed lines indicate the limit of detection of the assay. Panels represent at least two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant (p > 0.05).
Identification of CX3CR1hi PMs
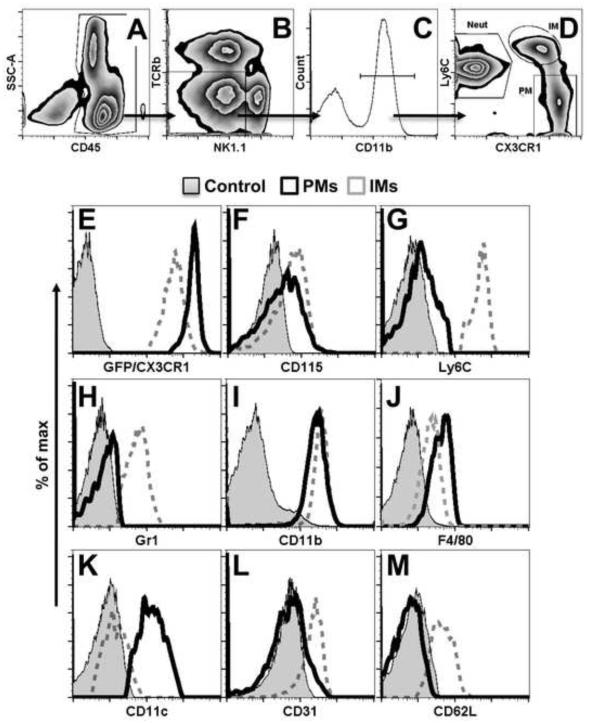
We took advantage of GFP expression combined with discriminatory cell surface markers to distinguish PMs from other CX3CR1-expressing PBL populations by flow cytometry. Singlet CD45+ PBLs were gated to exclude CX3CR1+ NK1.1+ natural killer (NK) and CD3+ T cells (Stievano et al., 2004). GFPhi PMs were identified as CD115+Ly6C−Gr1−CD11b+F4/80+ cells that expressed CD11c but lacked the adhesion molecules, CD31 and CD62L. In contrast, GFPint IMs were identified as CD115+Ly6C+Gr1loCD11b+F4/80lo cells with the adhesion molecules but without CD11c (Figure 2). These markers provide a definitive identification of PMs (Auffray et al., 2009).
Figure 2.
Characterization of CX3CR1hi PMs in peripheral blood. (A -D) Gating strategy for PM (patrolling monocyte), IM (inflammatory monocyte), and, Neut (neutrophil) subpopulations. CD45+CD11b+ PBLs, gated to exclude T cells (TCRβ−) and NK cells (NK1.1−), were subjected to evaluation for Ly6C and CX3CR1 levels characteristic of these subpopulations. (E – M) Detection of monocyte subset-defining phenotypic markers on PMs (black, solid line), IMs (gray, dashed lines) and negative controls (filled histogram). PBLs were obtained from naïve Cx3cr1gfp/+ mice and phenotyped by flow cytometry following doublet exclusion. Plots are of data from one mouse representative of a group of four mice.
Viral chemokine promotes biphasic recruitment of PMs to local sites of infection
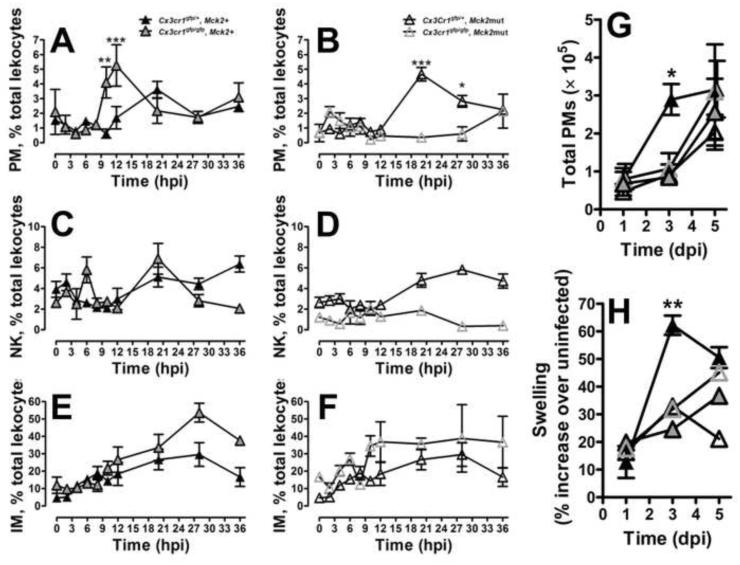
The MCK2-enhanced myelomonocytic cell recruitment responsible for MCMV dissemination peaks in numbers at 3 dpi (Noda et al., 2006). PMs respond very early, within a few hours of bacterial inoculation (Auffray et al., 2007). Given that the cell that disseminates MCMV must be recruited early, we evaluated the impact of MCK2 on PMs. PM recruitment to inoculated FPs was influenced by host CX3CR1 and viral MCK2 function. Except for CD115 downmodulation, infection did not alter the CD115+Ly6C−Gr1−CD11b+F4/80+CD11c+ phenotype of PMs. PMs were initially recruited between 8 and 20 hpi in Cx3cr1gfp/+ or Cx3cr1gfp/gfp FPs exposed to Mck2+ virus (Figure 3A). A similar pattern of early recruitment was observed in Cx3cr1gfp/+ mice exposed to Mck2mut virus (Figure 3B). In contrast, when host CX3CR1 and viral MCK2 signaling were disrupted together, recruitment failed. Thus, these two chemokine pathways independently promoted early PM recruitment. MCK2 was sufficient to drive robust recruitment independent of CX3CR1 (Figure 3A), suggesting that viral chemokine elaboration ensures early PM recruitment even when the CX3CL1-CX3CR1 axis is not engaged.
Figure 3.
