Abstract
This paper presents the characterization and comparison of physiological tremor for pointing tasks in multiple environments, as a baseline for performance evaluation of microsurgical robotics. Previous studies have examined the characteristics of physiological tremor under laboratory settings as well as different operating conditions. However, different test methods make the comparison of results across trials and conditions difficult. Two vitroretinal microsurgeons were evaluated while performing a pointing task with no entry-point constraint, constrained by an artificial eye model, and constrained by a rabbit eye in vivo. For the three respective conditions the 3D RMS positioning error was 144 μm, 258 μm, and 285 μm, and maximum 3D error was 349 μm, 647 μm, and 696 μm. A spectral analysis was also performed, confirming a distinct peak near in the 6–12 Hz frequency range, characteristic of hand tremor during tasks in all three environments.
Keywords: tremor, accuracy, microsurgery, physiological tremor, medical robotics, smart instruments
I. Introduction
Microsurgical tasks, such as those performed in the field of ophthalmology, are some of the most difficult tasks for surgeons due to limitations in human performance. Procedures dealing with retinal vein occlusions [1] or internal limiting membrane (ILM) peeling require micrometer positioning accuracy, as typical thickness of the ILM is around 5–10 μm [2], while retinal vasculature is less than 100 μm in diameter. Accuracy during the procedure must be on the order of these feature sizes, and preferably better, in order to successfully avoid damaging other tissue within the eye. Meanwhile, hand tremor has been shown to have an RMS amplitude on the order of 50–200 μm [3], [4] at a frequency commonly in the 8–12 Hz band [5–7].
Several groups have developed robotic systems with the aim of improving positioning accuracy and reducing hand tremor, such as the Steady Hand Eye Robot [8] and Micron, a fully active handheld micromanipulator [9] and others [10]. It is important to characterize hand tremor during microsurgery in vivo in order to provide accurate system design parameters for these systems and to quantify the degree to which tests in vitro provide a realistic environment for evaluation of tremor-cancellation performance by such systems.
To this end, hand tremor has been characterized while performing simple tasks within the laboratory setting using inertial sensing [11][12], optical tracking [13][14], and handwriting analysis [15]. However, the main interest of these studies has been toward developing models to quantify pathological tremor such as Parkinson’s disease or essential tremor [5], rather than characterizing normal physiological tremor under realistic surgical conditions. Only a few attempts have been made to characterize tremor during surgical procedures in vivo [11], [16].
There has been no investigation of tremor directly comparing such laboratory tests to surgical tasks in vivo. This is possibly due to the difficulty of measuring the instrument tip accurately during the surgery without interfering with the task. In an ongoing effort to quantify the three dimensional accuracy during microsurgery, this paper presents some of the first comparisons between results in different environments.
Tremor is typically defined as a quasi-periodic oscillation of varying amplitude in a limb [17]. For the purpose of evaluating microsurgical systems, we shall define tremor as any unwanted or involuntary motion of the hand. This includes such occurrences as low frequency wander or drift, involuntary jerk, and quasi-periodic oscillatory motion. This methodology is used since any unwanted deviation during microsurgical tasks has the potential for damaging tissue and should be mitigated.
II. Materials and Methods
Tremor was evaluated under three different conditions in order to examine how the effects of interacting with different environments influences tremor characteristics. Conditions such as the elevation of the hand, posture, and positioning of the instrument were kept as similar as possible across all trials in order to provide accurate comparison between testing conditions. The surgical setups for testing within an artificial eye and in vivo are shown in Fig. 2 and Fig. 3 to demonstrate their similarity. To evaluate tremor in each condition, two surgeons performed a holding-still task in which they attempted to hold the tip of the instrument fixed in 3D space above a visible target point for a 20-second duration. The task was then repeated three times for each condition, for a total of six trials per surgeon per condition. All the tasks were performed under magnification through a stereo surgical microscope with the same instrument.
