Abstract
Human decision-making is significantly modulated by previously experienced outcomes. Using event-related potentials (ERPs), we examined whether ERP components evoked by outcome feedbacks could serve as biomarkers to signal the influence of current outcome evaluation on subsequent decision-making. In this study, eighteen adult volunteers participated in a simple monetary gambling task, in which they were asked to choose between two options that differed in risk. Their decisions were immediately followed by outcome presentation. Temporospatial principle component analysis (PCA) was applied to the outcome-onset locked ERPs in the -200 – 1000 ms time window. The PCA factors that approximated classical ERP components (P2, feedback-related negativity, P3a, & P3b) in terms of time course and scalp distribution were tested for their association with subsequent decision-making strategies. Our results revealed that a fronto-central PCA factor approximating the classical P3a was related to changes of decision-making strategies on subsequent trials. The decision to switch between high- and low-risk options resulted in a larger P3a relative to the decision to retain the same choice. According to the results, we suggest the amplitude of the fronto-central P3a is an electrophysiological index of the influence of current outcome on subsequent risk decision-making. Furthermore, the ERP source analysis indicated that the activations of the frontopolar cortex and sensorimotor cortex were involved in subsequent changes of strategies, which enriches our understanding of the neural mechanisms of adjusting decision-making strategies based on previous experience.
Keywords: decision-making, outcome evaluation, event-related potential (ERP), principal components analysis (PCA), P3 component
INTRODUCTION
Decision-making requires the ability to choose between competing options that produce diverse outcomes (Yang & Zhang, 2011). Human brain makes decisions by assigning incentive values to different options under consideration and then comparing those values (Litt, Plassmann, Shiv, & Rangel, 2011). The value assignment can be modeled by the probabilistic relationship between each option and its corresponding outcome (e.g., reward or punishment) according to past experience (Wunderlich, Symmonds, Bossaerts, & Dolan, 2011). An option that consistently leads to hedonic outcomes becomes the preferred selection if the option-outcome association is successfully learned (Morrison, Saez, Lau, & Salzman, 2011). Discovering the optimal decision-making strategy from previous outcomes is critical for humans and animals because it plays an important role in environmental adaptation and survival (Lecrubier, Weiller, Andersen, & Baldwin, 2006; Ohira, et al., 2010; Peterson, Lotz, Halgren, Sejnowski, & Poizner, 2011). Cognitive systems that rapidly construct option-outcome associations provide high levels of behavioral flexibility (Wallis, 2011). In the field of experimental psychology, a trial-by-trial decision-making task paradigm is helpful for investigating the impact of current outcomes on future decision-making behavior (Peterson, et al., 2011). For instance, during the classical Iowa gambling task, in which participants received feedback immediately after making a choice, most normal participants (i.e., participants without brain injury) change their selection preference dramatically within just a few trials (Bechara, 2004; Bechara, Damasio, & Damasio, 2000).
High-temporal resolution techniques, most notably the event-related potential (ERP), have provided valuable knowledge about how current outcomes shape future behavior. Below we summarize some of the most relevant findings. Johnson & Donchin (1982) discovered that when asking participants to count the number of specific tones, the P3 amplitude revealed the efficiency of adopting the new rules of tone generation after “probability transitions” (see also Aleksandrov & Maksimova, 1985; Pritchard, Houlihan, & Robinson, 1999). Polezzi (2008) found that higher P2 amplitudes were associated with a lower frequency of uncertain choices in subsequent trials, suggesting that the P2 component represented a distinction between predictability and unpredictability (see also Chen, Qiu, Li, & Zhang, 2009; Schuermann, Endrass, & Kathmann, 2012). In a strategic economic game against a computer opponent, Cohen & Ranganath (2007) observed that a feedback-locked ERP component, namely the feedback-related negativity (FRN), predicted whether participants would change decision behavior, with the FRN following monetary loss being more negative-going when participants chose a different option on the subsequent trial (see also Cavanagh, Frank, Klein, & Allen, 2010; Cohen, Wilmes, & van de Vijver, 2011; van der Helden, Boksem, & Blom, 2010).
Risk decision-making (e.g., monetary gambling) is a major type of decision-making involving uncertainty and has been one of the most active research topics (Bechara, Damasio, Tranel, & Damasio, 2005; Hertwig & Erev, 2009). In a risk decision-making task, participants have to choose between low- and high-risk options (Krain, Wilson, Arbuckle, Castellanos, & Milham, 2006). Past outcomes significantly modulate risk-taking preferences (Hertwig, Barron, Weber, & Erev, 2004; Thaler & Johnson, 1990). For instance, decision-makers become more risk-avoidant after monetary gains, but more risk-seeking after monetary losses (Gu, Ge, Jiang, & Luo, 2010; Isen, Nygen, & Ashby, 1988). To our knowledge, the underlying electrophysiological mechanism of how individual experience influences risky behavior remains largely unknown, since limited ERP studies that focus on the option-outcome association had utilized risk decision-making paradigms until very recently (Polezzi, Sartori, Rumiati, Vidotto, & Daum, 2010; San Martín, Appelbaum, Pearson, Huettel, & Woldorff, 2013; Schuermann, et al., 2012; Yang & Zhang, 2011). In most of these studies, the targeted ERP component following outcome presentation was correlated with overall risk-taking tendency (Polezzi, et al., 2010), or was sensitive to risk levels of the decision that leads to the current outcome (Schuermann, et al., 2012; Yang & Zhang, 2011). Nevertheless, the relationship between ERP components elicited by outcome feedback and subsequent risk decision-making still remains to be explored.
When analyzing the ERP components of interest (the P2, FRN, P3, etc.), a troublesome problem is their temporal overlap. This phenomenon may weaken the reliability of the results obtained (Nelson, Patrick, Collins, Lang, & Bernat, 2011; Yu, Zhou, & Zhou, 2011). Principal components analysis (PCA) is a useful tool for statistical decomposition of ERPs and may help to solve this issue (Dien & Frishkoff, 2005; Donchin & Heffley, 1979; Foti, Weinberg, Dien, & Hajcak, 2011; Macnamara, Ochsner, & Hajcak, 2011). This method mainly uses orthogonal transformation to convert ERP data of possibly-correlated components into a set of relatively uncorrelated principal components (Dien, 2010a). Numerous studies have demonstrated that compared with the conventionally-averaged ERPs, the PCA technique significantly enhances source analysis effects of a high-density ERP data set (Carretie, et al., 2004; Debener, Makeig, Delorme, & Engel, 2005; Dien, 2010b; Dien, Beal, & Berg, 2005; Richards, 2004). The combination of temporal and spatial PCA processes utilizes the observed data in both temporal and spatial domains, thereby further facilitating ERP components isolation from overlapping responses (Spencer, Dien, & Donchin, 1999).
The current work aims to examine the association between ERP components elicited by outcome presentation and subsequent strategies in risk decision-making scenarios. A classical monetary gambling task was applied while electroencephalogram (EEG) data were recorded from the participants. In general, the task design was consistent with the description of Gehring & Willoughby (2002), in which participants faced a two-alternative forced choice between a high- and low-risk option in each trial (see next section for details). We chose this paradigm because of its elegant design and its reliability in eliciting the ERP components of interest (Gu, et al., 2011; Nieuwenhuis, Yeung, Holroyd, Schurger, & Cohen, 2004). Temporospatial PCA was utilized to isolate ERP signals that were sensitive to subsequent decision-making strategies from temporally overlapping potentials and to identify the likely neural generator of this activity. Our goal is to find whether one or more ERP components (the P2, FRN, or P3) that were most often investigated by previous literatures could be regarded as potential indices of the impact of monetary outcomes on subsequent risk decisions.
