Abstract
Rationale
Preclinical models of cocaine addiction in the rodent have shown that cocaine induces both positive and negative affective states. These observations have led to the notion that the initial positive/euphoric state induced by cocaine administration may be followed by an opposing, negative process. In the rodent, one method for inferring positive and negative affective states involves measuring their ultrasonic vocalizations (USVs). Previous USV recordings from our laboratory suggested that the transition between positive and negative affect might involve decaying or sub-satiety levels of selfadministered cocaine.
Objectives
In order to explicitly test the role of cocaine levels on these affective states, the present study examined USVs when calculated body levels of cocaine were clamped (i.e. held at a constant level via experimenter- controlled infusions) at, below, or above subjects’ self-determined drug satiety thresholds.
Results
USVs indicated that 1) positive affect was predominantly observed during the drug loading period, but declined quickly to near zero during maintenance and exhibited little relation to calculated drug level, and 2) in contrast, negative affect was observed at sub-satiety cocaine levels, but was relatively absent when body levels of cocaine were clamped at or above subjects’ satiety thresholds.
Conclusions
The results reinforce the opponent-process hypothesis of addiction and suggest that an understanding of the mechanisms underlying negative affect might serve to inform behavioral and pharmacological therapies.
Keywords: Cocaine, Addiction, Affect, Ultrasonic Vocalization, Drug Abuse
1.0-Introduction
Cocaine produces a number of physical and psychological symptoms (NIDA 1985; Schank et al., 2008; Levinthal 2010 pp 90–115). Subjective reports of cocaine’s effects on affect are routinely taken from human participants; gauging affect in animal studies has the potential to improve the understanding of cocaine abuse. One preclinical method for inferring affect during drug use, withdrawal, and relapse involves the measurement of rat ultrasonic vocalizations (USVs; Barker et al., 2010, 2013; Browning et al., 2011; Ma et al., 2010; Maier et al., 2010, 2012; Mu et al., 2009; Mutschler & Miczek 1998a, 1998b).
USVs in adult rats are divided into two frequency ranges. USVs in the 18–33 kHz range are referred to as “22-kHz” USVs, while calls in the higher frequency range span 38–80 kHz and are referred to as “50-kHz” USVs. Twenty-two kHz USVs are further subdivided into “long” (300–3400 ms) or “short” duration emissions (20–300 ms; Brudzynski et al., 1991, 1993; Barker et al., 2010). Twenty-two kHz USVs are thought to be indicative of a negative affective state, whereas the 50-kHz range is thought to be indicative of a positive affective state (Brudzynski, 2013).
USVs are increasingly used as a tool in drug abuse research (Panksepp et al., 2002; Covington and Miczek 2003; Thompson et al., 2006; Simola et al., 2010; Maier et al., 2010; Wright et al., 2010, 2012; Taracha et al., 2012; Meyer et al., 2012), demonstrating that USVs can be used to derive rats’ affective state during anticipation of drug (Ma et al., 2010), drug administration (Barker et al., 2010; Ahrens et al., 2009; Mahler et al., 2013), drug withdrawal (Barros & Miczek 1996; Mutschler & Miczek 1998a, 1998b), presentation of drug cues (Browning et al., 2011), and drug reinstatement (Browning et al., 2011; Mahler et al., 2013; Barker et al., 2013).
During cocaine self-administration (SA), rats emitted proportionately more short 22-kHz than 50-kHz USVs when access to cocaine was restricted, preventing subjects from attaining drug levels analogous to those achieved during continuous reinforcement (Barker et al., 2010). In contrast, subjects with more liberal access to cocaine emitted proportionately more 50-kHz than 22-kHz USVs. This suggests that USVs may be modulated by cocaine levels, consistent with animals’ ability to assess interoceptive drug cues as cocaine levels fluctuate (Wise et al., 1995).
Indeed, early observations by Pickens and Thompson (1968) led to the subsequent insight that animals do not self-administer cocaine when body concentrations exceed a certain threshold (Pickens & Thompson 1971; Yokel & Pickens 1974; Dougherty & Pickens 1973; Wise 1987; Wise et al., 1995; Tsibulsky & Norman 1999; Lynch & Carroll., 2001; Norman & Tsibulsky 2006; Olmstead et al., 2000), leading to the notion that a “satiety threshold” for abused psychostimulants may exist (Wise, 1987; Yokel and Pickens, 1974; Norman & Tsibulsky 2006).
The findings of Barker et al. (2010) suggested that affect might be modulated as a function of drug level, but this was not explicitly tested. In the present study, we tested the effects of calculated drug levels on USV production by maintaining animals’ calculated drug levels at 1) their self-determined satiety thresholds, 2) 50% below their satiety thresholds, or 3) 25% above their satiety thresholds using a series of “drug-clamps” following two weeks of long-access SA training. A drug-clamp consisted of a series of computer controlled micro-infusions (analogous to an i.v. drip) designed to maintain an animal’s calculated body-level of cocaine at a fixed value for an extended period of time.
2.0-Materials and Methods
2.1-Subjects and Surgery
Singly-housed male Long-Evans rats (n=11; Charles River, Wilmington, MA) were catheterized and cared for as described previously (Root et al., 2011). Subjects were housed on a 12 hour: 12 hour light: dark cycle with dawn at 10:30AM. All protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals and have been approved by the Institutional Animal Care and Use Committee, Rutgers University.
2.2-Experimental Apparatus
Experiments were conducted in Plexiglas chambers which were housed inside wooden, sound-attenuating chambers. Animals were attached to an intravenous fluid delivery system consisting of a syringe pump (Razel Scientific, St. Albans, VT, USA) connected to a fluid swivel. A spring leash connected to the bottom of the swivel and extended to the head of the animal through the top of each chamber. The intravenous catheter was contained inside the spring leash and continued through a steel cannula on the animal’s head and into the right jugular vein. For SA sessions, a glass lever (4.7cm × 2.5 cm) was inserted through a hole 4 cm off the floor of the SA chamber and 6 cm from the door of the chamber. The lever was set so that 0.049 Newtons of force were required to produce a response. The cocaine HCl used in experiments was provided by the National Institute on Drug Abuse (Rockville, MD). Cocaine was dissolved in sterile saline and passed through a 0.2µm Nalgene microfilter. All experimental apparatuses were controlled by a PC running MED-Associates hardware and software (St. Albans, VT). White noise was produced through a speaker positioned outside of the animal’s sound-attenuating chamber at ~72 dB (1724 Noise Generator; Grason-Stadler, Eden Prairie, MN).