Viral MCK2 chemokine regulates PM recruitment to MCMV-inoculated FPs. (A − F) Initial cell recruitment patterns of CX3CR1+ PMs (A and B), NK cells (C and D) and IMs (E and F) shown as frequencies along with statistical comparison of groups at the same time points. (G) The second phase of MCK2-dependent PM recruitment shown as total PMs at 1, 3 and 5 dpi. CD45+ cells were isolated from Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice inoculated with either Mck2+ or mutant virus and evaluated by flow cytometry. (H) FP swelling measured by digital caliper. Symbols show mean values ± SE for n = 5 mice. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant (p > 0.05). See also Figures S1 and S2.
The initial appearance of PMs may influence recruitment of other immune cells to sites of infection. NK cell frequencies increased after 12 hpi in all settings, paralleling the rise in PMs, with a similar dependence on host CX3CR1 and viral MCK2 signaling (Figure 3C and D). In contrast, IM recruitment prior to 24 hours (hpi) proceeded independent of host or viral chemokine signaling (Figure 3E and F); a pattern shared by neutrophils and T cells (not shown). Although additional mechanisms may be involved, the data suggest that the early MCK2-driven PM migration initiates an NK cell response that is sustained by IMs later during infection (Crane et al., 2009; Hokeness et al., 2005). Though potentially important in orchestration of the innate response, this complex relationship will require further study.
Maximal inflammation coincides with peak viral titers in FPs at 3 to 5 dpi (Saederup et al., 2001). When the contribution of CX3CR1hi monocytes to the inflammatory infiltrate was followed up to 5 dpi, a second wave of PM recruitment into FPs was observed at 3 dpi. Unlike the early recruitment pattern (Figure 3A), the 3 dpi pattern required a combination of host and viral chemokine signaling (Figure 3G). In all but one setting, PM numbers mirrored swelling such that host and viral chemokine signaling together were necessary to produce full inflammation (Figure 3G and H). Thus, MCK2 seems to influence levels of PM recruited to the inoculated site, ensuring optimal numbers of susceptible cells are present when viral progeny are produced. Consistent with PM recruitment data, combined MCK2 and CX3CR1 function contribute to optimal virus dissemination to salivary glands. These titers were 1000-fold higher at 14 dpi than when either chemokine system was compromised (Figure S1). In contrast to a mutant virus used to implicate a role for MCK2 in mediating macrophage infection (Wagner et al., 2013), our MCK2-deficient virus efficiently infected primary BM-derived macrophages (BMDMs) as well as J774 macrophage cells (Figure S2). Thus, the K181 MCMV strain used here does not rely on MCK2 to facilitate entry into macrophages, in contrast to the behavior of the bacmid derivative of MCMV strain Smith. While further comparisons are beyond the scope of this work, extensive genome sequence differences between viral strains likely underlie the biological differences that have been observed.
PMs are the vehicles of MCMV dissemination
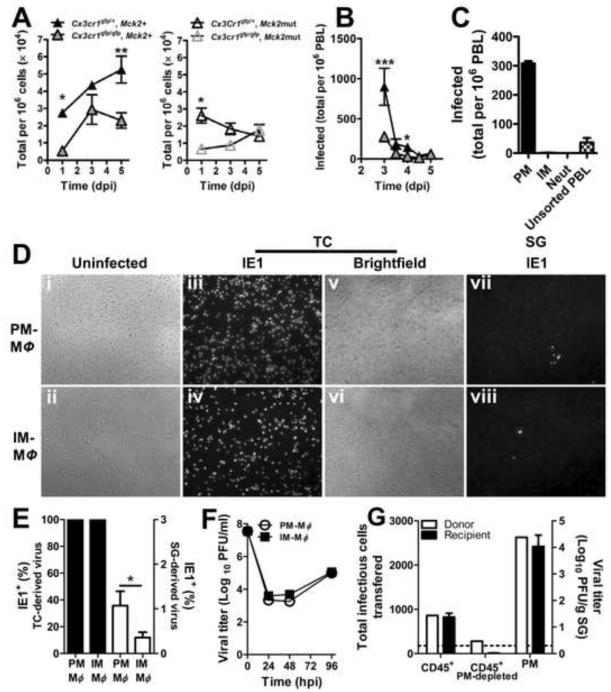
Given the evidence that viral MCK2 and host CX3CR1 collaborate to recruit PMs to inoculation sites, we next investigated PM contribution to cell-associated viremia that peaks by 3 to 5 dpi (Noda et al., 2006; Saederup et al., 2001; Saederup et al., 1999). Viremia was not detected following FP inoculation despite high assay sensitivity (1 infected cell in 2 × 106 total PBLs), but was readily detected following systemic (ip) inoculation. This is likely due to the ip route circumventing the robust NK response induced at a local inoculation site. In control Mck2+ virus-infected Cx3cr1gfp/+ mice, PM numbers increased from 1 dpi to 5 dpi whether absolute numbers (data not shown) or proportion of PBL (Figure 4A, left panel) was assessed. In contrast, viremia in Cx3cr1 gfp/gfp mice was lower and did not rise after 3 dpi. PM accumulation was much lower during Mck2mut virus infection of both Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice (Figure 4A, right panel). These results prompted us to focus specifically on CX3CR1-dependent PM function. When virus-positive PBL numbers were compared, three times more virus-positive cells were detected at 3 dpi in Cx3cr1gfp/+ compared to Cx3cr1gfp/gfp mice (Figure 4B). Thus, virus dissemination was strongly tied to CX3CR1+ PM availability. To identify the virus-positive subset, myeloid cells were sorted and subjected to infectious centers assay. Consistent with the CX3CR1-dependent pattern of viremia, MCMV was detected exclusively in PMs (Figure 4C), and was absent in IMs or neutrophils. Even though systemic administration overcomes a CX3CR1-dependent step in seeding salivary glands (see Figure 1A), the virus still targets PMs. Thus, MCK2-responsive, CX3CR1-dependent PMs emerge as the sole myelomonocytic lineage functioning as vehicles to support viral dissemination.
Figure 4.
