Abstract
Glial cell-line derived neurotrophic factor (GDNF) has demonstrated robust effects on dopamine (DA) neuron function and survival. A post-translational processing model of the human GDNF proprotein theorizes the formation of smaller, amidated peptide(s) from the proregion that exhibit neurobiological function, including an 11-amino-acid peptide named dopamine neuron stimulating peptide - 11 (DNSP-11). A single treatment of DNSP-11 was delivered to the substantia nigra in the rat to investigate effects on DA-neuron function. Four weeks after treatment, potassium (K+) and d-amphetamine evoked DA release were studied in the striatum using microdialysis. There were no significant changes in DA-release after DNSP-11 treatment determined by microdialysis. Dopamine release was further examined in discrete regions of the striatum using high-speed chronoamperometry at 1-, 2-, and 4-weeks after DNSP-11 treatment. Two weeks after DNSP-11 treatment, potassium-evoked DA release was increased in specific subregions of the striatum. However, spontaneous locomotor activity was unchanged by DNSP-11 treatment. In addition, we show that a single treatment of DNSP-11 in the MN9D dopaminergic neuronal cell line results in phosphorylation of ERK1/2, which suggests a novel cellular mechanism responsible for increases in DA function.
Keywords: dopamine, glial cell line-derived neurotrophic factor, Parkinson’s disease, striatum, peptide
1.0 Introduction
The potential of neurotrophic factors to combat neurodegenerative disease has led to intense investigation of their function (for review see [46]). In particular, glial cell-line derived neurotrophic factor (GDNF) has shown profound effects on nigrostriatal dopamine (DA) neuron function in normal, aged [26], and lesioned [5, 29] rat models. Changes in neurochemical function due to GDNF treatment are thought to contribute to improved behavioral performance in rodents, which is also seen in GDNF treated non-human primates [15, 22, 23]. Effects of GDNF in rodent and non-human primate dopaminergic systems provided the necessary evidence to warrant further investigation of the therapeutic value of GDNF. Phase I and II clinical trials using GDNF for the treatment of Parkinson’s disease (PD) produced conflicting results that highlight delivery issues related to the poor bioavailabilty and/or distribution of GDNF [21, 39, 48, 51]. Effective delivery and diffusion to target areas in the brain were thought to be hindered by the large size and heparin-binding characteristics of GDNF [25, 39, 48]. The limitations of GDNF in the clinic have stimulated new efforts to find and characterize small mimetic molecules [3] that avoid previous administration issues.
Recently, biological activity has been reported from highly conserved sequences from the proregion of GDNF [8, 34]. A post-translational processing scheme for human GDNF was described by our group and others [8, 34]. In particular, the processing scheme predicts the liberation of an 11-amino acid amidated peptide from the proregion of GDNF through a series of endoproteolytic cleavage events and peptide amidation enzymes [8]. Bioactvitiy of the rat homolog (LLEAPAEDHSL-NH2) was confirmed in studies in which the peptide, named Brain Excitatory Peptide (BEP), demonstrated binding affinity for CNS-related tissues and increased excitatory post-synaptic potentials in rat hippocampal brain slices [34]. Bradley et al, 2010 [8] introduced the successful synthesis and evaluation of the human homolog of the 11-amino acid peptide (PPEAPAEDRSL-NH2), DA neuron stimulating peptide-11 (DNSP-11). Briefly, in vitro studies support that DNSP-11 demonstrates trophic-like actions in dopaminergic fetal mesencephalic cells by increasing cell morphological features. The ERK1/2 pathway has been associated with neurite outgrowth induced by nerve growth factor (NGF) and GDNF [47, 54]. In the MN9D dopaminergic neuronal cell line, DNSP-11 appears to confer neuroprotection against 6-hydroxydopamine (6-OHDA). Additionally, neuroprotective effects against staurosporine and gramicidin cytotoxicity, including a reduction in cytochrome c release from mitochondria in B65 dopaminergic neurons, were reported [8]. Investigation of DNSP-11’s in vivo properties revealed that dopaminergic neurons internalize exogenously applied DNSP-11 and this treatment subsequently alters dopaminergic neuron function with increased basal levels of DA and DA metabolites 4 weeks after a single treatment to the substantia nigra (SN) [8]. ERK1/2 is implicated in many cell processes that mediate gene expression through histone kinase proteins and nuclear transcription factors [12, 41].
To gain a further understanding of DNSP-11’s effects [8, 38], we investigated changes in DA neuron function at the terminal projections in the striatum and motor behavior following a single administration of DNSP-11 to the SN in Fischer 344 (F344) rats. We also examined DNSP-11’s neurotrophic-like effects on ERK1/2 activation in MN9D dopaminergic neurons. Collectively our studies illustrate the similarities and differences between GDNF and DNSP-11.
2.0 Methods
2.1 Ethics Statement
Animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee (ID number: 882M2005) and were in strict agreement with AAALACI guidelines.
2.2 Materials
All chemicals were purchased from Fisher Scientific (Fisher Chemical Fairlawn, NJ) or Sigma-Aldrich (St. Louis, MO). DNSP-11 peptide was synthesized and purified by W.M. Keck Foundation (Yale University, New Haven, CT). The control peptide, containing identical amino acid constituents in a random order (scrambled 11mer), was obtained from AC Scientific (Duluth, GA). Solutions of DNSP-11 or control peptide (6 μg/μL) were dissolved in sterile citrate buffer (10 mM Sodium Citrate + 150 mM NaCl, pH 5) as described previously [8]. MN9D dopaminergic cells were provided as a gift by Michael Zigmond (University of Pittsburgh).