MATERIALS AND METHODS
Participants
Eighteen Chinese students (9 females; mean age 21.61 ± 2.50 years) were recruited from Beijing Normal University as paid volunteers. All participants were free of regular use of medication or other nonmedical substances that might affect the central nervous system. All had normal vision (with or without correction), and none had history of neurological disease. All participants gave their informed consent prior to the experiment. The experimental protocol was approved by the local Ethics Committee (Beijing Normal University) and was in compliance with the ethical guidelines of the American Psychological Association (Cayoun, 2006).
Behavioral procedure
Before the task, participants were told that they would be involved in a monetary gambling game. They were informed about the rules and the meanings of symbols in the task and were asked to respond in a way that would maximize the total score amount (the instruction indicated that an optimal strategy existed in the task). The higher the score they earned, the more bonus money they would receive at the end of the experiment.
During the formal task, participants sat comfortably in an electrically-shielded room approximately 100 cm in front of a computer screen. The experimental procedure is illustrated in Fig. 1. Each trial began with the presentation of a central fixation point (white against a black background). After 1200 ms, two white rectangles (2.5 degree × 2.5 degree of visual angle) appeared on the left and right sides of the fixation point, displaying the numbers “9” and “99” (indicating the gambling points). The sides of presentation of these two numbers were counterbalanced across the trials. Participants gambled by selecting the option displayed in the left or right rectangle by pressing the “F” or “J” button on a conventional computer keyboard with their left or right index finger. The chosen rectangle was highlighted by a red outline for 500 ms, followed by a time jitter between 800 and 1200 ms. Finally, the outcome valence of the participants’ choice was presented in the chosen rectangle for 1000 ms. Positive valence (“+”) indicated that participants won the points that were chosen in this trial, while negative valence (“−”) indicated they lost the points. The formal task consisted of four blocks of 128 trials each (512 trials in total). Stimulus display and behavioral data acquisition were conducted using E-Prime software (Version 1.1, Psychology Software Tools, Inc., Pittsburgh, PA). Unbeknownst to the participant, the probabilities of winning and losing were equal regardless of participants’ task performance.
Figure 1. Schematic diagram of two adjacent experimental trials in the monetary gambling task.
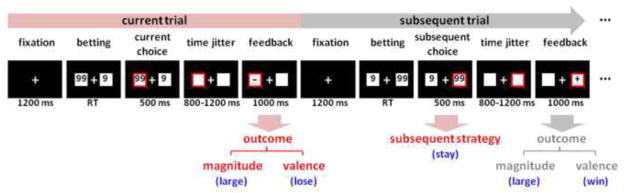
The three major factors (outcome valence, outcome magnitude, and subsequent strategy) in data analysis are indexed in red. In this example, the participant has chosen “99” (high-risk option) in the current trial, and the outcome valence is “−” (negative), so the outcome in the current trial is “-99” (lose 99 points). The participant’s choice in the subsequent trial is “99”, which means he/she decided to stay in the high-risk option rather than switch to the low-risk option. RT = response time.
Behavioral measures
The “9” is defined as the low-risk option (small potential win or loss) while the “99” is defined as the high-risk option (large potential win or loss). The tendency to choose the low-risk option indicates a preference for risk-avoidant strategy. This preference was measured as the “risk-avoidant ratio,” by dividing the number of risk-avoidant choices by the total number of choices. It was unnecessary to calculate the “risky ratio,” because the value of risky ratio was equal to one minus the risk-avoidant ratio in each condition.
Inspired by previous studies (Boorman, Behrens, Woolrich, & Rushworth, 2009; Cohen & Ranganath, 2007; Daw, O’Doherty, Dayan, Seymour, & Dolan, 2006), the current study was also interested in whether participants switched their decision-making strategies (i.e., risky or risk-avoidant) in adjacent trials. The frequency of “strategy-switching” (either from the low- to high-risk option or the reverse) was measured as the “switch ratio,” by dividing the number of strategy-switching by the total number of trials.
ERP recording and preprocessing
Electroencephalogram (EEG) activity was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap, with an online reference to the left mastoid and off-line algebraic re-referencing to the average of the left and right mastoids (NeuroScan Inc., Herndon, USA). Horizontal electrooculogram (HEOG) was recorded from electrodes placed at the outer canthi of both eyes. Vertical electrooculogram (VEOG) was recorded from electrodes placed above and below the left eye. Impedance levels were set at less than 5 kΩ. EEG and EOG signals were continuously sampled at 500 Hz and filtered within 0.05 – 100 Hz. Ocular artifacts were removed from EEGs using a regression procedure implemented with Neuroscan software (Scan 4.3) (Semlitsch, Anderer, Schuster, & Presslich, 1986).
Data were preprocessed using Matlab R2011a (MathWorks, Natick, USA). The recorded EEG data were down-sampled to 250 Hz and filtered with a 0.05 – 30 Hz finite impulse response filter with zero phase distortion. Filtered data were segmented beginning 200 ms prior to the onset of outcome and lasting for 1200 ms. All epochs were baseline-corrected with respect to the mean voltage over the 200 ms preceding the onset of outcome, followed by averaging in association with three major factors (see the Statistics section).
Average ERPs analysis
Since the P2 component could not be visually detected in the conventionally-averaged ERPs (see Fig. 2), two ERP components (the FRN and P3) were chosen for analysis. Based on both previous literature (e.g., Bellebaum & Daum, 2008) and visual inspection, the FRN amplitude was measured as the mean amplitude within a 240 – 300 ms time window, while the P3 amplitude was measured as the mean amplitude within a 320 – 400 ms time window. The averaged data were derived from all electrodes, but only the electrodes at which the components reached their peak values were entered into statistical analysis (Gu, et al., 2011; Long, Jiang, & Zhou, 2012).
Figure 2. The illustration of the main effects of the three major factors on the conventionally-averaged ERPs evoked by outcome presentation (from left to right: outcome magnitude, outcome valence, subsequent strategy).
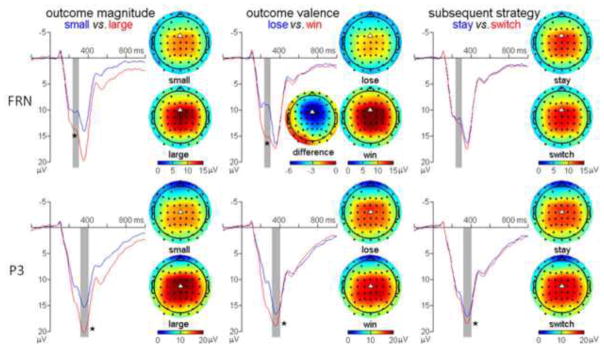
The scalp topographies of every condition are available on the right side of the corresponding ERP waveforms. For the FRN component, the scalp topography of the “loss-win” difference wave is also provided, so as to demonstrate that the spatial distribution of the FRN is similar to reports from classical studies (Holroyd & Krigolson, 2007). The waveforms of FRN and the P3 were derived from Cz and FCz, respectively (as indexed by the white triangles in the topographies). The light grey areas indicate the time window for the analyses on the FRN and the P3. Asterisks indicate significant effects (* p < 0.05).
