Abstract
Ursolic acid (UA), a pentacyclic triterpenoid widely found in medicinal herbs and fruits, has been reported to possess a wide range of beneficial properties including anti-hyperglycemia, anti-obesity, and anti-cancer. However, the molecular mechanisms underlying the action of UA remain largely unknown. Here we show that UA inhibits leucine-induced activation of the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway in C2C12 myotubes. The UA-mediated inhibition of mTORC1 is independent of Akt, tuberous sclerosis complex 1/2 (TSC1/2), and Ras homolog enriched in brain (Rheb), suggesting that UA negatively regulates mTORC1 signaling by targeting at a site downstream of these mTOR regulators. UA treatment had no effect on the interaction between mTOR and its activator Raptor or inhibitor Deptor, but suppressed the binding of RagB to Raptor and inhibited leucine-induced mTOR lysosomal localization. Taken together, our study identifies UA as a direct negative regulator of the mTORC1 signaling pathway and suggests a novel mechanism by which UA exerts its beneficial function.
Introduction
Ursolic acid (UA) is a triterpene compound derived from certain traditional medicinal plants [1]. UA has been found to possess a wide range of health benefits such as anti-obesity-related diseases [2], anti-hyperglycemia [3] and anti-cancer [4]. However, the molecular mechanisms underlying the beneficial effects of UA remain largely unknown. UA has been shown to ameliorate hepatic fibrosis by decreasing Akt phosphorylation and blocking NFκB nuclear localization in mouse hepatocytes [5]. Very recently, UA has also been found to inhibit the initiation and progression of prostate cancer by down-regulation of various pro-inflammatory mediators including NF-κB, STAT3, Akt and IKKα/β [6]. However, whether UA has additional targets remains to be determined.
The mechanistic target of rapamycin (mTOR) is a serine-threonine kinase that plays important roles in the regulation of protein and lipid synthesis, autophagy, as well as mitochondrial metabolism and biogenesis [7], [8]. mTOR interacts with several proteins to form two distinct multiprotein complexes named mTORC1 and mTORC2 [9]. While mTOR, Deptor, and mLST8 are present in both complexes, Raptor and Rictor are present in mTORC1 and mTORC2, respectively. Among these components, Raptor and Deptor positively and negatively regulate mTOR activation, respectively [10], [11]. mTORC1 signaling is regulated by a variety of upstream signals including growth factors, amino acids, energy status and oxygen concentration. Insulin regulates mTORC1 activity by activation of the class I PI3K/Akt signaling pathway [9]. Amino acids, on the other hand, have been shown to activate mTORC1 by signaling through the class 3 PI3K, hVps34 [12]. Insulin-stimulated activation of the PI3K/Akt signaling pathway leads to the inhibition of TSC1/TSC2, resulting in activation of small GTPase Rheb and subsequent activation of mTORC1 [13], [14], [15]. The mechanisms by which mTORC1 is activated by amino acids are less clear but a number of studies have shown that the binding of Raptor to the Rag GTPases, a family of four related small GTPases [16], is necessary for mTOR to localize onto the lysosome where mTOR is activated by its upstream activator Rheb [16], [17], .
While the mTORC1 signaling pathway has been well established as a key component to promote growth and accelerate cancer, it also plays an important role in regulating metabolism [20], [21], [22]. Skeletal muscle is the major site of glucose disposal and impairment in muscle glucose uptake contributes to type 2 diabetes [23]. Diet-induced over-activation of mTORC1 in muscle has been shown to inhibit insulin signaling via a feedback mechanism involving S6K-mediated serine phosphorylation of IRS-1, which reduces the ability of IRS1 to activate PI3K, leading to impaired glucose uptake and systemic insulin resistance [24]. Preventing over-activation of the mTORC1 signaling pathway in muscle could thus provide an effective treatment for obesity-induced insulin resistance and metabolic dysfunction.
In the current study, we investigated the effect of UA on mTOR signaling, we found that UA inhibits leucine-stimulated mTOR activation in C2C12 myotubes by inhibiting mTOR from targeting to lysosome, uncovering a novel mechanism underlying the negative regulation of the mTORC1 signaling pathway by UA, a small molecule that possess a wide range of beneficial properties including anti-hyperglycemia, anti-obesity, and anti-cancer.