2.3-Shaping/Self –Administration
SA training began daily at 10:30 AM, immediately following the commencement of the light cycle. At the start of each session, a single-non retractable glass lever was affixed to the side wall of the SA chamber. Animals were shaped by rewarding approximations of the lever response (e.g., touching but not fully depressing the lever) during the first 1–3 sessions. For shaping and training, drug was available on a fixed-interval 40 s schedule with a 2 minute limited hold for the first 10 infusions, following which drug was available on a variable-interval 1–6 minute schedule with a 2 minute limited hold. Responses that occurred after the fixed- or variable-interval had elapsed produced a 0.24 mg/0.2ml infusion of cocaine (~0.67–0.77mg/kg) and initiated the subsequent interval. Sessions lasted for 6 hours or 80 infusions, whichever occurred first. Subjects received post-session feeding in order to maintain body weights of approximately 320–340 grams. Water was available ad libitum except during SA sessions. All animals were trained for 14 days (7 days/week) with no breaks between training sessions. No priming infusions were given to initiate SA behavior during training sessions.
2.4-Drug-Clamp Tests
2.4.1-Drug Clamp (n=8)
Following 14 days of training, the role of calculated drug level in USV production was tested by administering two drug clamps: a sub-satiety clamp and circa-satiety clamp. For clamp conditions, drug satiety was defined specifically for each subject as the individual’s peak calculated drug level. Peak drug level was operationally defined as the highest calculated drug level (mg/kg) that an animal volitionally administered during the second week of training. All drug levels were calculated using the formula reported in Root et al. (2011). Work in our laboratory suggests that the peak calculated drug level correlates highly with cessation of SA responding (Root et al., 2011), and it has been suggested by others that response cessation is a reliable measure of “drug satiety” (Wise, 1987; Norman & Tsibulsky 2006). For the circa-satiety condition, each subject’s calculated drug level was clamped (i.e. held constant) at 0.1 mg/kg above its peak drug level, while the sub-satiety conditions involved clamping drug levels at 50% of the subject’s circa-satiety drug level. On clamp tests, subjects were allowed to self-administer under their training contingencies for the first 90 minutes of the session. Subsequently, the response manipulandum was removed for the remainder of the session and subjects began a transition period lasting no longer than 30 minutes. During the transition, subjects’ calculated drug levels were slowly increased or decreased in order to reach their clamp levels. Upon stabilizing at the clamp level, subjects’ calculated drug levels were fixed for the remaining 240 minutes of the six hour session. Calculated drug levels were maintained at a fixed drug level by delivering computer-controlled intravenous microinfusions of cocaine with a constant inter-infusion interval. Microinfusions consisted of recurring 200 ms infusions (0.006mg/5µl/infusion; 0 .018–0.021 mg/kg) with a custom inter-infusion interval (described above). Subjects were counterbalanced such that half of the animals received the sub-satiety condition first, while the other half received the circa-satiety condition first. All subjects were then tested in the opposite clamp condition following a 1–3 day return to regular training contingencies.
2.4.2-Drug Clamps with Lever (n=7)
Four animals that had received the drug clamps along with an additional three animals were given a series of sub- and circa- satiety clamp sessions in which the lever remained in the chamber during the clamp period (i.e. was not removed after 90 minutes) but had no programmed consequence. The mix of a within- and between-subjects design was used in order to benefit from 1) within-subjects comparisons, which reduce the amount of inter-individual variability in USVs and 2) between-groups comparisons of animals with and without the lever. For animals participating in the drug clamp sessions with and without lever, drug clamps with lever always occurred after the drug clamp sessions without lever. All other contingencies for clamp sessions with the lever were congruent to those described in section 2.4.1. Drug clamps with the lever were counterbalanced such that half the subjects received the sub-satiety clamp with the lever or circa-satiety clamp with the lever first. Animals were always returned to normal SA for 1–3 days before any subsequent clamp test was administered.
2.4.3-Supra-Satiety Test (n=7)
In order to ensure that body levels of cocaine during the circa-satiety condition did in fact reach or slightly exceed subjects’ satiety threshold, the seven animals from the drug clamps with the lever were tested in a supra-satiety clamp condition following their participation in the circa- and sub-satiety clamps with the lever and 1–3 days’ normal SA. Supra-satiety drug levels were explicitly designed to be well above each subject’s self-determined satiety threshold. Thus, supra-satiety was defined as 25% above each subject’s circa-satiety drug level. For the supra-satiety clamp, the contingencies were identical to those in the sub- and circa-satiety clamps with the lever (i.e., the lever remained in the chamber throughout the session).
2.5-USV Recordings
A condenser microphone (CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany) was used to record USVs. The microphone was suspended ~2.5 cm above a cluster of small holes in the top of the SA chamber and was enclosed in a Plexiglas tube (5 cm diameter, 5 cm height, with 3mm thick wall). At the start of the session, recordings were triggered by a signal sent from a computer program to the recording hardware (Ultrasound Gate 116H, Avisoft Bioacoustics, Berlin, Germany). Sonorous activity was recorded at a 250-kHz sampling frequency (16-bits) using Avisoft Recorder software (Avisoft Bioacoustics, Berlin, Germany) throughout the session and stored for offline analysis. Recorded .wav files were then analyzed using Avisoft SASLab Pro (Avisoft Bioacoustics, Berlin, Germany).
2.5.1-Baseline Recordings
Prior to surgery, subjects were allowed to live in the SA chamber for 4 days. On the fourth day, USVs were recorded for all subjects during a 6-hour baseline period beginning at 10:30 AM, termed the “baseline” recording, which acted as a time-of-day control for SA.
2.5.2-Self-administration Recordings
One USV recording was taken during SA training on day 10 (henceforth termed the week 2 recording). This recording session characterized USV activity from well-trained animals before clamp contingencies were implemented.
