Abstract
Exposure of human skin to low doses of solar UV radiation (UVR) causes increased pigmentation, while chronic exposure is a powerful risk factor for skin cancers. The mechanisms mediating UVR detection in skin, however, remain poorly understood. Our recent studies revealed that UVR activates a retinal-dependent G protein-coupled signaling pathway in melanocytes. This phototransduction pathway leads to the activation of transient receptor potential A1 (TRPA1) ion channels, elevation of intracellular calcium (Ca2+) and rapid increase in cellular melanin content. Here we report that physiological doses of solar-like UVR elicit a retinal-dependent membrane depolarization in human epidermal melanocytes. This transient depolarization correlates with delayed inactivation time of the UVR-evoked photocurrent and with sustained Ca2+ responses required for early melanin synthesis. Thus, the cellular depolarization induced by UVR phototransduction in melanocytes is likely to be a critical signaling mechanism necessary for the protective response represented by increased melanin content.
Keywords: melanocyte, ultraviolet, phototransduction, calcium signaling, UV
Introduction
Melanocytes are neural crest derived cells that migrate during development to the skin, ear, brain, and heart.1-5 All of these melanocytes share the ability to produce melanin and, in the case of skin, to transfer it to neighboring keratinocytes.6 Melanin production is essential for melanocyte function; defects in this process can lead to skin pigmentation disorders, increased susceptibility to melanoma, as well as visual and hearing defects.7,8 Unlike the skin of most other vertebrates, human skin contains melanocytes within the basal epidermal layer that are responsible for the skin’s pigmentation response to solar UV radiation (UVR). Several functional ion channels are expressed at the plasma membrane of human epidermal melanocytes (HEMs).9-12 Changes in melanocyte plasma membrane potential can control cellular functions such as migration, proliferation and morphology.13
UVR, a constant presence in our environment, has damaging effects on human skin, causing skin cancers and photoaging.14 Exposure to low doses of UVR stimulates a protective pigmentation response mediated by melanocytes present in the epidermis. UVR causes DNA damage in the epidermis and results in melanin synthesis in melanocytes and transfer to neighboring keratinocytes, leading to increased skin pigmentation within one day after exposure.15 Considering the time course and activation mechanism of the delayed pigmentation response, it is likely that other signaling pathways in skin contribute to earlier UVR detection to initiate protection prior to DNA damage.
We recently found that human epidermal melanocytes (HEMs) have a retinal-dependent and G protein-mediated UVR-activated pathway that leads to a rapid intracellular Ca2+ response and activation of an ionic current, suggesting the presence of a phototransduction cascade in melanocytes.12,16 We found that UVR activates transient receptor potential A1 (TRPA1) ion channels in the plasma membrane, which, in response to prolonged UVR stimuli, mediate sustained Ca2+ responses necessary for early increases in cellular melanin content.12,16
How does TRPA1 mediate the long-lasting, sustained Ca2+ response in response to a transient stimulus? Here we show that physiological UVR doses induce a retinal-dependent membrane depolarization in HEMs. We hypothesize that the change in membrane potential delays the inactivation of UVR-activated TRPA1 channels, thus allowing Ca2+ influx for an extended period of time. Our findings demonstrate that the equivalent of seconds to minutes of sun exposure depolarizes melanocytes and that melanocyte depolarization contributes to the elevated intracellular Ca2+ required for early melanin synthesis.
Results and Discussion
Our recent work showed that exposure of cultured primary human epidermal melanocytes (HEMs) to UVR with spectral characteristics (90% UVA and 10% UVB) similar to solar UVR leads to activation of TRPA1 ion channels.12 Because opening of TRPA1, a nonselective cation channel, allows primarily Ca2+ and Na+ ions to enter the cell at resting membrane potentials, we investigated whether the UVR-mediated activation of TRPA1 leads to a change in membrane potential that might regulate downstream events.