2.3 Infusion Delivery of DNSP-11 to the Substantia Nigra
Male F344 rats (3–6 months of age) were used in all studies. Rats were obtained from Harlan Laboratories Inc. (Indianapolis, IN) and were housed on a 12 hr light/dark cycle with food and water provided ad libitum.
Animals were treated with either DNSP-11 (30 μg), control peptide (30 μg) or citrate buffer vehicle (equivalent volume) to the right SN as described previously [28]. This DNSP-11 dose reflects a half-log increase from the most effective dose of GDNF determined in vivo [28, 33]. All studies were conducted double-blinded to treatment groups.
2.4 Spontaneous Locomotor Activity
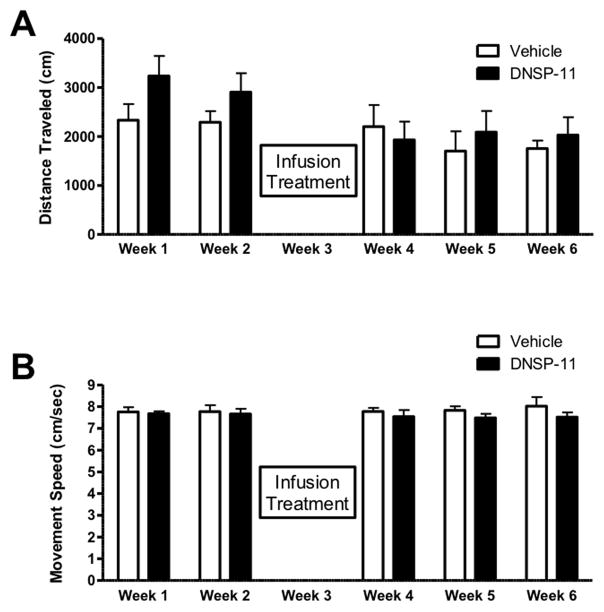
Animals were habituated to the activity chambers through weekly testing sessions, 4 weeks before treatment. Animals were subsequently divided into two groups with comparable values for movement velocity. Animals received DNSP-11 or vehicle as described above. Activity parameters (total distance and movement speed (velocity)) were assessed weekly for three weeks after treatment as described previously [26]. Each testing session consisted of a total of 60 minutes in the activity chamber averaged every ten minutes. Motor activity was represented by the total distance traveled (cm) and average movement speed (the distance traveled per time (cm/sec)).
2.5 In vivo (Striatal) Microdialysis
CMA 11 microdialysis probes (4 mm membrane length, CMA Microdialysis, Stockholm, Sweden) prepared according to manufacturer instructions. The percent recovery rate for the neurochemicals of interest was determined by perfusing the probes with artificial cerebral spinal fluid (aCSF) (124 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM NaH2PO4) while submerged in a solution containing standardized concentrations of the neurochemical(s) of interest. The percentage of neurochemicals in the collected solution relative to the standard solution yields the neurochemical percent recovery.
Four weeks after treatment, in vivo microdialysis was performed in the right striatum. The microdialysis and HPLC-EC protocols were modified from previous protocols [24, 26, 28]. CMA 11 microdialysis probes were stereotaxically placed in the striatum: from bregma (mm) AP: +1.5 ML: −2.3 DV: −8.0 (tip of microdialysis probe) [26, 28, 45]. Thus, the sampling area of the probe spanned the dorsal-ventral orientation of the striatum from 4 mm to 8 mm [45]. Perfusion solutions included: 1) aCSF followed by 2) 100 mM K+ aCSF (26 mM NaCl, 100 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM NaH2PO4) and 3) 250 μM d-amphetamine aCSF (pH 7.2 – 7.4, all). All solutions were perfused at a rate of 1 μL/min with a sampling interval of 20 minutes. Dialysate samples were immediately frozen (−70 °C) after collection and thawed before analysis by high performance liquid chromatography coupled with electrochemical detection (HPLC-EC). Due to probe variability, neurochemical concentrations from all dialysate samples were corrected for individual probe recovery before data analysis.
2.6 In vivo (Striatal) Chronoamperometry
Carbon fiber microelectrodes coupled with high-speed chronoamperometry were used to measure evoked DA release in the rat striatum 1, 2, and 4 weeks following intranigral treatment. Oxidation and reduction currents were digitized using the FAST-16 mkII recording system (Quanteon, LLC, Nicholasville, Kentucky) as outlined in previous work from our laboratory [20, 28]. For a detailed review of underlying principles see Gerhardt and Burmeister [17] and Cahill et al.[9].
Before experiments, microelectrodes were coated with Nafion® (50 mg/mL, Sigma-Aldrich Chemical Co., St. Louis, MO) to increase sensitivity and selectivity for dopamine versus electroactive species in the brain (e.g. ascorbic acid). Before animal recordings, carbon fiber microelectrodes were calibrated in vitro. Before animal recordings, carbon fiber microelectrodes were calibrated in vitro in a slowly stirred 0.05 M phosphate buffered saline solution [19]. Microelectrodes used in the described experiments exhibited linear responses to serial DA additions (R2 ≥ 0.96) with LODs of 39 ± 6 nM (mean ± SEM) n = 68 (microelectrodes) with a signal to noise ratio ≥ 3.
A glass micropipette (1 mm o.d., 0.58 i.d., A-M Systems, Inc., Everett, WA) was pulled and bumped to an inner diameter of 5–12 μm. The micropipette was affixed flush with the carbon fiber tip and 280–320 μm from the recording surface and was filled with an isotonic potassium chloride solution (120 mmol/L KCl, 29 mmol/L NaCl, 2.5 mmol/L CaCl2•2H2O, pH = 7.2–7.4) used to locally depolarize striatal terminals [20].
