Abstract
Influenza virus and Streptococcus pneumoniae are two major pathogens that lead to significant morbidity and mortality in the elderly. Since both pathogens enter the host via the mucosa, especially the upper respiratory tract (URT), it is essential to elicit pathogen-specific secretory IgA (SIgA) antibody (Ab) responses at mucosal surfaces for defense of the elderly. However, as aging occurs, alterations in the mucosal immune system of older individuals result in a failure to induce SIgA Abs for protection from these infections. To overcome mucosal immunosenescence, we have developed a mucosal dendritic cell targeting, novel double adjuvant system which we show to be an attractive and effective immunological modulator. This system induces a more balanced Th1- and Th2- type cytokine response which supports both mucosal SIgA and systemic IgG1 and IgG2a Ab responses. Thus, adaptation of this adjuvant system to nasal vaccines for influenza virus and S. pneumoniae could successfully provide protection by supporting pathogen-specific SIgA Ab responses in the URT in the mouse model of aging. In summary, a double adjuvant system is considered to be an attractive and potentially important strategy for the future development of mucosal vaccines for the elderly.
Introduction
Vaccines that provide protection of the mucous membranes in addition to the systemic tissues are an ideal strategy for preventing infectious diseases, most of which are initiated by the invasion of pathogens through the mucosa. In this regard, the development of effective mucosal vaccines requires a complete understanding of the intricacies within the mucosal immune system itself. The mucosae can be divided anatomically and functionally into organized lymphoid tissues where initial induction of immunity occurs and more diffuse sites where actual effector immune responses take place (Kiyono et al., 2008; Fujihashi et al., 2009; Fujihashi et al., 2013). Both nasopharyngeal- and gut-associated lymphoid tissues (NALT and GALT) serve as major inductive sites for mucosal immunity in the upper respiratory (UR) and gastrointestinal (GI) tracts. NALT and GALT are covered by a lymphoepithelium containing microfold (M) cells which are most proficient in the uptake of luminal antigens (Ags) (Kiyono et al., 2008; Fujihashi et al., 2013). The subepithelium is especially enriched in Ag-presenting cells (APCs) including dendritic cell (DC) subsets. Underneath this APC enriched subepithlium lies two distinct areas, a B cell zone with germinal centers enriched in surface IgA+ B cells and a separate but adjacent T cell area with both naïve and memory CD4+ and CD8+ T cell phenotypes (Kiyono et al., 2008; Fujihashi et al., 2013). Thus, the GALT and NALT contain all of the necessary immunnocompetent cells for the initiation of Ag-specific T and B cell responses.
Secretory IgA (SIgA) is the primary antibody (Ab) involved in protecting mucosal surfaces and is locally produced by plasma cells in mucosal effector sites such as the lamina propria of the GI, UR and reproductive tracts, originally induced in the distant organized inductive sites (e.g., GALT and NALT) (Kiyono et al., 2008; Fujihashi et al., 2013). For the formation of SIgA, Ag-specific IgA Abs produced by cellular cooperation between IgA+ B cells and helper T (Th) cells interact with polymeric Ig receptor (pIgR) expressed by epithelial cells. Similarly, mucosal cytotoxic T cells are initiated at mucosal inductive tissues and exhibit their actual function at effector sites (Kiyono et al., 2008; Fujihashi et al., 2013). In this regard, the majority of T and B cells in mucosal effector tissues are activated and express a memory phenotype (Kiyono et al., 2008; Fujihashi et al., 2013).
It has been shown that Ag-specific SIgA Abs play a central role in the induction of mucosal immunity to infectious diseases (Kiyono et al., 2008; Fujihashi et al., 2013). However, protective mucosal immunity is dramatically affected in aging and thus, the GI tract in the elderly is deficient in SIgA Ab synthesis and is particularly susceptible to infectious diseases (Powers, 1992; Schmucker et al., 1996). In this regard, it has been shown that Ag-specific mucosal IgA Ab responses were significantly altered in aged experimental animals including non-human primates (Schmucker et al., 1988; Taylor et al., 1992; Enioutina et al., 2000; Thoreux et al., 2000). Further, it has been shown that age-associated immune dysregulation occurs in mucosal immune compartments as early as 12-14 months of age in mice (Koga et al., 2000; Fujihashi et al., 2009). Thus, both Ag-specific Ab and cytokine responses, induced by oral ovalbumin (OVA) and native cholera toxin (nCT) as mucosal adjuvant were markedly reduced in one-year-old mice, and were essentially the same as those seen in fully aged two-year-old mice (Koga et al., 2000; Fujihashi et al., 2009). Since it has been shown that NALT shares common immunological and functional features with Peyer’s patches (PPs), a major component GALT, one could assume that NALT would follow a similar immunosenescence process as seen in GALT. However, the duration of immunosenescence in NALT markedly differs from that seen in GALT (Hagiwara et al., 2003; Fujihashi et al., 2009). Furthermore, it has been shown that the longevity, organogenesis and cell trafficking of these two inductive tissues are distinctly regulated (Kunisawa et al., 2008). In this regard, in contrast to oral immunization, nasal immunization effectively induced Ag-specific mucosal and systemic immune responses in one-year old mice (Koga et al., 2000; Hagiwara et al., 2003; Fujihashi et al., 2009). More importantly, one-year old mice given nasal tetanus toxoid (TT) vaccine and nCT as adjuvant were protected from tetanus toxin challenge (Hagiwara et al., 2003). These results suggest that a distinct immune aging process exists between the two sites; this occurs earlier in GALT (than in NALT) accounting for differences in Ag-specific mucosal SIgA and parenteral IgG Ab responses (Fujihashi et al., 2009).
