Abstract
The intracellular aminopeptidases that process antigenic epitopes have been recently shown to be valid targets for eliciting cell-mediated antitumor immune responses. Here, we discuss the recent development of potent, low molecular weight inhibitors of these enzymes and how such compounds may constitute a promising tool for cancer immunotherapy.
Keywords: antigen, inhibitor, enzyme, peptide, presentation, cytotoxicity, immunotherapy, epitope
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells can elicit strong antitumor immune responses based on changes in the antigenicity or immunogenicity of (pre)malignant cells. In turn, cancer cells can evolve mechanisms that alter antigen presentation, thus evading immune responses and establishing neoplastic lesions.1 Tumor-associated antigenic peptides, like all antigenic peptides presented by MHC class I molecules, are generated by the proteolytic degradation of intracellular proteins via the ubiquitin-proteasome pathway. Generally, the last step of this proteolytic cascade is the trimming of the N-terminal extensions found on antigenic peptide precursors inside the endoplasmic reticulum, which is catalyzed by aminopeptidases such as endoplasmic reticulum aminopeptidase 1 (ERAP1) and ERAP2. Throughout the last decade, an intense wave of investigation has highlighted the importance of these enzymes in both the generation of several antigenic peptides and the destruction of others, effectively affecting immunodominance.2 Furthermore, the lack of ERAP1 activity was found to be sufficient to elicit both classical and non-classical MHC-related immune responses.3
The analysis of ERAP1 and ERAP2 expression by malignant cells has demonstrated that the levels of these enzymes undergo significant changes that may correlate with the ability of cancer cells to evade immune responses.4,5 Interestingly, these changes are not restricted to a “simple” downregulation but include various imbalances in expression levels that affect the antigenic peptide repertoire.5 This strategy may be highly efficient for cancer cells to escape immunosurveillance, since potentially detrimental antigens can be eliminated while a near-to-normal antigen presentation can be preserved to avoid recognition by NK cells.
It has recently been investigated whether ERAP1 might constitute a valid target for cancer immunotherapy.6,7 Two distinct research groups demonstrated that the genetic downregulation of ERAP1 in cancer cells is sufficient to elicit potent anticancer immune responses in rodent models, block disease development and improve the short-term survival of tumor-bearing animals. Interestingly, the cytotoxic responses documented in these two studies originated from different types of immune effectors. In the first study, ERAP1 downregulation stimulated the cytotoxic activity of NK cells. Most likely, this reflected a decline in the levels of MHC class I molecules on the surface of cancer cells, in turn resulting from the defective production of antigenic peptides.6 Conversely, in the second study, the lack of ERAP1 elicited a CTL response specific for a cryptic tumor-associated antigen that was normally destroyed by ERAP1.7 These findings highlight both the importance and the multifaceted role of ERAP1 in antitumor immunity.
Although these studies have established the potential value for manipulating antigen processing for cancer immunotherapy, the tools for the pharmacological control of ERAP1 or ERAP2 activity have been subpar. Indeed, although the compound leucinethiol, a modest and non-selective inhibitor of aminopeptidases, successfully mimicked some of the effects of ERAP1 downregulation in cancer cells, no potent ERAP1-specific inhibitors have been described until recently. Much to our dismay, almost every available aminopeptidase inhibitor tested was a poor inhibitor of ERAP1. This may relate to the unique structural properties of this enzyme: ERAP1 exists in at least two highly distinct conformational states, one of which has a deformed specificity pocket at the active site, making targeted inhibition difficult.8
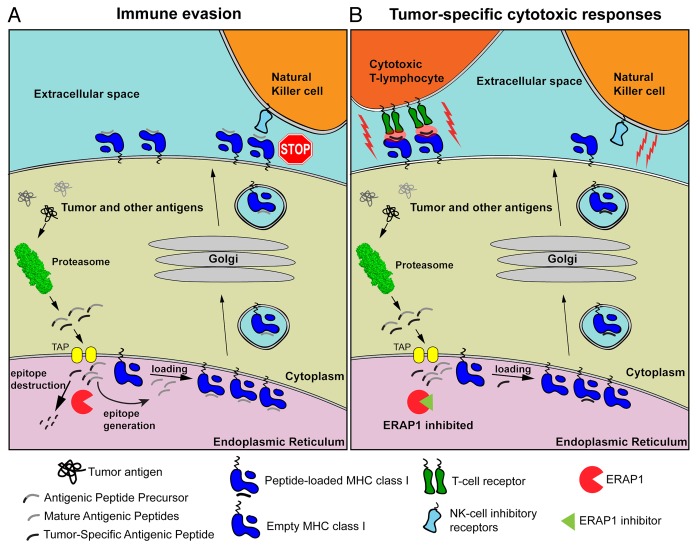
To develop potent ERAP1 inhibitors we decided to go back to the drawing board armed with information from several years of structural and biochemical analysis of the enzyme.8 Using a structure-guided rational design approach and focusing on the Zn-containing active site of the enzyme, we combined known and novel Zn-binding groups with side-chains customized to occupy the specificity pockets of ERAP1. Some of the first-generation inhibitors designed by this approach – pseudopeptides that contain a phosphinic group that acts as a transition state-analog – has already yielded highly encouraging results. In our first proof-of-principle study, one of those compounds, DG013A, was found to be a potent (nM range) inhibitor of ERAP1 as well as ERAP2, to enhance antigen presentation in HeLa cells and to elicit potent CTL responses against murine colon carcinoma cells.9 The effectiveness of this compound in vitro and ex vivo suggests that we now have an important tool to manipulate antigen processing, effectively reprogramming cancer cells to reveal themselves to the immune system (Fig. 1).