Animals were prepared for electrochemical recordings at the designated time points after unilateral DNSP-11, vehicle, or control peptide treatment as previously described [19]. The carbon fiber/micropipette assembly was stereotaxically placed and lowered into the striatum (from bregma (mm)): AP +1.0, ML −2.3, DV −3.5 to −7.5 (incisor bar: −2.3) [45]. The potassium solution was locally-applied by pressure ejection (10–30 psi) of nitrogen gas to elicit a single peak from each recording depth (500 μm increments; DV: −3.5 to −7.5) [44]. The solution volume ejected (75–125 nL) was monitored [14] and the resulting amplitude was quantified as a transient and robust increase (delta) from the baseline oxidation current (nA). The change (delta) was then divided by the electrode slope (nA/μM) to calculate the concentration change in DA (μM) [28]. All peaks used for analysis exhibited reduction/oxidation ratios of 0.746 ± 0.004 (mean ± SEM), n = 509 (peaks) consistent with the established profile for DA [18].
Following electrochemical recordings, animals were euthanized while under heavy anesthesia and brains were frozen until cryosectioning. Brains were cryo-sectioned (20 μM) and stained with cresyl violet to confirm microelectrode placement in the striatum.
2.7 ERK1/2 signaling
To test the phosphorylation of ERK1/2 (pERK1/2), MN9D cells were plated in 35 mm dishes at least 48 hours before experiment. The cells were then incubated in serum-free medium containing 0.2% bovine serum albumin (BSA) for 20 hours prior to the experiment. Cells were then treated with GDNF or DNSP-11. Cell lysates were denatured in the sample buffer at 95°C, resolved by electrophoresis, and probed by Western blotting with ERK1/2 and pERK1/2 antibodies (Cell Signaling Technologies; Boston, MA) followed by chemiluminescence detection as described previously [35, 52]. Several x-ray films were analyzed to determine the linear range of the chemiluminescence signals, and the quantifications were performed using densitometry analysis mode of the QuantityOne software (Bio-Rad, Inc).
2.8 Statistics
For in vivo data (microdialysis and electrochemistry) outliers (values > 2 standard deviations from the mean) were removed from data sets before statistical analyses. Evoked DA release microdialysis data were analyzed by a two-tailed unpaired t test comparing treatment groups. A two-way ANOVA with Bonferroni’s post hoc tests was used to analyze the spontaneous locomotor activity and in vivo chronoamperometric data. In ERK1/2 studies a one-way ANOVA was used to determine significance among groups followed by Tukey’s post hoc analysis. Statistical significance was defined as p < 0.05 for all analyses.
3.0 Results
3.1 Locomotor Activity Parameters are Not Altered by DNSP-11
Analogous to previous studies with GDNF, we assessed possible behavioral changes associated with DNSP-11 treatment. Movement speed and distance traveled were determined before treatment and weekly after treatment (1-, 2-, and 3 weeks after treatment). Total distance traveled showed no significant changes (F(1,70) = 2.734, p = 0.1027) at any of the time points examined after DNSP-11 treatment (Figure 1A). Also, movement speed was not significantly changed (F(1,70) = 2.652, p = 0.1079) by DNSP-11 treatment (Figure 1B) at any of the examined time points. In this initial investigation of the effect of DNSP-11 on DA release and motor behavior, electrochemical studies were carried out at the 4-week time in lieu of behavioral measures in order to provide a more direct durational comparison to the in vivo microdialysis experiments performed 4-weeks after a single treatment [8]
Figure 1. Assessment of Spontaneous Motor Movement.
Spontaneous movement assessed weekly after unilateral DNSP-11 treatment (shown as mean ± SEM, n=8). No significant differences in the spontaneous locomotor parameters, distance traveled (A) (F(1,70) = 2.734, p = 0.1027) or movement speed (B) (F(1,70) = 2.652, p = 0.1079) were observed.
3.2 In vivo (Striatal) Microdialysis Measures Indicate DNSP-11 Does Not Affect Dopamine Release
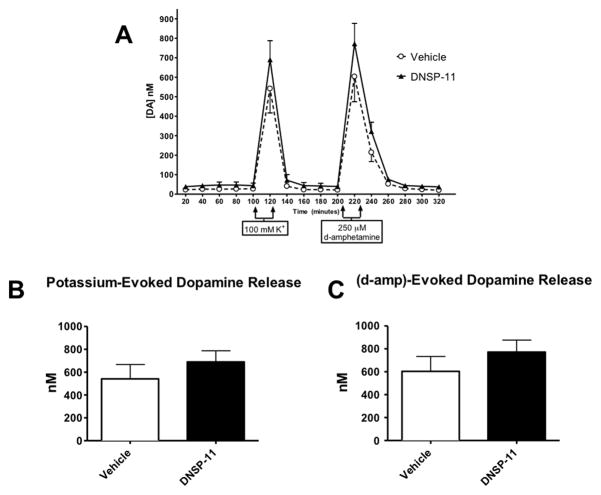
Because prior studies showed modulation of extracellular basal levels of striatal DA after GDNF [26, 28] and DNSP-11 treatment we further investigated DNSP-11’s effects on nigrostriatal dopaminergic function by investigating DA release. Four weeks after treatment (DNSP-11 or vehicle) striatal DA release was determined. A total of 16 fractions were collected (Figure 2A) while varying the perfusion solution: fractions 1–5 – aCSF, fraction 6 – 100 mM K+ aCSF, fractions 7–10 – aCSF, fraction 11 – 250 μM d-amphetamine aCSF, fractions 12 –16 aCSF. Calcium-dependent vesicular release of DA was stimulated by reverse microdialysis of an isotonic high potassium/aCSF solution (fraction 6). Transporter-mediated release was investigated using the d-amphetamine/aCSF solution (fraction 11), which caused DA release through the reversal of the dopamine transporter [10, 37]. Neither potassium- nor d-amphetamine-evoked DA release was significantly different between treatment groups (p > 0.05, both (Figure 2B,C)).