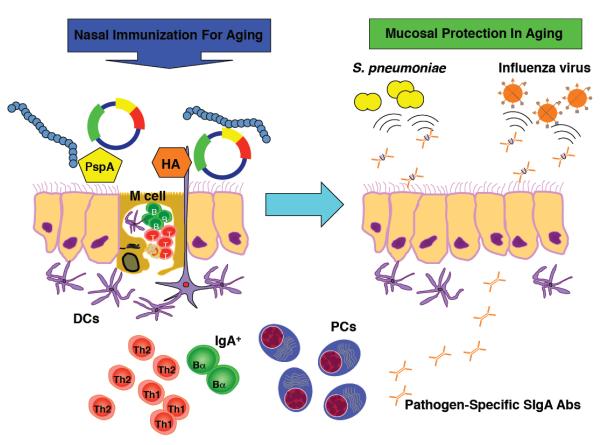
It is well established that respiratory influenza viral and Streptoccocus pneumoniae bacterial infections are sharply increased in the elderly and are often fatal (Fujihashi et al., 2009). To this end, a cellular and molecular understanding of the impaired mucosal immune response to these pathogens and the development of a novel way to enhance specific immunity in the elderly would be clinically relevant and important. Lamina propria plasma cells secrete polymeric (largely dimeric) IgA (pIgA) Abs which are taken up by a pIgA/pIgM receptor (pIgR) for transport across epithelial cells with ultimate release into the lumen as SIgA Abs. Pathogen-specific SIgA Abs in the nasal mucosa and URT block invasion of and/or reduce growth of pathogenic viruses and bacteria (Figure 1). This can result in the inhibition of disease development, less severe clinical damage and early recovery even when these pathogens partially invade the host. In this regard, one should strongly consider developing a new generation of mucosal adjuvants that could overcome immunosenescence of mucosal immunity and induce universal pathogen-specific SIgA Ab responses in the UR tract. In this brief review, we will focus on several nasal vaccination strategies and a two component adjuvant system that may have the potential to correct altered mucosal immune responses in aging and provide protection from infectious pathogens such as influenza virus and S. pneumoniae in the UR tract.
Figure 1. Nasal vaccines given with a double adjuvant system elicit protective mucosal immunity in aging.
Nasal immunization with hemagglutinin (HA) or pneumococcal surface protein A (PspA) plus pFL and CpG ODN induces HA- or PspA-specific SIgA Ab responses in aged mice. Thus, nasal challenge with influenza virus or S. pneumoniae resulted in the complete protection of the nasal mucosa of aged mice.