PMs harbor infectious MCMV in blood. (A) Time course of PM levels in PBL of Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice infected with Mck2+ (left panel) or mutant (right panel) virus. (B) Time course of PBL-associated viremia assessed by infectious centers assay. (C) Identification of PBL harboring infectious virus. PM, patrolling monocytes; IM, inflammatory monocytes; Neut, neutrophils. Mice were inoculated via the FP route and blood was collected at stated time points. PBLs or flow cytometry-sorted subsets were assessed by infectious centers assays. Symbols are mean values ± SE for n = 5 - 10 mice. (D – F) Infection of PM- or IM- derived macrophages with tissue culture (TC; D – F) or salivary gland (SG; D and E) stock virus. (E) Frequencies of IE1+ cells. (F) Virus replication. Data points are mean values ± SE. (G) Transfer of infection by CX3CR1gfp/+ CD45+ PBLs, PM-depleted CD45+ PBLs or sorted PMs to naïve Cx3cr1gfp/gfp recipient mice. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant (p > 0.05).
Once placed in culture, PMs and IMs isolated from blood differentiated into macrophages that were equally susceptible to tissue culture stock MCMV; a type of virus preparation that reflects the quality of virus produced in host tissues outside of salivary glands. At high m.o.i., all macrophages were 100% IE1+ by 24 hpi (Figure 4D and E). At lower m.o.i., both macrophage preparations were also equally susceptible to infection (data not shown). Consistently, macrophages generated from both monocyte lineages supported similar levels of viral replication (Figure 4F). As expected (Mims and Gould, 1978), macrophages were less susceptible to infection with salivary gland stock virus (Figure 4D and E). Thus, the process of natural dissemination is mediated by CX3CR1-dependent PMs, highly susceptible cells recruited to initial infection sites serve as dissemination vehicles capable of seeding tissue-resident macrophages that may arise from either monocyte subset.
PMs disseminate virus to promote systemic infection
PBLs mediate transfusion-transmitted CMV infection in humans (Prince et al., 1971; Yeager, 1974). Horizontal transmission has also been demonstrated in mouse models of blood transfusion (Cheung and Lang, 1977; Roback et al., 2006). We employed a transfusion model to investigate whether PM-mediated MCMV dissemination is sufficient to promote systemic infection. Cx3cr1gfp/gfp naïve recipients were transfused with either non-depleted, PM-depleted or the sorted PM components of CD45+ PBLs isolated from Cx3cr1gfp/+ mice at the peak in viremia. Recipient salivary glands were harvested and titered at 14 days post-transfer (Figure 4G). Non-depleted CD45+ cells (0.068% infectivity) transmitted infection to 100% of the recipients (Figure 4G). PM-depletion decreased infectivity to 42% of the non-depleted population. In contrast, sorted PMs were enriched with infected cells (5.25% infectivity) that transferred infection with high efficiency, yielding 1000-fold higher titers in salivary glands versus non-depleted PBLs. Remarkably, engrafting Cx3cr1gfp/gfp mice with infected donor Cx3cr1gfp/+ PMs resulted in comparable virus levels in the salivary glands as in directly infected Cx3cr1gfp/+ mice (Figure 1). The data here provide further evidence bolstering the PM contribution s MCMV dissemination.
PM lineage cells dictate peak splenic titers and influence latency
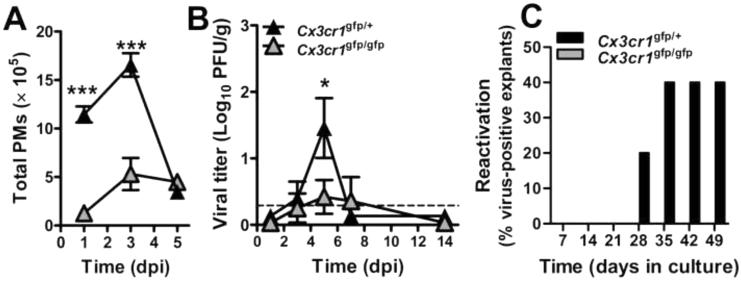
Macrophages and myeloid progenitors that give rise to these cells have been implicated as sites of HCMV (Sinclair, 2008a) as well as MCMV latency (Mitchell et al., 1996; Pollock et al., 1997). Spleens were collected to investigate the contribution of PMs to acute infection. Total numbers of CX3CR1hi PMs recruited to spleens at 3 dpi were significantly compromised in the absence of CX3CR1; although, levels were comparable at 5 dpi (Figure 5A). MCMV dissemination to and infection of the spleen correlated with PMs such that higher peak titers of virus were detected in Cx3cr1gfp/+ mice two days after PM numbers were elevation (5 dpi); a time when titers in Cx3cr1gfp/gfp mice remained low (Figure 5B). As replication levels early in infection directly correlate with latency outcome, spleens were harvested from mice at 63 dpi when no ongoing lytic infection in tissues, including salivary glands, is detected (data not shown). Virus reactivation was not detected in explants from Cx3cr1gfp/gfp mice whereas Cx3cr1gfp/+ tissues became positive by 28 days in culture (Figure 5C), and when evaluated in salivary glands, twice as many Cx3cr1gfp/+ explants reactivated. Differences in spleen and salivary gland outcomes may stem from the gross differences in antiviral mechanisms that function in these sites (Reddehase et al., 2008). All lung, liver, and BM explants remained negative (data not shown). Together, the data here show a consistent pattern of viral biology that depends on PMs for optimal systemic infection in the natural host. Cells that traffic virus during acute infection also act either as vehicles or direct host cells during latency, an area of investigation that may now be pursued more directly as a result of our observations. Thus, MCMV employs MCK2 to regulate recruitment of CX3CR1-dependent PMs to infected tissues that are exploited by the virus for dissemination, peak viral titers in spleen, and persistent infection of salivary glands that ultimately influences patterns of latency that may be detected in spleen and salivary glands.
Figure 5.
Impact of CX3CR1 on splenic PM and viral levels during acute infection as well as reactivation from latency. (A) Total PMs and (B) viral titers in spleens of Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice during acute infection. Tissues were collected at 1, 3, 5, 7 and 14 dpi. Horizontal line represents assay threshold. PMs were enumerated by flow cytometry and viral titers were obtained by plaque assay. Symbols are mean values ± SE for groups of 5 – 8 mice. *p < 0.05; ***p < 0.001.(C) Reactivation of latent MCMV in explanted spleen collected at 63 dpi. Tissues were sectioned into three portions and supernatant sampled at weekly intervals were titered by plaque assay. Bars show the percentage of groups of 5 animals whose splenic tissue yielded virus.