Figure 2. In vivo Microdialysis used to Determine Evoked Dopamine Release in the Striatum.
A) Extracellular levels of DA were determined 4 weeks after unilateral treatment with 30 μg of DNSP-11 or vehicle to the SN. Microdialysis methods were used, collecting samples at 20-minute intervals. A high-potassium (K+) solution was used to cause local depolarization and vesicular release of DA. Also, a high- d-amphetamine solution was used to determine transporter-mediated release of DA. Neither (B) potassium- nor (C) d- amphetamine-evoked DA release was changed by DNSP-11 treatment (p > 0.05, both). Analyzed using a two-tailed unpaired t test. Data are shown as mean ± SEM (nmol/L), n = 5–6.
3.3 DNSP-11 Increased Striatal Evoked Dopamine Release 2 weeks after Treatment in the Dorsal and Intermediate but not Ventral Subregions
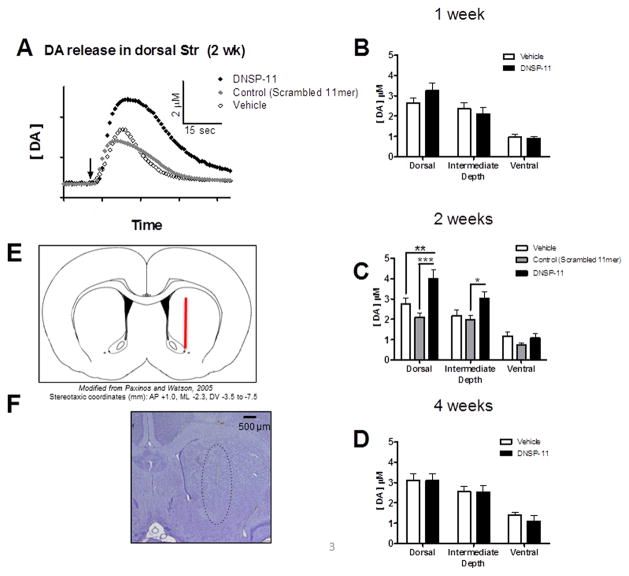
In vivo DA release was investigated using electrochemical methods that have been shown to reliably measure catecholamine release by local application of a potassium chloride solution [20]. Subregional effects on potassium-evoked DA release within the dorsal-ventral orientation of the striatum were investigated as well as the duration of effects from a single treatment. Experiments were performed 1-, 2-, and 4-weeks after a single unilateral treatment (DNSP-11, vehicle, or control peptide) to the SN. The control peptide became available during these studies and was added to the 2-week time point. Release measurements were taken every 500 μm along the dorsal-ventral orientation of the striatum – generating a striatal depth profile of DA release at each time point. Stereotaxic placement of the recording assembly is represented in Figure 3E. The brains from animals used in electrochemical recordings were collected for histochemical determination of appropriate electrode placement after recordings (Figure 3F).
Figure 3. Evoked Dopamine Release at Multiple Depths within the Striatum at 1, 2, and 4 weeks.
A) Representative traces of DA release amplitude in the dorsal striatum evoked by a local application of potassium (arrow) in vehicle, control and DNSP-11 treatment groups (2 weeks after treatment)(inset: axes units). DNSP-11 treatment (black) increased the amplitude of dopamine release evoked by potassium relative to the amplitude of evoked DA in vehicle (open) and control (gray) treated animals. B–D) Electrochemical measures of DA at three subregions within the striatum were taken at three time points after intranigral treatment with DNSP-11 or vehicle: (B) 1 week (F(1,147)= 0.2540, p = 0.6150), (C) 2 weeks (F(2,203)= 13.04, p <0.0001), and (D) 4 weeks (F(1,121)= 0.2236, p = 0.6371). Two weeks after treatment (A)(C) there was a significant increase in average DA-release amplitude in DNSP-11 treated groups versus vehicle (**p<0.01) and control peptide (***p < 0.001) in the dorsal striatum (mean ± SEM (μM), vehicle: 2.77 ± 0.28; control: 2.11 ± 0.18; DNSP-11 4.00 ± 0.44). In the intermediate striatum (C) there was also a significant increase (*p < 0.05) in potassium-evoked DA in DNSP-11 treated groups versus control only (mean ± SEM (μM), Control: 1.99 ± 0.20; DNSP-11 3.02 ± 0.34). Data were analyzed using a two-way ANOVA with Bonferroni’s post hoc tests. Data are shown as mean ± SEM, n = 23–27 (number of peaks). Averages were calculated from 7–8 animals (per treatment group). Carbon fiber microelectrodes were stereotaxically placed at coordinates corresponding to the rat striatum – (E) represented in the illustration. Following electrochemical recordings, brains were cryosectioned and stained to confirm electrode placement in the striatum. The electrode tract is highlighted inside the dashed outline (F).