Temporospatial PCA
ERP PCA Toolkit (EP Toolkit, version 2.23) was employed to conduct PCA in this study (Dien, 2010a). The EP Toolkit is a MATLAB-based software package that provides a semi-automatic routine for a two-step sequential PCA procedure. The first step is temporal PCA performed on all time points from each participant’s average ERPs. After capturing the variances in time domain, spatial PCA is conducted for each of the resultant temporal factors using all recording electrodes, such that the variances of the spatial distribution of the ERP data were obtained. According to the comparative study of Dien (2010b), Promax rotation and Infomax rotation were used in temporal and spatial PCA, respectively.
Finally, ten temporal factors × three spatial factors were extracted from our ERP data based on the Scree plot, yielding 30 temporospatial factor combinations. These combinations were arranged in a descending order according to the amount of variances accounted for in the original ERPs.
Statistics
This study focused on the potential influence of current outcomes on subsequent decision-making strategies. Thus, the “risk-avoidant ratio” and “switch ratio” were analyzed with two factors that were defined in current trials. Outcome valence refers to the valence of the outcome (win vs. loss) of the current trial. Outcome magnitude refers to the magnitude of the outcome (small vs. large), which depended upon participants’ choices (low-risk vs. high-risk) in current trials. The third factor, subsequent strategy, was taken into account in the definition of these measures (see the Behavioral measures subsection).
The analyses of electrophysiological data focused on three factors: outcome valence, outcome magnitude, and subsequent strategy (see Fig. 1). Subsequent strategy denotes the selection in the following trials (stay vs. switch); “stay” means the same option being chosen in the current and the next trial while “switch” means different options being chosen in two trials. (Note: the subsequent strategy factor may alternatively have been analyzed using a small/large classification instead of the stay/switch classification; see supplementary materials for more details.)
For all the analyses listed below, the significance level was set at 0.05. Greenhouse–Geisser correction for ANOVA tests was used when appropriate. Post-hoc testing of significant effects was conducted using the Bonferroni method. Significant interactions were analyzed using simple effects models. Partial eta-squared ( ) was reported to demonstrate the effect size in ANOVA tests, where 0.05 represents a small effect, 0.10 equals a medium effect, and 0.20 represents a large effect (Pfabigan, Alexopoulos, Bauer, & Sailer, 2010). Statistical analysis was performed using SPSS Statistics 19.0 (IBM, Somers, USA). For the sake of brevity, effects that did not reach significance have been omitted.
RESULTS
Behavioral Results
Risk-avoidant ratio
The average risk-avoidant ratio was 58.4 ± 10.1 % (mean ± SD) in 508 trials (the first trial of each block was not calculated), which was significantly greater than chance (50 %) according to the result of a one-sample t test (t (17) = 3.55, p < 0.01). A repeated-measures 2 (outcome valence) × 2 (outcome magnitude) ANOVA was performed on the risk-avoidant ratio in each condition. The main effect of outcome valence was significant (F (1, 17) = 12.1, p < 0.01, ); participants selected the low-risk option (“9”) more frequently following wins (61.9 ± 15.3 %) than following losses (51.4 ± 14.9 %). The main effect of outcome magnitude was also significant (F (1, 17) = 7.37, p < 0.05, ); participants selected the low-risk option more frequently following small outcomes (61.9 ± 16.5 %) than following large outcomes (51.5 ± 13.5 %).
Switch ratio
The average switch ratio was 42.2 ± 10.1 % in 508 trials (the first trial of each block was not calculated), which was significantly less than chance according to the result of a one-sample t test (t (17) = −3.24, p < 0.01); participants were more likely to repeat the same strategy than to switch strategies between individual trials. A repeated-measures 2 × 2 ANOVA was performed on the switch ratio in each condition. The main effect of outcome magnitude was significant (F (1, 17) = 12.8, p < 0.01, ); the switch ratio was larger following large outcomes (51.6 ± 9.2 %) than following small outcomes (38.1 ± 13.4 %). In other words, participants were more likely to switch away from (rather than stay on) risky strategy. The outcome valence × outcome magnitude interaction was significant (F (1, 17) = 12.1, p < 0.01, ). Simple effect analysis indicated that the switch ratio was significantly affected by outcome magnitude only when the outcome valence was win (F (1, 17) = 19.0, p < 0.001), with the strategy-switching happening more frequently following a large win (56.9 ± 14.1 %) than following a small win (33.0 ± 15.1 %).
ERP Results: Conventionally-averaged ERPs
FRN component
The amplitude of the FRN was most prominent at the fronto-central area and reached its minimum (negative peak) at electrode position FCz (see Fig. 2). A repeated-measures 2 (outcome valence) × 2 (outcome magnitude) × 2 (subsequent strategy) ANOVA was performed on the FRN at electrode FCz. The main effects of outcome valence (F (1, 17) = 63.0, p < 0.001, ) and outcome magnitude (F (1, 17) = 21.3, p < 0.001, ) were significant; the FRN was more negative-going following losses (9.1 ± 5.2 μV) than following wins (15.0 ± 5.9 μV) (see also Holroyd, Hajcak, & Larsen, 2006) and was more negative-going following small outcomes (10.2 ± 4.5 μV) than following large outcomes (13.9 ± 7.2 μV) (see also Gu, et al., 2011). The outcome valence × outcome magnitude interaction was significant (F (1, 17) = 26.7, p < 0.001, ). Simple effect analysis indicated that the effect of outcome magnitude on the FRN was stronger following wins (F (1, 17) = 32.4, p < 0.001; large outcome = 17.4 ± 6.4 μV, small outcome = 12.7 ± 4.2 μV; difference = 4.7 μV) than following losses (F (1, 17) = 9.94, p < 0.01; F (1, 17) = 9.94, p < 0.01; large outcome = 10.3 ± 5.1 μV, small outcome = 7.8 ± 3.9 μV; difference = 2.5 μV).
P3 component
The amplitude of the P3 was most prominent at the central area and reached its maximum at Cz (see Fig. 2). A repeated-measures 2 × 2 × 2 ANOVA was performed on the P3 at electrode Cz. The main effects of outcome valence (F (1, 17) = 29.4, p < 0.001, ) and outcome magnitude were significant (F (1, 17) = 47.9, p < 0.001, ); the P3 was larger following wins (18.2 ± 5.1 μV) than following losses (15.9 ± 5.7 μV) (see also Hajcak, Holroyd, Moser, & Simons, 2005) and was larger following large outcomes (19.4 ± 5.8 μV) than following small outcomes (14.6 ± 3.9 μV) (see also Yeung & Sanfey, 2004). Most notably, the main effect of subsequent strategy was significant (F (1, 17) = 10.1, p < 0.01, ); the P3 associated with subsequent strategy-switching (17.5 ± 5.4 μV) was larger than that associated with subsequent strategy-staying (16.6 ± 5.6 μV). The outcome magnitude × subsequent strategy interaction was significant (F (1, 17) = 5.15, p < 0.05, ). Simple effect analysis indicated that the P3 was larger in the strategy-switching condition than in the strategy-staying condition when the outcome magnitude was large (20.2 ± 5.6 μV vs. 18.6 ± 6.0 μV; F (1, 17) = 14.3, p < 0.01), but this effect failed to reach significance when the outcome magnitude was small (14.8 ± 3.9 μV vs.14.4 ± 4.2 μV; p > 0.05).