Materials and Methods
Plasmids and Reagents
Plasmids encoding FLAG-tagged wild-type and Q99L mutant of RagB, the lentiviral vector pWPI, the lentiviral envelope plasmids pMD2.G and psPAX2 were obtained from Addgene. The plasmids encoding wild-type and S16H mutant of Rheb were gifts of Dr. Bo Xiao (Johns Hopkins University School of Medicine) and were described previously [25]. Leucine, UA, the FLAG M2 antibody, and Cocktail 3 were from Sigma Aldrich. The Anti-LAMP1 antibody was from ABCAM. Alexa Fluor 488 Donkey anti-Mouse IgG (H+L) antibody and Alexa Fluor 568 Donkey Anti-Rabbit IgG antibody were from Invitrogen. Protein A-Sepharose beads were from GE Healthcare Life Sciences. Clarity Western ECL substrate was from bio-rad. HRP labeled anti-mouse and anti-rabbit secondary antibodies were from Promega. Normal rabbit IgG and normal mouse IgG were from Millipore; the RagB antibody was from Proteintech. All other antibodies were from Cell Signaling Technology.
Cell Culture
C2C12 cells (American Type Culture Collection) were cultured in DMEM (ATCC 30-2002) supplemented with 10% newborn calf serum and 1% penicillin/streptomycin. TSC1/2−/− and wild-type mouse embryonic fibroblasts (MEFs) (generous gifts of Dr. Kunliang Guan, University of California at San Diego) were grown in DMEM (Gibco 11995-065) supplemented with 10% new born calf serum and 1% penicillin/streptomycin [26]. PDK1 Knock-out MEF cells, Deptor-suppressed C2C12 cells and their wild-type control cells were described previously [26].
Generation of C2C12 Cells Expressing RagBwt, RagBQ99L Mutant, Rhebwt, Or RhebS16H Mutant
cDNAs encoding wild-type RagB, Q99L mutant of RagB, wild-type Rheb, S16H mutant of Rheb were subcloned into the lentiviral vector pWPI. Lentiviral particles were produced by transfecting 293T cells with the pMD2.G plasmid, the psPAX2 plasmid, and the pWPI plasmid encoding green fluorescent protein (GFP) vector, RagBwt, RagBQ99L , Rhebwt, or RhebS16H. The medium was changed to growth medium eight hours post transfection and collected after 48 hours. The collected medium was filtered via a 0.45-µm filter and stored at −80°C until needed. For cell infection, C2C12 cells were incubated with the lentiviral medium in the presence of 8 µg/ml polybrene for 72 hours. Cells were visually scored for GFP intensity. Control cells were infected with lentiviruses encoding only GFP. No notable difference in proliferation and behave was detected between infected and uninfected parental cells.
Western Blot and Immunoprecipitation (IP)
Cells were rinsed once with ice-cold PBS and lysed in ice-cold lysis buffer (40 mM HEPES [pH 7.4], 2 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, and 0.3% CHAPS, 1 mM sodium orthovanadate, 10 ug/ml leupeptin, 10 ug/ml aprotinin, 1 mM phenylmethanesulfonyl fluoride, and phosphatase inhibitor mixtures). Cell lysates were centrifuged at 13,000 rpm for 10 minutes and supernatants were collected. For IP experiments, primary antibodies were added to the lysates and incubated with rotation overnight at 4°C. After addition of 30 µl of 50% slurry of the protein A-sepharose, the incubation was continued for 2 more hours. Immunoprecipitates were washed three times with ice-cold lysis buffer containing 150 mM NaCl. Immunoprecipitated proteins were detected by Western blot using 8%–12% SDS-PAGE as described [27].
In-cell Cross-linking Reaction
The in-cell cross-linking experiments were performed according to the procedure described previously [16]. In brief, DSP (Dithiobis[succinimidyl propionate]) was dissolved in DMSO (Dimethyl sulfoxide) to a final concentration of 250 mg/ml to make a 250X stock solution for the in-cell cross-linking assay. C2C12 cells transiently expressing FLAG-tagged RagB grown on 100-mm plates were serum starved and pretreated with or without 50 µM UA for 60 minutes, followed with or without 10 mM leucine for 60 minutes. At the end of the treatment, cells were rinsed once with cold PBS and incubated with 4 ml of PBS containing 1 mg/ml DSP for 7 minutes at room temperature. The cross-linking reaction was quenched by the addition of 1 M Tris buffer (pH 8.5) to a final concentration of 100 mM. After one-minute incubation, the cells were rinsed once with ice-cold PBS and lysed with lysis buffer containing 40 mM HEPES (pH 7.4), 2 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 0.3% CHAPS, 1 mM sodium orthovanadate, 10 ug/ml leupeptin, 10 ug/ml aprotinin, 1 mM phenylmethanesulfonyl fluoride, and phosphatase inhibitor mixtures. Immunoprecipitations using antibodies to Raptor or the FLAG tag were performed as described above.