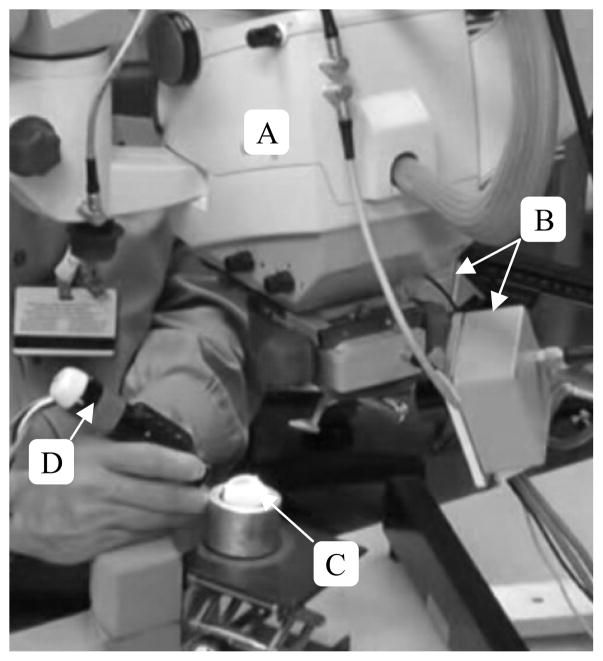
Figure 2.
Surgical setup for holding-still tasks in the eye phantom. A) Surgical microscope B) Apparatus to Sense Accuracy of Position (ASAP) C) Eye phantom D) Micron handheld micromanipulator
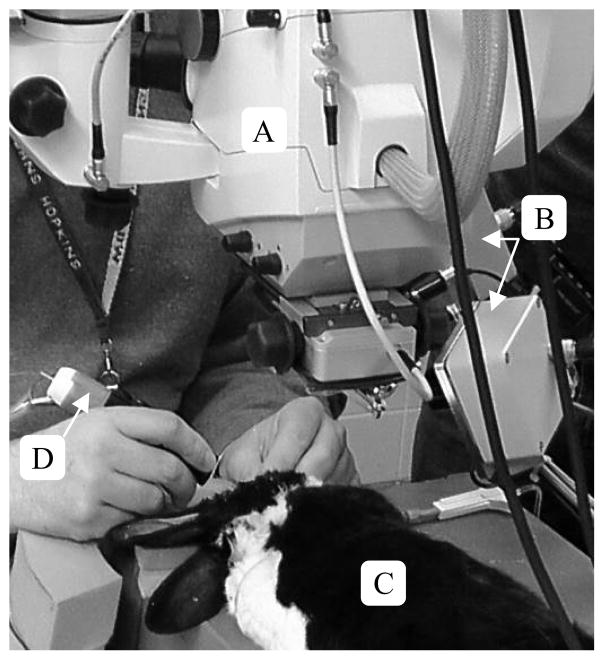
Figure 3.
Surgical setup for holding-still tasks in vivo. A) Surgical microscope B) Apparatus to Sense Accuracy of Position (ASAP) C) Rabbit D) Micron handheld micromanipulator.
Position sensing across trials was obtained through the use of Micron, a fully handheld active micromanipulator [9] and a custom optical tracking system known as ASAP (Apparatus to Sense Accuracy of Position) [18]. ASAP consists of two position sensitive detectors (PSDs) which track six LEDs located on the Micron handpiece, shown in Fig. 1. The LEDs provide three degree of freedom (3DOF) tracking of the handpiece and tip orientation. The position measurements are sampled at a rate of 1 kHz and are accurate to within 6 3m RMS. The tip position is then reconstructed using the known dimensions of the actuator. During trials, the data was logged to a workstation over Ethernet using User Datagram Protocol (UDP) to be analyzed later.
Figure 1.

