Abstract
Background
A stationary association between climate factors and epidemics of Mycoplasma pneumoniae (M. pneumoniae) pneumonia has been widely assumed. However, it is unclear whether elements of the local climate that are relevant to M. pneumoniae pneumonia transmission have stationary signatures of climate factors on their dynamics over different time scales.
Methods
We performed a cross-wavelet coherency analysis to assess the patterns of association between monthly M. pneumoniae cases in Fukuoka, Japan, from 2000 to 2012 and indices for the Indian Ocean Dipole (IOD) and El Niño Southern Oscillation (ENSO).
Results
Monthly M. pneumoniae cases were strongly associated with the dynamics of both the IOD and ENSO for the 1–2-year periodic mode in 2005–2007 and 2010–2011. This association was non-stationary and appeared to have a major influence on the synchrony of M. pneumoniae epidemics.
Conclusions
Our results call for the consideration of non-stationary, possibly non-linear, patterns of association between M. pneumoniae cases and climatic factors in early warning systems.
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is a major cause of upper and lower respiratory tract infection and respiratory tract disease in humans, particularly in children [1]. This agent is estimated to be responsible for 15%–20% of all cases of community-acquired pneumonia (CAP), up to 40% of cases of CAP among children [2], and more than one-third of childhood cases of CAP requiring hospitalization [3].
As with M. pneumoniae infection, a recent study found quantitative evidence that the incidence of M. pneumoniae pneumonia increased significantly with increased average temperature and relative humidity [4]. However, it remains unclear whether local weather factors have non-stationary (not consistent over time) associations with M. pneumoniae infection. In this regard, interannual global climatic events related to the El Niño Southern Oscillation (ENSO) and Indian Ocean Dipole (IOD) have been associated with the transmission of a number of infectious diseases, including dengue [5], [6], malaria [7]–[9] and cholera [10]–[12]. However, the interannual cycles of M. pneumoniae infection have not been examined, and the possible clinical impact of these events on CAP remains unclear.
Wavelet analysis is used to identify non-stationarity associations between disease dynamics and exposure covariates, and is useful in the analysis of epidemiological time series [13]. In particular, wavelet analysis has been used to determine whether the presence of a particular periodic cycle at a given time in the incidence of a disease corresponds to the presence of the same periodic cycle at the same time in an exposure covariate [13]. Wavelet analysis is increasingly used in epidemiology to explore the spatial and temporal dynamics of diseases [14], [15].
M. pneumoniae pneumonia remains a major health risk in many parts of the world. A better understanding of its sensitivity to climate might aid in the development of a reliable climate-based prediction system for M. pneumoniae pneumonia epidemics, which might in turn lead to improvements in the current disease control program in Japan and other countries where M. pneumoniae pneumonia is endemic.
Here, we explored the time-varying relationships between climate variations and the monthly incidence of M. pneumoniae pneumonia between 2000 and 2012 in Fukuoka, Japan. To our knowledge, this is the first report to quantify the time-varying impact of climate factors on the number of M. pneumoniae pneumonia cases using cross-wavelet analysis.
Materials and Methods
Data Sources
The number of M. pneumoniae pneumonia patients in Fukuoka Prefecture, located in the southwest of Japan (Fig. S1), is reported on a weekly basis from 120 sentinel medical institutions [4]. Cases are defined by clinical factors in combination with laboratory tests. Clinical manifestations include fever, cough, malaise, and headache, sometimes with tracheobronchitis with fever and nonproductive cough [2], [16]. Several types of laboratory tests are used for the diagnosis such as culture, PCR, and serological methods. Serological methods include passive agglutination, complement fixation and ELISA, and a combination of PCR and serology is recommended for reliable diagnosis [17], [18]. We obtained clinical data that were recorded and reported by sentinel volunteers to the Fukuoka Institute of Health and Environmental Sciences, the municipal public health institute of the Fukuoka Prefectural Government. Monthly cases for M. pneumoniae pneumonia were calculated from the daily records based on the day of diagnosed. The population number and structure have changed little during the study period [19]. Additionally, the reporting system or case definition (diagnosis criteria) has not been changed.