Roles for SIgA in upper respiratory tract infections
It is well accepted that specific Abs with neutralizing activity play a major role in protection from influenza virus infections. Furthermore, pathogen-specific SIgA Abs are a key player as a first line of defense against infectious diseases (Kiyono et al., 2008; Fujihashi et al., 2013). Although it has been shown that some protection can be provided by influenza-specific systemic IgG without mucosal SIgA Ab responses (Harriman et al., 1999), influenza-specific SIgA Ab responses are thought to be a necessary component for providing the most effective type of protective immunity. To support this view, it was reported that influenza hemagglutinin (HA)-specific SIgA Ab responses play a key role in protection against influenza infections in the URT and provide cross-protection against infection with a variant influenza virus within the same subtype (Liew et al., 1984). Current influenza vaccines which are licensed for parenteral administration in humans are highly effective against homologous virus infection (Wright et al., 2007). Unfortunately, these vaccines do not elicit SIgA Abs and are less effective against heterologous drift virus infection despite inducing neutralizing IgG Abs against one surface glycoprotein HA. In contrast, influenza-specific IgA Abs can bind not only to the vaccine strain, but also to some drift influenza virus strains for cross-protection (Tamura et al., 2004). Further, passive transfer of influenza-specific, purified SIgA Abs from nasal washes (NWs) of immunized mice provided protection against lethal infection in naïve mice (Tamura et al., 1991). Thus, an influenza-specific, IgG-independent Ab type of immunity could provide protection from influenza virus infection (Tamura et al., 1991). Moreover, IgA deficient (IgA−/−) mice given HA-based influenza vaccine resulted in high virus titers in the lung washes (LWs) despite high titers of HA-specific IgG Abs (Asanuma et al., 2012). These results clearly show that influenza-specific SIgA Abs at the surface of the nasal mucosa block invasion of and/or reduce growth of influenza viruses in the URT. Thus, induction of influenza-specific SIgA Abs would result in the inhibition of disease development, less severe clinical damage and early recovery even when this pathogen successfully invades the host.
It has been shown that IgA also plays a central role in immune defense, controlling the spread of respiratory bacterial pathogens such as S. pneumoniae. Indeed, it was reported that S. pneumoniae capsular polysaccharide-specific pIgA Abs more efficiently initiated complement-mediated killing of the bacterium (Janoff et al., 1999). A superior approach, which would be expected to eliminate virtually all nasal colonization, involves use of cross-reactive surface proteins that could protect against colonization of strains regardless of their capsular types. In this regard, it has been shown that vaccines containing pneumococcal surface protein A (PspA) can provide protective immunity against pneumococcal colonization (Arulanandam et al., 2001; Briles et al., 2003; Ogunniyi et al., 2007). Nasal immunization with PspA and nCT as mucosal adjuvant elicited PspA-specific mucosal SIgA Ab responses and provided protection against carriage of S. pneumoniae and subsequent pneumococcal infection (Wu et al., 1997; Yamamoto et al., 1998; Briles et al., 2000). Further, recent studies demonstrated that pIgA/pIgM receptor (pIgR)-deficient (pIgR−/−) mice which lack the ability to actively transport pIgA onto mucosal surfaces as SIgA Abs, failed to provide protection against nasal challenge with S. pneumoniae despite being given a mucosal vaccine which induced SIgA Abs in normal mice (Sun et al., 2004; Park et al., 2008; Ferreira et al., 2009). Although studies with pIgR−/− mice have suggested that SIgA is the most important Ab isotype in mediating protection against pneumococcal infection, the work failed to provide direct evidence for a role for S. pneumoniae-specific IgA Ab responses, since pIgR−/− mice possessed detectable levels of IgA Abs in their mucosal secretions (Park et al., 2008). Further, some of these studies did not focus on the regulation and prevention of nasal carriage of S. pneumoniae, which is of central importance in the spread of pneumococci from human to human (Park et al., 2008; Ferreira et al., 2009). In this regard, recent studies have provided more direct evidence for a central role for S. pneumoniae-specific IgA Ab responses in nasal carriage of S. pneumoniae. Thus, although IgA−/− mice given nasal PspA plus plasmid encoding Flt3 ligand cDNA (pFL) had significantly high levels of PspA-specific IgG Abs, high numbers of pneumococcal colony-forming units (CFUs) were still detected in NWs and nasal passages (NPs) (Fukuyama et al., 2010). In contrast, IgA+/+ mice given the same PspA-based nasal vaccine resulted in high levels of PspA IgA Abs and essentially no bacteria were detected in the nasal cavity (Fukuyama et al., 2010).
Correction of mucosal aging by use of a double adjuvant strategy
It has been shown that nasal immunization may provide a better means to induce protective immunity in aging when compared with oral vaccine delivery (Hagiwara et al., 2003). However, when completely aged (two-year old) mice were nasally immunized with OVA plus the most potent mucosal adjuvant nCT, significantly lower levels of OVA-specific SIgA Ab responses were noted in all external secretions (NWs, saliva and fecal extracts) than those seen in identically immunized, young adult or one-yr old mice (Fukuiwa et al., 2008). In contrast, plasma IgG anti-OVA Ab levels were comparable in all three age groups (Fukuiwa et al., 2008). These results suggest that although mucosal immunosenescence is more delayed in NALT, immunological deterioration ultimately occurs in the NALT-based immune system as well.