UVR exposure causes retinal-dependent cellular depolarization
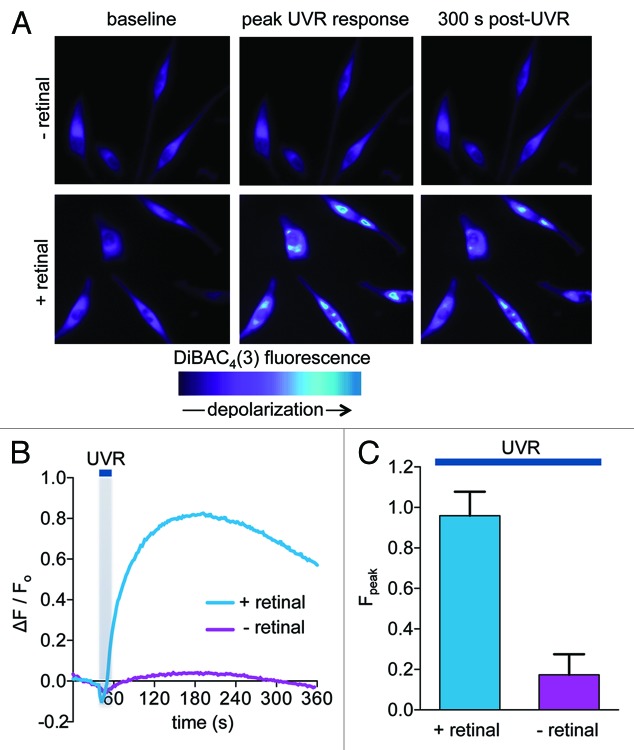
To measure UVR-induced changes in HEM membrane potential we used the voltage sensitive dye bis-(1,3-dibutylbarbituric acid) [DiBAC4(3)] and monitored the fluorescence intensity as a function of time of HEMs loaded with DiBAC4(3) and exposed to solar-like UVR doses equivalent to 240 sec of full sun exposure16 (240 mJ/cm2). An UVR-induced increase in fluorescence intensity was observed only when cells were preincubated with all-trans retinal (12 μM) (Fig. 1A, top panels vs. bottom panels), suggesting that UVR elicits a retinal-dependent membrane depolarization in HEMs (Fig. 1A–C). The change in DiBAC4(3) fluorescence that reflects the retinal-dependent depolarization was sustained, persisting for longer than 300 sec (Fig. 1A and B).
Figure 1. UVR induces a retinal-dependent increase in the fluorescence of the voltage-sensitive dye DiBAC4(3). (A) Pseudochrome fluorescence images of human epidermal melanocytes (HEMs) loaded with DiBAC4(3). HEMs preincubated with all-trans retinal (lower images) or vehicle (upper images) were imaged before (baseline, left images) or after exposure to 240 mJ/cm2 (12-sec exposure to 20 mW/cm2) UVR. The images (peak UVR response, middle) were recorded 120 sec after exposure, at the peak UVR-induced DiBAC4(3) fluorescence response and at 300 sec post-UVR (300 sec post-UVR, right images). (B) Relative changes in the DiBAC4(3) fluorescence intensity of representative HEMs preincubated with retinal (+ retinal) or vehicle (- retinal) and stimulated with UVR (240 mJ/cm2), as a function of time. (C) Mean amplitudes (Fpeak) of DiBAC4(3) relative fluorescence intensity of HEMs preincubated with retinal or vehicle and stimulated with 240 mJ/cm2 UVR. Bars represent average ± SEM. Fpeak = 0.96 ± 0.11 for retinal, Fpeak = 0.17 ± 0.10 for vehicle, p < 0.0006. n = 6 experiments per condition.
To our knowledge, direct measurements of HEM membrane potentials have not yet been reported. To further investigate the UVR-induced depolarization, we directly measured membrane potential (Vm) using whole-cell current clamp recordings with an internal solution containing physiological ionic concentrations. Current clamp measurements determined that unstimulated HEMs have a resting membrane potential of about −40 mV that is not altered by retinal preincubation (Vrest = −40.14 ± 1.94 mV, n = 7 in the absence of retinal and Vrest = −39.85 ± 1.27 mV, n = 11 in cells preincubated with retinal). UVR exposure did not significantly change Vrest in the absence of retinal (Fig. 2A, - retinal). However, in retinal-treated HEMs, UVR exposure induced a depolarization that became measurable during the UVR pulse, reached its maximum value within 20 sec after UVR exposure (ΔVm = 16.5 ± 1.06 mV), and then slowly repolarized to Vrest after more than 1 min following UVR stimulation (Fig. 2A and B).