The strength of the IOD was measured by the Dipole Mode Index (DMI), defined as the difference in sea surface temperature (SST) anomalies between the western (10°S–10°N, 50°–70°E) and eastern (10°S–0°, 90°–110°E) tropical Indian Ocean [20]. The DMI data were obtained from the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) (http://www.jamstec.go.jp/frcgc/research/d1/iod/). The ENSO indices (i.e. Multivariate ENSO Index (MEI), Niño 1+2, Niño 3, Niño 4 and Niño 3.4) were also extracted from the National Oceanic and Atmospheric Administration (NOAA) Climate Prediction Center data (http://www.cpc.ncep.noaa.gov). The MEI is the six main observed variables over the tropical Pacific that predicts the occurrence of the ENSO; sea level pressure, zonal and meridional components of the surface wind, sea surface temperature, surface air temperature, and total cloudiness fraction of the sky [21]. Data of SSTs the tropical Pacific has been divided into a number of regions named Niño 1, 2, 3, 4, and 3.4; Niño 1 is the area defined by 80°–90°W and 5°–10°S, Niño 2 by 80°–90°W and 0°S–5°S, Niño 3 by 90°–150°W and 5°N–5°S, Niño 4 by 150°W–160°E and 5°N–5°S, Niño 3.4 by 120°–170°W and 5°N–5°S [21].
We also obtained data on daily average temperature, relative humidity and rainfall in the prefecture from the Japan Meteorological Agency. Monthly means for average temperatures, relative humidity and rainfall were calculated from the daily records.
The ethics committee of the Fukuoka Prefectural Government Health and Environmental Research Advancement Committee approved this study on December 27, 2006 (Reference Number: 18–3515). Patient records/information was anonymized and de-identified prior to analysis.
Statistical Analysis
We examined the periodicity in the M. pneumoniae pneumonia incidence time series using cross-wavelet coherency analysis [13], [15]. This analysis provides the possibility of investigating and quantifying whether the presence of a particular frequency at a given time in M. pneumoniae pneumonia corresponds to the presence of the same frequency at the same time in a climate covariate. Cross-wavelet coherence significance was estimated using the method of Maraun and Kurths for a minimum time scale [22]. The Morlet Wavelet was used and the choice of minimum period of interest in the cycles was set at 6 months. A total smoothing window of 15 months was used to compute the cross-wavelet coherence. All statistical analyses were conducted using R statistical software, version 3.0.1.
Results
A total of 23,325 (100%) cases of M. pneumoniae from 2000 to 2012 were included in our analyses, with 9,149 (39.2%) in children aged 0–4 years, 9,751 (41.8%) in those aged 5–9 years, 3,059 (13.1%) in those aged 10–14 years, and 1,366 (5.9%) in those aged 15 years or older.
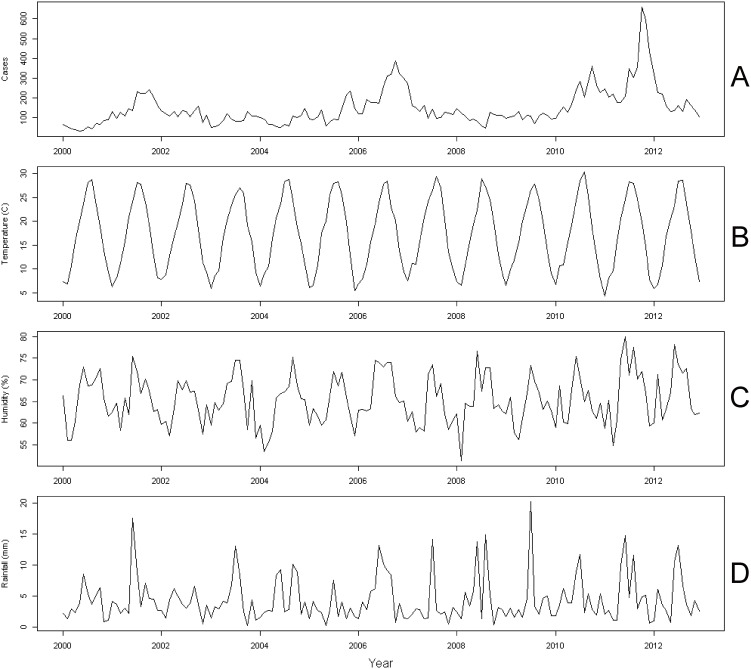
The time series for the number of M. pneumoniae cases per month, ambient temperature, relative humidity and rainfall during the study period are shown in Figure 1. Analysis of reported cases of M. pneumoniae pneumonia by month revealed that the seasonal peak in cases was not identical from year to year (Fig. 1).