Figure 1. Mechanisms by which the inhibition of ERAP might activate cell-mediated antitumor immune responses. (A) Tumor-associated as well as normal intracellular proteins are catabolized by the proteasome, generating mature antigenic peptides as well as several antigenic peptide precursors. In the endoplasmic reticulum (ER) lumen, ER aminopeptidase 1 (ERAP1) can destroy some antigenic epitopes and at the same time process peptide precursors to generate mature antigenic epitopes for presentation by MHC class I molecules on the cell surface. Since some-tumor associated antigens are also destroyed by ERAP1, tumor-specific cytotoxic T lymphocyte (CTL) responses are evaded. In addition, peptide-loaded MHC class I molecules inhibit the cytotoxic activity of natural killer (NK) cells. (B) In the presence of ERAP1 inhibitors, tumor-associated antigens are spared from degradation and rather loaded onto MHC class I molecules. These complexes are normally translocated on the cell surface, where they can be recognized by CTLs and hence drive cytotoxic antitumor responses. Alternatively, the decline in the abundance of mature antigenic peptides due to reduced ERAP1 activity can inhibit the translocation of MHC class I molecules on the cell surface, or result to empty or misfolded MHC class I molecules that are unable to engage the inhibitory NK-cell receptors (i.e., Ly49), hence promoting NK cell-mediated cytotoxic responses.
Still, much more work is needed before this approach can be translated to the clinic. The best inhibitor currently available has a limited (about 3-fold) selectivity for ERAP2 over ERAP1. Although this relatively low selectivity is almost certainly due to our design strategy, which exploited structural features that are conserved in ERAP1 and ERAP2, the selective inhibition of either of these enzymes may be highly desirable because it may allow for targeting specific subsets of antigenic peptides. A different design approach has recently yielded inhibitors that show promise in terms of selectivity, but are not as potent as DG013A.10 It should be noted that the complete inhibition of antigen processing needs to be approached with caution since it could stimulate autoimmune responses against bystander cells. Furthermore, the ability of this strategy to enhance the long-term survival of tumor-bearing animals needs to be thoroughly explored. The effects of molecules that alter antigen processing on the immunopeptidome needs also to be carefully analyzed, since even the partial inhibition of ERAP1 or ERAP2 may result in the desired phenotype while minimizing side effects. Regardless of these concerns, modulators of antigen processing constitute novel tools that can guide the development of innovative approaches to cancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Stratikos E. Modulating antigen processing for cancer immunotherapy. OncoImmunology 2013; 2:e27568; 10.4161/onci.27568
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27568
References
- 1.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 2.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202–7. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagarajan NA, Shastri N. Immune surveillance for ERAAP dysfunction. Mol Immunol. 2013;55:120–2. doi: 10.1016/j.molimm.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamphausen E, Kellert C, Abbas T, Akkad N, Tenzer S, Pawelec G, Schild H, van Endert P, Seliger B. Distinct molecular mechanisms leading to deficient expression of ER-resident aminopeptidases in melanoma. Cancer Immunol Immunother. 2010;59:1273–84. doi: 10.1007/s00262-010-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruci D, Giacomini P, Nicotra MR, Forloni M, Fraioli R, Saveanu L, van Endert P, Natali PG. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J Cell Physiol. 2008;216:742–9. doi: 10.1002/jcp.21454. [DOI] [PubMed] [Google Scholar]
- 6.Cifaldi L, Lo Monaco E, Forloni M, Giorda E, Lorenzi S, Petrini S, Tremante E, Pende D, Locatelli F, Giacomini P, et al. Natural killer cells efficiently reject lymphoma silenced for the endoplasmic reticulum aminopeptidase associated with antigen processing. Cancer Res. 2011;71:1597–606. doi: 10.1158/0008-5472.CAN-10-3326. [DOI] [PubMed] [Google Scholar]
- 7.James E, Bailey I, Sugiyarto G, Elliott T. Induction of protective antitumor immunity through attenuation of ERAAP function. J Immunol. 2013;190:5839–46. doi: 10.4049/jimmunol.1300220. [DOI] [PubMed] [Google Scholar]
- 8.Stratikos E, Stern LJ. Antigenic peptide trimming by ER aminopeptidases--insights from structural studies. Mol Immunol. 2013;55:212–9. doi: 10.1016/j.molimm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zervoudi E, Saridakis E, Birtley JR, Seregin SS, Reeves E, Kokkala P, Aldhamen YA, Amalfitano A, Mavridis IM, James E, et al. Rationally designed inhibitor targeting antigen-trimming aminopeptidases enhances antigen presentation and cytotoxic T-cell responses. Proc Natl Acad Sci U S A. 2013;110:19890–5. doi: 10.1073/pnas.1309781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papakyriakou A, Zervoudi E, Theodorakis EA, Saveanu L, Stratikos E, Vourloumis D. Novel selective inhibitors of aminopeptidases that generate antigenic peptides. Bioorg Med Chem Lett. 2013;23:4832–6. doi: 10.1016/j.bmcl.2013.07.024. [DOI] [PubMed] [Google Scholar]