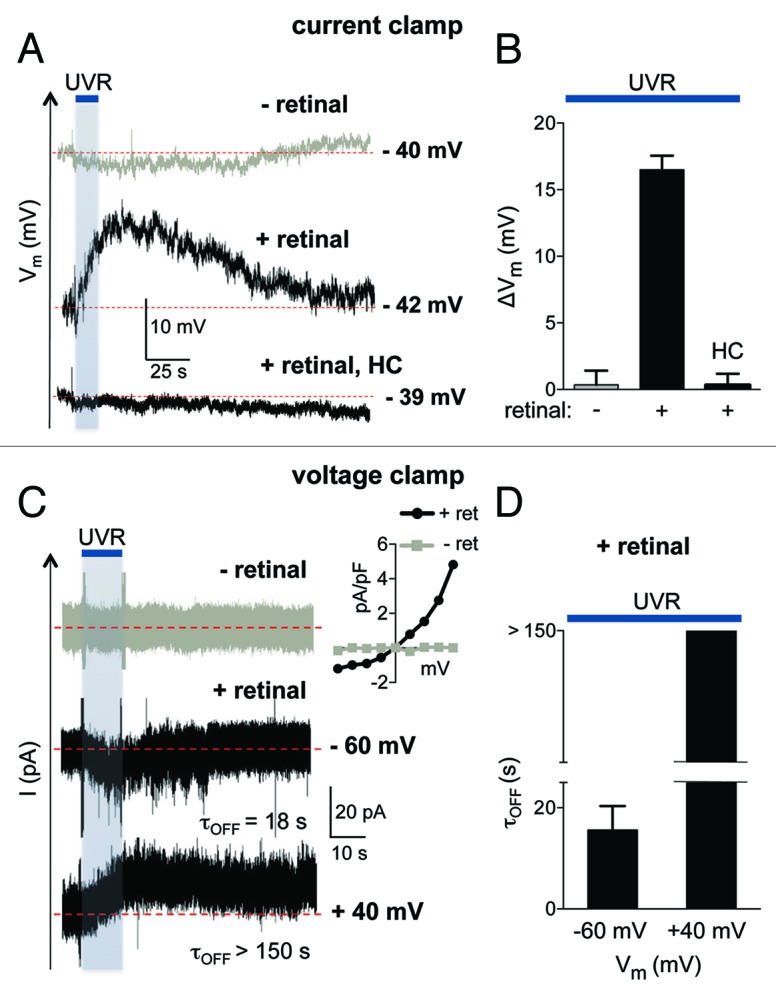
Figure 2. UVR exposure leads to cellular depolarization and sustained photocurrents. (A) In current clamp experiments, exposure of a representative HEM in whole-cell configuration to 240 mJ/cm2 UVR induced a retinal-dependent cellular depolarization that was abolished in the presence of the TRPA1 antagonist HC-030031 (HC, 100 μM). (B) The maximal change in mean membrane potential (ΔVm) elicited by UVR was significant only when HEMs were preincubated with retinal, but not in the absence of retinal. The retinal-dependent depolarization was abolished in the presence of HC-030031 (HC, 100 μM). ΔVm = 16.5 ± 1.06 mV for retinal, 0.35 ± 1.05 mV in the absence of retinal and 0.33 ± 0.84 mV when cells were treated with HC and retinal. n = 6 − 11 cells per condition. p < 0.0001 for retinal vs. no retinal and for retinal vs. HC. (C) The retinal-dependent UVR (240 mJ/cm2) photocurrent of a representative HEM inactivated completely with a time constant (τOFF) of 18 sec when the voltage was clamped at hyperpolarized potentials (−60 mV). In voltage-clamp recordings at a sustained depolarized potential (+40 mV), the UVR photocurrent inactivated incompletely, with a time constant (τOFF) > 150 sec. No photocurrents were measured in response to UVR in the absence of retinal. Inset: Current-voltage relationship of the UVR-activated whole-cell current, obtained using a voltage step protocol (−80 mV to +80 mV in 20 mV increments). (D) The average time constant (τOFF) for the decay of UVR photocurrents was τOFF = 15.62 ± 4.72 sec at −60 mV, while at +40 mV the current decay was incomplete, with a τOFF > 150 sec. n = 5 cells per condition.