Micron, a handheld micromanipulator with positioning LEDs is shown above. Orthogonal coordinate axes used for analysis are defined along the instrument tip as shown. The z-axis is oriented axially along the tip with the positive direction pointing towards the distal end. The z-axis is defined as orthogonal to the x-axis in the plane defined by the three LEDs closest to the tip. The y-axis then extends downward normal to the same LED plane.
All of the data collected is oriented with respect to the LEDs mounted on the handpiece. It is possible to transform the position data from the tool space into the workspace; however, this manipulation decreases the accuracy of the data. For this reason the results presented herein are oriented with respect to the handpiece coordinate system shown in Fig. 1.
1) Unconstrained model
The unconstrained model has been the most common method for studying the characteristics of tremor. During this trial condition the hand was supported in the same or similar posture assumed for surgery. However, the tip of the instrument is unconstrained by any structure such as the eyeball (vitreoretinal surgeons commonly refer to this as “open sky”). In order to maintain a similar target for the task as in other trials, a fundus image was placed in the microscope view. The surgeon was then asked to locate a feature on the surface of the image and hold the instrument tip a fixed distance above the feature for the duration of the trial.
2) Constrained model
The constrained model is tested using an artificial eye model developed by Johns Hopkins University, known as the eye phantom. The eye phantom is a hollow soft silicone sphere, roughly 25mm in diameter, that mimics the sclera of the eye. The interior of the eye phantom is painted to resemble the retinal vasculature and provide a consistent target across trials. The setup for the eye phantom is shown in Fig. 2, and was created to mimic the conditions in vivio. Rotation of the eye in the orbit was created by placing the eye phantom in a machined aluminum cup filled with a water-based lubricant (K-Y Jelly). During trials a cannula was inserted through the eye phantom wall, and the instrument tip then inserted through the cannula for the holding-still task.
3) Rabbit model
Trials in vivo were performed in the rabbit eye with the setup shown in Fig. 3. The rabbit was anesthetized and the vitreous humor was removed via pars plana vitrectomy under a board-approved protocol. When necessary, the lens was also removed from the eye in order to achieve sufficient visualization of the retina. The instrument was then inserted through a cannula in the sclera [2] and the surgeon was asked to locate a feature on the surface of the retina then hold the position for the duration of the trial.
B. Data Processing
The RMS and maximum error for tremor in the holding-still tasks was determined by calculating the mean position of data collected for each trial. The mean position was then used as the surgeon’s intended target (goal) position. Data were then compared across trials with respect to their calculated goal position for each individual axis and in three dimensions. While calculating RMS amplitude this method provides a good comparison of the overall magnitude of tremor across trials, it downplays the role of the larger variations due to tremor. These larger excursions can potentially cause the most harm to tissue, and for this reason it is also important to assess the maximum error. For each trial the maximum error was found for each axis and in three dimensions relative to the goal position, the mean was then calculated across similar trials.
In addition to assessing the RMS and maximum tremor magnitude, the frequency of occurrence was also investigated by combining data sets across each axis in similar holding-still trials to generate histograms for each condition. The number of occurrences was then normalized for each axis and trial for comparison.
A spectral analysis was also performed across all trials of similar type to confirm the source of the tremor and investigate the type of tremor contributing to positioning error. Hand tremor was already understood to contribute within the 8–12 Hz range as mentioned previously, however other sources of low frequency noise below 20 Hz could also contribute to the tremor magnitude. For comparison, spectral data were calculated for each trial and averaged at each frequency in three dimensions and for each individual axis.