NK and CD8 T cell antiviral control proceeds independent of CX3CR1
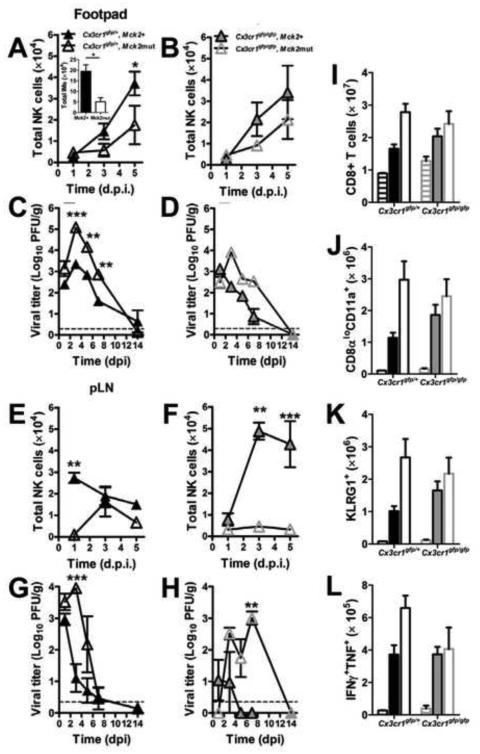
CX3CR1 is expressed on NK and CD8 T cells in addition to PMs. Both lymphocyte types are central to control of MCMV in C57BL/6 mice. Although CX3CR1 deficiency compromises PM mobilization (Landsman et al., 2009), the impact on NK and CD8 T cells has not been fully elucidated. MCK2-recruited IMs contribute to a potent NK cell response to MCMV (Crane et al., 2009; Hokeness et al., 2005). We observed the expected (Daley-Bauer et al., 2012) robust MCK2-dependent recruitment of IMs by 3 dpi in FPs (Figure 6A, inset) as well as efficient NK cell recruitment unaffected by CX3CR1 deficiency (Figure 6A and B). Thus, NK cell recruitment was more dependent on viral MCK2 and host IMs than on CX3CR1 or PMs, a pattern that continued through 5 dpi. Importantly, this pattern of NK cell recruitment resulted in more rapid control when MCK2 was expressed (Figure 6C and D). In the absence of MCK2, viral titers were higher and clearance was slower, consistent with NK cell function being more responsive to MCK2 than to CX3CR1 signaling. A similar impact on function was observed in draining, popliteal lymph nodes (Figure 6E – H). These data reinforce the dominance of MCK2-recruitment of IMs in NK cell responses (Crane et al., 2009; Hokeness et al., 2005) and suggest that CX3CR1 does not contribute markedly to NK cell response parameters during MCMV infection.
Figure 6.
CX3CR1 deficiency does not impair antiviral immune response. (A – D) NK cell levels (A and B) and viral titers (C and D) in inoculated FPs. Inset: IM numbers at 3 dpi. (E – H) NK cell levels (E and F) and viral titers (G and H) in draining popliteal lymph nodes. (I – L) Systemic CD8 T cell response in spleens at 7 dpi. Shown are (I) total CD8 T cells and, (J) the Ag-experienced population indentified as CD8αloCD11a+ cells. Ag-experienced cells were further characterized as effector T cells based (K) KLRG1 expression and (L) intracellular IFNγ and TNF following MCMV M45 peptide stimulation. Bars show mean values ± SE for n = 5 Cx3cr1gfp/+ (black) or Cx3cr1gfp/gfp (gray) mice subjected to mock (striped), Mck2+ (filled) or Mck2mut (open) virus infection. Cells were isolated from respective tissues and quantified by flow cytometry. Homogenized tissues were assessed by plaque assay for viral load. Data points are mean values ± SE for n = 5 mice and horizontal lines represent assay threshold. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant (p > 0.05).
In addition to the host defense benefit of the IM-driven NK response, NK-mediated suppression of the effector CD8 T cell response has been observed in C57BL/6 mice (Andrews et al., 2010; Su et al., 2001). The contribution of CX3CR1 to the systemic antiviral CD8 T cell response was evaluated in the spleen. Mck2+ virus-infected Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice mounted comparable responses in all parameters assessed (Figure 6I – L): proliferation, assessed as increase in cell numbers relative to naïve mice (Figure 6I); expansion within the Ag-experienced compartment detected as CD8loCD11a+ cells (Masopust et al., 2007) (Figure 6J); differentiation, based on increased KLRG1 and decreased CD62L and CD127 expression (Figure 6K and data not shown); and MCMV M45 peptide-induced cytokine production (Figure 6L). The MCK2- and IM-dependent pattern of CD8 T cell suppression was observed only in Cx3cr1gfp/+ but was absent in Cx3cr1gfp/gfp mice (Figure 6L). Given the apparent immune competence of CX3CR1-deficient mice, the defect in MCMV dissemination and latency appears to result from an intrinsic compromise of PM function rather than from any system-wide immune deficiency.
DISCUSSION
Here, CX3CR1-dependent PMs are identified as the myelomonocytic cell type responsible for CMV dissemination in the natural host. We show that MCMV-encoded MCK2 recruits PMs as well as IMs, exploiting both major monocyte populations. IMs modulate antiviral immunity, primarily by impairing CD8 T cells (Daley-Bauer et al., 2012). PMs acquire virus from initial infection sites and mask the virus from the immune response ensuring delivery to distal organs via the blood. In this capacity, PMs function as dissemination vehicles dictating maximal viral loads in spleen and persistence in salivary glands. This pattern dictates latency outcome in these tissues. Monocytes are well-recognized primary targets during HCMV infection (Daley-Bauer and Mocarski, 2012; Sinclair, 2008a), although focus has been on CD14+ subsets that behave like mouse CCR2+ IMs. The biological parallels between CX3CR1hi mouse and CD16+ human PMs suggests studies focused on this less abundant population in HCMV pathogenesis and latency might be fruitful.