In this regard, it would be of significance to develop a novel adjuvant which would elicit vaccine Ag-specific protective mucosal SIgA Ab responses in the elderly. One approach would involve use of a DC targeting strategy in order to correct mucosal immunosenescence. However, conflicting reports concerning functional DC subsets in aged mice have been put forth (Tesar et al., 2006; Kovacs et al., 2009; Shaw et al., 2010). Those suggesting impaired DC effects have included the reduced expression of CCR7 involved in cell tracking, interferon (IFN)-α production after herpes simplex virus-2 infection and IFN regulatory factor-7 (IRF-7) synthesis following CpG ODN activation (Kovacs et al., 2009; Shaw et al., 2010). In contrast, myeloid type DCs were shown to exhibit intact APC functions and TLR expression in aging (Tesar et al., 2006). In order to broadly stimulate potentially weakened DC functions in aging, a double adjuvant system has been developed using a combination of plasmid encoding Flt3 ligand cDNA (pFL) and CpG ODN (Fukuiwa et al., 2008) (Figure 2). Flt3 ligand (FL), a type 1 transmembrane protein, binds either fetal liver kinase 2 (flk2) or fms-like tyrosine kinase 3 (flt3) receptors (Rosnet et al., 1991; Lyman et al., 1993). It has been shown that administration of FL to mice resulted in marked increases in DCs in the bone marrow, the peritoneal cavity, spleen, and thymus (Maraskovsky et al., 1996; Pulendran et al., 1999; Williamson et al., 1999). Nasal delivery of pFL as mucosal adjuvant preferentially expanded CD8+ DCs and subsequently induced Ag-specific, mucosal immune responses mediated by IL-4-producing CD4+ T cells (Kataoka et al., 2004) (Figure 2). CpG ODN exhibits mucosal adjuvant activity through direct activation of TLR9-expressing, plasmacytoid DCs (pDCs) (Krieg et al., 1995; Halpern et al., 1996; Klinman et al., 1996; Chu et al., 1997; McCluskie et al., 1999; Boyaka et al., 2003). When pDCs are activated through TLR9, pDCs secrete a variety of cytokines, including IFN-α, IL-6, IL-12 and TNF-α (Halpern et al., 1996; Klinman et al., 1996). In this regard, it has been shown that Th1-type cytokine-mediated Ag-specific IgG2a Ab responses were induced when CpG ODN was co-administered as an adjuvant in mice (Chu et al., 1997; McCluskie et al., 1999; Boyaka et al., 2003) (Figure 2). Taken together, a combined double adjuvant system could activate two different types of DCs and expand them in the NALT for the induction of full blown immune responses in the URT of the elderly. Further, this adjuvant system could also induce a more balanced Th1- and Th2- type response which would avoid unnecessary inflammatory and/or allergic immune responses. Finally, since it has been shown that high frequencies of IL-17-producing cells are present in mucosal tissues and that some mucosal adjuvants can induce Th17 responses, a balanced Th1- and Th2-type cytokine responses would be suitable for the induction of ideal mucosal immunity without an exaggerated Th17-type cytokine response.
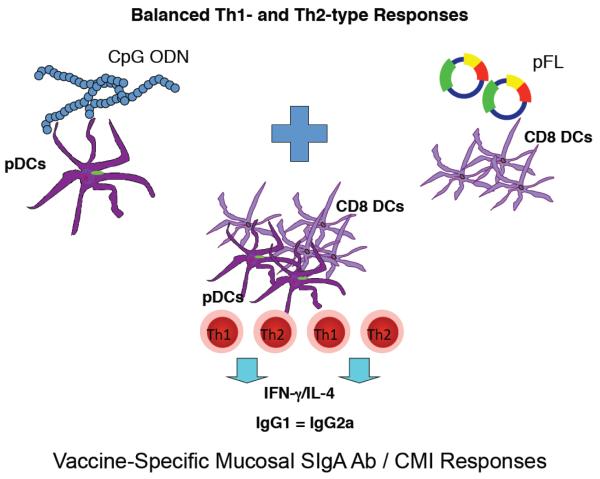
Figure 2. Nasal dendritic cell-targeting double adjuvant system for protective mucosal immunity in aging.
A combination of pFL and CpG ODN induces a more balanced Th1- and Th2-type of immune response to nasally delivered vaccines. Nasal delivery of CpG ODN activates plasmacytoid, B220+ DCs (pDC) and subsequently induces Th1-type, cell-mediated immunity (CMI) and cytotoxic T lymphocyte (CTL) activity in addtion to Ag-specific SIgA Ab responses. In contrast, pFL as nasal adjuvant preferentially expands the CD8+ DC-mediated Th2-type cytokine and Ag-specific SIgA Ab responses. Thus, a combined nasal CpG ODN and pFL stimulates both CD8+ DCs and pDCs and successfully induces Ag-specific SIgA Ab responses.