Immunofluorescence Assays
Cells were grown on round cover slips in 12-well plates to 60–80% confluence. Cells were serum starved, treated with or without leucine and/or UA, rinsed with PBS, and fixed with 4% paraformaldehyde for 5 minutes at room temperature. The coverslips were rinsed twice with PBS and permealized with 0.2% Triton X-100 in PBS for 15 minutes. After rinsing two more times with PBS, the coverslips were blocked for one hour in blocking buffer (0.25% BSA in PBS) and incubated with primary antibody overnight at 4°C. After rinsing with blocking buffer, cells were incubated with secondary antibodies for one hour at room temperature. The coverslips were mounted on glass slides using glycerol and the fluorescence images were detected by a Zeiss LSM780 NLO fluorescence microscope with a 63 x objective.
Statistical Analysis
Quantification of protein phosphorylation and total levels was performed by analyzing Western blots using the NIH Image J program. Statistical analysis of the data was performed using the analysis of variance (ANOVA). Data were presented as mean ± S.E.M. Statistical significance was set at P values of *<0.05, **<0.01, and ***<0.001.
Results
UA Inhibits Leucine-stimulated mTORC1 Signaling via a PI3K/Akt-independent Mechanism
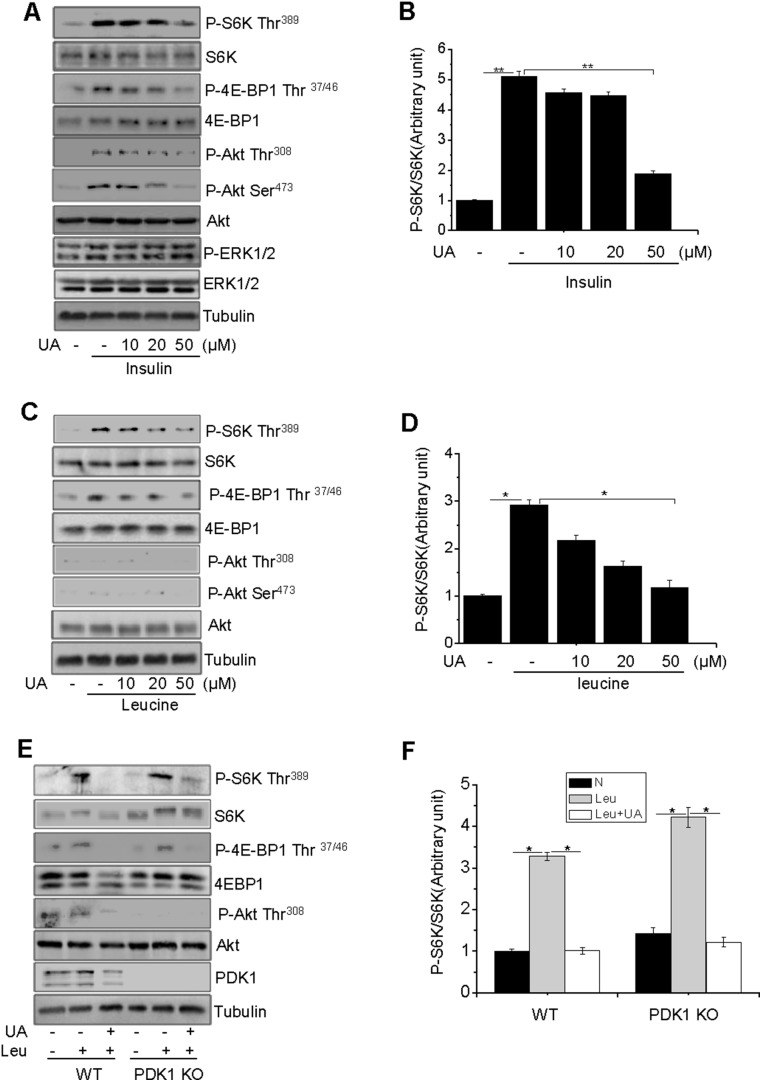
To determine the potential effect of UA on mTOR activity, differentiated C2C12 myotubes were treated with UA at different doses, followed with insulin or leucine treatment. Insulin treatment greatly stimulated the phosphorylation of S6K, 4EBP1, and Akt, and this phosphorylation was suppressed by UA treatment in a dose-dependent manner (Figs 1A and 1B). Insulin also stimulated the phosphorylation of ERK1/2, but this phosphorylation was not inhibited by UA (Fig. 1A). Unlike insulin, leucine stimulated the phosphorylation of S6K and 4EBP1, but had little effect on Akt phosphorylation (Figs. 1C and 1D), which is consistent with the finding that leucine had no effect on Akt phosphorylation in skeletal muscle cells [28], [29], [30], [31]. In addition, UA inhibited leucine-stimulated S6K phosphorylation in PDK1 knockout MEFs (Figs. 1 E and 1F). Pretreatment of C2C12 myotubes with UA had little effect on the protein levels of Raptor, Rictor, and mTOR (data not shown). Taken together, these results suggest that the UA is able to inhibit mTORC1 signaling via a novel mechanism.