Based on the heterogeneity of the origin of DA terminals in the striatum [1] and appreciable variability in amplitude among dorsal and ventral recording depths, data were grouped and analyzed by striatal subregion: dorsal, intermediate, and ventral. Each subregion included DA release data from three depths i.e. −3.5, 4.0, −4.5 (dorsal), −5.0, −5.5, −6.0 (intermediate), −6.5, −7.0, −7.5 (ventral) (Figure 3B–D). Because these data demonstrated greater variability within an individual animal (between recording depths) than between animals (at the same recording depth), each recording point (depth) was treated as an individual sample (n) in subregional analyses [11, 28]. Release amplitude was analyzed for each striatal subregion comparing DNSP-11 to vehicle treatment (Figure 3B–D) at 1-, 2-, and 4 weeks after treatment. Dopamine release amplitude was significantly increased at 2 weeks after DNSP-11 treatment (Figure 3A,C, Figure 4) and this effect was restricted to the dorsal striatum (p < 0.01, versus vehicle). Striatal DA release at all subregions was unchanged at earlier (1-week) and later (4-week) time points (p > 0.05, both). Thus, the peak effect of DNSP-11 occurred 2 weeks after treatment, with a 44% increase in potassium-evoked DA release in the dorsal striatum (versus vehicle).
Figure 4. Striatal Depth Profile of Potassium-Evoked Dopamine Release (2 Weeks).
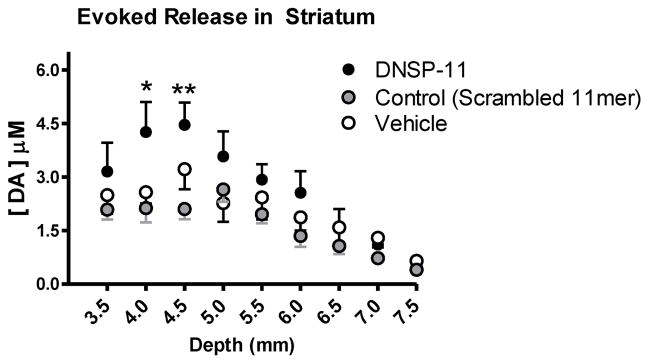
Measurements of DA release after local application of potassium were taken every 500 μm in the rat striatum. A significant effect of treatment was observed (F(2,185) = 12.88, p < 0.0001). Post hoc analysis indicates a significant increase in evoked DA release in DNSP-11 treated groups versus control peptide at two individual recording depths in the striatum (mean ± SEM (μM), DV: 4.0 mm, Control: 2.13 ± 0.40; DNSP-11: 4.26 ± 0.84; DV: 4.5 mm, Control: 2.11 ± 0.30; DNSP-11: 4.46 ± 0.62). Analyzed by a two-way ANOVA with Bonferroni’s post hoc tests (mean ± SEM, n = 7–8), *p < 0.05 **p <0.01.
A control peptide (scrambled 11mer) became available during these studies and was added as an additional treatment group to the 2-week time point studies (Figure 3A, C, Figure 4). This group was included to investigate the potential effects of a scrambled peptide on DA release. Subregional analysis shows a significant increase in amplitude of DNSP-11 treated groups in dorsal (p < 0.001) and intermediate (p < 0.05) striatal subregions versus control peptide. The ipsilateral striatum was used as the control recording in all experiments. Although the contralateral striatum may be viewed as an alternative within animal control, because the bilateral effects of DNSP-11 have not been established the ipsilateral striatum was used, exclusively, to compare DNSP-11 to control treatments (control/vehicle). Neurochemical analysis of nigral tissues from DNSP-11 treated animals do not indicate bilateral effects (data not shown).
An appreciable decline (data not quantified) in amplitude was evident in more ventral recording depths (Figure 4) compared to dorsal subregions. This decline was consistent with previous studies using the same methodology and reflects a corresponding decrease in DA nerve terminal density ventrally [20, 43]. Of greater interest than the depth variable was the treatment effect of DNSP-11. The striatal depth profile of individual recording depths (shown for the 2-week time point, Figure 4) indicated elevated evoked release of DA after DNSP-11 treatment, which was more apparent at dorsal recording depths. The potassium-evoked DA release amplitudes from DNSP-11 treated groups were significantly increased compared to control peptide groups at individual recording depths (Figure 4, DV: 4.0 mm, p < 0.05, DV: 4.5 mm, p < 0.01). No significant differences (p > 0.05) were observed for potassium-evoked DA release amplitudes between control peptide and vehicle treatment groups for any individual recording depth or striatal subregion.
3.4 DNSP-11 Activates ERK1/2 signaling pathway in the MN9D Neuronal Cell Line
To gain insight into the signaling mechanisms responsible for the neurotrophic-like activity of DNSP-11, we examined the capacity of DNSP to activate the ERK1/2 signaling pathway in MN9D dopaminergic neuronal cell culture. The ERK1/2 pathway has been associated with neuroprotective and neurotrophic effects of GDNF [42]. MN9D cells treated for 20 minutes with a previously determined neuroprotective dosage (100 nM) of DNSP-11 [8, 38], and increased dosage (1 μM), showed significant increases (> 300% and 400% respectively, compared to control) in ERK1/2 phosphorylation (Figure 5). These increases in activation of ERK1/2 in MN9D cells after 20 minutes of treatment, were similar to those observed for a 1.5 nM dosage (approximately 50 ng/mL) of GDNF (Figure 5) and previously reported results of GDNF in MN9D cells and rat organotypic hippocampal slices [7, 53].
Figure 5. DNSP-11 Increases ERK1/2 Phosphorylation in MN9D Dopaminergic Neuronal Cell Culture.