When fully aged (2 yr-old) mice were nasally immunized with OVA and a combination of pFL and CpG ODN, significant Ag-specific SIgA Ab responses were noted in external secretions, which were essentially equivalent to those observed in young adult mice (Fukuiwa et al., 2008). In addition, plasma IgG and IgA Ab responses were also noted in two-year-old mice immunized with OVA and double DNA adjuvants (Fukuiwa et al., 2008) (Figure 2). These results clearly indicate that the combination of pFL and CpG ODN as a nasal adjuvant system would be superior to the classical and well characterized nCT for the induction of Ag-specific mucosal SIgA Ab responses in aged mice.
Potential influenza vaccines for the elderly
Influenza virus infection remains a serious respiratory disease since the virus often escapes from pre-existing host immunity by altering the antigenic properties of its surface hemagglutinin (HA). In addition, new types of viruses including highly pathogenic avian influenza (HPAI; H5N1) as well as swine influenza (H1N1) viruses cause disease in humans (Wright et al., 2007; Beigel et al., 2008; Dawood et al., 2009). Therefore, effective vaccine development is essential for the prevention of seasonal and pandemic influenza. However, it is difficult to achieve effective protection by using currently available influenza vaccines in all age groups. Influenza virus infections have caused ~ 36,000 deaths annually during the 1990-1999 decade with an annual average of 226,000 hospitalizations over a 1979-2001 period in the USA (Tobita et al., 1975; Thompson et al., 2003). Over 90 % of these deaths and over 50 % of hospitalizations occurred among persons older than 65 years of age. Furthermore, persons over 85 years of age showed substantially higher rates of death than any other age group (Tobita et al., 1975; Pawelec, 2006; Pawelec et al., 2006). In addition, an impaired immune response to influenza infection and vaccination in the elderly may be the clinically most relevant fact associated with infectious diseases in aging (Castle, 2000; Thompson et al., 2004; Targonski et al., 2007). Even when the antigenic match between influenza vaccine and circulating virus is close, vaccination provides only 30–40 % protection in subjects aged > 65 years, whereas 70–90 % protection was seen in those < 65 years of age (Fukuda et al., 1999). The currently available trivalent inactivated influenza vaccines are particularly ineffective in preventing deaths among the elderly with associated chronic conditions (Gross et al., 1995; Fukuda et al., 1999; Goodwin et al., 2006; Pawelec, 2006).
A cellular and molecular understanding of the impaired immune response to pathogens and the development of novel vaccines for the elderly would be clinically relevant and important. Adjuvant systems have provided significant improvement in the development of influenza viral vaccines in the elderly (Galli et al., 2009; Jackson et al., 2012). Thus, an H5N1 vaccine with MF59 adjuvant (oil-in-water influenza vaccine adjuvant) induced a rapid rise in broadly cross-reactive Abs after boosting long-lived human memory B cells (Galli et al., 2009). More recently, the AS03 adjuvant system (Squalene, DL-α-tocopherol and polysorbate 80, GlaxoSmithKline) improved the immune response to inactivated 2009 H1N1 influenza vaccine in both healthy adults (18–64 years old) and older adults (> 65 years of age) (Jackson et al., 2012). Despite these attractive developments, this approach has failed to induce influenza virus-specific mucosal SIgA Ab responses. To address this, our study was designed to determine whether a nasal influenza vaccine together with double adjuvant system pFL and CpG ODN would enhance influenza virus-specific immunity for the prevention of influenza infection in aged mice. A double adjuvant system plus A/Puerto Rico/8/34 (PR8)-HA induced increased numbers of CD11b+ CD11c+ DCs and both CD4+ Th1- and Th2-type responses in mucosal inductive tissues and subsequently elicited PR8-HA-specific SIgA Ab responses in the UR tract of aged mice (Asanuma et al., 2012) (Figure 1). Thus, when mice were challenged with PR8 virus via the nasal route, both aged and young adult mice given nasal vaccine exhibited complete protection (Asanuma et al., 2012) (Figure 1). It should be emphasized that the influenza vaccine given with the double adjuvant system induced high titers of influenza-specific SIgA and plasma IgG Ab responses which provided protective immunity in fully aged mice. These results indicate the potential for a double adjuvant system for future human use.