Figure 1. Monthly time series data in Fukuoka, Japan, 2000–2012.
(A) M. pneumoniae pneumonia cases; (B) temperature; (C) relative humidity; and (D) rainfall.
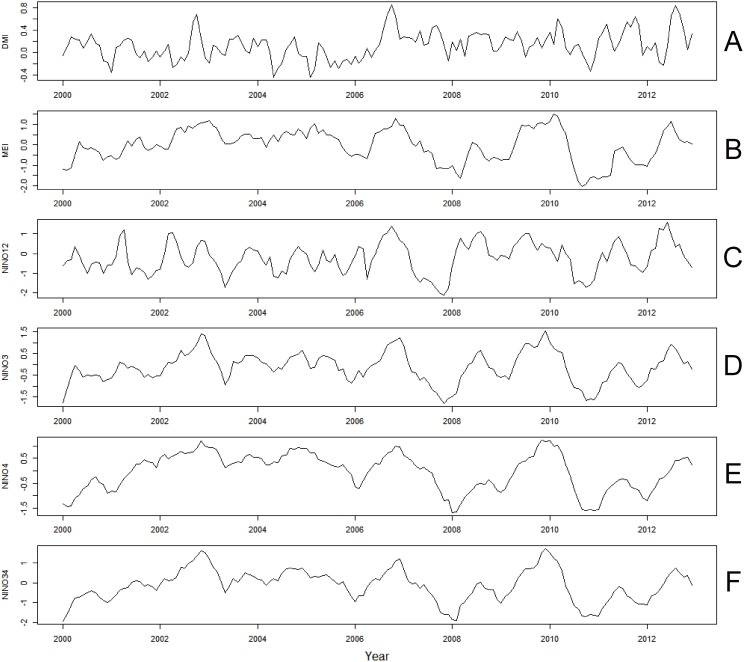
The time series for DMI and ENSO indices (MEI, Niño 1+2, Niño 3, Niño 4 and Niño 3.4) during the same period are shown in Figure 2. Strong positive IOD events (indicated by large DMI values) occurred in 2006 and 2012 and, in these years, the DMI peaked in October and August, respectively. Strong ENSO events (indicated by large MEI index values) were observed in 2006 and again in 2009–2010 (Fig. 2).
Figure 2. Monthly time series data for the global climatic indices (2000 to 2012).
(A) DMI (dipole mode index); (B) MEI (multivariate ENSO index); (C) Niño 12 (ENSO index); (D) Niño 3 (ENSO index); (E) Niño 4 (ENSO index); (F) Niño 34 (ENSO index).
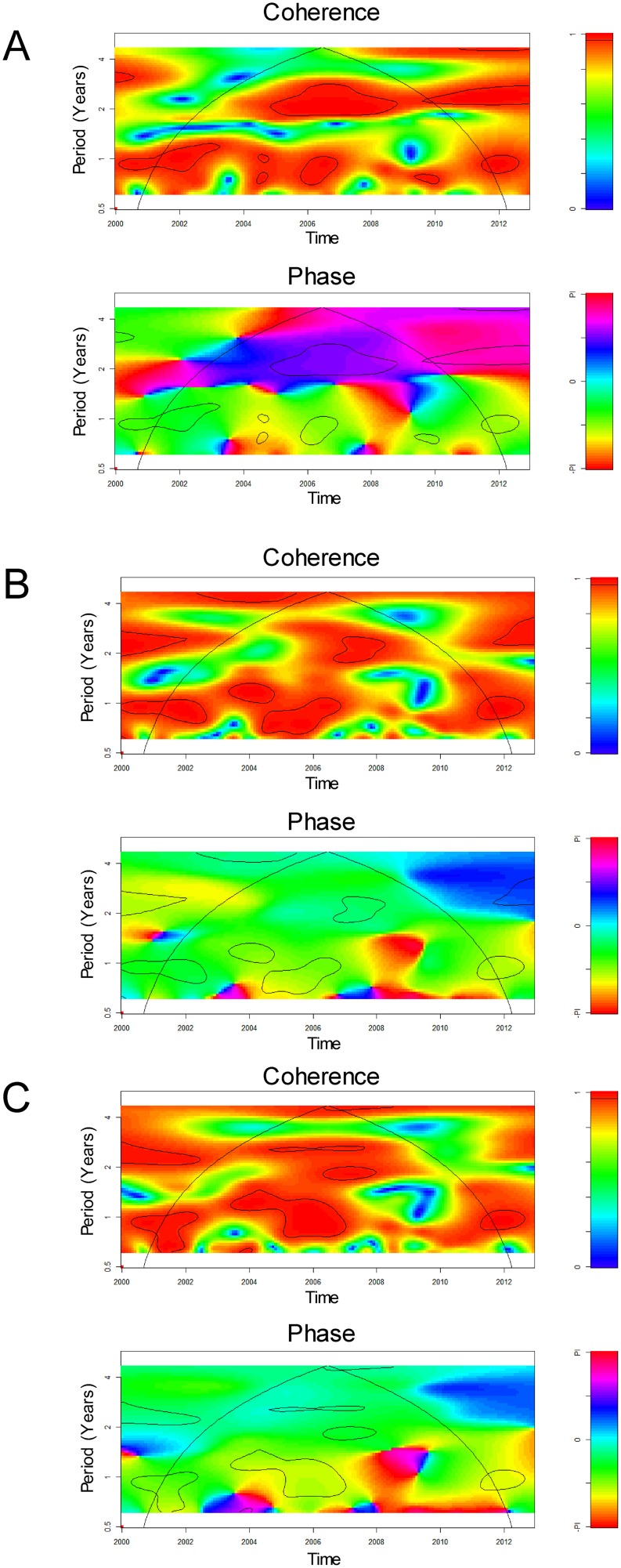
Cross-wavelet coherence and cross-wavelet phase analysis of the global climatic time series (DMI and ENSO indices) with M. pneumoniae cases are shown in Figure 3. Cross-wavelet coherency analysis revealed that M. pneumoniae cases were significantly (p< 0.05) coherent with DMI and ENSO indices at periods of around 1–2 years in 2005–2007 and 2010–2011, with DMI and ENSO indices leading the disease data, as shown by the cross-wavelet phases (Fig. 3).
Figure 3. Cross-wavelet coherency and phase of the M. pneumoniae pneumonia time series with the global climatic indices.
(A) DMI (dipole mode index); (B) MEI (multivariate ENSO index); (C) Niño 12 (ENSO index); (D) Niño 3 (ENSO index); (E) Niño 4 (ENSO index); (F) Niño 34 (ENSO index). Red regions in the upper part of the plots indicate frequencies and times for which the two series share variability. The cone of influence (within which results are not influenced by the edges of the data) and the significant coherent time-frequency regions (p<0.05) are indicated by solid lines. In cross-wavelet phase plots, colors correspond to different lags between the variability in the two series for a given time and frequency, measured in angles from -PI to PI. A value of PI corresponds to a lag of 16 months.