Because during voltage clamp recordings UVR exposure of HEMs leads to retinal-dependent TRPA1 activation,12 we tested the contribution of TRPA1 ionic currents to the observed depolarization, using the specific TRPA1 antagonist HC-030031 (HC). In the presence of 100 μM HC, 240 mJ/cm2 UVR did not elicit a retinal-dependent depolarization in HEMs (Fig. 2A and B). Our data suggest that TRPA1 activation is necessary for the UVR-induced depolarization.
UVR photocurrent inactivation is dependent on Vm
Because TRPA1-mediated UVR photocurrents are outward rectifying (Fig. 2C), depolarization will have little effect on current amplitudes near physiological potentials. However, previous work showed that depolarized membrane potentials alter TRPA1-mediated inactivation kinetics in response to chemical agonists.17 To determine whether sustained depolarization of HEMs might similarly alter the kinetics of TRPA1-mediated currents, we monitored the retinal-dependent UVR-induced current as a function of time at hyperpolarized vs. depolarized potentials (Fig. 2C, inset).
At a constant negative potential (Vm = −60 mV), UVR evoked an inward photocurrent that inactivated shortly after the stimulus ceased, with the time constant τoff = 18 sec (Fig. 2C, middle trace). In contrast, at a constant positive potential (Vm = +40 mV), the same UVR dose evoked an outward current that persisted for many seconds after the stimulus, inactivating very slowly and often incompletely (τOFF > 150 sec, Fig. 2C, bottom trace). Thus, the time course of the UVR photocurrent inactivation in HEMs is dependent on membrane potential: currents inactivated relatively quickly and completely at a hyperpolarized potential, and slowly and often incompletely under strong depolarization (Fig. 2C and D).
UVR-induced depolarization contributes to sustained Ca2+ responses
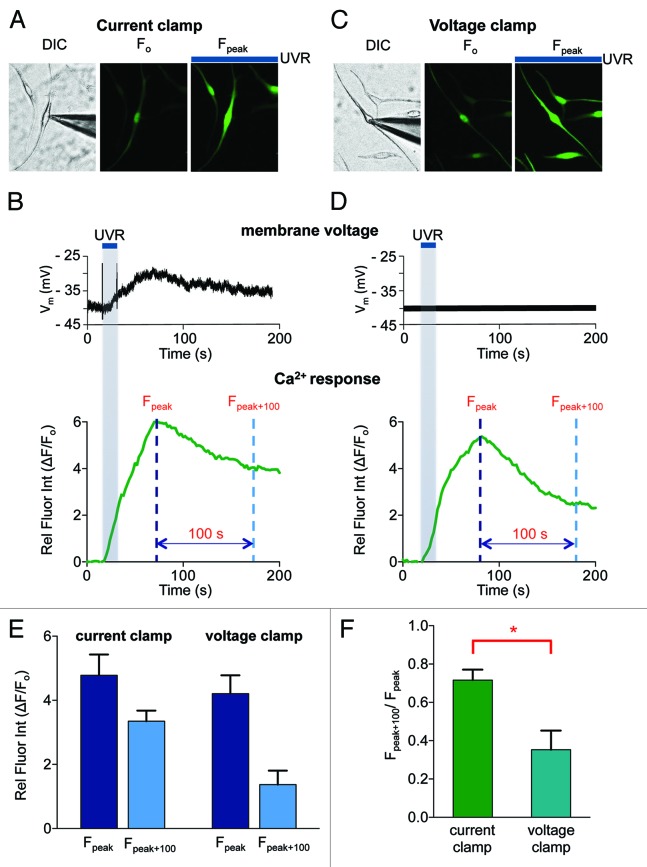
We previously found that in HEMs UVR induced a TRPA1-mediated sustained Ca2+ response that was critical for cellular melanin increase.12 To investigate whether the observed UVR-induced membrane depolarization can account for the persistent Ca2+ response, we performed Ca2+ imaging with the fluorometric Ca2+ indicator Fluo-4, while simultaneously monitoring the membrane potential. HEMs loaded with Fluo-4 and preincubated with retinal were stimulated with 240 mJ/cm2 UVR in whole-cell current clamp configuration (Fig. 3A and B). The membrane potential depolarized by ~10 mV and remained elevated for more than 200 sec (Fig. 3B, top panel). The intracellular Ca2+ concentration ([Ca2+]ic) of the monitored cell, as measured by the relative change in fluorescence intensity, increased with a similar time course and decreased very slowly after reaching the peak (Fig. 3B, bottom panel). We then monitored [Ca2+]ic while maintaining the membrane potential constant near the resting potential (Vm = −40 mV) (Fig. 3C). The time course and amplitude of the increase in [Ca2+]ic was similar to that seen in the current clamp experiment, but after reaching a peak, the [Ca2+]ic decreased more rapidly (Fig. 3D, bottom panel).