A pneumococcal mucosal vaccine for the elderly
Pneumococcal infection is a major disease affecting the URT and subsequently causes severe illness, resulting in over 40,000 deaths in the United States each year (Murray et al., 1997). Most notably, severe illness and mortality caused by pneumococci have sharply increased in the elderly (Janssens et al., 2004). Infections with pneumococci are thought to always be preceded by nasal colonization (Gray et al., 1980). As a result, optimal protection against pneumococci is thought to require protection against colonization at the mucosal surface in addition to protection from the invasive type of infection. The effectiveness of the currently licensed pneumococcal polysaccharide vaccine (PPV) for adults consists of capsular polysaccharides derived from 23 serotypes (PPV23). In fact, PPV23 elicited predominant polysaccharide-specific polymeric IgA2 Ab responses in human serum, saliva and tears (Lue et al., 1988; Tarkowski et al., 1990). This vaccine was effective in young adults; however, its effectiveness was reduced at ages above 75 years (Shapiro et al., 1991; Schenkein et al., 2008). It has previously been reported that older adults have a less effective Ab response to PPV23 than do their younger counterparts (Rubins et al., 1998; Romero-Steiner et al., 1999; Rubins et al., 1999). These observations clearly indicate that the development of a new generation of vaccines will be essential in order to provide effective protection against S. pneumoniae in the elderly. Since pneumococcal infection is a major URT disease which occurs on mucosal surfaces, one must take into account a mucosal vaccine strategy for developing an effective vaccine that could induce pathogen-specific immunity in the URT of the elderly.
In addition to PPVs, PspA has also been employed as Ag in experimental nasal vaccines in order to induce S. pneumoniae-specific mucosal protective immunity. Nasal administration of PspA-based vaccines has been shown to preferentially induce PspA-specific Ab responses in the URT as well as in other mucosal lymphoid tissues (Yamamoto et al., 1998; Arulanandam et al., 2001; Briles et al., 2003; Ogunniyi et al., 2007), whereas oral immunization is limited to Ab induction, largely in the GI tract (Rudin et al., 1998; Rudin et al., 1999). Indeed, it has been shown that nasal immunization with PspA-based vaccines in mice provided effective protective immunity against pneumococcal colonization and invasive infection (Yamamoto et al., 1998; Arulanandam et al., 2001; Briles et al., 2003; Ogunniyi et al., 2007). In order to further expand the potential of PspA-based vaccines for the elderly, a double adjuvant system was also tested in order to prevent pneumococcal colonization and invasive infection. High levels of PspA-specific mucosal SIgA and plasma IgG Abs were detected in aged mice given a nasal vaccine consisting of PspA with a combination of pFL and CpG ODN (Fukuyama et al., 2011) (Figure 1). Similar to other studies (Fukuiwa et al., 2008; Asanuma et al., 2012), increased numbers of DCs and a more balanced Th1- and Th2-type cytokine response by mucosal CD4+ T cells were seen in fully aged mice (Fukuyama et al., 2011) (Figure 1). Importantly, aged mice given PspA plus a combination of pFL and CpG ODN showed protective immunity against nasal S. pneumoniae colonization. These results demonstrate that nasal delivery of a combined DNA adjuvant system offers an attractive possibility for protection of the elderly from S. pneumoniae infection.
Future applications for these double adjuvant and mucosal delivery systems for the induction of protective immunity in aging
The elderly have already experienced viral and bacterial pathogens and thus possess pathogen-specific Abs, whose titers are both too low and too clonally restricted to effectively combat reinfection. It would be of great benefit to the aged population if one could use an innate adjuvant system alone, without Ag, in order to enhance mucosal immunity against past respiratory infections. Thus, it will be interesting to investigate whether nasal delivery of the innate molecules pFL and CpG ODN enhances pre-existing host memory Ab responses against influenza and the pneumococcus in the elderly. In addition to a nasal delivery system, one should consider targeting other mucosal inductive tissues which potentially induce URT immune responses. To this end, recent studies have shown that vaccine delivery through eye-drops effectively induce Ag-specific SIgA Ab responses (Nagatake et al., 2009; Seo et al., 2010). Further, sublingual application of influenza vaccine successfully elicited protective mucosal immunity (Song et al., 2008; Park et al., 2012). It was demonstrated that a nanometer-sized hydrogel (nanogel) consisting of a cationic cholesteryl group-bearing pullulan (cCHP) is also an effective nasal vaccine delivery vehicle for the induction of protective immunity without co-administration of biologically active adjuvant (Nochi et al., 2010). Nasal vaccination with the nanogel containing PspA induced Ag-specific SIgA responses which provided protective immunity against S. pneumoniae infection (Kong et al., 2013). Nevertheless, we still need to understand the precise cellular and molecular mechanisms for mucosal immunosenescence in order to develop novel mucosal vaccines for the elderly which can overcome their immunodeficiency.