III. Results
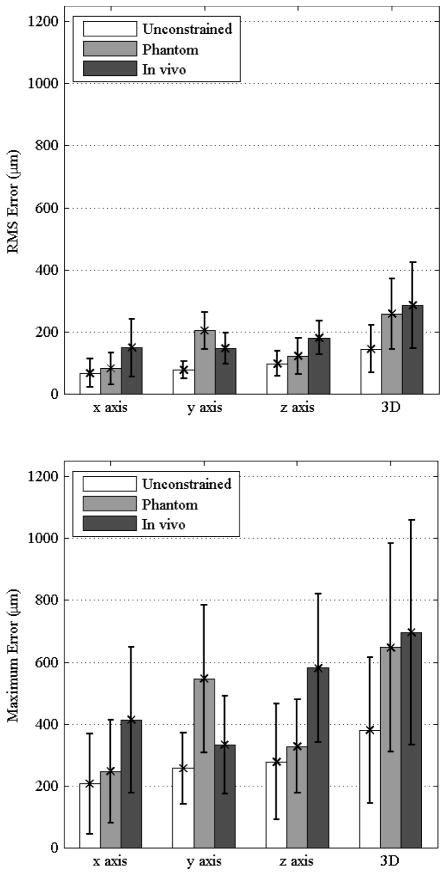
The results of the RMS error and maximum error for all conditions are presented in Fig. 4 in the upper and lower graphs, respectively. The three-dimensional RMS error was 144±67 μm, 258±144 μm, 285±146 μm when unconstrained, in the eye phantom, and in vivo, respectively. Maximum error was observed to be roughly three times larger than RMS error in all cases, with the three dimensional maximum error calculated to be 379±143 μm, 647±309 μm, 696±332 μm when unconstrained, in the eye phantom, and in vivo, respectively.
Figure 4.
(upper) RMS error is shown for each individual axis and in three dimensions across all trials. (lower) The maximum error is shown with error bars indicating one standard deviation.
Similar trends of increasing error across conditions can be observed across each axis in all conditions. However, significant results can only be observed when differentiating between some of the conditions depending on the axis as seen in Fig. 4. A unique outlier is that the error is significantly larger along the y axis for the phantom trials, which correspondingly increases the three-dimensional maximum error.
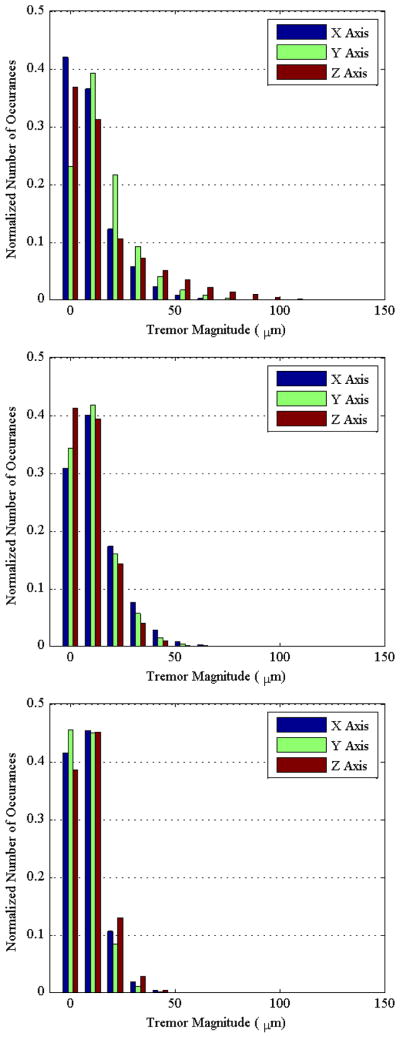
The histograms shown in Fig. 5 present the normalized frequency of occurrence for varying tremor magnitudes under each condition. The frequency of higher-magnitude tremor shows a decreasing trend from the unconstrained condition, to conditions in the eye phantom, and then to conditions in vivo. This decreasing trend in the frequency remains fairly constant in all three axes across trial conditions.
Figure 5.
Histograms are shown for the conditions while unconstrained (upper), constrained (middle), and in vivo (lower). The number of occurrences in each data set is normalized along the vertical axis for comparison. Frequencies for higher-magnitude tremor can be seen to decrease from unconstrained to constrained conditions to conditions in vivo.