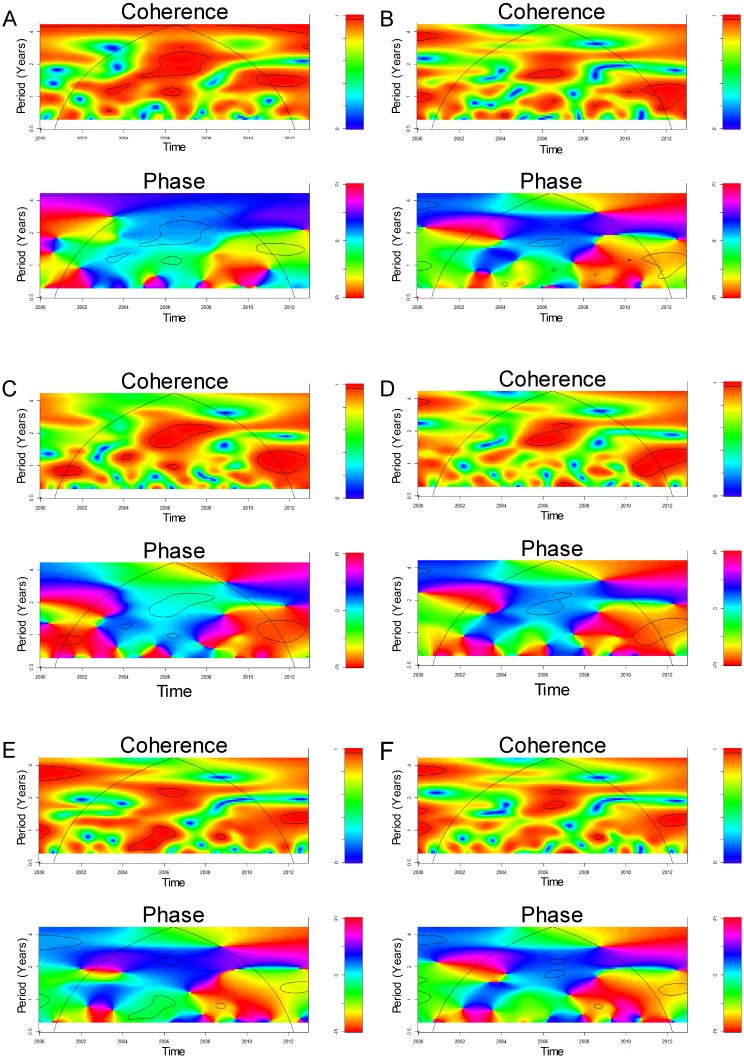
Cross-wavelet coherence and cross-wavelet phase analysis of the local climatic time series (temperature, relative humidity and rainfall) with M. pneumoniae cases are shown in Figure 4. Cross-wavelet coherency analysis revealed that M. pneumoniae cases were significantly (p<0.05) coherent with temperature, relative humidity and rainfall at periods of around 1–2 y, with temperature leading the disease data, as shown by the cross-wavelet phases (Fig. 4). All local climatic factors had non-stationary relationships with both DMI and ENSO indices (Fig. S2).
Figure 4. Cross-wavelet coherency and phase of the M. pneumoniae pneumonia time series with local weather indices.
(A) Temperature; (B) relative humidity; and (C) rainfall. Red regions in the upper part of the plots indicate frequencies and times for which the two series share variability. The cone of influence (within which results are not influenced by the edges of the data) and the significant coherent time-frequency regions (p<0.05) are indicated by solid lines. In cross-wavelet phase plots, colors correspond to different lags between variability in the two series for a given time and frequency, measured in angles from -PI to PI. A value of PI corresponds to a lag of 16 months.
Discussion
In this study, we found evidence that the monthly incidence of M. pneumoniae pneumonia cases was significantly associated with the dynamics of both the IOD and ENSO for the 1–2-year periodic mode in 2005–2007 and 2010–2011. This association was non-stationary and appeared to have a major influence on the synchrony of M. pneumoniae epidemics. These findings indicate that epidemic early warning systems should consider non-stationary, and possibly non-linear patterns of association between M. pneumoniae cases and climatic factors.
