Abstract
Hyperpolarization-activated, cyclic nucleotide-sensitive (HCN4) channels produce the “funny current,” If, which contributes to spontaneous pacemaking in sinoatrial myocytes (SAMs). The C-terminus of HCN channels inhibits voltage-dependent gating, and cAMP binding relieves this “autoinhibition.” We previously showed 1) that autoinhibition in HCN4 can be relieved in the absence of cAMP in some cellular contexts and 2) that PKA is required for β adrenergic receptor (βAR) signaling to HCN4 in SAMs. Together, these results raise the possibility that native HCN channels in SAMs may be insensitive to direct activation by cAMP. Here, we examined PKA-independent activation of If by cAMP in SAMs. We observed similar robust activation of If by exogenous cAMP and Rp-cAMP (an analog than cannot activate PKA). Thus PKA-dependent βAR-to-HCN signaling does not result from cAMP insensitivity of sinoatrial HCN channels and might instead arise via PKA-dependent limitation of cAMP production and/or cAMP access to HCN channels in SAMs.
Keywords: sinoatrial node, If, hyperpolarization-activated cyclic nucleotide-sensitive channel, HCN4, cyclic nucleotide-binding domain, protein kinase A
Introduction
Hyperpolarization-activated, cyclic nucleotide-sensitive (HCN) channels produce the cardiac “funny current,” If, which contributes to spontaneous pacemaker activity in sinoatrial myocytes (SAMs). HCN channels have a conserved cyclic nucleotide binding domain (CNBD) in the C-terminus which inhibits voltage-dependent gating. cAMP binding to the CNBD relieves this “autoinhibition,” causing a depolarizing shift in the voltage dependence of activation.1 We recently observed that autoinhibition of HCN4 (the predominant sinoatrial HCN isoform) can be relieved in the absence of ligand in some cellular contexts, rendering the channels insensitive to cAMP.2
β adrenergic receptor (βAR) stimulation potentiates If via a depolarizing shift in the voltage dependence of activation. It is generally assumed that direct cAMP binding to HCN4 mediates this βAR activation of If. However, we previously showed that βAR signaling to HCN channels in SAMs requires PKA activity, and that PKA phosphorylation of heterologously-expressed HCN4 channels causes a depolarizing shift in voltage dependence, which is similar in magnitude to the shifts produced by βAR stimulation or cAMP binding.3 These results suggest a model in which βAR-generated cAMP activates If via PKA-dependent phosphorylation of the native sinoatrial HCN channels. However, indirect, mechanisms for PKA-dependent regulation of If are also possible, and the mechanistic basis for the PKA requirement in βAR-to-HCN signaling in SAMs is not known.
Taken together our findings of tunable cAMP sensitivity of HCN4 and of PKA-dependence in βAR-to-HCN signaling raise the possibility that native HCN channels in mouse SAMs may be insensitive to direct activation by cAMP. In this short follow-up study, we evaluated the ability of cAMP to activate If in mouse SAMs in the absence of PKA activity.
Results and Discussion
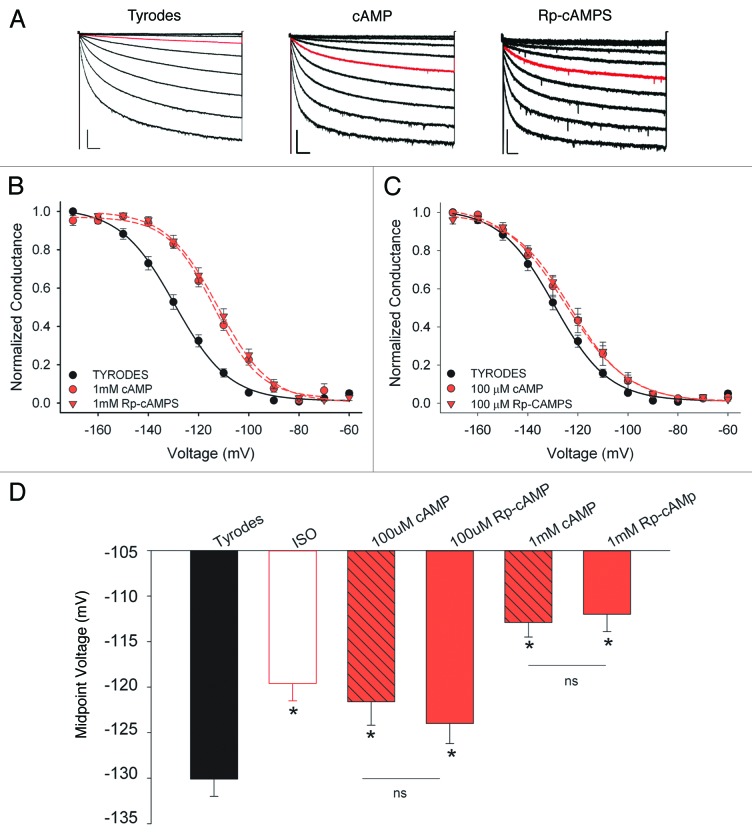
If was recorded from acutely dissociated mouse SAMs in whole cell voltage clamp recordings. Cells were held at−50 mV, and If was elicited by 3-sec test pulses from −60 to −170 mV in 10 mV increments (Fig. 1A). To determine whether cAMP can activate native HCNs in SAMs independent of PKA activity, we compared the effects on the midpoint activation voltage (V1/2) of If in response to intracellular dialysis with cAMP or Rp-adenosine cyclic 3′5′-phosphorothioate (Rp-cAMPS), a cAMP analog that cannot activate PKA4 but can activate If in excised inside-out membrane patches from rabbit SAMs.5 We found that cAMP and Rp-cAMPS produced nearly identical depolarizing shifts in the midpoint activation voltage (V1/2) of If in mouse SAMs when applied at either a saturating (1 mM) or sub-saturating (100 μM) concentration (Fig. 1B and C; Table 1). The V1/2 values for 1 mM cAMP or Rp-cAMPS were significantly more depolarized than the V1/2 produced by the βAR agonist, isoproterenol (ISO; 1 μM), whereas V1/2 values in 100 μM Rp-cAMPS or cAMP were statistically similar to those produced by ISO (Table 1).