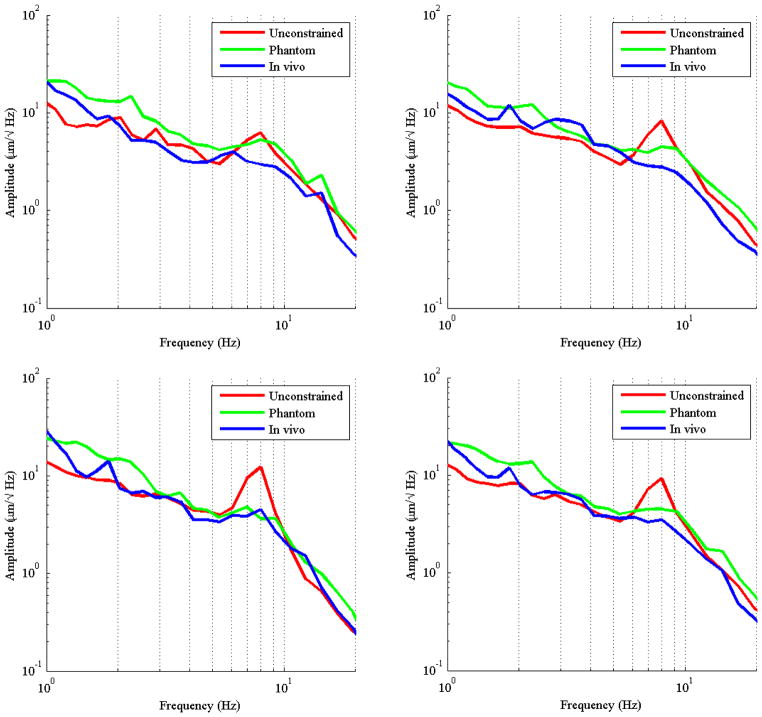
A comparison of averaged spectral data across trials for the frequency range from 0.1 Hz to 20 Hz is presented in Fig. 6 for all conditions. Distinct peaks can be observed in the 1–3 Hz frequency range and the 6–12 Hz range in all axes and conditions. However, the peak in the 6–12 Hz region in the unconstrained condition is much higher as compared to the condition in vivo. The conditions in the eye phantom and in vivo have a peak that is much flatter and distributed over a larger frequency range than the unconstrained condition.
Figure 6.
Spectral analysis is shown across all three trials for the x-axis (top left), y-axis (top right), z-axis (bottom left), and 3D (bottom right). The hand tremor peak is distinctly visible for the unconstrained condition in the range of 6–12Hz. The lower frequency drift may also be observed in the range of 1–3 Hz for the conditions when constrained and in vivo. The third higher frequency peak in the 20–40 Hz range along the x-axis for the conditions when constrained and in vivo is associated with finger tremor.
IV. Discussion
By controlling for variability between surgical conditions it is possible to assess the comparison between tremor in the unconstrained laboratory, a constrained condition within an eye phantom, and their feasibility with respect to conditions in vivo. To this end the RMS and maximum error presented in Fig. 4 demonstrate that there is an increased positioning error during holding-still tasks along all axes during operation in vivo. However, this error in vivo is not significantly different than the error for the constrained condition, suggesting that the additional kinematic constraint at the sclerotomy may increase tip position error, presumably due to the awkwardness of the kinematic configuration for the human operator.
Within the spectral data, distinct peaks can be observed in the 1–3 Hz frequency range that can be attributed to voluntary corrections through visual human-in-the-loop feedback [19]. The larger frequency peak identifiable at the range of 6–12 Hz, which matches well with prior literature showing the tremor peak related to the hand to occur in the range of 8–12 Hz [6], [11], [19]. When the hand tremor peak across conditions, it is more widely distributed in the eye phantom and in vivo. Though there is similar energy from the oscillations in all conditions, the energy from the hand is distributed over a larger frequency range for these conditions. As hand position was kept relatively constant during all trials, the shift in amplitude between conditions can be attributed to the constraint imposed by the entry point to the eye.