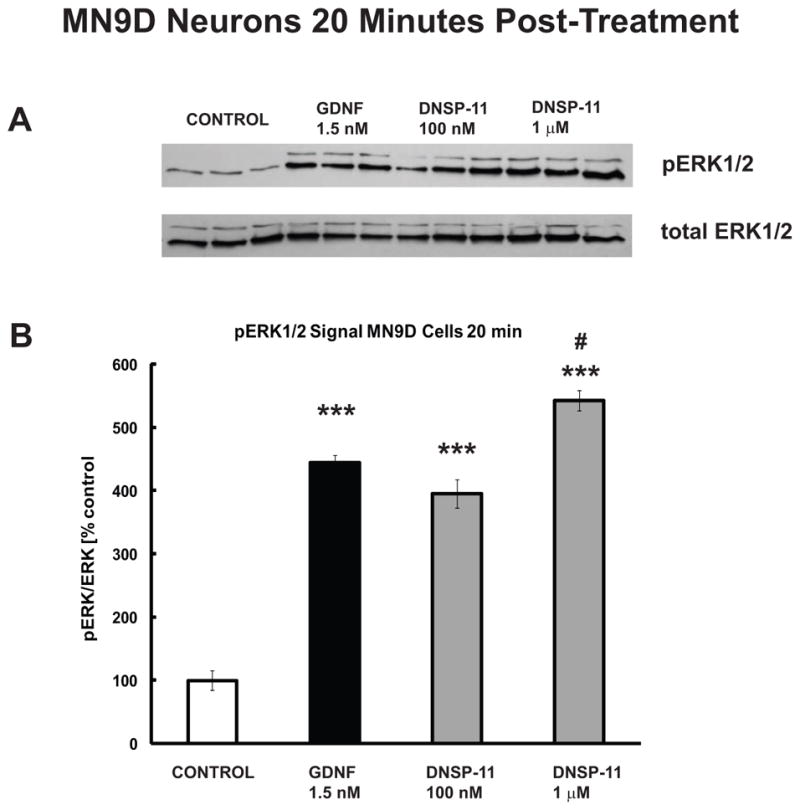
A) Western blot analysis showing pERK1/2 levels 20 minutes after treatment with GDNF (1.5 nM) and two dosages (100 nM and 1 μM) of DNSP-11. Experiments were performed in triplicate. B) Quantification shows that significant activation of pERK1/2 following treatment with GDNF (black bar) and both dosages of DNSP-11 (gray bars) at 20 minutes versus control (open bar). Data are presented as % control of the mean +/− SEM ratio of pERK1/2:ERK1/2 (n=3). One-way ANOVA was used to determine significance among groups, followed by Tukey’s post hoc analysis. (***p<0.001 vs control; #p<0.05 vs GDNF).
4.0 Discussion
These studies are the first to characterize multiple in vivo changes by DNSP-11, in the rat using measures of DA-neuron function and motor behavior. Microdialysis studies were performed to investigate DA-release dynamics at the 4-week time point because tonic microdialysis levels were increased by DNSP-11 in previously published work, also 4 weeks after a single treatment [8]. Following findings of no change in evoked DA release in the microdialysis studies, in vivo electrochemical methods were used to determine the duration of effects (time-course study) and potential subregional effects on DA release within the heterogeneous striatum. Although no significant change in d-amphetamine- or potassium-evoked DA release was apparent in the microdialysis studies (4-week time point), the major findings of these studies are the enhanced release of DA in the dorsal striatum 2 weeks after a single intranigral treatment. Electrochemical studies designed to characterize evoked DA release in discrete striatal subregions indicated a significant increase in evoked DA release in the dorsal striatum (44% increase versus vehicle, 90% versus control peptide) and the intermediate striatal subregion (52% increase versus control peptide). These effects were present only at the earlier time point (2 weeks). Thus, the lack of effect on DA release in microdialysis studies may be due to: 1) selective subregional effects of DNSP-11 that are diluted by the larger sampling area of microdialysis methods and/or 2) negligible release differences, as determined using both microdialysis and electrochemical methods at the 4-week time point. In summary, these results indicate a functional change in DA terminals in the striatum that we believe to be relevant to neurodegenerative disorders like PD where striatal DA levels decline precipitously with disease progression [2, 30–32].
Surprisingly, measures of spontaneous locomotor behavior did not indicate significant changes following DNSP-11 treatment like previous studies using GDNF. Of note, the effects of GDNF on motor behavior occurred relatively acutely (7–10 days after treatment) and subsequently returned to baseline levels [33]. Although behavior was examined in DNSP-11-treated animals as early as 1 week following treatment, in the future earlier time points will be evaluated. Despite the lack of changes in motor activity in these studies, we believe DNSP-11 has the potential to modulate motor function. However, because non-lesioned, young F344 rats do not exhibit motor impairment [27], behavioral endpoints would be more appropriately examined in a model with a behavioral deficit. Indeed, in rats with 6-hydroxydopamine lesions, DNSP-11 has shown improved neurochemical function with corresponding behavioral improvement [8]. Thus, the behavior modifying properties of DNSP-11 will be further examined in systems with a neurochemical deficiency of the nigrostriatal pathway and corresponding motor deficit.