Highlights.
Pathogen-specific SIgA Abs are the central player for mucosal Immunity in aging.
A nasal DC-targeting, double adjuvant system overcomes mucosal immunosenescence.
A double adjuvant system induces a more balanced Th1- and Th2-type cytokine response.
Acknowledgments
This work is supported by National Institutes of Health (NIH) grants AG025873 as well as the Japan Society of the Promotion of Science (JSPS) program entitled “Young Researcher Overseas Visits Program for Vitalizing Brain Circulation”, Global Center of Excellence (GCOE) Program “Center of Education and Research for the Advanced Genome - Based Medicine - For personalized medicine, the control of worldwide infectious diseases - “MEXT” Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 2001;69:6718–6724. doi: 10.1128/IAI.69.11.6718-6724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Zamri NB, Sekine S, Fukuyama Y, Tokuhara D, Gilbert RS, Fukuiwa T, Fujihashi K, Sata T, Tashiro M, Fujihashi K. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine. 2012;30:803–812. doi: 10.1016/j.vaccine.2011.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J, Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008;78:91–102. doi: 10.1016/j.antiviral.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 2003;170:5636–5643. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, Ferguson LM, Nahm MH, Nabors GS. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 2000;182:1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- Briles DE, Hollingshead SK, Paton JC, Ades EW, Novak L, van Ginkel FW, Benjamin WH., Jr. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 2003;188:339–348. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- Enioutina EY, Visic VD, Daynes RA. Enhancement of common mucosal immunity in aged mice following their supplementation with various antioxidants. Vaccine. 2000;18:2381–2393. doi: 10.1016/s0264-410x(00)00008-6. [DOI] [PubMed] [Google Scholar]
- Ferreira DM, Darrieux M, Silva DA, Leite LC, Ferreira JM, Jr., Ho PL, Miyaji EN, Oliveira ML. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 2009;16:636–645. doi: 10.1128/CVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K, Boyaka PN, McGhee JR. Host defenses at mucosal surfaces. In: Rich RT, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology. Mosby Elsevier; Philadelphia, PA: 2013. pp. 287–304. [Google Scholar]
- Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–343. doi: 10.1016/j.it.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Fukuda F, Bridges CB, Brammer TL. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm. Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- Fukuiwa T, Sekine S, Kobayashi R, Suzuki H, Kataoka K, Gilbert RS, Kurono Y, Boyaka PN, Krieg AM, McGhee JR, Fujihashi K. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. 2008;26:4849–4859. doi: 10.1016/j.vaccine.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Hollingshead SK, Briles DE, Fujihashi K. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J. Immunol. 2011;186:2454–2461. doi: 10.4049/jimmunol.1002837. [DOI] [PubMed] [Google Scholar]
- Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Oishi K, Hollingshead SK, Briles DE, Fujihashi K. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J. Immunol. 2010;185:1755–1762. doi: 10.4049/jimmunol.1000831. [DOI] [PubMed] [Google Scholar]
- Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, Del Giudice G, Banzhoff A, Brauer V, Montomoli E, Zambon M, Katz J, Nicholson K, Stephenson I. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl. Acad. Sci. (USA) 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Gray BM, Converse GM, 3rd, Dillon HC., Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann. Intern. Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, Etani Y, Kweon MN, Tamura S, Kurata T, Takeda Y, Kiyono H, Fujihashi K. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 2003;170:1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-γ production by stimulation of interleukin-12 and tumor necrosis factor-α. Cell. Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- Jackson LA, Chen WH, Stapleton JT, Dekker CL, Wald A, Brady RC, Edupuganti S, Winokur P, Mulligan MJ, Keyserling HL, Kotloff KL, Rouphael N, Noah DL, Hill H, Wolff MC. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J. Infect. Dis. 2012;206:811–820. doi: 10.1093/infdis/jis427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Invest. 1999;104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect. Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+ CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 2004;172:3612–3619. doi: 10.4049/jimmunol.172.6.3612. [DOI] [PubMed] [Google Scholar]
- Kiyono H, Kunisawa J, McGhee JR, Mestecky J. The Mucosal Immune System. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 983–1030. [Google Scholar]
- Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. (USA) 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J. Immunol. 2000;165:5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- Kong IG, Sato A, Yuki Y, Nochi T, Takahashi H, Sawada S, Mejima M, Kurokawa S, Okada K, Sato S, Briles DE, Kunisawa J, Inoue Y, Yamamoto M, Akiyoshi K, Kiyono H. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 2013;81:1625–1634. doi: 10.1128/IAI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr., Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- Lue C, Tarkowski A, Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J. Immunol. 1988;140:3793–3800. [PubMed] [Google Scholar]
- Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth LT, Picha KS, McKenna HJ, Fletcher FA, Maraskovsky E, Farrah T, Foxworthe D, Williams DE, Beckmann MP. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18:231–237. doi: 10.1016/s0264-410x(99)00194-2. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Nagatake T, Fukuyama S, Kim DY, Goda K, Igarashi O, Sato S, Nochi T, Sagara H, Yokota Y, Jetten AM, Kaisho T, Akira S, Mimuro H, Sasakawa C, Fukui Y, Fujihashi K, Akiyama T, Inoue J, Penninger JM, Kunisawa J, Kiyono H. Id2-, RORγt-, and LTβR-independent initiation of lymphoid organogenesis in ocular immunity. J. Exp. Med. 2009;206:2351–2364. doi: 10.1084/jem.20091436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 2007;75:350–357. doi: 10.1128/IAI.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Ferko B, Byun YH, Song JH, Han GY, Roethl E, Egorov A, Muster T, Seong B, Kweon MN, Song M, Czerkinsky C, Nguyen HH. Sublingual immunization with a live attenuated influenza A virus lacking the nonstructural protein 1 induces broad protective immunity in mice. PLoS One. 2012;7:e39921. doi: 10.1371/journal.pone.0039921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Ko HJ, Shim DH, Yang JY, Park YH, Curtiss R, 3rd, Kweon MN. MyD88 signaling is not essential for induction of antigen-specific B cell responses but is indispensable for protection against Streptococcus pneumoniae infection following oral vaccination with attenuated Salmonella expressing PspA antigen. J. Immunol. 2008;181:6447–6455. doi: 10.4049/jimmunol.181.9.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G. Immunity and ageing in man. Exp. Gerontol. 2006;41:1239–1242. doi: 10.1016/j.exger.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann. N. Y. Acad. Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- Powers DC. Immunological principles and emerging strategies of vaccination for the elderly. J. Am. Geriatr. Soc. 1992;40:81–94. doi: 10.1111/j.1532-5415.1992.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. (USA) 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991;6:1641–1650. [PubMed] [Google Scholar]
- Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect. Immun. 1999;67:5979–5984. doi: 10.1128/iai.67.11.5979-5984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J. Infect. Dis. 1998;178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- Rudin A, Johansson EL, Bergquist C, Holmgren J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin A, Riise GC, Holmgren J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 1999;67:2884–2890. doi: 10.1128/iai.67.6.2884-2890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal vaccination for patients with COPD: current practice and future directions. Chest. 2008;133:767–774. doi: 10.1378/chest.07-0996. [DOI] [PubMed] [Google Scholar]
- Schmucker DL, Daniels CK, Wang RK, Smith K. Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology. 1988;64:691–695. [PMC free article] [PubMed] [Google Scholar]
- Schmucker DL, Heyworth MF, Owen RL, Daniels CK. Impact of aging on gastrointestinal mucosal immunity. Dig. Dis. Sci. 1996;41:1183–1193. doi: 10.1007/BF02088236. [DOI] [PubMed] [Google Scholar]
- Seo KY, Han SJ, Cha HR, Seo SU, Song JH, Chung SH, Kweon MN. Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. J. Immunol. 2010;185:3610–3619. doi: 10.4049/jimmunol.1000680. [DOI] [PubMed] [Google Scholar]
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr. Opin. Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. (USA) 2008;105:1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Johansen FE, Eckmann L, Metzger DW. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 2004;173:4576–4581. doi: 10.4049/jimmunol.173.7.4576. [DOI] [PubMed] [Google Scholar]
- Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur. J. Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 2004;57:236–247. [PubMed] [Google Scholar]
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J. Immunol. 1990;144:3770–3778. [PubMed] [Google Scholar]
- Taylor LD, Daniels CK, Schmucker DL. Ageing compromises gastrointestinal mucosal immune response in the rhesus monkey. Immunology. 1992;75:614–618. [PMC free article] [PubMed] [Google Scholar]
- Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, Kaech S, Goldstein DR. Murine myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Thoreux K, Owen RL, Schmucker DL. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology. 2000;101:161–167. doi: 10.1046/j.1365-2567.2000.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med. Microbiol. Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 1999;163:3668–3675. [PubMed] [Google Scholar]
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses: Influenza in humans - past pandemics and the H5N1 epidemic. In: Knipe DN, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Streds SE, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1691–1740. [Google Scholar]
- Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Briles DE, Yamamoto S, Ohmura M, Kiyono H, McGhee JR. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J. Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]