Since the instrument is passed through a cannula inserted through the eye wall, the shaft of the instrument may translate axially more or less unimpeded, manifesting as the larger tremor peak along the z axis. The substantial impedance encountered in the lateral directions by the tissue (or silicone) results in a damping effect, as the axes of the instrument are no longer free to oscillate independently as in the unconstrained condition.
In addition, once the constraint imposed from the eye phantom or rabbit eye is present the manipulations can no longer be dominated by the arm. The fingers must be recruited for performing microscale positioning. This recruitment can be observed in the higher-frequency tremor along the x-axis, in the range of 20–40Hz [20], [21].
An interesting relation is observed across the three conditions. The unconstrained condition has been shown to have more common occurrence of higher-magnitude tremor oscillation; however, the RMS and maximum values show the unconstrained condition to consistently have the lowest position error. When examining the spectral data it can be observed that in all three axes the unconstrained condition also has the lowest energy in the 1–3 Hz region, corresponding to low-frequency drift.
The error in the other two conditions was consistently shown to be higher in both RMS and maximum error. Since these conditions also contain higher spectral amplitude in the lower 1–3 Hz band, the larger error magnitude can be attributed to drift error, and not necessarily larger error from physiological tremor.
The results presented are consistent with the current quantification of tremor obtained from laboratory settings and confirm the overall characterization of hand tremor remains within the range of 8–12 Hz. It should be noted that it is important to consider the constraints that shall be placed on
We have compared the characteristics of tremor across three conditions during a holding-still task. As this is an ongoing effort to analyze tremor, we are seeking to gather data from a wider pool of surgeons to compensate for the minor variation among surgeries and surgeons. The holding-still task presented is also limited as a static condition for evaluating tremor; other useful conclusions are expected to be drawn from examining dynamic tasks. We anticipate being able to gather dynamic tremor evaluations through the use of a visual cue injection system [22], which would provide a path within the surgical environment for the surgeon to trace. This method would also be able to enforce a fixed reference point across trials to increase the repeatability.
Acknowledgments
Research supported by the U.S. National Institutes of Health (grant nos. R01EB007969 and R01EB000526) and the Kwanjeong Educational Foundation.
References
- 1.Tang WM, Han DP. A study of surgical approaches to retinal vascular occlusions. Archives of Ophthalmology. 2000;118:138–143. doi: 10.1001/archopht.118.1.138. [DOI] [PubMed] [Google Scholar]
- 2.Davson H. The Eye. New York: Academic Press; 1962. [Google Scholar]
- 3.Peral-Gutierrez F, Liao AL, Riviere CN. Static and dynamic accuracy of vitreoretinal surgeons. Proc 26th Annu Int Conf IEEE Eng Med Biol Soc. 2004:2734–2737. doi: 10.1109/IEMBS.2004.1403783. [DOI] [PubMed] [Google Scholar]
- 4.Hotraphinyo L, Riviere CN. Three-dimensional accuracy assessment of eye surgeons. Proc 22nd Annu Int Conf IEEE Eng Med Biol Soc. 2001:3458–3461. [Google Scholar]
- 5.Mansur HP, Cury LK, Andrade AO, Pereira AA, Miotto GAA, Soares AB, Naves ELM. A review on techniques for tremor recording and quantification. Crit Rev Biomed Eng. 2007;35(5):343–362. doi: 10.1615/critrevbiomedeng.v35.i5.10. [DOI] [PubMed] [Google Scholar]