The pattern of enhanced DA release by DNSP-11 is of particular interest in the context of PD where DA levels are depleted most severely in the human putamen [2, 30–32] – the analogous subregion to the lateral component of the dorsal striatum in rodents [16, 36]. However, it is unlikely that the dorsal striatum, the subregion showing positive effects these studies, is comprised of nigrostriatal projections exclusively with consideration of stereotactic placement variation among animals, striatal heterogeneity and diffuse anatomical boundaries of mesolimbic and nigrostriatal projections [1, 13, 16]. Importantly, the spatial presentation of increased evoked DA release for DNSP-11 contrasts with previous studies using GDNF reported by Hebert et al., (1996) [28] that showed a uniform increase in DA release at nearly all recording depths. Thus, effects of GDNF appear to involve both nigrostriatal (A9) and mesolimbic (A10) DA projections, while DNSP-11 appears to more selectively affect the dorsal striatum[36]. The selectivity of subregional effects of DA release may be interpreted as merely a consequence of experimental design – using stereotaxic coordinates targeting the SN. However, studies using GDNF and this treatment protocol have shown a uniform increase in evoked DA release in nearly all recording regions of the striatum [28] – supporting modulation of DA-neuron projections from the SN as well as the adjacent DA-neuron nucleus the ventral tegmental area (VTA) [1].
Enhanced DA release 2 weeks after a single treatment supports a robust effect on synaptic function and may implicate plastic changes in the nigrostriatal pathway [4]. Recently, we demonstrated a marked decline in immunohistochemical labeling of DNSP-11 occurring 3 hours after treatment and near undetectable levels 24 hours after treatment [8]. This rapid (hours) clearance of DNSP-11 relative to presentation of neurochemical effects (weeks) support that DNSP-11 induces long-term changes in DA neuron function – characteristic of transforming growth factors like GDNF [33, 40]. Because DNSP-11 is cleared rapidly, additional dosing may be required to maintain neurochemical changes. Additional studies to investigate continuous- or repeated-dosing paradigms are ongoing. Importantly, these studies also indicate that treatment with an 11-amino acid control peptide, of identical composition but different sequence than DNSP-11, does not cause comparable effects on DA release.
The ERK1/2 phosphorylation studies provide insight into a potential mechanism underlying changes in DA release. Indeed, increases in DA synthesis and storage and/or DA metabolism are possible contributing factors to the increased release. GDNF increases DA release by increasing DA synthesis/storage through stimulating actions on tyrosine hydroxylase (TH) activity [50] – a modification thought to be mediated by ERK signaling [49]. GDNF has been previously shown to activate the ERK1/2 pathway in MN9D and organotypic primary cell culture [7, 53]. Here we show that DNSP-11 also shows similar significant activation of ERK1/2 in MN9D cells. However, the apparent differences in potency between DNSP-11 and GDNF are further evidence of a novel DNSP-11 mechanism(s). Previously we have shown that DNSP-11 does not interact with the putative GDNF receptor, GFRα1 (GDNF Family receptor-α1), a differential response to staurosporine protection, and pull down analysis showing interacting protein partners unique from GDNF [8]. It is also possible that the release enhancing effects of DNSP-11 may be due to other presynaptic release mechanisms. For example, an increase in the number of DA-releasing synapses in vivo may be possible as increased morphological complexity of DA-neuron cell cultures has been demonstrated with DNSP-11 treatment [8]. The potential synaptic mechanisms and the mechanistic cellular aspects of DNSP-11 function are currently under investigation.
The presented studies add that processing of the proregion may also result in the generation of bioactive peptides with other unique, long–lasting in vivo functions. To our knowledge, DNSP-11 is the first peptide derived from the prodomain of the human GDNF family to demonstrate in vivo trophic-like actions [8, 34, 38]. Because of it’s relative size and less complex structure, DNSP-11 may provide specific advantages over larger neurotrophic molecules used to target neurodegenerative diseases including: ease of synthesis [8], long-term storage [46], improved biodistribution/lack of heparin binding [8] and potential for increased bioavailabilty and/or modification lending to alternative (less invasive) delivery routes [3, 6]. These possibilities merit further investigation to confirm the potential of DNSP-11 to reach target sites and confer therapeutic benefit.
Highlights.
A peptide (DNSP-11), derived from the proregion of GDNF, was investigated.
DNSP-11 was delivered to the substantia nigra to investigate in vivo effects.
DNSP-11 caused increased dopamine release in the dorsal, but not ventral, striatum.
The release effects occurred 2 weeks after treatment but not at 1- or 4-weeks.
DNSP-11 increased ERK1/2 phosphorylation in neuronal cells.
Acknowledgments
This work is supported by funding sources:
GAG: USPHS NS39787; DA017186; AG13494; NSF EEC-0310723; NIH Training Grants: 5T32 AG000242-14 and 1T32 DA022738 (OML/JLF)
DMG: T32-AG00242; PO1-13494; NINDS P50-NSO39787; endowed funds
LHB: NIH COBRE Pilot P20RR20171; PhRMA Foundation; Columbus Foundation; University of Kentucky College of Medicine Startup Funds; NIH NSO75694; NIH CCTS Pilot UL1TR000117.