Anticipating the potential effects of changes in climate on the incidence and distribution of M. pneumoniae pneumonia requires a close understanding of the relationship between climate and disease dynamics [23]. Our present results show that the dynamics of M. pneumoniae pneumonia incidence are strongly associated with those of climate variables, including DMI and ENSO indices, with coherent cycles of around 1–2 years in 2005–2007 and 2010–2011. Consistent with this, an epidemiological study has suggested that El Niño events are associated with viral pneumonia hospitalizations [24]. In addition, a more recent study suggested that warming of the Indian Ocean relative to the Pacific might play an important role in modulating Pacific climate changes in the 20th and 21st centuries [25]. Another recent study has revealed a unique coupled mode over the Indo-Northwestern Pacific (NWP) warm pool during the boreal summer that arises from interaction between the Indian Ocean (IO) sea surface temperature and the Pacific-Japan (PJ) teleconnection pattern, and that this PJIO coupled mode can exist without ENSO but is efficiently excited by it [26]. Although childhood pneumonia, which is responsible for 17% of childhood deaths worldwide [27], is rarely mentioned in the context of climate change [28], our combined IOD and ENSO results demonstrate the importance of global climate factors in the prevalence of M. pneumoniae pneumonia infections.
Our study also found that the dynamics of M. pneumoniae pneumonia cases are strongly associated with local climate variables, temperature, relative humidity and rainfall, with coherent cycles of around 1–2 y in 2004–2007. Our results are consistent with a previous study which suggested that higher temperature and relative humidity might influence the increase in M. pneumoniae pneumonia cases after adjustment for potential confounding by seasonal and interannual patterns [4]. However, this previous study assumed that the association of temperature and relative humidity with M. pneumoniae pneumonia incidence was consistent over the study period. In contrast, our present findings suggest that the previous finding based on this assumption can be improved by assuming a non-stationary (not consistent over time) association, and by more accurately evaluating the possibility of a non-linear association between climatic covariates and M. pneumoniae pneumonia incidence.