Figure 1. Similar effects of cAMP and Rp-cAMPS on If in sinoatrial myocytes. (A) Representative If whole cell current families recorded from SAMs in control (Tyrodes), 1 mM camp, or 1 mM Rp-cAMPS. Red traces indicate currents at −100 mV to illustrate similar shift in voltage dependence in the presence of cAMP or Rp-cAMPS. Scale bars, 250 ms 200 pA for control and 1 mM camp, 250 ms and 100 pA for 1 mM Rp-cAMP. (B) Average normalized conductance-voltage plots for If in Tyrodes (black circles), 1 mM cAMP (red circles), or 1 mM Rp-cAMPS (red triangles). (C) Average normalized conductance-voltage plots for If in Tyrodes (black circles), 100 μM cAMP (red circles), or 100 μM Rp-cAMPS (red triangles). (D) Average midpoint activation voltages for If in Tyrodes, 1 μM ISO, or the indicated concentrations of cAMP or Rp-cAMPS. Asterisks indicate p < 0.05 vs. Tyrodes, ns indicates p > 0.05.
Table 1. Midpoint activation voltages for If in mouse sinoatrial myocytes.
| V1/2 control (mV) |
n | |
|---|---|---|
| Tyrodes | −130.1 ± 1.9 | 17 |
| ISO | −119.6 ± 1.9* | 17 |
| 3mM cAMP | −114.1 ± 1.9*,† | 7 |
| 1mM cAMP | −112.9 ± 1.6*,† | 10 |
| 1mM Rp-cAMP | −112.0 ± 1.9*,† | 10 |
| 100 uM cAMP | −121.6 ± 2.6* | 14 |
| 100 uM Rp-cAMP | −124.0 ± 2.2* | 14 |
p < 0.05 vs. control, †p < 0.05 in comparison with each other. One-way ANOVAs with Student-Newman-Keuls post-test.
These data demonstrate that PKA activity is not required for cAMP stimulation of native HCN channels in mouse sinoatrial myocytes under the same recording conditions in which we observed that PKA activity is required for βAR-mediated activation of If. Thus, the requirement for PKA in βAR-to-HCN signaling in SAMs does not result from cAMP insensitivity of sinoatrial HCN channels. Rather, it most likely arises via limitation of cAMP production and/or restriction of cAMP access to HCN channels. A simple model for PKA-dependent cAMP production is difficult to conceive given that the known PKA-sensitive adenylate cyclase (AC) isoforms are inhibited rather than activated by PKA6 (although a more complicated scenario is suggested by the observation that Ca2+-activated ACs have been shown to regulate If in guinea pig SAMs7). On the other hand, PKA activates the cardiac phosphodiesterases, PDE3 and PDE48 and PDEs have been shown to form restricted cAMP signaling domains in a variety of cellular systems9-12 and to regulate both If13 and sinoatrial pacemaking rate.14-16 Quantitative description of the roles of ACs and PDEs in βAR regulation of If in SAMs awaits future studies
Materials and Methods
Animal procedures were performed in accordance with protocols approved by the IACUC at the University of Colorado Denver, Anschutz Medical Campus. SAMs were isolated from adult male C57BL/6J mice as previously described.3,17
Whole-cell voltage-clamp recordings of If from SAMs were conducted as previously reported.3,17 Cells were perfused (1–2 ml/min) with Tyrode’s solution (in mM, 140 NaCl, 5.4 KCl, 1.2 KH2PO4, 5 HEPES, 5.55 glucose, 1 MgCl2, 1.8 CaCl2 ; pH adjusted to 7.4 with NaOH) containing 1 mM BaCl2. Recording pipettes had resistances of ~1.5–3.0 MΩ when filled with an intracellular solution consisting of (in mM) 135 potassium aspartate, 6.6 sodium phosphocreatine, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 EGTA, 4 Mg-ATP; pH adjusted to 7.2 with KOH. cAMP (Sigma-Aldrich A6885) or Rp-adenosine-3′5′-cyclic monophosphorothioate sodium salt (Rp-cAMPS; BioLog A 002 S) were added to the intracellular solution at the indicated concentrations. Reported voltages were corrected for a calculated −14 mV junction potential error. Conductance was calculated from inward currents using the equation G = I/(V-Vr), where G is conductance, I is the time-dependent inward current at a given voltage, V, and Vr is the reversal potential for If (-30 mV3,18). Conductances were subsequently plotted as a function of voltage and fit with a Boltzmann equation to determine midpoint activation voltages (V1/2).