Figure 1. UA inhibits leucine-stimulated mTOR activation via a PI3K–Akt-independent manner.
(A), Serum-starved C2C12 myotubes were pretreated with or without UA at the indicated concentrations for 60 min, followed with or without 10 nM insulin (Ins) for 10 min (A) or 10 mM leucine (Leu) for 60 min (C). The phosphorylation and protein levels of Akt, S6K, 4EBP1, and ERK1/2 were determined by Western blot with indicated antibodies. (B) and (D), S6K phosphorylation in (A) and (C) was semi-quantified using the NIH Image J Program and normalized to S6K protein levels. (E), Serum-starved wild type and PDK1-knockout (KO) MEFs were pretreated with or without 50 µM UA for 60 minutes, followed with or without 10 mM leucine (Leu) for 60 minutes. The phosphorylation and protein levels of the interesting signaling molecules were determined by Western blot using specific antibodies as indicated. (F), S6K phosphorylation in (E) was semi-quantified using the NIH Image J Program and normalized to S6K protein levels. Tubulin was used as a loading control for all experiments, differences between groups were examined for statistical significance using ANOVA. Data are presented as mean±S.E.M. from three independent experiments. *, P<0.05, **P<0.01, N, no addition.
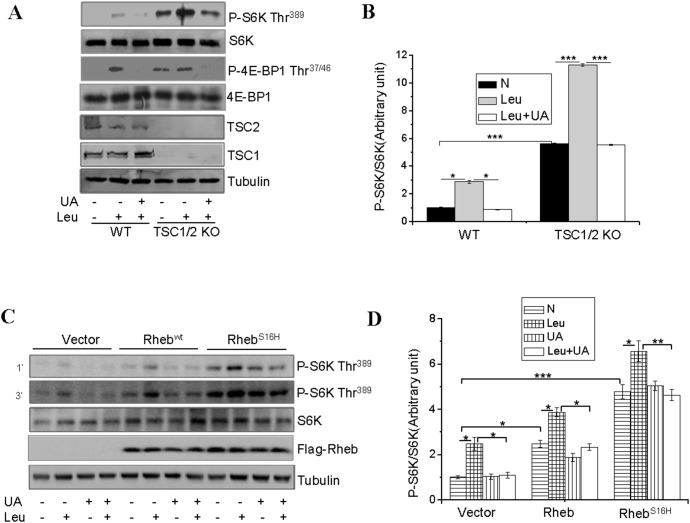
UA Inhibits Leucine-stimulated, mTOR Activation in a TSC1/2 and Rheb-independent Manner
Branched-chain amino acids such as leucine have been found to activate mTOR complex 1 (mTORC1) via inhibition of TSC1/TSC2 [32], [33]. To determine whether UA inhibits leucine-stimulated mTOR signaling by targeting TSC1/2, we examined whether knockout of TSC1/2 affects the ability of UA to suppress mTORC1 signaling. Basal and leucine-stimulated S6K and 4E-BP1 phosphorylation is increased in TSC1/2 knockout MEFs compared to control MEFs (Figs. 2A and 2B). UA treatment inhibited leucine-stimulated phosphorylation of S6K and 4E-BP1 in not only control MEFs but also TSC1/2-null MEFs (Figs. 2A and 2B), suggesting that UA inhibits leucine-stimulated mTOR signaling by acting at a site downstream of TSC1/2 in the mTORC1 signaling pathway.
Figure 2. UA inhibits leucine-stimulated mTOR activation in a TSC1/2 and Rheb-independent manner.
(A), Serum-starved TSC1/2+/+ (WT) and TSC1/2−/− (KO) MEF cells were pretreated with or without 50 µM for 60 min and then treated with or without 10 mM leucine (Leu) for 60 min. The phosphorylation and protein levels of S6K, 4E-BP1 and the protein levels of TSC1 and TSC2 in cell lysates were determined by Western blot with the indicated antibodies. (B), S6K phosphorylation shown in (A) was semi-quantified and normalized to S6K protein levels. (C), serum-starved C2C12 cells transiently expressing Rheb or RhebS16H were treated with or without 50 µM UA for 1 hour, followed with or without 10 mM leucine (Leu) for 60 min. The phosphorylation and protein levels of S6K, and the protein levels of FLAG-Rheb in cell lysates were determined by Western blot using indicated antibodies. (D), S6K phosphorylation in (C) was semi-quantified using the NIH Image J Program and normalized with S6K protein levels. Differences between groups were examined for statistical significance using ANOVA. Data are presented as mean ± S.E.M. from three independent experiments. *, P<0.05, **, P<0.01, and ***, p<0.001. N, no addition.