2.5.3-Drug Clamp Recordings
For all drug clamps (with and without the lever) and the supra-satiety test, USV recordings were initiated by a computer signal from the behavioral computer at the start (time zero) of the session and were recorded continuously for the entire 6 hours of all clamp tests.
2.6-Characterization of USVs
Avisoft SASLab Pro (Avisoft Bioacoustics, Berlin, Germany) was used for post-hoc analysis of calls as described previously (Barker et al., 2010). While USVs can be differentiated into several call-types based on patterns of frequency modulation (Wright et al., 2010; Wöhr et al., 2007), a preliminary analysis revealed no trends related to call-type in the present data. Thus, statistical models of USVs collapsed call type (fixed frequency, frequency modulated, and trill vocalization types).
2.7-Data Analysis
2.7.1 Self-administration and clamp behaviors
Behavioral measures included the number of lever presses, number of infusions earned, total drug consumption (mg/kg), peak drug level (mg/kg) and bodyweight (g). SA behavioral data were analyzed using a repeated-measures ANOVA (SAS PROC GLIMMIX, SAS Institute Inc., 2005, Cary, NC) with Sidak adjustments for post-hoc comparisons. Pairwise comparisons were made between session 1 and each subsequent session of training (2–14). Lastly, a Sidak adjusted “plateau contrast” was designed to test whether behavioral measures fit an asymptotic curve typical of long-access training in this paradigm (e.g., Root et al., 2011; contrast coefficients: [−6, −2.25, −1, −0.1, 0.6, 0.75, 1, 1, 1, 1, 1, 1, 1, 1]). Lever responses during the drug clamps with the lever were analyzed using a 3 × 6 repeated-measures ANOVA with 3 levels of condition (sub-, circa- and supra-satiety clamps with the lever) and 6 levels of hour.
2.7.2 Analysis of USVs during drug loading
Ultrasonic vocalizations were examined across the drug loading period (defined as the first nine self-administered infusions). Given that contingencies for all USV recordings were the same during the first 90 minutes, data from all load-up recordings were analyzed together. The data were analyzed using a repeated-measures ANOVA in SAS PROC GLIMMIX. The 2 × 10 model utilized a change score (B−A)/(B+A) to examine changes in USVs during loadup (‘A’) when compared to baseline (‘B’). The 2 × 10 repeated-measures ANOVA also compared USVs in the 22- and 50-kHz frequency ranges (2 levels of frequency) across 10 levels of infusion (0–9 infusions earned).
2.7.3 Analysis of USVs during drug clamps
Preliminary analysis revealed no differences in USVs emitted between the circa- and sub-satiety clamps with and without the lever. Thus, data from these conditions were combined for analysis. USVs during the clamp period were analyzed using a 3 × 5 repeated-measures ANOVA in SAS Proc GLIMMIX. The model included 3 levels of Call Frequency and 5 levels of Condition. The model specified a gamma distribution with a log link, and a constant of one was added to accommodate the logarithmic transform by eliminating zeroes from the dataset.
Ultrasonic vocalizations in each frequency range were compared between conditions during the final four hours of each session using Tukey-Kramer adjusted post-hoc tests. Results demonstrated that there were no differences between any conditions in the 33-kHz frequency range. Thus, no further results for this frequency range are reported.
2.7.4 Analysis of USVs during maintenance of SA
During the maintenance period of the week 2 recording, self-administered drug levels increased upon each infusion and then decayed until the next infusion. To assess the relationship between calculated drug levels and the emission of 22-kHz vocalizations, USVs during maintenance were sorted into 4 quartiles based on the calculated drug level at the time of their emission. Thus, quartiles corresponded to the number of 22-kHz USVs emitted at the lowest 25% of calculated drug levels through the highest 25%. The use of quartiles served to normalize differences in absolute drug consumption across animals. These data were analyzed with a repeated measures ANOVA in SAS Proc GLIMMIX (SAS Institute Inc.). A logarithmic transform of the data was used and a negative binomial distribution was specified based on the data structure. Post-hoc comparisons of all pairwise comparisons amongst quartiles were Tukey-Kramer corrected.
3.0-Results
3.1-Behavior
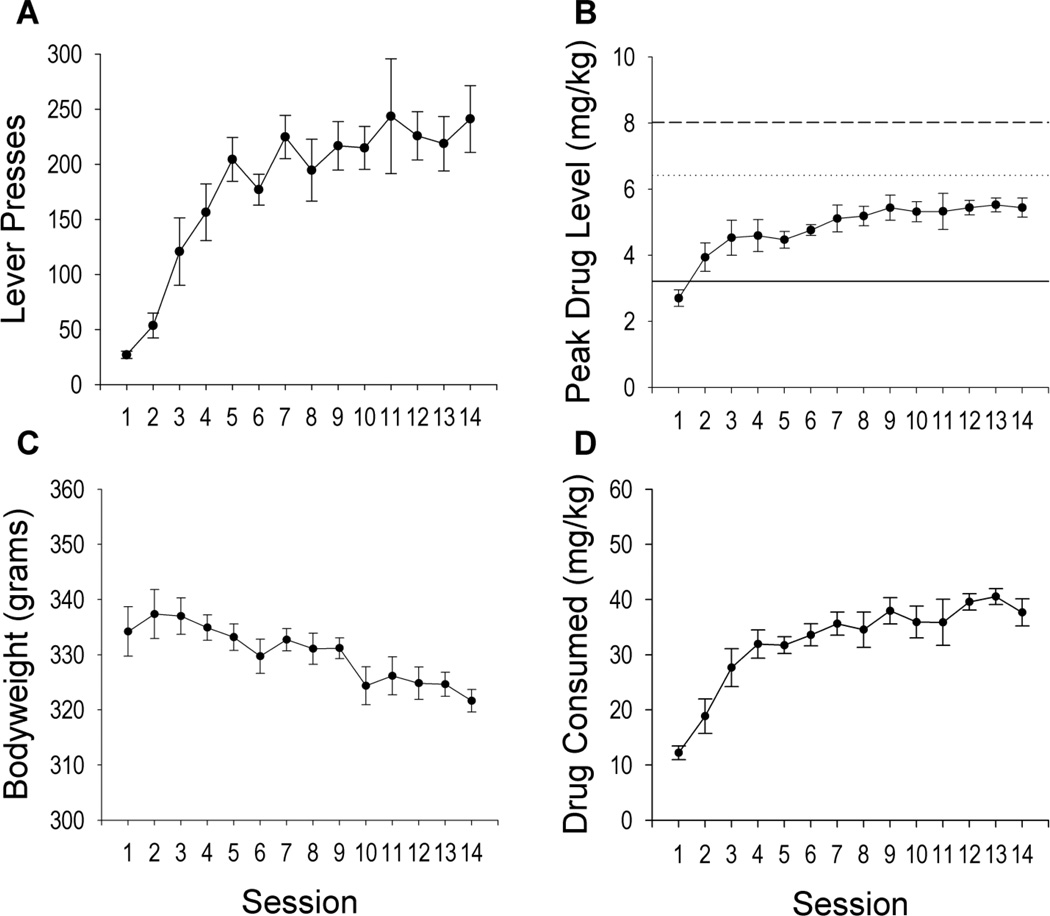
Rats acquired stable cocaine SA behavior. Over training days, animals increased their number of lever presses (F(10,129) = 28.07, p < 0.001; Figure 1A), cocaine infusions earned (F(10,129) = 27.66, p < 0.001), and total drug consumption (F(10,129) = 52.71, p < 0.001; Figure 1D), likely as functions of escalating intra-session peak drug levels (F(10,129) = 12.15, p < 0.001; Figure 1B) and a slight decrease in bodyweights (F(10,129) = 4.22, p < 0.001; Figure 1C).