Figure 3. UVR-induced depolarization of HEMs modulates Ca2+ responses. (A) Images of a representative HEM loaded with the Ca2+ indicator Fluo-4 and stimulated with 240 mJ/cm2 UVR. In whole-cell current clamp experiments the fluorescence intensity of the recorded HEM (DIC, left image and F0, middle image) increased in response to UVR (Fpeak, right image). (B) In current clamp conditions the time course of the UVR-induced change in membrane voltage (top panel) and intracellular Ca2+ measured by the relative fluorescence intensity of the cell (ΔF/F0) (lower panel) were measured simultaneously for the HEM shown in (A). (C) Images of a representative HEM in whole-cell voltage clamp experiments near the resting membrane potential of HEMs (−40 mV) loaded with the Ca2+ indicator Fluo-4 (DIC, left image, and F0, middle image). UVR stimulation (240 mJ/cm2) leads to increased fluorescence intensity of the recorded HEM and neighboring cells (Fpeak, right image). (D) The membrane potential of the representative HEM shown in (C) was maintained at −40 mV (top panel). The Ca2+ response, as measured by the change in relative fluorescence intensity (ΔF/F0) as a function of time was determined in response to UVR stimulation (240 mJ/cm2) (lower panel). (E) Comparison of the peak (Fpeak) and delayed (Fpeak + 100) Ca2+ responses elicited by UVR (240 mJ/cm2) when the membrane was allowed to depolarize (current clamp) or was maintained constant (voltage clamp). The peak Ca2+ responses were not affected by membrane potential (dark blue bars, n = 5 cells per condition) and were reduced 100 sec after the peak response (Fpeak + 100) (light blue bars). Fpeak = 4.78 ± 0.07 for current clamp and 4.07 ± 0.46 for voltage clamp. Fpeak + 100 = 3.35 ± 0.33 for current clamp and 1.37 ± 0.44 for voltage clamp. n = 5 cells per condition. (F) The ratio of the peak and delayed Ca2+ responses (Fpeak+100/Fpeak) measured under current and voltage clamp conditions, respectively, reflects that the concentration of intracellular Ca2+ decays more rapidly under voltage clamp conditions than in current clamp, when the membrane is allowed to depolarize. (Fpeak + 100) / Fpeak = 0.72 ± 0.05 for current-clamp and 0.35 ± 0.10 for voltage-clamp. n = 5 cells per condition. p < 0.03.
To compare Ca2+ responses under the two recording conditions, we measured the change in fluorescence intensity at the peak (Fpeak) and 100 sec after the peak (Fpeak+100) (dashed lines in Fig. 3B and D, bottom panels). The average UVR-induced peak Ca2+ responses (Fpeak) were very similar when the membrane potential was allowed to depolarize (current clamp) and when it was maintained constant (voltage clamp). In contrast, sustained Ca2+ responses (Fpeak+100) were reduced when the cell was voltage clamped at −40 mV, when compared with current clamp conditions (Fig. 3E, light blue bars). To directly compare the sustained Ca2+ responses, we represented the ratio Fpeak+100/Fpeak and found that it was reduced by ~51% in voltage clamp, as compared with the current clamp configuration (Fig. 3F), suggesting that membrane depolarization contributes to the persistent Ca2+ response.