The small GTPase Rheb has been shown to be necessary for amino acid-dependent mTORC1 activation [16]. To determine the potential involvement of Rheb in UA-induced suppression of mTORC1 signaling, we examined S6K phosphorylation in C2C12 cells stably expressing wild-type Rheb and the constitutively active RhebS16H. S6K phosphorylation was enhanced in cells overexpressing RhebWT and RhebS16H and the increase was augmented by treatment of the cells with leucine (Figs. 2C and 2D). While pre-treatment of the C2C12 cells with UA had little effect on Rheb overexpression-induced phosphorylation of S6K, it greatly inhibited the leucine-stimulated phosphorylation of S6K (Fig. 2C and 2D), suggesting that UA inhibits leucine-stimulated mTORC1 signaling via a Rheb activity-independent mechanism.
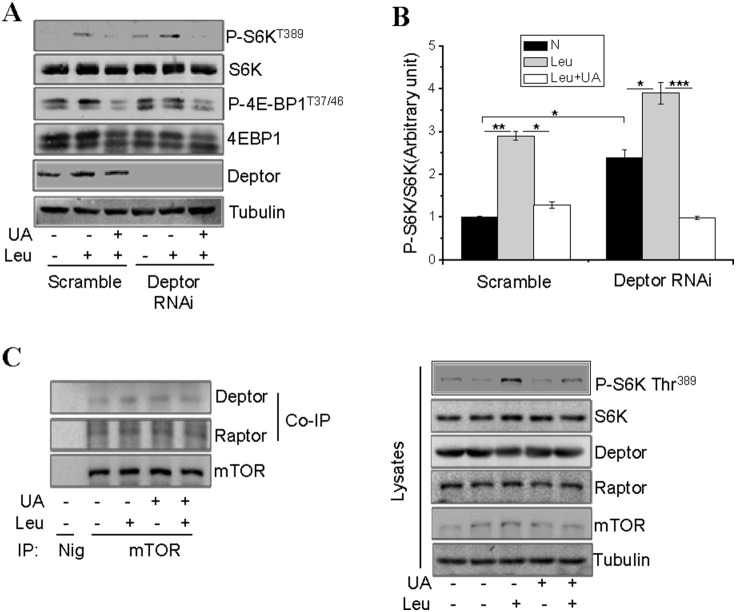
UA-mediated Inhibition of the mTORC1 Signaling Pathway is Independent of the Interaction between mTOR and Raptor/Deptor
To determine the potential involvement of the negative regulator Deptor in UA-induced inhibition of the mTORC1 signaling, we examined the effect of UA on leucine-stimulated S6K phosphorylation in C2C12 cells in which the expression levels of Deptor were suppressed by RNAi. As expected, mTORC1 signaling was increased in Deptor-suppressed cells, as demonstrated by increased basal S6K and 4EBP-1 phosphorylation (Figs. 3A and 3B). Leucine treatment further enhanced S6K phosphorylation in Deptor-suppressed cells and the stimulatory effect of leucine was reduced by UA treatment (Figs. 3A and 3B). Consistent with this, UA treatment had little effect on the interaction between mTOR and Deptor (Fig. 3C). Taken together, these results suggest that the interaction between Deptor and mTOR does not mediate the inhibitory effect of UA on leucine-stimulated mTOR activation. Since mTORC1 is activated by interaction with Raptor [34], we also examined the effect of UA on the association between mTOR and Raptor. We found that UA treatment had no significant effect on the association between mTOR and Raptor (Fig. 3C), suggesting that UA inhibits leucine-stimulated mTOR signaling by a distinct mechanism.
Figure 3. UA does not inhibit the associations between mTOR and Raptor or Deptor.
(A), Deptor-suppressed and scrambled C2C12 cells were serum starved and pretreated with or without 50 µM UA for 60 min, followed with or without 10 mM leucine (Leu) for 60 min. The phosphorylation of S6K, 4EBP1 and the protein levels of S6K, 4EBP1, Deptor, and Tubulin were determined by Western blot using specific antibodies. (B), S6K phosphorylation in (A) was semi-quantified using the NIH Image J Program and normalized with S6K protein levels. (C), C2C12 cells were serum starved and pretreated with or without leucine (Leu) for 60 min, followed with or without 50 µM UA for 60 min. mTOR was immunoprecipitated from cell lysates using an anti-mTOR antibody and the co-immunoprecipitaed Raptor or Deptor was determined by Western blot. Differences between groups were examined for statistical significance using ANOVA. Data are presented as mean ± S.E.M. from three independent experiments. NIg, normal immunoglobulin. *, P<0.05, **, P<0.01, and ***, p<0.001. N, no addition.