Figure 1. Animals acquire and stabilize SA behaviors across two weeks of SA training.
A) Lever responding across training significantly increased before stabilizing B) Peak drug level (mg/kg) across training increased over sessions. The average drug levels during the sub, circa, and supra-satiety conditions are shown using the solid, dotted, and dashed lines, respectively. C) Bodyweight (g) decreased across SA. D) Total drug consumption (mg/kg) increased across training. All data points are shown as the Mean ± SEM. Session is represented on the x-axes while individual dependent measures are represented on the y-axes.
The acquisition of SA occurred rapidly over the first 3–5 days and then stabilized to fit asymptotic curves. Significant increases in lever presses (Session 1 vs. 3–14: |t(129)| ≥ 4.54, p < 0.001), the number of earned infusions, peak drug level, and total drug consumption (Session 1 vs. 2–14: all |t(129)| ≥ 2.03, p< 0.05) were found using Sidak-adjusted post-hoc tests comparing session 1 to each subsequent session. The aforementioned behaviors (all |t(129)| ≥ 5.38, p< 0.001) and bodyweight (|t(129)|= 2.45 p< 0.05) stabilized prior to clamp testing as evidenced by significant plateau contrasts for asymptotic curves (section 2.7.1). These results demonstrate that animals stabilized their bodyweight and behavior prior to clamp testing and learned to titrate and stabilize their daily drug intake.
3.2-Lever responding during clamp sessions
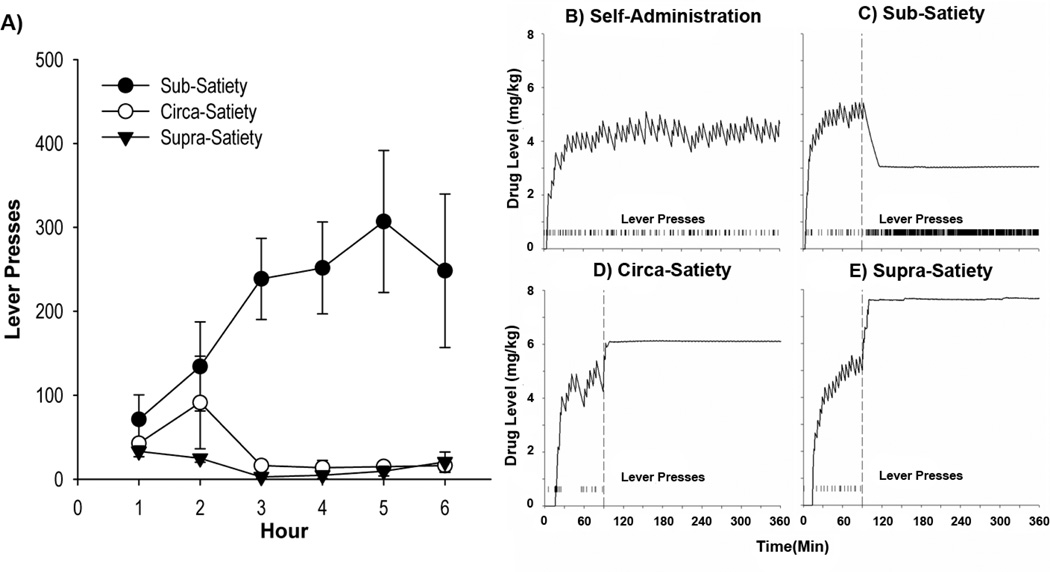
During clamp conditions with the lever, response patterns depended on the calculated drug level (clamp condition×hour interaction [F (10,60)= 3.785, p<0.05]). Post-hoc comparisons revealed no differences in lever responding during hours 1 & 2 (i.e., prior to drug clamps) nor during the clamp transition (Figure 2A). In contrast, subjects performed significantly more lever responses during the sub-satiety clamp than the circa- or supra-satiety clamps [all |t(60)|≥2.47, p<0.05] (Figure 2A). As can be seen in single animal example drug curves, compared with normal SA (Figure 2B), sub-satiety drug levels robustly increased responding (Figure 2C) whereas circa and supra satiety drug levels induced response cessation (Figures 2D–E).
Figure 2. Drug levels above satiety attenuate lever responding while drug levels below satiety increase responding.
A) Lever responses across hours in the sub-, circa- and supra-satiety drug clamps with the lever. Example calculated cocaine from one animal during B) self-administration C) the sub-satiety clamp with a lever D) the circa-satiety clamp with a lever and E) the supra-satiety clamp with a lever. The vertical dashed lines at 90 minutes represent the start of computer-controlled micro-infusions for each of the clamp conditions (C–E) and the tick marks along the bottom of each graph represent lever presses performed by the animal across the session.