Our results show that UVR phototransduction in HEMs leads to persistent membrane depolarization, which delays the inactivation of TRPA1-mediated photocurrents, thus allowing for sustained Ca2+ responses. In whole-cell voltage clamp experiments, inactivation of agonist-induced TRPA1 currents occurs within seconds,17-20 the time-course of this inactivation is modulated by Ca2+ via an unknown mechanism,17,20 and has also been reported to depend on membrane potential.17 UVR-induced activation of TRPA1 ion channels is associated with an initial transient increase in intracellular Ca2+ mediated by both opening of the TRPA1 channels and by release from internal stores,12 which could regulate TRPA1 channel inactivation by directly binding to the cytosolic region of the channel21,22 or via an indirect mechanism. At resting membrane potential the initial Ca2+ response decays with a τ1/2 < 100 sec (Fig. 3D). We discovered, however, that simultaneous with the UVR-induced Ca2+ response, melanocytes exhibit a shift in their membrane potential toward positive values (Fig. 3B), which correlates with slower inactivation of TRPA1 channels, in a similar fashion as reported for agonist-dependent activation of TRPA1.17 When membrane depolarization occurs, the initial transient Ca2+ response is followed by a persistent response, which is required for the melanin increase induced by UVR in melanocytes.12
What conductances mediate the UVR-induced depolarization? Blocking TRPA1 precludes depolarization, suggesting that TRPA1 is required. The relatively small inward currents through TRPA1 channels (Fig. 2C) alone might not account for the measured depolarization; other channels activated downstream of UVR-signaling could contribute to voltage changes. The initial depolarization caused by cation flux through TRPA1 could activate voltage-gated Na+ or Ca2+ channels that would further depolarize the cell. Despite being non-excitable cells, primary human melanocytes might express voltage-gated Na+ channels9 and Ca2+ channels,23 but the characterization and function of these channels in melanocytes remains unknown. Alternatively, other Ca2+-dependent mechanisms could contribute to the observed depolarization. While TRPA1 contributes to both the initial transient and sustained phase of the Ca2+ response,12 membrane depolarization only contributes to the persistent Ca2+ response (Fig. 3B–F). Depolarization cannot occur in the absence of TRPA1, but TRPA1 will open in the absence of depolarization, as shown in voltage clamp experiments.
Short-term depolarization of excitable cells is a commonly used mechanism for intra- and intercellular signaling. Melanocytes have not been shown to function as excitable cells; persistent depolarization of human melanocytes with a depolarizing agent reportedly altered cellular morphology,13 but the physiological significance of this finding is not clear. Here we provide evidence that physiological doses of UVR are able to transiently depolarize human melanocytes in order to modulate the Ca2+ responses that lead to increased cellular melanin. Based on our results, we propose that UVR exposure leads to TRPA1-dependent depolarization, which acts to delay TRPA1 inactivation, leading to the sustained Ca2+ responses necessary for melanin synthesis (Fig. 4A and B).