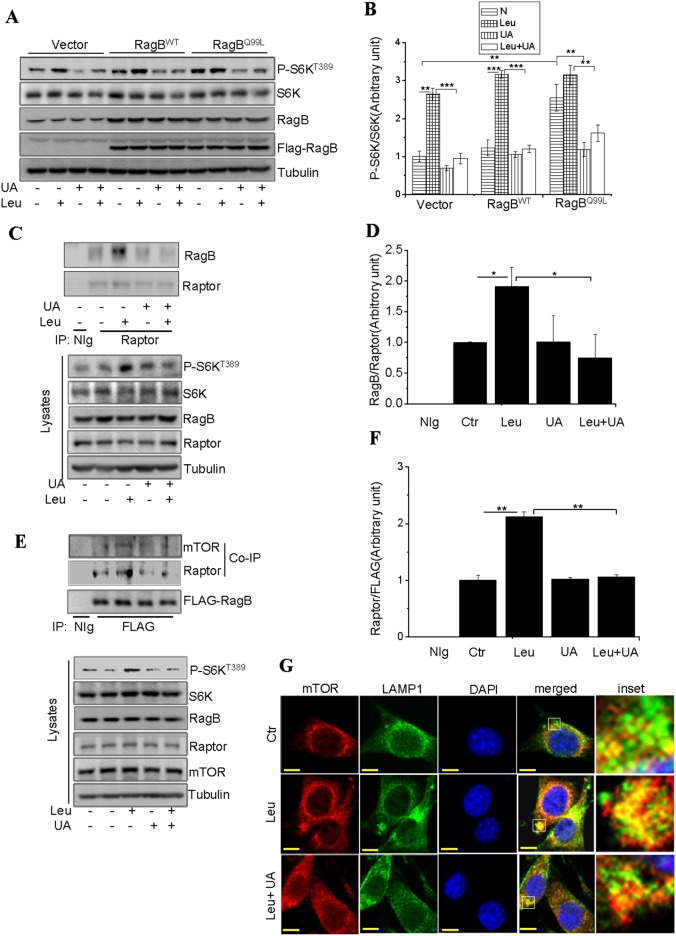
UA Inhibits Leucine-stimulated mTORC1 Signaling by Disrupting mTOR Lysosomal Localization
The small GTPase Rag has been shown to be critical for leucine-stimulated mTORC1 activation [16], [35]. To determine if UA inhibits mTORC1 signaling by targeting Rag GTPase, wild-type RagB or the constitutively active RagBQ99L were transiently expressed in C2C12 cells. Overexpression of RagB per se had no significant effect on S6K phosphorylation but potentiated leucine-stimulated S6K phosphorylation (Figs. 4A and 4B). Overexpression of RagBQ99L, on the other hand, significantly promoted basal S6K phosphorylation, which was not further enhanced by leucine treatment (Figs. 4A and 4B). Treatment of the cells with UA inhibited leucine-stimulated mTORC1 signaling in cells overexpressing either RagB or RagBQ99L (Figs. 4A and 4B), suggesting that UA acts at RagB or at a site downstream of RagB. To further test this, we examined the effect of UA on the association between RagB and Raptor, the later has been shown to mediate the interaction between RagB and mTORC1 [16]. Co-IP experiments showed that leucine treatment significantly stimulated the interaction between RagB and Raptor (Figs. 4C–4D). Similar result was obtained by reciprocal Co-IP experiments (Fig. 4E and 4F). However, the leucine-stimulated association of RagB with Raptor and mTOR was inhibited by UA (Figs. 4C–4F).
Figure 4. UA inhibits leucine-stimulated mTORC1 activity by disrupting mTOR lysosomal localization.