3.3- USVs during drug loading
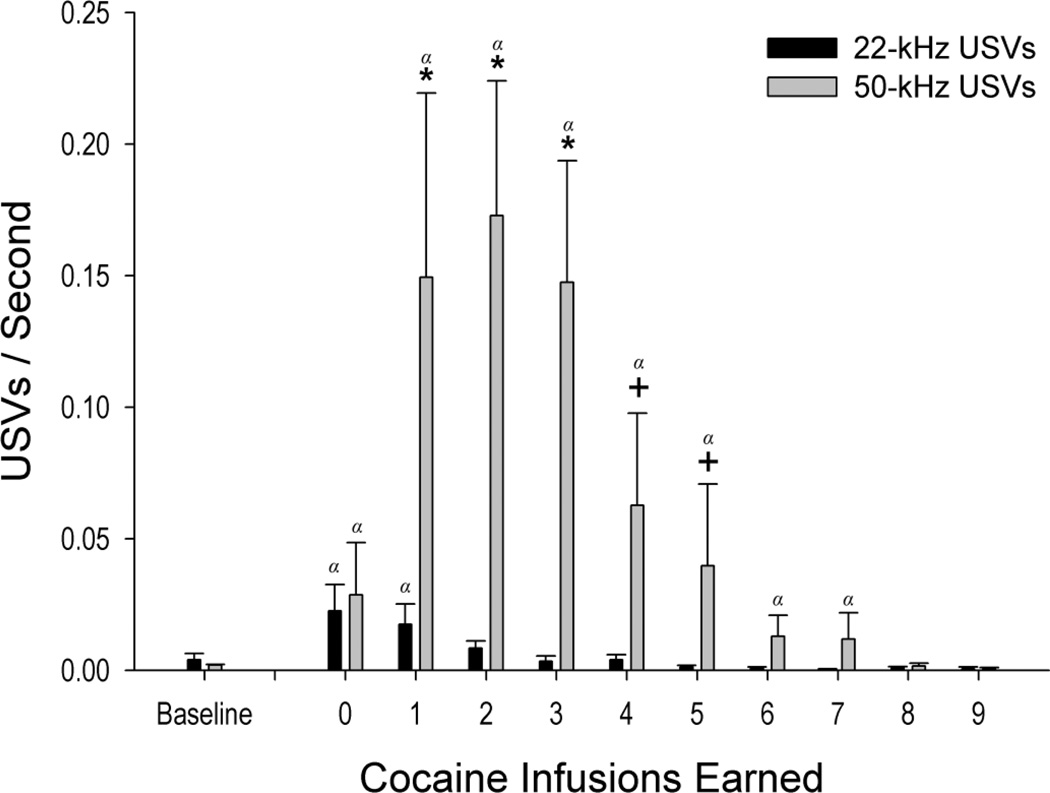
The observed pattern of vocalizations emitted during load-up changed across earned infusions (Call Frequency × Infusion interaction [F(9,190) =51.67, p<0.001]). Results showed that the highest numbers of short 22-kHz USVs (mean duration = 47.9 ± 10.8 ms) were emitted prior to the first infusion (0.023 ± 0.01 USVs/sec) and decayed by the third infusion. Compared to baseline levels, 22-kHz USVs were emitted at higher rates prior to the first infusion of cocaine and after animals had received a single infusion of cocaine (all |t(190) >2.10|, p < 0.05). As levels of cocaine increased across subsequent infusions (i.e. 2–9 infusions earned; Figure 3) 22-kHz USVs decayed to baseline levels. Conversely, 50-kHz USVs (mean duration = 51.4 ± 21.1 ms) increased nearly six-fold from 0.029 ± 0.02 USVs/sec prior to the first infusion, to 0.173 ± 0.05 USVs/sec between the second and third infusions. 50-kHz USVs were emitted at greater rates than baseline prior to the first self-administered infusion and remained elevated over baseline until the seventh earned infusion (all |t(190) > 2.29|, p <0.05) before returning to baseline. When compared to the 22-kHz USVs, more 50-kHz USVs were emitted once animals received the first self-administered infusion (t (190)= 2.03, p<0.05) and remained significantly or marginally elevated through the fifth infusion (infusions 2–3, all p<0.05; infusions 4–5 all p<0.10).
Figure 3. Fifty-kHz ultrasonic vocalizations increase following cocaine administration (load-up) before subsequently decaying.
The average number of ultrasonic vocalizations (USVs; mean ± SEM) is shown as a function of the number of infusions earned. Twenty-two kHz USVs were highest prior to cocaine SA and decayed following the first infusion of cocaine. Fifty-kHz USVs rapidly increased following the first infusion but decayed as SA continued. During the pre-drug period (i.e., ‘0’ infusions earned) individual subjects emitted exclusively 22- or 50-kHz USVs during 85% of observations. The remaining observations were characterized by baseline rates of USV emission or a mix of 22- and 50-kHz USVs with one call category always predominating (Mean=15-fold difference). Nine of eleven subjects exhibited increases in 50-kHz USVs following the first infusion of cocaine while the remaining two subjects exhibited primarily decreases in 22-kHz USVs over the same period. +: p < 0.10, *: p <0.05 when compared to the 22-kHz range. α: p< 0.05 when compared within frequency to baseline.