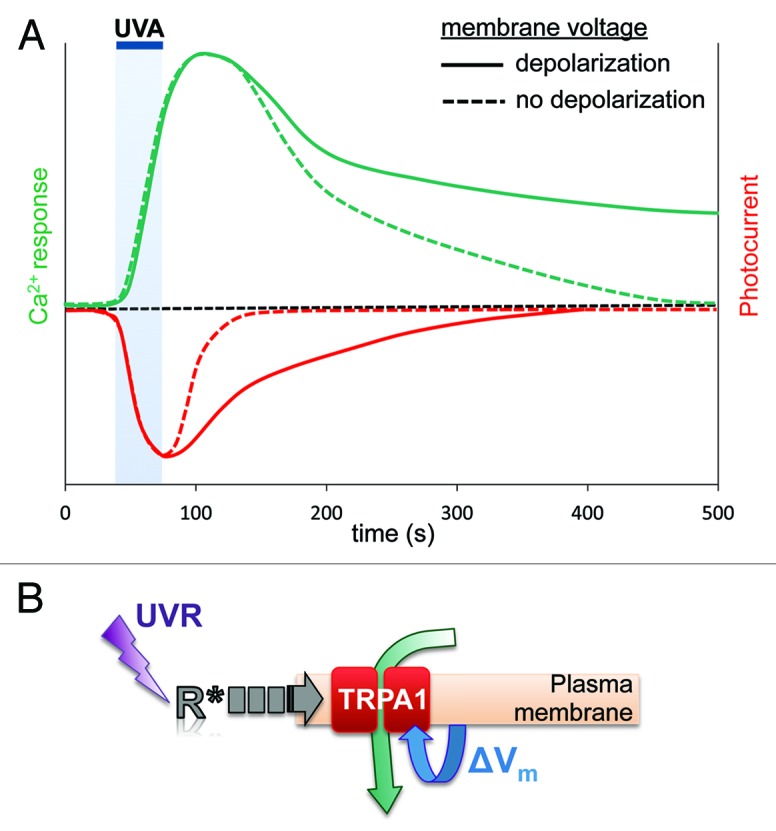
Figure 4. Membrane voltage modulates UVR phototransduction in human melanocytes. (A) Graphic representation of typical UVR-induced Ca2+ and photocurrent responses when the plasma membrane is allowed to depolarize or maintained at constant Vm (no depolarization). UVR exposure leads to HEM depolarization, which delays inactivation of UVR photocurrents (red traces), resulting in prolonged Ca2+ influx and increased duration of Ca2+ responses (green traces). Under voltage clamp, in the absence of membrane depolarization, UVR photocurrents inactivate faster and Ca2+ responses decay more rapidly. (B) Schematic representation of the proposed membrane voltage regulation of the UVR phototransduction pathway in human melanocytes. UVR activates a retinal-dependent G protein-coupled receptor, which initiates a signaling cascade to activate TRPA1. TRPA1 activation depolarizes the plasma membrane to modulate TRPA1-mediated UVR photocurrents by delaying channel inactivation. Prolonged photocurrents lead to the persistent Ca2+ responses required for early melanin synthesis.
Materials and Methods
UVR stimulation
UVR stimulation was performed as previously described,12,16 using a Mercury-Xenon Arc lamp (Newport) with filtered output (90% UVA and 10% UVB) comparable to solar UVR. Each 1 sec of experimental irradiance of 10 mW/cm2 equates to 10-sec exposure to bright solar UVR (with a UV index of 10). Light was filtered using 280-nm long-pass (LP) / 400-nm short-pass (SP) filters for UV radiation (UVR).
Electrophysiology
Electrophysiological experiments were performed as previously described.12 All-trans retinal was stored, solubilized, and applied as previously described.16 All experiments were performed under dim-red or infrared illumination. Whole-cell patch clamp recordings were carried-out using micropipettes with 3–6 MΩ resistance at room temperature using an EPC 10 amplifier (HEKA Instruments Inc.) with PatchMaster software (HEKA Instruments Inc.), filtered at 2.9 kHz and digitized at 20 kHz. Modified Ringer’s extracellular solution contained (in mM): 150 NaCl, 1.8 CaCl2, 1.2 MgCl2, 10 D-glucose, 25 HEPES; pH 7.4, 310 mOsm/L. Voltage clamp recording internal pipette solution contained (in mM): 140 CsCl, 1 MgCl2, 4 MgATP, 10 EGTA, 10 HEPES; pH 7.2, 290 mOsm/L. Current clamp recording internal pipette solution contained (in mM): 120 K-gluconate, 4 NaCl, 6 Na-gluconate, 2 MgATP, 0.02 EGTA, 10 HEPES; pH 7.2, 290 mOsm/L. Current clamp pipette solution was used for simultaneous voltage clamp and Ca2+ imaging experiments. Whole-cell current values were plotted as a function of time and fitted with a single-exponential function in Prism 6 (GraphPad) to calculate the time constants of inactivation.