ERP Results: Temporospatial PCA Factors
Five factor combinations that consisted of the first five temporal factors and the first spatial factor in each of the five temporal factors were recognized to closely correspond to the ERP components P2, FRN, P3a, P3b, and slow wave (SW) in terms of time course and scalp distribution (see Table 1). Thereafter, the waveforms for the five factor combinations were reconstructed back into voltage space. These PCA factor combinations were statistically analyzed as the mean amplitudes within different time windows (160 – 200 ms for PCA-P2, 240 – 300 ms for PCA-FRN, 340 – 400 ms for PCA-P3a, 430 – 530 ms for PCA-P3b, and 800 – 1000 ms for PCA-SW).
Table 1.
Temporospatial PCA factors selected for data analysis.
| PCA factor a | associated ERP component | variance explained (%) | peak latency (ms) | peak channel |
|---|---|---|---|---|
| TF1SF1 | P3b | 25.8 | 480 | CPz |
| TF2SF1 | FN | 17.3 | 268 | FCz |
| TF3SF1 | P3a | 8.69 | 372 | FCz |
| TF4SF1 | SW | 6.79 | 912 | FCz |
| TF5SF1 | P2 | 2.75 | 180 | FCz |
TF = temporal factor, SF = spatial factor.
The data were derived from all electrodes, but only the electrodes at which the components reached their peak values were entered into analysis. A repeated measures 2 (outcome valence) × 2 (outcome magnitude) × 2 (subsequent strategy) ANOVA was performed on each PCA factor (see Table 2 for a summary of the statistical results).
Table 2.
Summarization of the statistical results.
| factors | behavior
|
average ERP
|
PCA-derived ERP
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| RAR | SR | P3 | FRN | P3b | FRN | P3a | SW | P2 | |
| A | * | ** | *** | *** | *** | *** | *** | ** | |
| B | ** | *** | *** | *** | ** | ||||
| C | × | × | ** | ** | |||||
| A×B | ** | *** | * | ||||||
| A×C | × | × | * | * | |||||
| B×C | × | × | |||||||
| A×B×C | × | × | |||||||
A: outcome magnitude (small vs. large); B: outcome valence (loss vs. win); C: subsequent strategy (stay vs. switch). RAR = risk-avoidant ratio; SR = switch ratio. “×”: no definition. Asterisks indicate significant effects (*p < 0.05, **p < 0.01, ***p < 0.001).
PCA factor corresponding to P3b component (TF1SF1)
The amplitude of the PCA-P3b was most prominent at the centro-parietal area and reached its maximum at CPz (see Fig. 3). The main effect of outcome magnitude was significant (F (1, 17) = 43.4, p < 0.001, ); the PCA-P3b was larger following large outcomes (11.2 ± 5.4 μV) than following small outcomes (7.93 ± 3.7 μV).
Figure 3. The illustration of the main effects of the three major factors on grand-mean PCA yielding factors, as well as the corresponding scalp topographies.
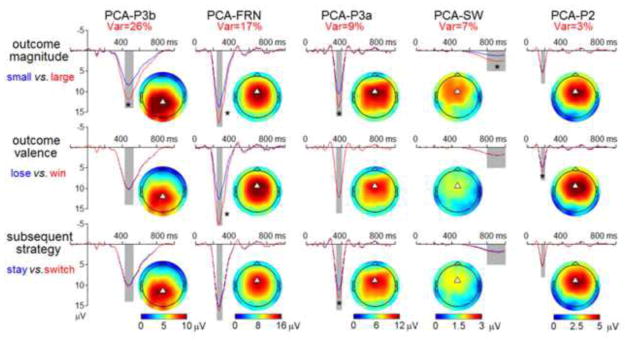
The waveforms of the PCA components were derived from FCz, except the PCA-P3b, which was derived from CPz (as indexed by the white triangles). Asterisks indicate significant effects (* p < 0.05).
PCA factor corresponding to FRN component (TF2SF1)
The amplitude of the FRN was most prominent at the fronto-central area and reached its minimum (negative peak) at FCz (see Fig. 3). The main effects of outcome valence (F (1, 17) = 58.7, p < 0.001, ) and outcome magnitude (F (1, 17) = 22.1, p < 0.001, ) were significant; the PCA-FRN was more negative-going following losses (11.7 ± 5.1 μV) than following wins (17.4 ± 5.7 μV) and was more negative-going following small outcomes (12.8 ± 4.7 μV) than large outcomes (16.3 ± 6.8 μV). The outcome valence × outcome magnitude interaction was significant (F (1, 17) = 5.87, p < 0.05, ). Simple effect analysis indicated that the effect of outcome magnitude was stronger following wins (F (1, 17) = 27.3, p < 0.001) than following losses (F (1, 17) = 11.7, p < 0.01). In sum, the results of the analysis on the conventional FRN and the PCA-FRN reveal the same pattern.
PCA factor corresponding to P3a component (TF3SF1)
The amplitude of the PCA-P3a was most prominent at the fronto-central area and reached its maximum at FCz (see Fig. 3). The main effect of outcome magnitude was significant (F (1, 17) = 44.5, p < 0.001, ); the PCA-P3a was larger following large outcomes (12.4 ± 4.3 μV) than following small outcomes (9.52 ± 3.2 μV). The main effect of subsequent strategy was also significant (F (1, 17) = 13.8, p < 0.01, ); the PCA-P3a associated with subsequent strategy-switching (11.6 ± 4.0 μV) was larger than that associated with subsequent strategy-staying (10.3 ± 4.0 μV). The outcome magnitude × subsequent strategy interaction was significant (F (1, 17) = 5.21, p < 0.05, ). Simple effect analysis indicated that a larger PCA-P3a was linked to strategy-switching rather than strategy-staying when the outcome magnitude was large (13.0 ± 4.2 μV vs. 12.0 ± 4.4 μ; F (1, 17) = 16.3, p < 0.01).
PCA factor corresponding to SW component (TF4SF1)
The amplitude of the PCA-SW was most prominent at the fronto-central area and reached its maximum at FCz (see Fig. 3). The main effect of outcome magnitude was significant (F (1, 17) = 15.3, p = 0.001, ); the PCA-SW was larger following large outcomes (2.44 ± 2.6 μV) than following small outcomes (1.16 ± 1.9 μV).
PCA factor corresponding to P2 component (TF5SF1)
The amplitude of the PCA-P2 was most prominent at the fronto-central area and reached its maximum at FCz (see Fig. 3). The main effect of outcome valence was significant (F (1, 17) = 11.2, p < 0.01, ); the PCA-P2 was larger following wins (5.33 ± 2.9 μV) than following losses (4.31 ± 2.6 μV). The outcome magnitude × subsequent strategy interaction was significant (F (1, 17) = 5.02, p < 0.05, ). Simple effect analysis indicated that the effect of subsequent strategy was significant only when the outcome magnitude was large (F (1, 17) = 4.71, p < 0.05).