A laboratory-based study has suggested that although the survival of airborne M. pneumoniae is determined by both temperature and relative humidity, the temperature response is mediated by relative humidity in that the effects of temperature are only observed if some water vapor is present [29]. Bacterial survival and virus stability in aerosols might be increased by higher humidity [28]. With respect to the effect of rainfall, an increase in time spent indoors because of increased rainfall will increase crowding and might lead to higher risk for contact transmission.
The finding for an association with a model which includes DMI and ENSO indices supports the recent proposal that large-scale climate indices may be more useful for forecasting than local climate variables [30]. Climate change is expected to have an enormous effect on the burden of infectious diseases due to the potential for increasing temperatures and changing rainfall patterns [31], and IOD and ENSO-related changes in temperature, rainfall, and other environmental factors might have both direct effects (e.g. extreme weather events) and indirect effects (e.g. changes in transmission and outbreaks of infectious diseases) on the incidence of M. pneumoniae pneumonia. Climate can affect the dynamics of infection in a host population through several linear and nonlinear pathways. Among these, it can affect several biological traits of parasitic organisms across their life cycle, from individual life histories to population dynamics [32]. Further, changes in climate and air quality substantially increase respiratory morbidity and mortality for patients with common chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD) and respiratory tract infections [33]. While local climate is more likely to affect only the biological components of disease transmission, large-scale climate patterns might also influence contextual components of disease dynamics, such as population susceptibility.
Using cross-wavelet coherence and cross-wavelet phase analysis, we found that the effects of global and local climatic indices appeared at periods of around 1–2 y. These results may relate to the long incubation period of M. pneumoniae, and its relatively low transmission rate and late detection of outbreaks [34]. These characteristics hinder or prevent the efforts of public health officials and health-care providers in detecting M. pneumoniae pneumonia infections, and indicate the importance of developing more accurate and precise models of any effects of climate factors on disease risk, as well as further discussion of disease-specific issues.
A few limitations of this study warrant mention. First, not all cases in the community are represented in the surveillance data. This under-reporting can occur anywhere in the reporting chain, from the initial decision of a patient not to seek health care to the failure to record cases in the disease registry due to the mildness or lack of symptoms [35], and commonly occurs in closed and semi-closed communities [36]. However, we consider that this would have been unlikely to result in substantial bias because the degree of under-reporting is not likely to vary over time. Second, the participating sentinel medical institutions were recruited on a voluntary basis; however, this is unlikely to have posed a threat to the validity of the comparisons over time, which is the subject of this study.
Finally, with regard to the practical implications of our present findings, understanding the effects of climate factors on the epidemiology of infectious diseases is important for the planning of health services. These observed associations of climate factors with adverse health effects might represent a possible model or analog for the potential health impacts of future climate changes. Health services may need to prepare for the effects of climate change on the epidemiology of M. pneumoniae pneumonia through the implementation of preventive public health interventions. For instance, a recent study described the containment of an M. pneumoniae outbreak thanks to public health interventions that included communicating to the public the importance of respiratory hygiene, providing hand sanitizer in schools, and informing health-care providers about macrolide resistance [37]. Another study suggested that effective communications, coupled with university policies that help students adopt preventive health behaviors, including proper hand and respiratory hygiene, staying home and seeking medical care when ill, and refining health messages and communication methods to improve awareness of disease outbreaks among students, might reduce transmission of M. pneumoniae and the severe complications that can go with it [38]. These interventions reference the possibility of changes in behavior, such as people coming into closer contact with each other and thereby increasing the chance of person-to-person transmission.
In conclusion, this study found quantitative evidence that non-stationary associations have a major influence on synchrony between the monthly incidence of M. pneumoniae cases and the dynamics of both the IOD and ENSO for the 1–2-year periodic mode in 2005–2007 and 2010–2011. Our results call for the consideration of non-stationary, possibly non-linear patterns of association between M. pneumoniae cases and climatic factors in epidemic early warning systems.
Supporting Information
Location of Fukuoka Prefecture on Kyushu Island, southwest of Tokyo, Japan.