HCMV dissemination may also be influenced by viral chemokines (Daley-Bauer and Mocarski, 2012; Penfold et al., 1999), although species specificity has not allowed elaboration of this contribution. The MCMV study here demonstrates that a viral chemokine can recruit and hijack both monocyte subsets to create a pathogen-host balance that promotes infection. MCK2 amplifies the biphasic chemotaxis of CX3CR1hi PMs to disseminate virus, and the CCR2+ IMs to dampen antiviral CD8 T cell immunity (Daley-Bauer et al., 2012). The first phase of PM recruitment contributes to the initial NK cell response (French et al., 2006), analogous to IM-mediated modulation of NK cells later in infection (Crane et al., 2009; Hokeness et al., 2005). The second PM recruitment phase is precipitated by a MCK2-CX3CR1 synergy that ensures optimal numbers of susceptible cells are present to acquire virus when viral levels peak. Studies suggested a CCR2-dependent IM effect on NK cell response (Crane et al., 2009; Hokeness et al., 2005) that impairs the adaptive immunity in MCMV-infected C57BL/6 mice (Andrews et al., 2010; Su et al., 2001). Here, we expand understanding of the immunomodulatory MCK2-IM axis to show that this drives the potent antiviral NK cell response limiting CD8 T cell priming. In BALB/c mice where the NK cell response is negligible, immunosuppression is achieved by a direct impact of IMs on the CD8 T cell response (Daley-Bauer et al., 2012). The importance of MCK2 in governing monocyte-driven activities ensuring successful infection has significant implications for studies that employ MCK2-deficient Smith strain bacmid-derived viruses (Jordan et al., 2011). Recent evidence implicating MCK2 as a mediator of viral entry into macrophages (Wagner et al., 2013) was not sustained by independently constructed, well-characterized K181 strain-derived mutant and repaired rescue viruses used in our studies. This draws into question any general role in entry but reinforces the contribution of MCK2 chemokine activity in viral dissemination (Noda et al., 2006; Saederup et al., 2001; Saederup et al., 1999). Thus, MCMV relies on viral chemokine-dependent strategies as an integral component of pathogenesis.
Our observations point to key contributions of PM-lineage cells in CMV pathogenesis, extending the understanding of events that lead to systemic infection (Daley-Bauer and Mocarski, 2012; Hsu et al., 2009; Manning et al., 1992; Noda et al., 2006; Sacher et al., 2008; Saederup et al., 2001; Stoddart et al., 1994). By comparing local and systemic inoculation, we show that a MCK2-responsive, CX3CR1-dependent, PM-associated viremia occurs independent of route whereas the requirement for CX3CR1 to support dissemination to salivary glands is very much inoculation route-dependent. As PMs retain the immature monocyte phenotype and circulate in blood, we speculate that they encounter and acquire virus through contact with infected vascular endothelial cells at local sites of infection; a step that is bypassed by systemic inoculation (Hsu et al., 2009; Sacher et al., 2008). Most importantly, our data support the crucial contribution of PMs for peak splenic titers as well as persistent levels of virus in salivary glands that correlate with the establishment of lifelong latency. We therefore propose a model for the natural process of MCMV infection: (i) replication in parenchymal and endothelial cells at primary infection sites (Hsu et al., 2009; Sacher et al., 2008); (ii) MCK2-enhanced PM recruitment s to these sites; (iii) infection of PMs; (iii) trafficking of PMs via the blood stream to distal organs; (iv) differentiation into permissive macrophages and dendritic cells capable of seeding virus-susceptible tissues; and, (v) establishment of a PM lineage-driven latent reservoir following immune control of acute infection. This pattern may parallel a process in HCMV pathogenesis where more information is needed on whether vCXCL1 chemokine (Penfold et al., 1999) recruits monocytes to enhance dissemination and/or latency (Sinclair, 2008a). Therapeutic disruption of this process would be predicted to cut short dissemination and preclude latency, as occurs in CX3CR1-deficient mice.
The ontogeny of murine monocytes is becoming more clearly understood. Under steady state conditions, BM resident, short-lived CX3CR1intLy6Chi IMs precursors give rise to CX3CR1hiLy6C− PMs (Varol et al., 2007; Yona et al., 2013). PMs may also arise from an IM-independent precursor (Hanna et al., 2011). Given the well-established relationship between differentiation state and susceptibility to CMVs, IMs may represent immature (F4/80lo) and PMs may represent sufficiently mature (F4/80+) cells even though both are able to differentiate into virus-susceptible macrophages. Further, IMs extravasate into tissues in response to inflammatory insult (Carlin et al., 2013). Differentiation of IMs into macrophages that support infection may occur in tissues and not in the bloodstream. Given that MCK2 amplifies IM recruitment to infected tissues (Daley-Bauer et al., 2012), macrophages that support viral replication may certainly be IM-derived.
The contribution of PMs to establishing a latent reservoir of MCMV has implications for investigations of HCMV pathogenesis and latency in vitro, typically when focused on myeloid lineage cells that give rise to monocytes, macrophages and dendritic cells (Keyes et al., 2012; Reeves et al., 2005; Riegler et al., 2000; Rossetto et al., 2013; Soderberg-Naucler et al., 2001; Taylor-Wiedeman et al., 1991). Blood monocytes, in particular, have contributed to understanding of host-pathogen mechanisms involved in both lytic and latent infections. CD14loCD16+ PMs are a minor population (~7%) of human blood monocytes where IMs represent the majority (~90%) and include two distinct phenotypes, CD14+CD16− and CD14+CD16+ (Cros et al., 2010). Although recent focus has been on CD14+-enriched monocytes (Avdic et al., 2013; Huang et al., 2012; Poole et al., 2013), PMs may also contribute to the biology of HCMV. Studies that have attributed CD14+ monocyte-derived lineage cells as sites of latency capable of supporting viral reactivation upon differentiation in vitro (Keyes et al., 2012; Riegler et al., 2000; Rossetto et al., 2013; Soderberg-Naucler et al., 2001; Taylor-Wiedeman et al., 1991) seem more remincent of the behavior of IMs in mice that play no physiologic role in dissemination or latency (Daley-Bauer et al., 2012) but can be infected in vitro. Given the low numbers of latent viral DNA positive cells in blood of healthy individuals (Slobedman and Mocarski, 1999b), additional investigation of CD16+ PMs may clarify mechanisms and relevant cell types in HCMV pathogenesis.