(A), C2C12 cells transiently expressing control vector, RagB or RagBGTP were serum starved and treated with or without 50 µM UA for 60 min, followed with or without 10 mM leucine (Leu) for 60 min. The phosphorylation and protein levels of S6K and the protein levels of RagB, FLAG, and Tubulin in cell lysates were determined by Western blot using specific antibodies as indicated. (B), S6K phosphorylation in (A) was semi-quantified using the NIH Image J Program and normalized to the levels of S6K in cells. (C), C2C12 cells transiently expressing RagB were serum starved and pretreated with or without with or without 50 µM UA for 60 min, followed with or without 10 mM leucine (Leu) for 60 min. Cells were then treated with the cross-linker chemical DSP and lysed. Raptor was immunoprecipitated from cell lysates and its associated proteins were detected by Western blot. (D), The cellular levels of RagB in (C) was semi-quantified using the NIH Image J Program and normalized to Raptor. (E), Serum-starved C2C12 cells transiently expressing RagB were pretreated with or without leucine (Leu) for 60 min and then with 50 µM UA for 60 min. Cells were then treated with DSP and lysed. FLAG-taged RagB was immunoprecipitaed from cell lysates and the associated proteins were determined by Western blot using antibodies to mTOR or Raptor. (F), the levels of Raptor in (E) was semi-quantified using the NIH Image J Program and normalized to FLAG-RagB. Differences between groups were examined for statistical significance using ANOVA. Data are presented as mean ± S.E.M. from three independent experiments. NIg, normal immunoglobulin. *, P<0.05, **, P<0.01, and ***, P<0.001. N, no addition. (G), serum-starved C2C12 cells were pretreated with or without 50 µM UA for 60 min, followed with or without 10 mM leucine (Leu) for 60 min. Cells were stained using antibodies specific to mTOR (red) and LAMP1 (green) and visualized by a confocal immunofluorescence microscope. Inset: Higher magnification to show colocalization (yellow) of mTOR and LAMP1. Scale bar, 10 µm.
To further elucidate the mechanism by which UA inhibits mTORC1 signaling, we examined RagB-mediated recruitment of mTORC1 to lysosome, which is critical for leucine-stimulated mTORC1 activation [18]. Confocal immunofluorescence experiments showed that treatment of the C2C12 cells with leucine promoted the co-localization of mTOR with the lysosomal marker LAMP1 (Fig. 4G). The co-localization of mTOR with LAMP1 was greatly suppressed by pre-treating the cells with UA (Fig. 4G), suggesting a mechanism by which UA inhibited leucine-stimulated activation of the mTORC1 signaling pathway.
Discussion
UA has been shown to exert many beneficial effects such as anti-diabetes [36], anti-obesity [37], and anti-cancer [38]. Several recent studies show that UA exerts its anti-tumor role through inhibition of the mTOR signaling pathway [39], suggesting a potential mechanism underlying the beneficial effects of UA. However, how UA inhibits mTORC1 signaling pathway remains unknown.
C2C12 myotubes possess morphological, biochemical and metabolic properties of the isolated skeletal muscle [40], [41] and are widely used for studying muscle cell function and physiology. Here we show that UA inhibits insulin- and leucine-stimulated mTORC1 signaling in C2C12 cells (Figs. 1A–1D). While UA is able to inhibit insulin-stimulated mTORC1 signaling by negative regulation of Akt, the inhibition of leucine-induced mTORC1 activation by UA appears to be mediated by an Akt-independent novel mechanism. As shown in figure 3, the UA-mediated inhibition of the mTORC1 signaling pathway is independent of the cellular levels of Raptor and Deptor, two key regulators of the mTORC1 signaling pathway [11], [34], [42]. On the other hand, treating C2C12 myoblasts with UA suppressed the interaction between Raptor and RagB (Figs. 4C–4F), and inhibited the recruitment of mTOR onto the lysosomal surface (Fig. 4G), a key step in the amino acid–induced activation of mTORC1 [16], [43]. Although UA has little effect on the association between mTOR and Raptor, the prevention of mTOR or Raptor from binding to RagB (Figs. 4C–4F) may provide a mechanism for UA to suppress the recruitment of mTOR to the lysosomal surface for activation.
How UA disrupts the binding of RagB to Raptor remains unknown. One possibility may be that UA directly interacts with RagB or mTORC1 components, leading to a conformational change of these proteins and thus impairs their interaction. Since the interaction between mTOR with the Rag proteins is critical for mTOR localization to the lysosome in response to amino acid stimulation [44], disruption the interaction between RagB and mTORC1 components could be the mechanism by which UA suppresses leucine-stimulated mTOR localization to the lysosome. Alternatively, UA may inhibit the conversion of GDP-bound inactive form of RagB to the GTP-bound active forms, thus preventing RagB interaction with mTOR. Future studies are needed to clarify these possibilities.
In summary, we have demonstrated for the first time that UA inhibits leucine-stimulated mTOR signaling in C2C12 cells. In addition, we show that UA inhibits mTORC1 signaling by disrupting the interaction between RagB-mTORC1. As aberrant activation of the mTORC1 signaling pathway is associated with impaired energy homeostasis and increased incidents of human tumors/cancers [45], [46], identification of small molecular inhibitors of this signaling pathway and elucidating their mechanisms of action are critical for the prevention and treatment of these diseases.