Comparison between Average ERPs and PCA Results
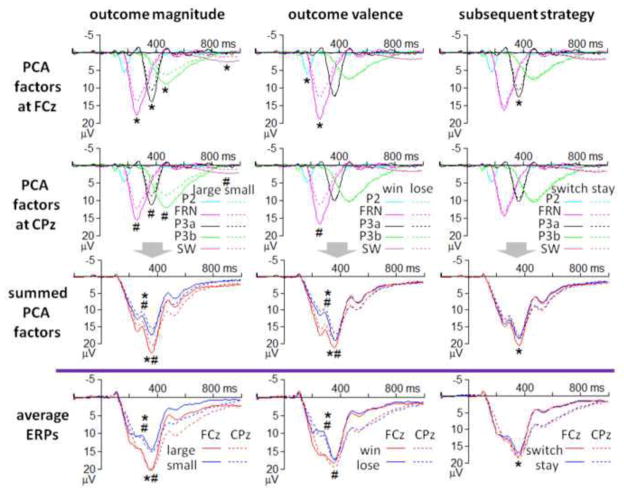
To compare the classical ERP components (see Fig. 2) and the PCA-yielded factor combinations (see Fig. 3), the waveforms of the five PCA factors were temporally and spatially summarized in Fig. 4. and Fig. 5, respectively.
Figure 4. Temporal summarization of the five PCA factors (upper panels) and the conventionally-averaged ERPs (the lowest panel; divided by the purple line).
* p < 0.05 at FCz; # p < 0.05 at CPz.
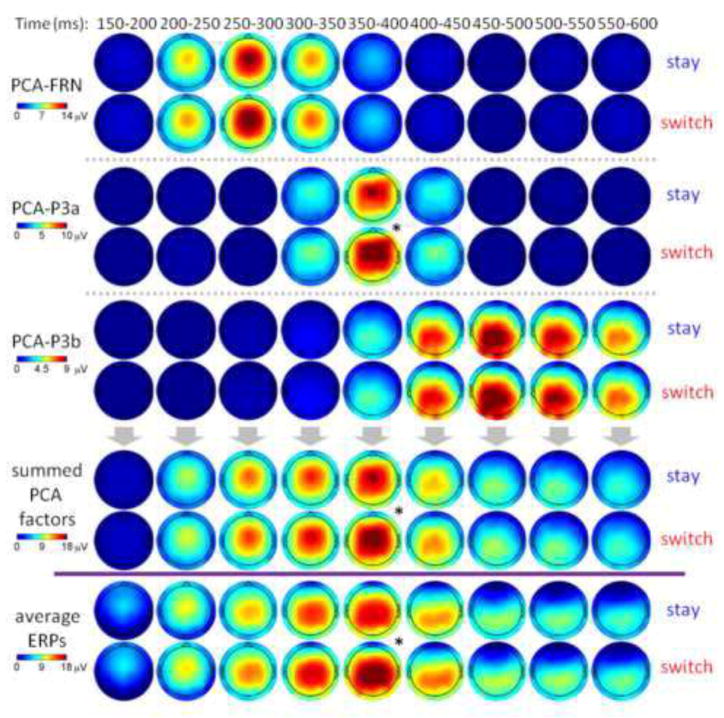
Figure 5. Spatial summarization of the PCA factors (upper panels) and the conventionally-averaged ERPs (the lowest panel; divided by the purple line) in response to the effect of subsequent strategy.
The first three PCA factors, which accounted for more than 50% variances in the ERP data, are summarized. Asterisks indicate significant main effects (*p < 0.05).
Superposition of PCA factors in time domain
According to Fig. 4, we suggest that (1) outcome valence affected the PCA-FRN, which corresponded to the effects on the conventional FRN and P3, but outcome valence did not affect PCA-P3a and PCA-P3b; (2) outcome magnitude affected the PCA-FRN, PCA-P3a, PCA-P3b, and PCA-SW, which corresponded to the effects on the conventional FRN and P3; (3) subsequent strategy selectively affected the PCA-P3a, which corresponded to the effect on the conventional P3.
Superposition of PCA factors in space domain
According to Fig. 5 (only the effect of subsequent strategy was illustrated), we suggest that (1) the topography of conventional ERP was similar with the summarization of the topographies of the PCA-FRN, PCA-P3a, and PCA-P3b; (2) the peak location of the conventional P3 was accounted for by the PCA-FRN, PCA-P3a, and PCA-P3b. Specifically, the peak location of the conventional P3 changed from FCz before 300 ms (the peak location of PCA-FRN and PCA-P3a) to Cz at approximately 300 – 400 ms, and finally to CPz after 400 ms (the peak location of PCA-P3b); (3) the conventional P3 variances associated with subsequent strategy was accounted for by the PCA-P3a variances at FCz.
sLORETA brain activity pattern at PCA-P3a latency
As reported above, the effect of subsequent strategy was evident on the PCA-P3a at the time interval 340 – 400 ms. The standardized low resolution brain electromagnetic tomography (sLORETA, the version updated on November 4th, 2008), downloaded from the official website (http://www.uzh.ch/keyinst/loreta.htm), was employed to explore the possible neuronal generators of this PCA-derived component (Pascual-Marqui, 1999, 2002; Pascual-Marqui, Michel, & Lehmann, 1994). Results suggested that (1) the highest level of activation at PCA-P3a latency was localized to the right frontopolar cortex (Brodmann area 10, MNI coordinates = [35, 60, −5]) (MNI: Montreal Neurological Institute), (2) the sensorimotor cortex showed a relatively weak activation in both hemispheres (mainly at Brodmann area 4: the primary motor cortex), and (3) subsequent strategy-switching elicited a higher activation than subsequent strategy-staying (see Fig. 6).
Figure 6. sLORETA images of the standardized current density maximum in response to the main effect of subsequent strategy.
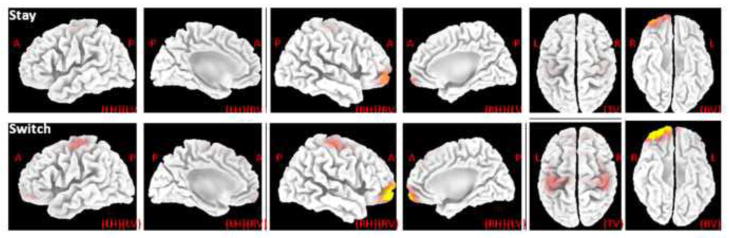
The results at the peak latency of PCA-P3a (372 ms) are illustrated. The color scale is equal in all the maps, of which the strongest activations are indexed in yellow. A = anterior; P = posterior; R = right; L = left; LH = left hemisphere; RH = right hemisphere; LV = left view; RV = right view; TV = top view; BV = bottom view.
DISCUSSION
Using a simple monetary gambling task, the current study investigated the potential relationship between monetary outcomes and subsequent risk decision-making strategies with behavioral and electrophysiological measures. Both the behavioral and ERP results indicated the influence of current outcomes on future risk decisions, but they revealed different aspects of this influence.