The specific cell types that are important in harboring latent virus have been a longstanding topic of interest. Monocytes and myelomonocytic progenitors are apparent sites of CMV latency in both mice (Reddehase et al., 2008) and humans (Sinclair, 2008a), and the role these cells play in dissemination or persistence would potentially disrupt the path to latency. The discrepancy between our data and a recently published study excluding CX3CR1hi PM lineage cells as sites of latency in lungs (Marquardt et al., 2011) may reflect: (i) the use of an attenuated MCK2 mutant with reduced ability to infect monocytes (Jordan et al., 2011; Wagner et al., 2013; Wagner et al., 1999); (ii) reliance on a systemic inoculation route that bypasses PM-associated steps in dissemination and latency; and/or, (iii) a specialized feature of lung infection that remains to be elaborated.
Collectively, we have shown that PM lineage cells contribute to viral dissemination, peak replication, persistent infection and detection of latency, defining a major monocyte population targeted by viral chemokine that contributes to the success of viral pathogenesis. Our approach reinforces the importance of infection route used in animal models in elucidating mechanisms involved in pathogenesis. The study reveals that CMV evolved a chemokine-driven modulatory strategy that exploits one arm of the monocyte compartment primarily to perpetuate infection and the other arm to modulate host immune control to achieve a pathogen-host balance. We predict the full appreciation of the contributions of monocyte lineages to HCMV pathogenesis will benefit from studies investigating CD16+ PMs in parallel with the more abundant CD14+ IMs. These findings will have bold implications for improving vaccine and therapeutic strategies targeting HCMV, as well as for preventing transmission to susceptible recipients by transfusion and transplantation.
MATERIALS AND METHODS
Viruses, Mice and Experimental Infection
MCMV K181+-derived recombinant Mck2 mutant (RM461) and rescued (RQ461) viruses (Stoddart et al., 1994) were propagated in NIH 3T3 murine fibroblasts (ATCC CRL-1658) (Manning et al., 1992). Virus was prepared from clarified infected cell medium (Saederup et al., 1999) and used to inoculate 8- to 16-week-old mice. Cx3cr1gfp/gfp C57BL6 mice (Jung et al., 2000) (JAX #05582) were crossed with WT C57BL/6 (JAX #00664) mice to produce Cx3cr1gfp/+ offspring used as controls. Mice were inoculated either ip (1 × 106 plaque-forming units; PFU) or in a hind FP (5 × 106 PFU) with virus diluted in medium. Mock infection was with virus-free medium. Mice were housed at the Emory University Division of Animal Resources and experiments were conducted in accordance with National Institutes of Health and Emory University Institutional Animal Care and Use Committee guidelines.
Plaque and Infectious Center Assays
Plaque assays were conducted on tissues homogenized in medium (Manning et al., 1992) or sonicated macrophages and supernatant harvested from cultures, and infectious assays on blood leukocyte suspensions overlaid onto 3T3 Swiss Albino (3T3-SA) murine fibroblasts (ATCC CCL-92). Virus titers were calculated at 4 days when cells were fixed with methanol and stained with Giemsa for plaque visualization.
Antibodies
Antibodies to CD16/32 (FcγRII/III; Clone 2.4G2), Ly6C (Clone AL21), Ly6G (Clone 1A8), TNF-a (Clone MP6-XT22), CD4 (Clone RM4-5), CD11b (Clone M1/70), CD3ε (Clone 500A2) and NK1.1 (Clone PK136) were purchased from BD PharMingen; CD45 (Clone 30F11), CD69 (H1.2F3), and F4/80 (Clone BM8) from Invitrogen; IFN-γ (Clone XMG1.2), CD62L (Clone MEL14), CD115 (Clone ASF98), and CD107a (Clone 1D4B) from Biolegend; and CD4 (Clone GK1.5), CD11c (Clone N418), Ly-6A/E (Sca-1; Clone D7), CD19 (Clone 1D3), CD31 (PECAM-1; Clone 390), CD8a (Clone 53-6.7), CD49b (Clone DX5), Siglec H (Clone eBio440c), CD3ε (Clone 17A2), and Ly6C/6G (Gr-1; Clone RB6.8C5) from eBioscience.
Tissue Collection, Leukocyte Preparation, and Flow Cytometry
Single-cell suspensions were prepared from tissues as follows: FPs were minced into ~3 mm sections and digested with collagenase D (1.5 mg/ml; Sigma) in PBS; spleens and pLNs were mashed through a metal sieve; and blood cells separated on Histopaque-1119 (Sigma) density medium. Following hypotonic lysis of erythrocytes, suspensions were filtered (40 mm mesh), and viable cell counts were performed with trypan blue dye exclusion on a hemacytometer. Cell surface FcγRII/III was blocked prior to incubating with Abs for multiparametric flow cytometric analyses. Data were acquired by flow cytometry (BD LSRII cytometer and FACSDiva Software; BD Biosciences), analyzed with FlowJo (TreeStar), and graphed with Prism (GraphPad).
Cells were sorted (purity >90%; FACSVantage, BD Biosciences) from blood of Cx3cr1gfp/+ mice for adoptive transfer experiments or in vitro differentiation of macrophages.
Adoptive Transfer of Infectivity
Mice were inoculated ip with 3.5 × 106 PFU virus and blood was collected at 3 dpi. Blood leukocytes were prepared, stained and sorted by flow cytometry. Purified leukocytes were injected into tail veins of naïve recipient mice and salivary glands were collected at 11 dpi. Viral titers were calculated by plaque assay.