(TIF)
Cross-wavelet coherency and phase of the temperature, humidity and rainfall time series with the DMI and with ENSO indices. (A) Temperature and DMI; (B) temperature and MEI; (C) temperature and Niño 1+2; (D) temperature and Niño 3; (E) temperature and Niño 4; (F) temperature and Niño 3.4; (G) relative humidity and DMI; (H) relative humidity and MEI; (I) relative humidity and Niño 1+2; (J) relative humidity and Niño 3; (K) relative humidity and Niño 4; (L) relative humidity and Niño 3.4; (M) rainfall and DMI; (N) rainfall and MEI; (O) rainfall and Niño 1+2; (P) rainfall and Niño 3; (Q) rainfall and Niño 4; and (R) rainfall and Niño 3.4. The coherency scale is from zero (blue) to one (red). Other technical details are presented in the legend of Fig. 3 and Fig. 4.
(TIF)
Acknowledgments
We thank the Fukuoka Prefectural Government, Department of Public Health and Medical Affairs, Division of Public Health for their painstaking efforts in infectious disease surveillance in Fukuoka, Japan.
Funding Statement
The study was supported by grants from the Ministry of Health, Labour and Welfare, Japan and budgets for research of the Department of Public Health and Medical Affairs, Fukuoka Prefectural Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Spuesens EB, Fraaij PL, Visser EG, Hoogenboezem T, Hop WC, et al. (2013) Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 10: e1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waites KB, Talkington DF (2004) Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17: 697–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, et al. (2011) The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53: e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Onozuka D, Hashizume M, Hagihara A (2009) Impact of weather factors on Mycoplasma pneumoniae pneumonia. Thorax 64: 507–511. [DOI] [PubMed] [Google Scholar]
- 5. Cazelles B, Chavez M, McMichael AJ, Hales S (2005) Nonstationary influence of El Niño on the synchronous dengue epidemics in Thailand. PLoS Med 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johansson MA, Cummings DA, Glass GE (2009) Multiyear climate variability and dengue-El Niño southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med 6: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou G, Minakawa N, Githeko AK, Yan G (2004) Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci U S A 101: 2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A (2003) El Niño and health. Lancet 362: 1481–1489. [DOI] [PubMed] [Google Scholar]
- 9. Hashizume M, Terao T, Minakawa N (2009) The Indian Ocean Dipole and malaria risk in the highlands of western Kenya. Proc Natl Acad Sci U S A 106: 1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reiner RC Jr, King AA, Emch M, Yunus M, Faruque AS, et al. (2012) Highly localized sensitivity to climate forcing drives endemic cholera in a megacity. Proc Natl Acad Sci U S A 109: 2033–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashizume M, Faruque AS, Terao T, Yunus M, Streatfield K, et al. (2011) The Indian Ocean dipole and cholera incidence in Bangladesh: a time-series analysis. Environ Health Perspect 119: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G (2005) Refractory periods and climate forcing in cholera dynamics. Nature 436: 696–700. [DOI] [PubMed] [Google Scholar]
- 13. Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bulletin of the American Meteorological Society 79: 61–78. [Google Scholar]
- 14. Cazelles B, Chavez M, McMichael AJ, Hales S (2005) Nonstationary influence of El Niño on the synchronous dengue epidemics in Thailand. PLoS Med 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chaves LF, Pascual M (2006) Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med 3: e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsiodras S, Kelesidis I, Kelesidis T, Stamboulis E, Giamarellou H (2005) Central nervous system manifestations of Mycoplasma pneumoniae infections. J Infect 51: 343–354. [DOI] [PubMed] [Google Scholar]
- 17. Dorigo-Zetsma JW, Zaat SA, Wertheim-van Dillen PM, Spanjaard L, Rijntjes J, et al. (1999) Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J Clin Microbiol 37: 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Templeton KE, Scheltinga SA, Graffelman AW, Van Schie JM, Crielaard JW, et al. (2003) Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae . J Clin Microbiol 41: 4366–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuoka Prefectural Government (2013) Fukuoka Prefecture Migration Survey. Available: http://www.pref.fukuoka.lg.jp/dataweb/search-1-1619.html. Accessed 2013 Aug 1.