- 6.Elble RJ, Randall JE. Motor-unit activity responsible for 8- to 12-Hz component of human physiological finger tremor. J Neurophysiol. 1976 Mar;39(2):370–383. doi: 10.1152/jn.1976.39.2.370. [DOI] [PubMed] [Google Scholar]
- 7.Stiles RN, Randall JE. Mechanical factors in human tremor frequency. J Appl Physiol. 1967 Sep;23(3):324–30. doi: 10.1152/jappl.1967.23.3.324. [DOI] [PubMed] [Google Scholar]
- 8.Uneri A, Balicki M, Handa JT, Gehlbach P, Taylor R, Iordachita I. New Steady-Hand Eye Robot with micro-force sensing for vitreoretinal surgery. Proc IEEE Int Conf Biomed Robot Biomechatron. 2010:814–819. doi: 10.1109/BIOROB.2010.5625991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLachlan RA, Becker BC, Cuevas Tabarés J, Podnar GW, Lobes LA, Riviere CN. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012;28(1):195–212. doi: 10.1109/TRO.2011.2169634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitcher JD, Wilson JT, Schwartz SD, Hubschman J. Robotic eye surgery3: past, present, and future. J Comput Sci Syst Biol. 2012;S3:1–4. [Google Scholar]
- 11.Singh SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. Proc 28th Northeast Bioeng Conf. 2002:171–172. [Google Scholar]
- 12.Caliguri MP, Tripp RM. A portable hand-held device for quantifying and standardizing tremor assessment. J Med Eng Technol. 2004 Dec;28(6):254–262. doi: 10.1080/03091900410001658111. [DOI] [PubMed] [Google Scholar]
- 13.Becker BC, Member S, Tummala H, Riviere CN. Autoregressive modeling of physiological tremor under microsurgical conditions. Proc 30th Annu Int Conf IEEE Eng Med Biol Soc. 2008:1948–1951. doi: 10.1109/IEMBS.2008.4649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latt WT, Tan UX, Veluvolu KC, Lin JKD, Shee CY, Ang WT. System to assess accuracy of micromanipulation. Proc 29th Annu Int Conf IEEE Eng Med Biol Soc. 2007:5743–5746. doi: 10.1109/IEMBS.2007.4353651. [DOI] [PubMed] [Google Scholar]
- 15.Vinjamuri R, Crammond D, Kondziolka D, Lee HN, Mao ZH. Extraction of sources of tremor in hand movements of patients with movement disorders. IEEE Trans Inf Technol Biomed. 2009 Jan;13(1):49–56. doi: 10.1109/TITB.2008.2006403. [DOI] [PubMed] [Google Scholar]
- 16.Riviere C, Jensen P. A study of instrument motion in vitreoretinal microsurgery. Proc 22nd Annu Int Conf IEEE Eng Med Biol Soc. 2000:1–2. [Google Scholar]
- 17.Elble RJ, Koller WC. Tremor. Baltimore: Johns Hopkins; 1990. [Google Scholar]
- 18.MacLachlan RA, Riviere CN. High-speed microscale optical tracking using digital frequency-domain multiplexing. IEEE Trans Instrum Meas. 2009 Jun;58(6):1991–2001. doi: 10.1109/TIM.2008.2006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAuley JH, Farmer SF, Rothwell JC, Marsden CD. Common 3 and 10 Hz oscillations modulate human eye and finger movements while they simultaneously track a visual target. J Physiol. 1999;515(3):905–917. doi: 10.1111/j.1469-7793.1999.905ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday DM, Conway BA, Farmer SF, Rosenberg JR, Rosenberg JAYR. Load-independent contributions from motor-unit synchronization to human physiological tremor. J Neurophysiol. 1999;82:664–675. doi: 10.1152/jn.1999.82.2.664. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto K, Nishada K, Zhou L, Itakura N, Seki K, Hamba S. Characteristics of physiological tremor in five fingers and evaluations of fatigue of fingers in typing. Ann Physiol Anthrop. 1992;11(1):61–68. doi: 10.2114/ahs1983.11.61. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez Palma S, Becker BC, Lobes LA, Riviere CN. Comparative evaluation of monocular augmented-reality display for surgical microscopes. Proc Annu Int Conf IEEE Eng Med Biol Soc. 2012:1409–1412. doi: 10.1109/EMBC.2012.6346203. [DOI] [PMC free article] [PubMed] [Google Scholar]