Membrane potential measurement and Ca2+ imaging
Image series were acquired at 2-sec intervals and the fluorescence intensity in a region of each cell was measured as a function of time using NIS Elements software (Nikon). The data were then analyzed with MatLab (MathWorks) and plotted using Prism 6 software. For membrane potential measurements, cells were incubated for 20 min in Ringer’s solution containing 5 μM DiBAC4(3) (Molecular Probes) and 250 μM sulfinpyrazone to prevent loss of DiBAC4(3) from cells. Fluorescent calcium imaging was performed using the fluorometric calcium indicator Fluo-4 AM (Invitrogen/Molecular Probes). Cells were incubated for 20 min in Ringer’s solution containing 2 μM Fluo-4 AM and 250 μM sulfinpyrazone, then washed and imaged at room temperature. DiBAC4(3) and Fluo-4 fluorescence intensities were quantified as ΔF / Fo (t) = [Fcell(t) − Fbaseline] / Fbaseline. UVR-induced sustained Ca2+ responses were measured 100 sec after peak responses and quantified as (Fsustained − Fo) / (Fpeak − Fo).
Statistical analyses
Numerical data are mean ± SEM and p-values were calculated using the two-tailed Studen’s t test and considered significant when p < 0.05. n refers to the number of cells for electrophysiology and Ca2+ imaging experiments and the number of independent experiments for membrane potential imaging.
Acknowledgments
This work was supported by grants from Brown University (to E.O.) and a National Science Foundation Graduate Research Fellowship (to N.W.B.). We thank Dr Anita Zimmerman, Dr Julie Kauer and Valerie Yorgan for helpful discussion and Sarah Pierce for assistance with experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/25322
References
- 1.Zecca L, Tampellini D, Gatti A, Crippa R, Eisner M, Sulzer D, et al. The neuromelanin of human substantia nigra and its interaction with metals. J Neural Transm. 2002;109:663–72. doi: 10.1007/s007020200055. [DOI] [PubMed] [Google Scholar]
- 2.Brito FC, Kos L. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res. 2008;21:464–70. doi: 10.1111/j.1755-148X.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 3.Yajima I, Larue L. The location of heart melanocytes is specified and the level of pigmentation in the heart may correlate with coat color. Pigment Cell Melanoma Res. 2008;21:471–6. doi: 10.1111/j.1755-148X.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana M. Sound needs sound melanocytes to be heard. Pigment Cell Res. 1999;12:344–54. doi: 10.1111/j.1600-0749.1999.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami A, Fisher DE. Key discoveries in melanocyte development. J Invest Dermatol. 2011;131(E1):E2–4. doi: 10.1038/skinbio.2011.2. [DOI] [PubMed] [Google Scholar]
- 6.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–94. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 7.Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, et al. What are melanocytes really doing all day long...? Exp Dermatol. 2009;18:799–819. doi: 10.1111/j.1600-0625.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekmehag B, Persson B, Rorsman P, Rorsman H. Demonstration of voltage-dependent and TTX-sensitive Na(+)-channels in human melanocytes. Pigment Cell Res. 1994;7:333–8. doi: 10.1111/j.1600-0749.1994.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 10.Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanisz H, Stark A, Kilch T, Schwarz EC, Müller CS, Peinelt C, et al. ORAI1 Ca(2+) channels control endothelin-1-induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol. 2012;132:1443–51. doi: 10.1038/jid.2011.478. [DOI] [PubMed] [Google Scholar]
- 12.Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A. 2013;110:2383–8. doi: 10.1073/pnas.1215555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heck DE, Gerecke DR, Vetrano AM, Laskin JD. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol Appl Pharmacol. 2004;195:288–97. doi: 10.1016/j.taap.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–64. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21:1906–11. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–61. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YY, Chang RB, Allgood SD, Silver WL, Liman ERA. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137:493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–5. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 20.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–9. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 22.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–9. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Pushparaj C, Bahí N, Sorolla A, Herreros J, Pamplona R, et al. Functional expression of voltage-gated calcium channels in human melanoma. Pigment Cell Melanoma Res. 2012;25:200–12. doi: 10.1111/j.1755-148X.2012.00978.x. [DOI] [PubMed] [Google Scholar]