3.4-Ultrasonic vocalizations during drug clamps
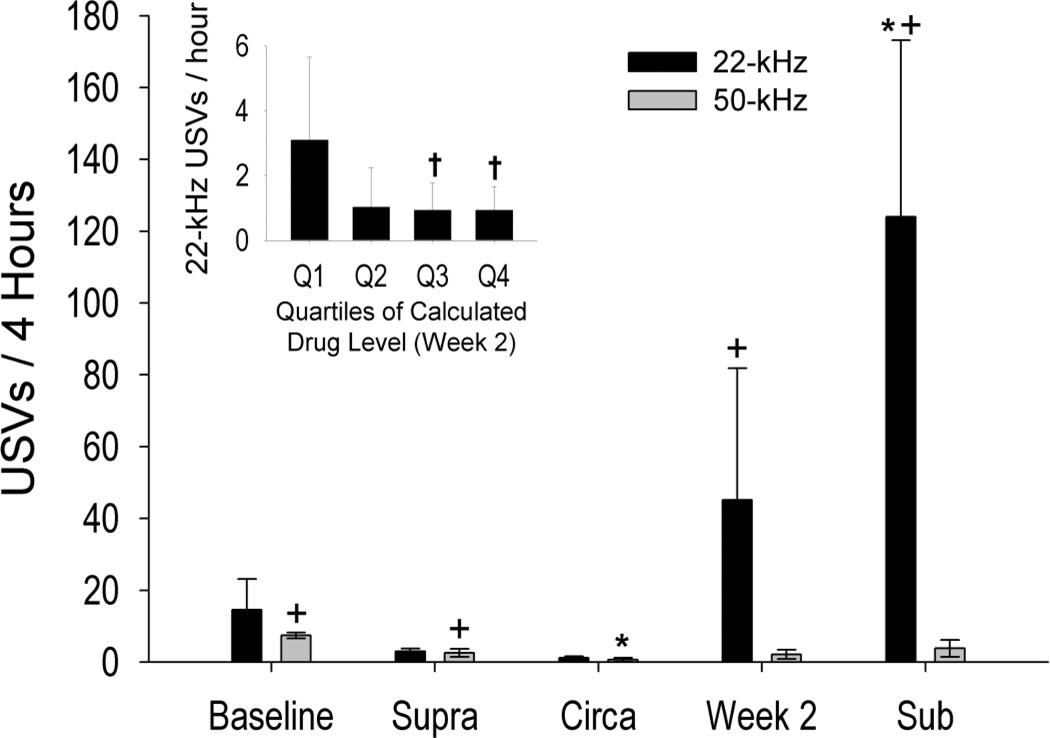
USVs varied significantly as a function of condition and call frequency (condition × call frequency interaction: F(8,143) = 15.74, p < 0.001). Twenty-two kHz USVs were emitted at greater rates when calculated drug levels were clamped below satiety than when drug levels were clamped at or above satiety and at greater rates than those observed at baseline (Figure 4; all |t(143) ≥ 3.60|, p ≤ 0.032). Furthermore, more 22-kHz USVs were observed during the maintenance period of week 2 than during the circa-satiety clamp (t(143) = 3.58, p< 0.05). Otherwise, 22- kHz USVs were not different between the week 2 recording versus any other condition. The observed pattern of 22-kHz USVs suggests that animals experienced negative affect when drug level was held at sub-satiety levels. Conversely, 22-kHz USVs were absent when calculated drug levels were clamped at or above satiety.
Figure 4. Sub-satiety drug levels produce increases in 22-kHz ultrasonic vocalizations.
The average number of ultrasonic vocalizations (USVs; mean ± SEM) emitted during the last four hours of each condition. Twenty-two kHz USVs were greatest when calculated drug levels were clamped below satiety (sub-satiety drug clamp). In comparison to 50-kHz USVs, all eleven subjects showed robust increases in short-22kHz USVs during the sub-satiety clamp. Twenty-two kHz calls were also observed during the maintenance period of SA (week 2) and were greatest when calculated drug levels were lowest (inset). Inset: 22-kHz USVs during the last four hours of the week 2 recording are shown as a function of drug level quartiles.Q1: 0–25th percentile; Q2: 25–50th percentile; Q3: 50–75th percentile; Q4: 75–100th percentile. †:P < 0.05 when compared to the lowest quartile (Q1). *: p <0.05 when compared within-frequency to the baseline condition. +: p<0.05 when compared within-frequency to the circa-satiety clamp. All other statistical comparisons are found in the text.
No differences in 50-kHz USVs were observed between the baseline, week 2, sub-satiety, and supra-satiety conditions (Figure 4). Similarly, no differences in 50-kHz USVs were observed between the week 2 and circa-satiety conditions or between the sub- and circa-satiety conditions. There were however more 50-kHz USVs during the baseline and supra-satiety conditions than in the circa-satiety condition [all |t(143) ≥ 4.38|, p ≤ 0.002]. Still, rates of 50-kHz vocalizations were extremely low in all clamp conditions (less than 2 calls/hour), but were confined mostly to the load-up period (Figure 3).
During the sub-satiety clamp, subjects emitted more 22-kHz than 50-kHz USVs (t (143) = 5.75, p<0.001). No other comparisons within clamp conditions revealed significant differences between rates of 22-kHz and 50 k-Hz calls.
3.5 Ultrasonic vocalizations during maintenance of normal SA
Comparing 22-kHz USVs between week 2 and the circa-satiety clamp revealed that more short 22-kHz USVs were emitted during maintenance of SA than when calculated drug levels were clamped at satiety. Taken together with the dose-response type relationship observed across the circa-satiety, week 2, and sub-satiety conditions (Figure 4), one would predict that short 22-kHz USVs would be most prevalent at the lowest calculated drug levels experienced by rats during the maintenance period of SA.
This prediction was confirmed by examining 22-kHz USVs as a function of drug level quartile (F (3, 21) =4.03, p < 0.05). Specifically, short 22-kHz USVs were most prevalent at the lowest 25% of drug levels (Q1; Figure 4 inset) when compared to the highest two quartiles of calculated drug levels (Q1 (0–25th percentile) vs. Q3 (50–75th percentile) & Q4 (75–100th percentile) all | t(21) > 3.18| p < 0.05). No other significant differences amongst quartiles were observed.
4.0-Discussion
The present data corroborate findings from our laboratory (Barker et al., 2010) and others (e.g., Mahler et al., 2013; Ettenberg, 2004; Ettenberg et al., 1999), suggesting that animals experience both positive and negative affect in association with psychostimulant SA. These results are also consistent with self-reports from human drug users, who describe a transition from positive to negative affect after psychostimulant use (Breiter et al., 1997; Volkow et al., 1997). Moreover, these results extend our previous findings to show that 1) 50-kHz USVs are limited to the loading period and do not persist throughout long- access sessions and 2) short 22-kHz USVs can be brought under experimental control by preventing satiety or producing sub-satiety states.