Acknowledgements
This work was supported by NIH grant HL088427 (to C.P.). E.D.L. was partially supported by NINDS T32NS007083.
Glossary
Abbreviations:
- βAR
β adrenergic receptor
- CHO
Chinese hamster ovary cell
- CNBD
cyclic nucleotide-binding domain
- HCN
hyperpolarization-activated, cyclic nucleotide-sensitive channel
- HEK
human embryonic kidney cell
- ISO
isoproterenol
- PDE
phosphodiesterase
- Rp-cAMPS
Rp-adenosine cyclic 3′5′-phosphorothioate
- SAM
sinoatrial myocyte
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/25293
References
- 1.Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–10. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- 2.Liao Z, Lockhead D, St Clair JR, Larson ED, Wilson CE, Proenza C. Cellular context and multiple channel domains determine cAMP sensitivity of HCN4 channels: ligand-independent relief of autoinhibition in HCN4. J Gen Physiol. 2012;140:557–66. doi: 10.1085/jgp.201210858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 2010;136:247–58. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothermel JD, Parker Botelho LH. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988;251:757–62. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bois P, Renaudon B, Baruscotti M, Lenfant J, DiFrancesco D. Activation of f-channels by cAMP analogues in macropatches from rabbit sino-atrial node myocytes. J Physiol. 1997;501:565–71. doi: 10.1111/j.1469-7793.1997.565bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 7.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582:1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira RF, Terrin A, Di Benedetto G, Cannon RC, Koh W, Kim M, et al. The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS One. 2010;5:e11725. doi: 10.1371/journal.pone.0011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terrin A, Monterisi S, Stangherlin A, Zoccarato A, Koschinski A, Surdo NC, et al. PKA and PDE4D3 anchoring to AKAP9 provides distinct regulation of cAMP signals at the centrosome. J Cell Biol. 2012;198:607–21. doi: 10.1083/jcb.201201059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes AP, Livera G, Huang P, Sun C, O’Neal WK, Conti M, et al. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem. 2005;280:7997–8003. doi: 10.1074/jbc.M407521200. [DOI] [PubMed] [Google Scholar]
- 12.Jurevicius J, Skeberdis VA, Fischmeister R. Role of cyclic nucleotide phosphodiesterase isoforms in cAMP compartmentation following beta2-adrenergic stimulation of ICa,L in frog ventricular myocytes. J Physiol. 2003;551:239–52. doi: 10.1113/jphysiol.2003.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua R, Adamczyk A, Robbins C, Ray G, Rose RA. Distinct patterns of constitutive phosphodiesterase activity in mouse sinoatrial node and atrial myocardium. PLoS One. 2012;7:e47652. doi: 10.1371/journal.pone.0047652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galindo-Tovar A, Kaumann AJ. Phosphodiesterase-4 blunts inotropism and arrhythmias but not sinoatrial tachycardia of (-)-adrenaline mediated through mouse cardiac beta(1)-adrenoceptors. Br J Pharmacol. 2008;153:710–20. doi: 10.1038/sj.bjp.0707631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaumann AJ, Galindo-Tovar A, Escudero E, Vargas ML. Phosphodiesterases do not limit beta1-adrenoceptor-mediated sinoatrial tachycardia: evidence with PDE3 and PDE4 in rabbits and PDE1-5 in rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:421–30. doi: 10.1007/s00210-009-0445-5. [DOI] [PubMed] [Google Scholar]
- 16.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–9. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, St Clair JR, Larson ED, Proenza C. Myristoylated peptides potentiate the funny current (I(f)) in sinoatrial myocytes. Channels (Austin) 2011;5:115–9. doi: 10.4161/chan.5.2.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52:51–64. doi: 10.1016/S0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]