Abstract
Tumor-associated macrophages (TAMs) provide a significant contribution to tumor growth and metastasis. We demonstrated the existence of two main TAM subsets, differing in activation state and localization. Of these, M2-like TAMs reside in hypoxic regions of the tumor mass and can be used as targets for hypoxia tracers. This said, hypoxia does not regulate the differentiation of TAMs but finely tunes the activity of the M2-like population.
Keywords: tumor-associated macrophage, M1, CD206, hypoxia, nanobody, PHD2, M2, molecular imaging
Introduction
The development of anticancer therapeutics over the past decades has been approached from a cancer cell-centric view, as the aim was to hit malignant cells while leaving untransformed cells largely unharmed. The features that discriminate cancer cells from most normal cells are an enhanced proliferation rate and the accumulation of mutated oncogenes and tumor suppressor genes. Hence, many of anticancer agents available to date were selected based on their ability to eradicate rapidly dividing cells and/or interfere with the function of oncogenes. However, the complexity of cancer reaches far beyond the biology of malignant cells alone, and includes several pathophysiological processes that have only recently been appreciated as important contributors to tumor progression. These processes, which might provide the targets for the next generation of anticancer therapeutics, include the influx of myeloid cells, in particular macrophages, within neoplastic lesions, and the hypoxic nature of the tumor microenvironment. Here, we will elaborate on the interplay between these two phenomena and the consequences for tumor growth and metastasis.
Tumor-Associated Macrophages Encompass Distinct Subpopulations
The importance of tumor-infiltrating macrophages for disease progression is highlighted by a recent meta-analysis of the literature, demonstrating a significant correlation between the density of CD68+ macrophages and reduced overall survival in patients affected by most solid tumors.1 Besides operating as inflammatory cells that detect and destroy invading pathogens through their phagocytic and cytotoxic activity, macrophages are also important guardians of tissue homeostasis. This trophic, anti-inflammatory role of macrophages is hijacked by developing tumors, which benefit not only from the local production of epithelial growth factors and angiogenic mediators but also from the avoidance of overt immunological responses. Consequently, tumor-associated macrophages (TAMs) contribute to tumor progression and metastasis by stimulating the ability of malignant cells to invade surrounding tissues, migrate and enter the circulation, by promoting angiogenesis and by subverting antitumor T-cell responses.2 Such a diversity of tasks suggests the existence of specialized TAM subpopulations. Moreover, the tumor topography is very dynamic and heterogeneous due to regional differences in the availability of oxygen and growth factors, in the organization of the extracellular matrix and in metabolic functions.3 The fact that macrophages display a remarkable adaptability to the microenvironment lends further support to the notion that distinct TAM subsets exist within a single neoplastic lesion.
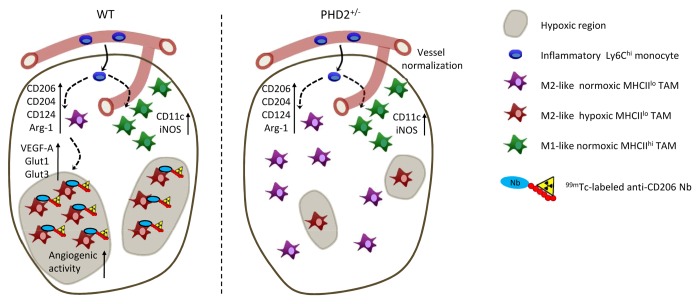
Employing several mouse tumor models, we found that neoplastic lesions are infiltrated with a heterogeneous population of myeloid cells comprising distinct immature and mature macrophage subsets.4 Based on the differential expression of MHC class II molecules, mature TAMs could be subdivided in 2 major subpopulations (Fig. 1). Interestingly, MHCIIlo TAMs resemble anti-inflammatory, alternatively activated M2 macrophages, as shown by elevated expression levels of mannose receptor, C type 1 (MRC1, also known as CD206), macrophage scavenger receptor 1 (MSR1, also known as CD204) and interleukin-4 receptor, α (IL4Rα, also known as CD124) and an increased arginase 1 enzymatic activity. These data were corroborated by an extensive gene expression analysis, confirming the M2-like nature of this TAM subset. Conversely, the genetic and surface marker expression profile of MHCIIhi TAMs are suggestive of an inflammatory M1-like phenotype. It could be hypothesized that MHCIIlo and MHCIIhi TAMs derive from different monocyte precursors. However, when we differentially labeled Ly6Chi vs. Ly6Clo blood monocytes, we found that the former differentiate into both TAM subsets. Hence, microenvironmental cues are responsible for skewing Ly6Chi monocyte differentiation into either MHCIIlo or MHCIIhi TAMs.