First, the behavioral data indicated that participants were more prone to choose the low-risk option after receiving monetary gains than losses (see also Gu, et al., 2010; Isen, et al., 1988; Masaki, Takeuchi, Gehring, Takasawa, & Yamazaki, 2006 for similar results), which supported the idea that the objective economic valence of an outcome modulates subsequent risk-taking behavior (Goyer, Woldorff, & Huettel, 2008; Loewenstein, Weber, Hsee, & Welch, 2001). According to previous research, monetary rewards elicited risk-avoidance by strengthening participants’ desire to maintain positive mood (Nygren, Isen, Taylor, & Dulin, 1996) and avoid anticipated regret (Zeelenberg & Beattie, 1997; Zeelenberg, Beattie, van der Pligt, & de Vries, 1996). We admit that it is unclear why the ERPs failed to capture the relation between outcome valence and subsequent behavior, and further studies which take individual parameters (e.g., personality) into account may address this issue more completely (Sitkin & Weingart, 1995; Smillie, Cooper, & Pickering, 2011).
Second, the ERP data elicited by outcome presentation indicated that a larger P3 component was more likely to be followed by strategy-switching on a subsequent trial, and this effect was more prominent when the current outcome magnitude was large (“99”). Further PCA discovered similar results in a fronto-central factor which approximates the classical P3a, thus providing more accurate temporospatial information of this effect. In most studies focusing on outcome evaluation, the P3 component is regarded as a measure of motivational significance, which refers to the potential impact of an outcome event on levels of motivation (Mangels, Butterfield, Lamb, Good, & Dweck, 2006; Nieuwenhuis, Aston-Jones, & Cohen, 2005; Wu & Zhou, 2009; Yeung & Sanfey, 2004). Since the probabilities of winning and losing were equal, the participants might have experienced that neither the high- nor the low-risk option consistently led to gains or losses during the task. Thus, in order to identify the optimal strategy indicated in the task instruction, occasionally switching to a different option would be necessary, regardless of participants’ risk propensity. However, since the participants only had knowledge about the outcome of the selected option while the outcome of the forgone option was not presented (i.e., the partial-feedback paradigm, see Hertwig & Erev, 2009 for details), stronger motivation was needed for strategy-switching to explore the unknown possibilities, indexed by increased P3 amplitudes (see also Polezzi, et al., 2010). This hypothesis helps to explain why the connection between the P3 component and subsequent decisions was stronger in the large outcome condition, because greater rewards and penalties are both related to enhanced motivational significance, and the P3 amplitude varies with outcome magnitude accordingly (Gu, et al., 2011; San Martin, 2012; Sato, et al., 2005; Yeung & Sanfey, 2004).
As an alternative, the P3a results might be interpreted in terms of attention allocation. The frontal P3a is traditionally associated with involuntary, transient allocation of attentional resources to novel stimuli or salient changes of stimuli (Knight, 1996; Polich & Comerchero, 2003). In this study, the PCA-P3a observation might indicate that when the actual outcome violated participants’ expectation, more attention was attracted and thus resulted in a larger chance of changing decision-making strategies. In harmony with this theory, San Martín et al. (2013) recently suggested that a larger P3a preceding behavioral adjustment indicated that additional cognitive resources were allocated to current outcomes. It is undetermined which theory explains our findings more appropriately and further investigation is needed on this issue.
The ERP source analysis based on the PCA results provided valuable information about the neural underpinnings of the influence of current outcomes on subsequent decisions. Most importantly, this analysis revealed significant activation in the right frontopolar cortex (BA 10) within the PCA-P3a time-window, indicating that this area was likely the main generator of the P3a waveform. Numerous studies have associated the frontal pole with attentional-, task-, and strategy-switching (for reviews, see Burgess, Dumontheil, & Gilbert, 2007; Koechlin & Hyafil, 2007). Remarkably, Daw et al. (2006) reported higher activations in the right frontopolar cortex when gamblers decided to switch to an alternative choice than when they continued to select more familiar options (see also Badre, Doll, Long, & Frank, 2012). A similar pattern was detected by Boorman et al. (2009) immediately before the switching actually occurred, indicating the importance of this region in implementing exploratory decisions on the forthcoming trial (see also Bunge & Wendelken, 2009). The current study extended these findings by providing evidence that the neural mechanism for future behavioral switch may be activated as early as the stage of outcome evaluation, especially when participants received motivationally-salient outcomes (e.g., outcomes with large magnitude) (San Martín, et al., 2013). This idea is supported by the discovery that frontal pole neurons in the primate brain encode information about stay or switch at the time of outcome presentation (Tsujimoto, Genovesio, & Wise, 2010; see also Wallis, 2010). We suggest that the frontopolar cortex might be the key of timely behavioral updating according to previous outcomes, which has important implications in effective behavioral adaptation to an uncertain environment (see also Bunge & Wendelken, 2009).
Moreover, the sensorimotor cortex also contributed to the generation of the PCA-P3a factor. Both actual and imaginary movements change cerebral electrical activity at the sensorimotor area (Wolpaw, Birbaumer, McFarland, Pfurtscheller, & Vaughan, 2002). According to the structure of the cortical motor homunculus, the light red area in Fig. 6 was approximately the anatomical divisions of hand and finger. Hence, the PCA-P3a source of sensorimotor cortex might be attributed to the brain’s imaginary finger movement for the next trial. We suggest this result supports our assumption that the evaluation of current outcomes involves considerations for future decision-making. In our opinion, the process of tracking the relative advantage of behavioral switching, in which the frontopolar cortex plays a key role, may be triggered when an outcome event is motivationally significant. Thereafter, if the motive to explore a foregone alternative overrides the tendency to maintain the current strategy (Daw, et al., 2006), the sensorimotor cortex would be directed to prepare for a decision-making “switch.” Further brain-imaging work should test this hypothesis to account for the relatively-limited accuracy of EEG source localization (Zhukov, Weinstein, & Johnson, 2000).
Finally, a few issues need to be addressed. Cohen & Ranganath (2007) reported that larger FRN magnitude indicated that participants would be more likely to choose a different option on the subsequent trial. However, in the current study, the ANOVA results reveal that only the PCA-P3a could be attributed to the consistent trend of amplitude variation associated with switch and stay strategies. In contrast, the PCA-FRN, as well as other PCA factors, failed to change consistently according to subsequent strategies (see Fig. 7). Very recently, San Martín et al. (2013) also reported that the frontal P3a elicited by outcome presentation, but not the FRN, predicted behavioral adjustment on subsequent trials. In our opinion, the difference in task demands might account for the discrepancy in ERP results between these studies. Specifically, Cohen & Ranganath (2007) used a strategic economic game in which the computer tried to find a pattern from the participant’s selections in the previous six trials. If the computer successfully discovered a strategy, the participant would be beaten in the next trial. Therefore, in that game, participants were forced not to use any continuous strategy. In contrast, participants were encouraged to discover the optimal strategy in both the current study and that of San Martín et al. (2013). This interpretation indicates that the frontal P3a is selectively associated with the built-up of option-outcome association, and further studies should verdict this issue.
Fig. 7. The influence of subsequent strategy on the PCA-P3b, PCA-FN, and PCA-P3a for each participant.