Funding Statement
This work was supported by an NIH RO1 grant (DK100697, http://grants.nih.gov/grants/funding/r01.htm), a grant from the National Nature Science Foundation of China (81130015, http://www.nsfc.gov.cn/nsfc/cen/00/download.html), and a grant from the National Basic Research Program of China (2014CB910500, http://www.most.gov.cn/kjjh/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liobikas J, Majiene D, Trumbeckaite S, Kursvietiene L, Masteikova R, et al. (2011) Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J Nat Prod 74: 1640–1644. [DOI] [PubMed] [Google Scholar]
- 2. Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, et al. (2012) Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One 7: e39332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee J, Yee ST, Kim JJ, Choi MS, Kwon EY, et al. (2010) Ursolic acid ameliorates thymic atrophy and hyperglycemia in streptozotocin-nicotinamide-induced diabetic mice. Chem Biol Interact 188: 635–642. [DOI] [PubMed] [Google Scholar]
- 4. Leng S, Hao Y, Du D, Xie S, Hong L, et al. (2013) Ursolic acid promotes cancer cell death by inducing Atg5-dependent autophagy. Int J Cancer 133: 2781–2790. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Ikejima K, Kon K, Arai K, Aoyama T, et al. (2011) Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J Hepatol 55: 379–387. [DOI] [PubMed] [Google Scholar]
- 6. Shanmugam MK, Ong TH, Kumar AP, Lun CK, Ho PC, et al. (2012) Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 7: e32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babcock JT, Quilliam LA (2011) Rheb/mTOR activation and regulation in cancer: novel treatment strategies beyond rapamycin. Curr Drug Targets 12: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 8. Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484. [DOI] [PubMed] [Google Scholar]
- 9. Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, et al. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871. [DOI] [PubMed] [Google Scholar]
- 11. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, et al. (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, et al. (2005) Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A 102: 14238–14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang J, Dibble CC, Matsuzaki M, Manning BD (2008) The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 28: 4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang J, Manning BD (2008) The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, et al. (2011) Hepatic Sirt1 deficiency in mice impairs mTORC2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest 121: 4477–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, et al. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, et al. (2010) Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, et al. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ben-Sahra I, Howell JJ, Asara JM, Manning BD (2013) Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, et al. (2013) The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153: 840–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, et al. (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeFronzo RA (1988) Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687. [DOI] [PubMed] [Google Scholar]
- 24. Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146: 1473–1481. [DOI] [PubMed] [Google Scholar]
- 25. Zou J, Zhou L, Du XX, Ji Y, Xu J, et al. (2010) Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell 20: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu M, Wilk SA, Wang A, Zhou L, Wang RH, et al. (2010) Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem 285: 36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang A, Liu M, Liu X, Dong LQ, Glickman RD, et al. (2011) Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J Biol Chem 286: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ (2010) Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 29. Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF (2001) Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab 281: E466–471. [DOI] [PubMed] [Google Scholar]
- 30. Kimball SR, Shantz LM, Horetsky RL, Jefferson LS (1999) Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274: 11647–11652. [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, Jahn LA, Wei L, Long W, Barrett EJ (2002) Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab 87: 5553–5558. [DOI] [PubMed] [Google Scholar]
- 32. Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, et al. (2002) Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 4: 699–704. [DOI] [PubMed] [Google Scholar]
- 33. Yang Q, Guan KL (2007) Expanding mTOR signaling. Cell Res 17: 666–681. [DOI] [PubMed] [Google Scholar]
- 34. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, et al. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189. [DOI] [PubMed] [Google Scholar]
- 35. Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, et al. (2013) A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alqahtani A, Hamid K, Kam A, Wong KH, Abdelhak Z, et al. (2013) The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem 20: 908–931. [PubMed] [Google Scholar]
- 37. Li Y, Kang Z, Li S, Kong T, Liu X, et al. (2010) Ursolic acid stimulates lipolysis in primary-cultured rat adipocytes. Mol Nutr Food Res 54: 1609–1617. [DOI] [PubMed] [Google Scholar]
- 38. Gao N, Cheng S, Budhraja A, Gao Z, Chen J, et al. (2012) Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br J Pharmacol 165: 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeh CT, Wu CH, Yen GC (2010) Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res 54: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 40. Yang TT, Liu IM, Wu HT, Cheng JT (2009) Mediation of protein kinase C zeta in mu-opioid receptor activation for increase of glucose uptake into cultured myoblast C2C12 cells. Neurosci Lett 465: 177–180. [DOI] [PubMed] [Google Scholar]
- 41. Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727. [DOI] [PubMed] [Google Scholar]
- 42. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175. [DOI] [PubMed] [Google Scholar]
- 43. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, et al. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howell JJ, Manning BD (2011) mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab 22: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD (2013) A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans 41: 906–912. [DOI] [PubMed] [Google Scholar]