MCMV infection of monocyte-derived macrophages
Blood cells were prepared for flow cytometry and monocyte subsets were isolated as described above. Purified PMs and IMs were suspended in complete DMEM medium containing 20% fetal calf serum supplemented with 10% L929-conditioned medium. After days of culture with fresh medium every three days, adherent, differentiated macrophages were inoculated with tissue culture-derived (RQ461) stock or salivary gland-derived (v70) stock at an m.o.i. of 10 for 24 or 96 h. Viral replication by plaque assay on supernatant collected every daily for 4 days.
Macrophages were washed with PBS then fixed with 4% formaldehyde (EMS), autofluorescence was quenched with 50 mM NH4Cl, cells were permeabilized with 0.5% Triton X-100 and non-specific binding was blocked with PBS containing 0.1% BSA and 5% normal donkey serum. Anti-IE1 (Croma101) was added and bound antibodies were detected with donkey anti-mouse IgG (Alexa Fluor 594; BD Bioscience). Images were obtained using a Zeiss Axio Imager A1 epifluorescence microscope.
Latent Infections and Explant Reactivation
Groups of five Cx3cr1gfp/gfp or Cx3cr1gfp/+ C57BL6 mice were inoculated as described above; the salivary glands, lung, liver, spleen and BM were collected at 63 dpi, divided into three portions, minced into ~3 mm pieces and placed into culture with supernatant collected from culture wells and replaced with fresh culture medium at weekly intervals for 5 weeks. Reactivation of virus was assessed by plaque assay of culture supernatants on 3T3-SA fibroblasts.
Statistical analysis
Experimental groups contained a minimum of five mice. Statistical differences between groups were calculated by Student′s t test, one-way ANOVA with Bonferroni′s or Dunnett′s Multiple Comparison post test or two-way ANOVA with Bonferroni′s Multiple Comparison post test where p ≤ 0.05 considered significant using the GraphPad Prism 5 software.
Supplementary Material
HIGHLIGHTS.
CX3CR1 deficiency disrupts MCMV dissemination from local infection site to salivary glands
Viral MCK2 chemokine recruits CX3CR1hi patrolling monocytes (PMs) to local infection sites
CX3CR1hi PMs harbor infectious MCMV in blood and mediate virus dissemination
CX3CR1hi PM-mediated dissemination promotes viral latency
ACKNOWLEDGEMENT
We thank J. Upton and D. Livingston-Rosanoff for assistance with animal husbandry, R. Ahmed and S. Speck for reagents, S. Aguirre for sharing unpublished observations and the Mocarski laboratory members for comments on the manuscript. This work was supported by Public Health Service Grant R01 AI020211 and in part by the Flow Cytometry Core Facility of the Emory Vaccine Center. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Avdic S, Cao JZ, McSharry BP, Clancy LE, Brown R, Steain M, Gottlieb DJ, Abendroth A, Slobedman B. Human cytomegalovirus viral IL-10 polarizes monocytes towards a deactivated M2c phenotype to repress host immune responses. J Virol. 2013 doi: 10.1128/JVI.00912-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JF, Jr., O'Neil ME. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol. 1989;63:2667–2673. doi: 10.1128/jvi.63.6.2667-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KS, Lang DJ. Transmission and activation of cytomegalovirus with blood transfusion: a mouse model. J Infect Dis. 1977;135:841–845. doi: 10.1093/infdis/135.5.841. [DOI] [PubMed] [Google Scholar]
- Collins TM, Quirk MR, Jordan MC. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol. 1994;68:6305–6311. doi: 10.1128/jvi.68.10.6305-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane MJ, Hokeness-Antonelli KL, Salazar-Mather TP. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands. J Immunol. 2009;183:2810–2817. doi: 10.4049/jimmunol.0900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley-Bauer LP, Mocarski ES. Myeloid cell recruitment and function in cytomegalovirus immunity and pathogenesis. In: Reddehase MJ, editor. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. Caister Scientific Press; Norfolk, United Kingdom: 2012. pp. 363–373. [Google Scholar]
- Daley-Bauer LP, Wynn GM, Mocarski ES. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity. 2012;37:122–133. doi: 10.1016/j.immuni.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P, Davis-Poynter N, Degli-Esposti M, Densley E, Papadimitriou J, Shellam G, Farrell H. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J Virol. 1999;73:6800–6809. doi: 10.1128/jvi.73.8.6800-6809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Sjolin H, Kim S, Koka R, Yang L, Young DA, Cerboni C, Tomasello E, Ma A, Vivier E, et al. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J Immunol. 2006;177:4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gilbert GL, Hayes K, Hudson IL, James J. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leucocytes. Neonatal Cytomegalovirus Infection Study Group. Lancet. 1989;1:1228–1231. doi: 10.1016/s0140-6736(89)92330-1. [DOI] [PubMed] [Google Scholar]
- Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel L, Lacaille VG, Strobl H, Mellins ED, Mocarski ES. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J Virol. 2003;77:7563–7574. doi: 10.1128/JVI.77.13.7563-7574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama WM. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol. 2009;90:33–43. doi: 10.1099/vir.0.006668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Kew VG, Jestice K, Wills MR, Reeves MB. Efficient human cytomegalovirus reactivation is maturation dependent in the Langerhans dendritic cell lineage and can be studied using a CD14+ experimental latency model. J Virol. 2012;86:8507–8515. doi: 10.1128/JVI.00598-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S, Krause J, Prager A, Mitrovic M, Jonjic S, Koszinowski UH, Adler B. Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2. J Virol. 2011;85:10346–10353. doi: 10.1128/JVI.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LR, Hargett D, Soland M, Bego MG, Rossetto CC, Almeida-Porada G, St Jeor S. HCMV protein LUNA is required for viral reactivation from latently infected primary CD14(+) cells. PLoS One. 2012;7:e52827. doi: 10.1371/journal.pone.0052827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- MacDonald MR, Burney MW, Resnick SB, Virgin HI. Spliced mRNA encoding the murine cytomegalovirus chemokine homolog predicts a beta chemokine of novel structure. J Virol. 1999;73:3682–3691. doi: 10.1128/jvi.73.5.3682-3691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, Mocarski ES. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992;66:3794–3802. doi: 10.1128/jvi.66.6.3794-3802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt A, Halle S, Seckert CK, Lemmermann NA, Veres TZ, Braun A, Maus UA, Forster R, Reddehase MJ, Messerle M, Busche A. Single cell detection of latent cytomegalovirus reactivation in host tissue. J Gen Virol. 2011;92:1279–1291. doi: 10.1099/vir.0.029827-0. [DOI] [PubMed] [Google Scholar]
- Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA, Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978;41:143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mitchell BM, Leung A, Stevens JG. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology. 1996;223:198–207. doi: 10.1006/viro.1996.0468. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Jr., Hahn G, White KL, Xu J, Slobedman B, Hertel L, Aguirre SA, Noda S. Myeloid cell recruitment and function in pathogenesis and latency. In: Reddehase MJ, editor. Cytomegaloviruses: Pathogenesis, Molecular Biology, and Infection Control. Caister Scientific Press; Norfolk, United Kingdom: 2006. pp. 465–482. [Google Scholar]
- Mocarski ES, Jr., Shenk T, Griffith P, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1960–2014. [Google Scholar]
- Noda S, Aguirre SA, Bitmansour A, Brown JM, Sparer TE, Huang J, Mocarski ES. Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood. 2006;107:30–38. doi: 10.1182/blood-2005-05-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Dairaghi DJ, Duke GM, Saederup N, Mocarski ES, Kemble GW, Schall TJ. Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci U S A. 1999;96:9839–9844. doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JL, Presti RM, Paetzold S, Virgin H.W.t. Latent murine cytomegalovirus infection in macrophages. Virology. 1997;227:168–179. doi: 10.1006/viro.1996.8303. [DOI] [PubMed] [Google Scholar]
- Poole E, Walther A, Raven K, Benedict CA, Mason GM, Sinclair J. The myeloid transcription factor GATA-2 regulates the viral UL144 gene during human cytomegalovirus latency in an isolate-specific manner. J Virol. 2013;87:4261–4271. doi: 10.1128/JVI.03497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM, Szmuness W, Millian SJ, David DS. A serologic study of cytomegalovirus infections associated with blood transfusions. N Engl J Med. 1971;284:1125–1131. doi: 10.1056/NEJM197105202842004. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:315–331. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:243–262. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- Reeves M, Sissons P, Sinclair J. Reactivation of human cytomegalovirus in dendritic cells. Discov Med. 2005;5:170–174. [PubMed] [Google Scholar]
- Revello MG, Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Rev Med Virol. 2010;20:136–155. doi: 10.1002/rmv.645. [DOI] [PubMed] [Google Scholar]
- Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis. 1998;177:1170–1175. doi: 10.1086/515277. [DOI] [PubMed] [Google Scholar]
- Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol. 2000;81:393–399. doi: 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- Roback JD, Su L, Newman JL, Saakadze N, Lezhava LJ, Hillyer CD. Transfusion-transmitted cytomegalovirus (CMV) infections in a murine model: characterization of CMV-infected donor mice. Transfusion. 2006;46:889–895. doi: 10.1111/j.1537-2995.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 2010;67:2685–2693. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto CC, Tarrant-Elorza M, Pari GS. Cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14 (+) monocytes and CD34 (+) cells. PLoS Pathog. 2013;9:e1003366. doi: 10.1371/journal.ppat.1003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher T, Podlech J, Mohr CA, Jordan S, Ruzsics Z, Reddehase MJ, Koszinowski UH. The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe. 2008;3:263–272. doi: 10.1016/j.chom.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Saederup N, Aguirre SA, Sparer TE, Bouley DM, Mocarski ES. Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J Virol. 2001;75:9966–9976. doi: 10.1128/JVI.75.20.9966-9976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saederup N, Lin YC, Dairaghi DJ, Schall TJ, Mocarski ES. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc Natl Acad Sci U S A. 1999;96:10881–10886. doi: 10.1073/pnas.96.19.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo AA, Corbett AJ, Rawlinson WD, Scott GM, Degli-Esposti MA. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol Cell Biol. 2007;85:46–54. doi: 10.1038/sj.icb.7100013. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol. 2008a;41:180–185. doi: 10.1016/j.jcv.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sinclair J. Manipulation of dendritic cell functions by human cytomegalovirus. Expert Rev Mol Med. 2008b;10:e35. doi: 10.1017/S1462399408000872. [DOI] [PubMed] [Google Scholar]
- Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999a;73:4806–4812. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999b;73:4806–4812. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol. 2001;75:7543–7554. doi: 10.1128/JVI.75.16.7543-7554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stievano L, Piovan E, Amadori A. C and CX3C chemokines: cell sources and physiopathological implications. Crit Rev Immunol. 2004;24:205–228. doi: 10.1615/critrevimmunol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA. NK cell functions restrain T cell responses during viral infections. Eur J Immunol. 2001;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. Pt 9. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FM, Brizic I, Prager A, Trsan T, Arapovic M, Lemmermann NA, Podlech J, Reddehase MJ, Lemnitzer F, Bosse JB, et al. The Viral Chemokine MCK-2 of Murine Cytomegalovirus Promotes Infection as Part of a gH/gL/MCK-2 Complex. PLoS Pathog. 2013;9:e1003493. doi: 10.1371/journal.ppat.1003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Jonjic S, Koszinowski UH, Messerle M. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol. 1999;73:7056–7060. doi: 10.1128/jvi.73.8.7056-7060.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom ME, Fleming P, Comerford I, McColl SR, Andoniou CE, Degli-Esposti MA. A chemokine-like viral protein enhances alpha interferon production by plasmacytoid dendritic cells but delays CD8+ T cell activation and impairs viral clearance. J Virol. 2013;87:7911–7920. doi: 10.1128/JVI.00187-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager AS. Transfusion-acquired cytomegalovirus infection in newborn infants. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26:445–454. [Google Scholar]
- Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98:281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.