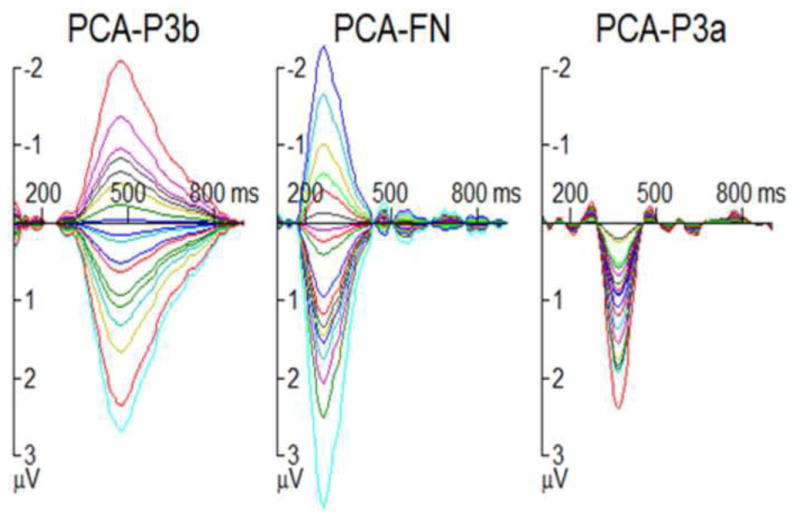
The difference waves between “switch” and “stay” conditions (switch waveform - stay waveform) are illustrated. Results from each of the 18 participants are displayed in distinct colors.
Moreover, the finding that the P3 or PCA-P3a component was associated with future decision behavior should be interpreted with caution, since the specific biophysical mechanisms that underlie the production of a given ERP components are always determined using some amount of inference (Luck, 2005). The correspondence between the five PCA factors and classic ERP components in this study was determined using an empirically-driven method and the interpretation of the current results requires caution (Foti, et al., 2011; Picton, et al., 2000; see supplementary materials for details). In addition, it is well-established that the ERP does not reflect the event-related brain dynamics comprehensively, since the traditional stimulus-locked ERP averaging technique filters out the contributions of those induced neural activities that are not phase-locked to the time-locked events by means of phase cancellation. Time-frequency analysis of event-related oscillation (ERO) before epoch averaging may provide complementary information on neural processing dynamics that is distinct from average ERP measurements (Makeig, Debener, Onton, & Delorme, 2004). By employing both the ERP and ERO measurements, it may be possible to obtain more information about the electrophysiological index in decision-making scenarios.
To sum up, the current study revealed that both the valence and magnitude of current outcomes were associated with subsequent risk decisions. Outcome valence directly modulated future decision-making such that participants’ risk-avoidance tendency was stronger after wins than losses, which may be described as emotionally-guided actions along an “approach-avoidance” dimension (Hastie, 2001). Meanwhile, the effect of outcome magnitude was observed in the relation between P3 amplitudes and subsequent strategy-switching. This relation became stronger when outcome magnitude was large, possibly due to enhanced motivational significance associated with large outcomes. Furthermore, the results of ERP source analysis indicated that a neural circuit including the frontopolar and sensorimotor cortices may be implicated in strategy-switching. In short, experienced outcome properties affected future decisions in the form of behavioral and electrophysiological representations based on different mechanisms, indicating that participants applied multiple strategies in the same scenario to minimize risk and cope with uncertainty (Sitkin & Weingart, 1995; Wunderlich, et al., 2011).
Supplementary Material
Participants chose between high- and low-risk options in a gambling task.
Larger ERP P3 was linked to the decision to switch between options.
The PCA revealed that the effect of P3 was accounted for by a fronto-central P3a.
The frontopolar cortex and sensorimotor cortex were involved in strategy-switching.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30930031, 91132704), National Key Technologies R&D Program (2009BAI77B01), the Ministry of Science and Technology (973 Program, 2011CB711000), the Global Research Initiative Program, United States National Institute of Health (Grants 1R01TW007897, P50DA05312, and 5 T32 AG 242-18), the National Center for Research Resources (UL1RR033173), the National Center for Advancing Translational Sciences (UL1TR000117), and Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (Y2CQ013005). The authors thank Blake LeMaster for help with grammar editing on the earlier version of this manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksandrov IO, Maksimova NE. P300 and psychophysiological analysis of the structure of behavior. Electroencephalography and Clinical Neurophysiology. 1985;61:548–558. doi: 10.1016/0013-4694(85)90974-5. [DOI] [PubMed] [Google Scholar]
- Badre D, Doll BB, Long NM, Frank MJ. Rostrolateral prefrontal cortex and individual differences in uncertainty-driven exploration. Neuron. 2012;73:595–607. doi: 10.1016/j.neuron.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends in Cognitive Sciences. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27:1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C. Comparing the bird in the hand with the ones in the bush. Neuron. 2009;62:609–611. doi: 10.1016/j.neuron.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Carretie L, Tapia M, Mercado F, Albert J, Lopez-Martin S, de la Serna JM. Voltage-based versus factor score-based source localization analyses of electrophysiological brain activity: a comparison. Brain Topography. 2004;17:109–115. doi: 10.1007/s10548-004-1008-1. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayoun BA. Towards a four-stage model of mindfulness-based cognitive behaviour therapy for children: A preliminary trial with generalised anxiety disorder. Australian Journal of Psychology. 2006;58:121–121. [Google Scholar]
- Chen P, Qiu J, Li H, Zhang Q. Spatiotemporal cortical activation underlying dilemma decision-making: an event-related potential study. Biological Psychology. 2009;82:111–115. doi: 10.1016/j.biopsycho.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. Journal of Neuroscience. 2007;27:371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Wilmes K, van de Vijver I. Cortical electrophysiological network dynamics of feedback learning. Trends in Cognitive Sciences. 2011;15:558–566. doi: 10.1016/j.tics.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Makeig S, Delorme A, Engel AK. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cognitive Brain Research. 2005;22:309–321. doi: 10.1016/j.cogbrainres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010a;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology. 2010b;47:170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principal components analysis of ERP data. In: Handy T, editor. Event Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. pp. 189–208. [Google Scholar]
- Donchin E, Heffley EF. The independence of the P300 and the CNV reviewed: a reply to Wastell. Biological Psychology. 1979;9:177–188. doi: 10.1016/0301-0511(79)90038-3. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goyer JP, Woldorff MG, Huettel SA. Rapid electrophysiological brain responses are influenced by both valence and magnitude of monetary rewards. Journal of Cognitive Neuroscience. 2008;20:2058–2069. doi: 10.1162/jocn.2008.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Ge Y, Jiang Y, Luo YJ. Anxiety and outcome evaluation: The good, the bad and the ambiguous. Biological Psychology. 2010;85:200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Lei Z, Broster L, Wu T, Jiang Y, Luo YJ. Beyond valence and magnitude: A flexible evaluative coding system in the brain. Neuropsychologia. 2011;49:3891–3897. doi: 10.1016/j.neuropsychologia.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hastie R. Problems for judgment and decision making. Annual Review of Psychology. 2001;52:653–683. doi: 10.1146/annurev.psych.52.1.653. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Barron G, Weber EU, Erev I. Decisions from experience and the effect of rare events in risky choice. Psychological Science. 2004;15:534–539. doi: 10.1111/j.0956-7976.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Erev I. The description-experience gap in risky choice. Trends in Cognitive Sciences. 2009;13:517–523. doi: 10.1016/j.tics.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research. 2006;1105:93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44:913–917. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Isen AM, Nygen TE, Ashby FG. Influence of positive affect on the subjective utility of gains and losses: It is just not worth the risk. Journal of Personality and Social Psychology. 1988;55:710–717. doi: 10.1037//0022-3514.55.5.710. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Donchin E. Sequential expectancies and decision making in a changing environment: an electrophysiological approach. Psychophysiology. 1982;19:183–200. doi: 10.1111/j.1469-8986.1982.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Weiller E, Andersen H, Baldwin D. Qualitative changes in symptomatology before and after treatment to remission in generalised anxiety disorder and major depression. International Journal of Neuropsychopharmacology. 2006;9:S232–S232. [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cerebral Cortex. 2011;21:95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Long Y, Jiang X, Zhou X. To believe or not to believe: trust choice modulates brain responses in outcome evaluation. Neuroscience. 2012;200:50–58. doi: 10.1016/j.neuroscience.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Macnamara A, Ochsner KN, Hajcak G. Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive Affective Neuroscience. 2011;6:348–358. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Butterfield B, Lamb J, Good C, Dweck CS. Why do beliefs about intelligence influence learning success? A social cognitive neuroscience model. Social Cognitive Affective Neuroscience. 2006;1:75–86. doi: 10.1093/scan/nsl013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H, Takeuchi S, Gehring WJ, Takasawa N, Yamazaki K. Affective-motivational influences on feedback-related ERPs in a gambling task. Brain Research. 2006;1105:110–121. doi: 10.1016/j.brainres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Saez A, Lau B, Salzman CD. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71:1127–1140. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011;218:419–428. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cerebral Cortex. 2004;14:741–747. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Nygren TE, Isen AM, Taylor PJ, Dulin J. The influence of positive affect on the decision rule in risk situations: Focus on outcome (and especially avoidance of loss) rather than probability. Organizational Behavior and Human Decision Processes. 1996;66:59–72. [Google Scholar]
- Ohira H, Ichikawa N, Nomura M, Isowa T, Kimura K, Kanayama N, Fukuyama S, Shinoda J, Yamada J. Brain and autonomic association accompanying stochastic decision-making. Neuroimage. 2010;49:1024–1037. doi: 10.1016/j.neuroimage.2009.07.060. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Review of methods for solving the EEG inverse problem. International Journal of Bioelectromagnetism. 1999;1:75–86. [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods & Findings in Experimental & Clinical Pharmacology. 2002;24D:5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Lotz DT, Halgren E, Sejnowski TJ, Poizner H. Choice modulates the neural dynamics of prediction error processing during rewarded learning. Neuroimage. 2011;54:1385–1394. doi: 10.1016/j.neuroimage.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan DM, Alexopoulos J, Bauer H, Sailer U. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology. 2010;48:656–664. doi: 10.1111/j.1469-8986.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polezzi D, Lotto L, Daum I, Sartori G, Rumiati R. Predicting outcome of decisions in the brain. Behavioural Brain Research. 2008;187:116–122. doi: 10.1016/j.bbr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Polezzi D, Sartori G, Rumiati R, Vidotto G, Daum I. Brain correlates of risky decision-making. Neuroimage. 2010;49:1886–1894. doi: 10.1016/j.neuroimage.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Polich J, Comerchero MD. P3a from visual stimuli: typicality, task, and topography. Brain Topography. 2003;15:141–152. doi: 10.1023/a:1022637732495. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Houlihan ME, Robinson JH. P300 and response selection: a new look using independent-components analysis. Brain Topography. 1999;12:31–37. doi: 10.1023/a:1022277506517. [DOI] [PubMed] [Google Scholar]
- Richards JE. Recovering dipole sources from scalp-recorded event-related-potentials using component analysis: principal component analysis and independent component analysis. International Journal of Psychophysiology. 2004;54:201–220. doi: 10.1016/j.ijpsycho.2004.03.009. [DOI] [PubMed] [Google Scholar]
- San Martín R, Appelbaum LG, Pearson JM, Huettel SA, Woldorff MG. Rapid brain responses independently predict gain-maximization and loss-minimization during economic decision-making. Journal of Neuroscience. 2013;33:7011–7019. doi: 10.1523/JNEUROSCI.4242-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin R. Event-related potential studies of outcome processing and feedback-guided learning. Frontiers in Human Neuroscience. 2012;6:304. doi: 10.3389/fnhum.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, Kuboki TL. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16:407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Schuermann B, Endrass T, Kathmann N. Neural correlates of feedback processing in decision-making under risk. Frontiers in Human Neuroscience. 2012;6:204. doi: 10.3389/fnhum.2012.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sitkin SB, Weingart LR. Determinants of risky decision-making behavior: A test of the mediating role of risk perceptions and propensity. The Academy of Management Journal. 1995;38:1573–1592. [Google Scholar]
- Smillie LD, Cooper AJ, Pickering AD. Individual differences in reward-prediction-error: extraversion and feedback-related negativity. Social Cognitive Affective Neuroscience. 2011;6:646–652. doi: 10.1093/scan/nsq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Thaler RH, Johnson EJ. Gambling with the house money and trying to break even: The effects of prior outcomes on risky choice. Management Science. 1990;30:643–660. [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nature Neuroscience. 2010;13:120–126. doi: 10.1038/nn.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helden J, Boksem MA, Blom JH. The importance of failure: feedback-related negativity predicts motor learning efficiency. Cerebral Cortex. 2010;20:1596–1603. doi: 10.1093/cercor/bhp224. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Polar exploration. Nature Neuroscience. 2010;13:7–8. doi: 10.1038/nn0110-7. [DOI] [PubMed] [Google Scholar]
- Wallis JD. The dynamics of learning and behavioral flexibility. Neuron. 2011;71:959–961. doi: 10.1016/j.neuron.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clinical Neurophysiology. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou XL. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Symmonds M, Bossaerts P, Dolan RJ. Hedging your bets by learning reward correlations in the human brain. Neuron. 2011;71:1141–1152. doi: 10.1016/j.neuron.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang QL. Electrophysiological correlates of decision-making in high-risk versus low-risk conditions of a gambling game. Psychophysiology. 2011;48:1456–1461. doi: 10.1111/j.1469-8986.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhou W, Zhou X. Rapid processing of both reward probability and reward uncertainty in the human anterior cingulate cortex. Plos One. 2011;6:e29633. doi: 10.1371/journal.pone.0029633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeelenberg M, Beattie J. Consequences of regret aversion 2: Additional evidence for effects of feedback on decision making. Organizational Behavior and Human Decision Processes. 1997;72:63–78. [Google Scholar]
- Zeelenberg M, Beattie J, van der Pligt J, de Vries NK. Consequences of regret aversion: Effects of Expected Feedback on risky decision making. Organizational Behavior and Human Decision Processes. 1996;65:148–158. [Google Scholar]
- Zhukov L, Weinstein D, Johnson C. Independent component analysis for EEG source localization. IEEE Engineering in Medicine and Biology Magazine. 2000;19:87–96. doi: 10.1109/51.844386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.