During the load-up period, animals emitted both 50-kHz and 22-kHz vocalizations. Examination of USVs as a function of the number of infusions earned demonstrated that 50-kHz USVs increased after the first cocaine infusion and were relatively short lived, decaying after the fourth or fifth subsequent infusion earned. This result mirrors the decay in 50-kHz calls observed by Browning and colleagues (2011). Twenty-two kHz USVs, on the other hand, were greatest prior to the first infusion and decayed once animals began SA. The presence of 22-kHz USVs observed prior to the first infusion in some animals, ~18 hours after the preceding SA session, corroborates findings from Miczek and colleagues, suggesting that long-access cocaine binges produce an aversive withdrawal state (Covington & Miczek 2003; Mutschler & Miczek, 1998a, 1998b). Indeed, it seems that—depending on the learned associations between the chamber and drug administration—either positive or negative affective reactions may be produced by cues preceding cocaine administration (Fig 3 caption; Ma et al., 2010; Wheeler et al, 2008; Wheeler et al., 2011).
The present study, using a long access model of SA, supplements results from many short access studies by demonstrating that the decay of 50-kHz USVs that increase during the initial load-up period is followed by a transition to short 22-kHz USVs during maintenance, perhaps suggesting the presence of an opponent negative process (Solomon & Corbit, 1978). Specifically, 22-kHz USVs observed during the maintenance of SA (week 2) were most prevalent when calculated drug levels were lowest. Moreover, 22-kHz USVs were emitted at even greater rates when calculated cocaine level was clamped below satiety, but were virtually absent when calculated drug level was clamped at or above satiety. Importantly, the presence or absence of the lever had no effect on 22-kHz USVs, suggesting that the increased amounts of unreinforced lever pressing (and thus energy expenditure) had no effect on the observed aversive reaction. These results corroborate findings from Ettenberg and colleagues (1999, 2004), which show a similar transition from positive to negative affect. While these opponent actions are generally observed following a single 0.75 mg/kg infusion of cocaine (e.g., Ettenberg et al., 1999), results in the present study suggest that aversive actions occur during an SA binge when calculated drug levels fall below satiety, even despite continued access to drug.
Several factors may account for the diminution of positive affect and subsequent transition to negative affect. First, repeated administration of cocaine causes more uptake sites to become blocked and subsequently more dopaminergic binding to autoreceptors and thus a tonic inhibition of dopamine neurons (Grace, 1995). This type of saturation results in decreases in dopamine release at the synaptic cleft and perhaps can account for the attenuation of the rewarding/positive effects of the drug. Alternatively, these changes might be accounted for by the up- or down-regulation of dopamine receptors that occurs as drug use transitions from acute to chronic (Nader et al., 2002)
Finally, it has been demonstrated that the lateral habenula and its projections to the rostromedial tegmental nucleus may play a role in cocaine’s transition from positive to aversive. Neurons in both structures receive projections from many of the same hypothalamic structures implicated in the production of aversive vocalizations (Brudzynski 2008). Furthermore, neurons in the rostromedial tegmental nucleus are capable of inhibiting dopamine neurons and, perhaps, positive affective responses to cocaine as well (Jhou et al., 2013). These types of persistent changes in the brain may account for the development of allostatic mechanisms that oppose cocaine’s euphoric effects and produce subsequent negative affect, drug tolerance and withdrawal (Koob 2008, 2009; Mutschler & Miczek 1998a, 1998b).
4.1- Limitations and Considerations
It is important to note that the role of short 22-kHz USVs is less understood than long 22-kHz USVs or 50-kHz USVs. Short 22-kHz USVs are often observed alongside long 22-kHz calls (e.g. Brudzynski et al., 1991), although this is not always the case (present data). These results suggest that short and long 22-kHz vocalizations perhaps represent similar but nonetheless discrete emotional states. In fact, to our knowledge, short 22-kHz USVs have been correlated with only aversive outcomes and are therefore interpreted in the present study as aversive vocalizations, as they have been interpreted previously (Mahler et al., 2013; Coffey et al., 2013; Barker et al., 2010, 2013). Moreover, data in the present study demonstrate that short 22-kHz USVs decay as the emission of 50-kHz USVs increases (Figure 3) and that 22-kHz USVs return following a decline in 50-kHz USVs. Notably, the suppression of 50-kHz USVs alone has been suggested to reflect a negative affective state (Wright & Panksepp, 2011) and the present data suggest that the suppression of 22-kHz USVs may give way to positive affect. Thus, the present study provides support that short 22- and 50-kHz USVs represent opposing states.
One should also note that drug in the present study was experimenter administered during the clamp sessions. Studies have demonstrated that yoked animals exhibit aversive reactions to drug administration (Dworkin et al., 1995). In contrast, intraperitoneal injections of stimulants—which are also experimenter administered—have been shown to produce positive affect as evidenced by their 50-kHz vocalizations (e.g., Mu et al., 2009). Thus, one cannot assume that stimulants are aversive simply because they are experimenter-administered, particularly in animals with an ongoing history of SA. Nor can one assume that stimulants are not aversive simply because they are self-administered. The present data indicate that schedules which prevent animals from adequately titrating their drug intake, which might include second-order schedules or paradigms with long-timeout periods, often accompanied by high rates of responding (consistent with sub-satiety drug levels), are capable of inducing a negative affective state. Alternatively, schedules that allow subjects to more freely control dose and infusion timing (e.g. Zimmer et al., 2013) would likely allow animals to more adequately titrate drug level and would likely produce affective responses similar to those observed in the circa-satiety condition, i.e., a spike in 50-kHz calls during load-up, followed by minimal calling during maintenance (except if drug level transiently falls below satiety level, prompting 22-kHz calls).
4.2.Conclusions
These results suggest critical interactions between drug dose and schedules of drug availability with operant responding and affective state. Accordingly, the negative relationship between calculated levels of cocaine and both rates of responding and negative affect, plus the paucity of positive affective calls during maintenance, suggest that responding during the maintenance phase of SA is perhaps more reliably driven by the motivation to escape falling or sub-satiety levels of the drug (negative reinforcement) rather than seeking out further bouts of euphoria. Without a doubt, this interaction plays an important role in determining what subjects learn about the myriad of drug-related cues and is important for treating drug dependence in humans. Appropriately, negative mood states are reported as one of the most common reasons for relapse in human addicts (Hodgins et al., 1995). Furthermore, many cocaine users report difficulties regulating their emotions (Fox et al., 2007). Thus, focusing preclinical research on the neuroanatomical changes that contribute to negative affect and emotional dysregulation in drug dependence (Koob, 2009b) may aid in the advancement of appropriate behavioral or pharmacological therapies.