Figure 1. Heterogeneity of tumor-associated macrophages. Ly6Chi inflammatory or classical monocytes enter primary tumors and differentiate into MHCIIlo and MHCIIhi tumor-associated macrophages (TAMs). MHCIIlo and MHCIIhi TAMs express high levels of M2-associated (i.e., CD124, CD204, CD206, and arginase 1) or M1-associated (i.e., CD11c, iNOS) markers, respectively. Notably, MHCIIlo TAMs associate with hypoxic tumor regions, while MHCIIhi TAMs are located in close proximity of blood vessels. Consequently, 99mTc-labeled anti-CD206 nanobodies that target MHCIIlo TAMs can be used as hypoxia tracers. In Egln1-haplodeficient mice, tumor oxygenation is improved as a result of vessel normalization. Data obtained in this model demonstrate that hypoxia does not alter the abundance of M2-like or M1-like TAMs, but increases the expression of angiogenic and metabolic proteins, including VEGFA, GLUT1, and GLUT3 specifically in hypoxic MHCIIlo TAMs.
Hypoxia as Driver of the Tumor-Supporting Functions of TAMs
We investigated whether hypoxia could be such a cue. Regions of chronic or cycling hypoxia (0.1–2% O2) are common in most solid tumors as a result of defective vascularization and intense metabolic activity.5 Upon O2 shortage, several intracellular prolyl hydroxylases (including those encoded by Egln1, Egln2, and Egln3) become inactive, which prevents the hydroxylation and degradation of the α chain of the transcription factor hypoxia-inducible factor 1 (HIF-1), a central mediator of hypoxic adaptation. Hypoxia promotes not only the invasiveness of malignant cells, but also chemo- and radioresistance, and is therefore very relevant from a clinical perspective.
Macrophages are well known to infiltrate hypoxic tumor regions,6 but to which extent hypoxia influences the TAM phenotype was not clear until recently. By staining hypoxic tumor regions with pimonidazole, we demonstrated that most MHCIIhi TAMs are located outside of hypoxic microenvironments, while MHCIIlo TAMs exhibit a predilection for these areas (Fig. 1).4,8 Interestingly, increasing the oxygenation of neoplastic lesions by vessel normalization in Egln1 haplodeficient mice7 does not alter the abundance, nor the M1/M2 distribution of TAM subsets.8 Rather, hypoxia regulates a subset of proteins involved in metabolism and angiogenesis, including vascular endothelial growth factor A (VEGFA), solute carrier family 2, member 1 (SCL2A1, also known as GLUT1), SCL2A3 (also known as GLUT3) and nitric oxide synthase 2, inducible (NOS2, also known as iNOS) specifically in MHCIIlo TAMs, thereby increasing their angiogenic functions.8 Hence, hypoxia is not the main driver of TAM differentiation, but rather fine tunes the biological activity of the M2-like subpopulation.
Applicability of These Findings
Hypoxia imaging is becoming increasingly important for the individualization of chemoradiotherapy,9 but hypoxia tracers need improvement. All hypoxia tracers available to date are nitroimidazoles, which become detectable below a specific threshold of partial O2 pressure (pO2) and thus only provide a discriminative binary (yes-or-no) signal. We considered the possibility of imaging TAMs that reside in hypoxic tumor regions, rather than hypoxia itself, (at least theoretically) providing quantitative data on local hypoxia. To this aim, we generated nanobodies (Nbs)-the smallest available antigen-binding fragments derived from Camelid heavy-chain-only antibodies (15 kDa)-against CD206, which is highly expressed by hypoxic MHCIIlo TAMs.10 We demonstrated that monovalent 99mTc-labeled anti-CD206 Nbs allow for the fast and specific detection of CD206+ cells within neoplastic lesions, as shown by pinhole SPECT/Micro-CT imaging.10 Importantly, bivalent anti-CD206 Nbs penetrate tumors less efficiently than their monovalent counterparts, but rapidly occupy all extratumoral binding sites. Hence, the administration of excess unlabeled bivalent anti-CD206 Nbs provides a novel strategy for eliminating extratumoral noise while preserving the detection of tumor-associated CD206+ TAMs. Altogether, 99mTc-labeled CD206-specific tracers were validated for the molecular imaging of CD206+ TAMs, representing a novel diagnostic (and perhaps therapeutic) approach targeting the tumor stroma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Hypoxia and tumor-associated macrophages: A deadly alliance in support of tumor progression. OncoImmunology 2013; 2:e27561; 10.4161/onci.27561
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27561
References
- 1.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 4.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res. 2010;70:10019–23. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 7.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–51. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1196. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Chitneni SK, Palmer GM, Zalutsky MR, Dewhirst MW. Molecular imaging of hypoxia. J Nucl Med. 2011;52:165–8. doi: 10.2967/jnumed.110.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72:4165–77. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]