- 20. Saji NH, Goswami BN, Vinayachandran PN, Yamagata T (1999) A dipole mode in the tropical Indian Ocean. Nature 401: 360–363. [DOI] [PubMed] [Google Scholar]
- 21. Wolter K, MS Timlin (2011) El Niño/Southern Oscillation behaviour since 1871 as diagnosed in an extended multivariate ENSO index (MEI.ext). Int J Climatol 31: 1074–1087. [Google Scholar]
- 22. Maraun D, Kurths J (2004) Cross wavelet analysis: significance testing and pitfalls. Nonlinear Processes in Geophysics 11: 505–514. [Google Scholar]
- 23. Patz JA, Hulme M, Rosenzweig C, Mitchell TD, Goldberg RA, et al. (2002) Climate change: Regional warming and malaria resurgence. Nature 420: 627–628 discussion 628. [DOI] [PubMed] [Google Scholar]
- 24. Choi KM, Christakos G, Wilson ML (2006) El Niño effects on influenza mortality risks in the state of California. Public Health 120: 505–516. [DOI] [PubMed] [Google Scholar]
- 25. Luo JJ, Sasaki W, Masumoto Y (2012) Indian Ocean warming modulates Pacific climate change. Proc Natl Acad Sci U S A 109: 18701–18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kosaka Y, Xie SP, Lau NC, Vecchi GA (2013) Origin of seasonal predictability for summer climate over the Northwestern Pacific. Proc Natl Acad Sci U S A 110: 7574–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization (WHO) (2008) The global burden of disease: 2004 update. Available: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004 update_full.pdf. Accessed 2013 Aug 1.
- 28. Paynter S, Ware RS, Weinstein P, Williams G, Sly PD (2010) Childhood pneumonia: a neglected, climate-sensitive disease? Lancet 376: 1804–1805. [DOI] [PubMed] [Google Scholar]
- 29. Wright DN, Bailey GD, Goldberg LJ (1969) Effect of temperature on survival of airborne Mycoplasma pneumoniae . J Bacteriol 99: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, et al. (2003) Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc Biol Sci 270: 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shuman EK (2010) Global climate change and infectious diseases. N Engl J Med 362: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 32. Hallett TB, Coulson T, Pilkington JG, Clutton-Brock TH, Pemberton JM, et al. (2004) Why large-scale climate indices seem to predict ecological processes better than local weather. Nature 430: 71–75. [DOI] [PubMed] [Google Scholar]
- 33. Bernstein AS, Rice MB (2013) Lungs in a warming world: climate change and respiratory health. Chest 143: 1455–1459. [DOI] [PubMed] [Google Scholar]
- 34. Ancel Meyers L, Newman ME, Martin M, Schrag S (2003) Applying network theory to epidemics: control measures for Mycoplasma pneumoniae outbreaks. Emerg Infect Dis 9: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Foy HM, Kenny GE, McMahan R, Kaiser G, Grayston JT (1971) Mycoplasma pneumoniae in the community. Am J Epidemiol 93: 55–67. [DOI] [PubMed] [Google Scholar]
- 36. Feikin DR, Moroney JF, Talkington DF, Thacker WL, Code JE, et al. (1999) An outbreak of acute respiratory disease caused by Mycoplasma pneumoniae and adenovirus at a federal service training academy: new implications from an old scenario. Clin Infect Dis 29: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention (CDC) (2012) Mycoplasma pneumoniae respiratory illness - two rural counties, West Virginia, 2011. MMWR Morb Mortal Wkly Rep 61: 834–838. [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention (CDC) (2013) Mycoplasma pneumoniae Outbreak at a University - Georgia, 2012. MMWR Morb Mortal Wkly Rep 62: 603–606. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Location of Fukuoka Prefecture on Kyushu Island, southwest of Tokyo, Japan.
(TIF)
Cross-wavelet coherency and phase of the temperature, humidity and rainfall time series with the DMI and with ENSO indices. (A) Temperature and DMI; (B) temperature and MEI; (C) temperature and Niño 1+2; (D) temperature and Niño 3; (E) temperature and Niño 4; (F) temperature and Niño 3.4; (G) relative humidity and DMI; (H) relative humidity and MEI; (I) relative humidity and Niño 1+2; (J) relative humidity and Niño 3; (K) relative humidity and Niño 4; (L) relative humidity and Niño 3.4; (M) rainfall and DMI; (N) rainfall and MEI; (O) rainfall and Niño 1+2; (P) rainfall and Niño 3; (Q) rainfall and Niño 4; and (R) rainfall and Niño 3.4. The coherency scale is from zero (blue) to one (red). Other technical details are presented in the legend of Fig. 3 and Fig. 4.
(TIF)