Acknowledgments
We thank Thomas Grace Sr., Jackie Thomas, and Kevin Coffey for excellent assistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All coauthors have seen and approve of the contents of the manuscript.
This study was supported by the National Institute on Drug Abuse Grants DA006886 (MOW), DA029873 (MOW), and DA032270 (DJB). This article is not currently under review by another journal.
Abbreviations
- USVs
Ultrasonic vocalizations
Footnotes
This article is dedicated to the memory of Linda King, a colleague whose character and commitment to students will not be forgotten.
The authors have no financial interests to be disclosed.
References
- [NIDA] National Institute on Drug Abuse (US) Research monograph series. Rockville (MD): Department of health and human services (US); 1985. Cocaine use in America: Epidemiologic and clinical perspectives. DHHS Pub. No. (ADM) 90–1414. [Google Scholar]
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Shallert T. Repeated intravenous amphetamine exposure: rapid and persistent of 50-kHz. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology. 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bercovicz D, Servilio LC, Simmons SJ, Ma S, Root DH, Pawlak AP, West MO. Rat ultrasonic vocalizations demonstrate that the motivation to contextually reinstate cocaine-seeking behavior does not necessarily involve a hedonic response. Addiction Biology. 2013 doi: 10.1111/adb.12044. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros HMT, Miczek KA. Withdrawal from oral cocaine in rats: ultrasonic vocalizations and tactile startle. Psychopharmacology. 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA. Positive affective vocalizations during cocaine and sucrose self-administration: a model for spontaneous drug desire in rats. Neuropharmacology. 2011;61(1–2):268–275. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalizations in rats. Curr. Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.014. In Press. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D, Bihari F. Comparison between cholinergically and naturally induced ultrasonic vocalization in the rat. J Psychiatr Neurosci. 1991;16:221–226. [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Bihari F, Ociepa D, Fu X. Analysis of 22 kHz ultrasonic vocalizations in laboratory rats: long and short calls. Physiol Behav. 1993;54:215–221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Communication of adult rats by ultrasonic vocalization: Biological, sociobiological, and neuroscience approaches. ILAR J. 2008;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- Coffey KC, Barker DJ, Ma S, Root DH, Martinez L, Horvitz JC, West MO. Effects of varying reinforcement probability on Pavlovian approach behavior and ultrasonic vocalizations in rats. Behav. Brain Res. 2013;237:256–262. doi: 10.1016/j.bbr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- Dougherty J, Pickens R. Fixed-interval schedules of intravenous cocaine presentation in rats. JEAB. 1973;20(1):111–118. doi: 10.1901/jeab.1973.20-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117(3):262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci. & Biochem Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BB. Evidence for opponent-process actions of intravenous cocaine. Pharmacol. Biochem. & Behav. 1999;64(3):507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug and Alcohol Dependence. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. Journal of Consulting and Clinical Psychology. 1995;63(3):400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu S, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J. Neurosci. 2013;33(17):7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic homeostatic dysregulation as a driver of drug seeking behavior. Drug Discov Today Dis Models. 2008;5(4):207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: Reward, antireward and emotional memory. Pharmacopsychiatry. 2009;42:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009b;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal CF. Drugs Behavior and Modern Society. Boston, MA: Allyn & Bacon; 2010. [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimenal and clinical psychopharmacology. 2001;9(2):131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle C. Repeated intravenous cocaine experience: Development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behavioral Brain Research. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE. A rodent “self-report” measure of methamphetamine craving? Ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behavioral Brain Research. 2013;236(1):78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ahrens AM, Ma ST, Schallert T, Duvachelle CL. Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behvaioral Brain Research. 2010;214(1):75–79. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvachelle CL. The missing variable: Ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology. 2012;219(4):1141–1152. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012;219(4):999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalizations in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from IV cocaine “binges” in rats: ultrasonic distress calls and startle. Psychopharmacology. 1998a;135:161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998b;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res. 2006;1116:143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Lafond MV, Everitt BJ, Dickinson A. Cocaine seeking by rats is a goal-directed action. Behav Neurosci. 2000;115(2):394–402. [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf The role of brain emotional system in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97(4):459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine reinforced behavior in rats: effects of reinforcement magnitude and fixed ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pickens R, Thompson T. Characteristics of stimulant reinforcement. In: Thompson T, Pickens R, editors. Stimulus Properties of drugs. New York: Appleton-Century-Crofts; 1971. pp. 172–192. [Google Scholar]
- Root DH, Barker DJ, Ma S, Coffey KR, Fabbricatore AT, West MO. Evidence for learned skill during cocaine self-administration in rats. Psychopharmacology. 2011;217:91–100. doi: 10.1007/s00213-011-2261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Cameron L, Weinshenker D. Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biological Psychiatry. 2008;63(11):1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N, Ma ST, Schallert T. Influence of acute caffeine on 50-kHz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int. J. Neuropsychopharm. 2010;13(1):123–132. doi: 10.1017/S1461145709990113. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. The American Economic Review. 1978;68(6):12–24. [Google Scholar]
- Taracha E, Hamed A, Krząścik P, Lehner M, Skórzewska A, Płaźnik A, Chrapusta SJ. Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology. 2012;222(4):619–632. doi: 10.1007/s00213-012-2658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Research. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69(11):1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120(1):10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psych. Review. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Wöhr M, Schwarting RW. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS ONE. 2007;2(12):e1365. doi: 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PBS. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology. 2010;211(1):1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Wright JM, Deng L, Clarke PBS. Failure of rewarding and locomotor stimulant doses of morphine to promote rat 50-kHz ultrasonic vocalizations. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2776-z. [DOI] [PubMed] [Google Scholar]
- Wright JS, Panksepp J. Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neuroscience and Biobehavioral Reviews. 2011;35:1902–1915. doi: 10.1016/j.neubiorev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Yokel AR, Pickens R. Drug level of D- and L-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, Roberts DCS. Examination of behavioral strategies regulating cocaine intake in rats. Psychopharmacology. 2013;225(4):935–944. doi: 10.1007/s00213-012-2877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]