WAC: USPHS Grants NS60924 and DA22314
The authors would like to thank Drs. Matt Joyce and Erin R. Hascup for their preliminary data related to this study. In part, this publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000117 (LHB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used
- DOPAC
3,4-dihydroxyphenylacetic acid
- 5-HT
5-hydroxytryptamine
- 5-HIAA
5-hydroxyindoleacetic acid
- DA
dopamine
- DNSP-11
dopamine neuron stimulating peptide- 11
- EC
electrochemical detection
- FAST-16
fast analytical sensing technology – 16
- GDNF
glial cell line-derived neurotrophic factor
- GFR
GDNF-family receptor
- HPLC
high performance liquid chromatography
- HVA
homovanillic acid
- PD
Parkinson’s disease
- SN
substantia nigra
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Conflict of interest:
GAG, DMG, LHB declare that 4 patents are pending related to these studies (University of Kentucky).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 2.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 3.Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 5.Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 6.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature neuroscience. 2002;5:514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 7.Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PloS one. 2009;4:e6486. doi: 10.1371/journal.pone.0006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley LH, Fuqua J, Richardson A, Turchan-Cholewo J, Ai Y, Kelps KA, et al. Dopamine neuron stimulating actions of a GDNF propeptide. PLoS One. 2010;5:e9752. doi: 10.1371/journal.pone.0009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–6. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- 10.Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–61. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 11.Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–63. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 12.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 13.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. The Journal of comparative neurology. 1978;180:545–80. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 14.Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–32. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 15.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–5. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–34. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhardt GA, Burmeister J, Meyers RA. Encyclopedia of Analytical Chemistry. Chichester: John Wiley & Sons Ltd; 2000. Voltammetry in vivo for chemical analysis of the nervous system. [Google Scholar]
- 18.Gerhardt GA, Hoffman AF. Effects of recording media composition on the responses of Nafion-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J Neurosci Methods. 2001;109:13–21. doi: 10.1016/s0165-0270(01)00396-x. [DOI] [PubMed] [Google Scholar]
- 19.Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–5. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt GA, Rose GM, Hoffer BJ. Release of monoamines from striatum of rat and mouse evoked by local application of potassium: evaluation of a new in vivo electrochemical technique. J Neurochem. 1986;46:842–50. doi: 10.1111/j.1471-4159.1986.tb13048.x. [DOI] [PubMed] [Google Scholar]
- 21.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–95. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 22.Grondin R, Zhang Z, Gerhardt GA, Gash DM. Dopaminergic therapy improves upper limb motor performance in aged rhesus monkeys. Ann Neurol. 2000;48:250–3. [PubMed] [Google Scholar]
- 23.Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 24.Hall ME, Hoffer BJ, Gerhardt GA. Rapid and Sensitive Determination of Catecholamines in Small Tissue Samples by High-Performance Liquid-Chromatography Coupled with Dual-Electrode Coulometric Electrochemical Detection. Lc Gc-Magazine of Separation. Science. 1989;7:258–65. [Google Scholar]
- 25.Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, et al. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol. 2001;168:155–61. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- 26.Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–8. [PubMed] [Google Scholar]
- 27.Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Research. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 28.Hebert MA, Vanhorne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: Presynaptic studies using in vivo electrochemistry and microdialysis. Journal of Pharmacology and Experimental Therapeutics. 1996;279:1181–90. [PubMed] [Google Scholar]
- 29.Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, et al. Glial-Cell Line-Derived Neurotrophic Factor Reverses Toxin-Induced Injury to Midbrain Dopaminergic-Neurons In-Vivo. Neuroscience Letters. 1994;182:107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 30.Hornykiewicz O. Brain monoamines and Parkinsonism. Psychopharmacology bulletin. 1975;11:34–5. [PubMed] [Google Scholar]
- 31.Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology. 1998;51:S2–9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 32.Hornykiewicz O. L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent. Amino acids. 2002;23:65–70. doi: 10.1007/s00726-001-0111-9. [DOI] [PubMed] [Google Scholar]
- 33.Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–32. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- 34.Immonen T, Alakuijala A, Hytonen M, Sainio K, Poteryaev D, Saarma M, et al. A proGDNF-related peptide BEP increases synaptic excitation in rat hippocampus. Exp Neurol. 2008;210:793–6. doi: 10.1016/j.expneurol.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Molecular biology of the cell. 2003;14:858–70. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–74. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 37.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. European journal of pharmacology. 2003;479:153–8. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 38.Kelps KA, Turchan-Cholewo J, Hascup ER, Taylor TL, Gash DM, Gerhardt GA, et al. Evaluation of the physical and in vitro protective activity of three synthetic peptides derived from the pro- and mature GDNF sequence. Neuropeptides. 2011;45:213–8. doi: 10.1016/j.npep.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Annals of Neurology. 2005;59:459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 40.Lin LFH, Doherty DH, Lile JD, Bektesh S, Collins F. Gdnf - A Glial-Cell Line Derived Neurotrophic Factor for Midbrain Dopaminergic-Neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 41.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 42.Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:3024–33. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oke A, Keller R, Adams RN. Dopamine and norepinephrine enhancement in discrete rat brain regions following neonatal 6-hydroxydopamine treatment. Brain Res. 1978;148:245–50. doi: 10.1016/0006-8993(78)90398-0. [DOI] [PubMed] [Google Scholar]
- 44.Palmer MR, Wuerthele SM, Hoffer BJ. Physical and physiological characteristics of micropressure ejection of drugs from multibarreled pipettes. Neuropharmacology. 1980;19:931–8. doi: 10.1016/0028-3908(80)90001-5. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2005. [Google Scholar]
- 46.Peterson AL, Nutt JG. Treatment of Parkinson’s disease with trophic factors. Neurotherapeutics. 2008;5:270–80. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. British journal of pharmacology. 2003;140:825–9. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. Journal of neurochemistry. 2001;79:349–60. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- 50.Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, et al. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90:245–54. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- 51.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. Journal of Neurosurgery. 2005;102:216–22. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 52.Sorkin A, Duex JE. Quantitative analysis of endocytosis and turnover of epidermal growth factor (EGF) and EGF receptor. In: Bonifacino Juan S, et al., editors. Current protocols in cell biology/editorial board. Unit 15. Chapter 15. 2010. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. Journal of neuroscience research. 2003;73:105–12. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- 54.Yoong LF, Wan G, Too HP. GDNF-induced cell signaling and neurite outgrowths are differentially mediated by GFRalpha1 isoforms. Mol Cell Neurosci. 2009;41:464–73. doi: 10.1016/j.mcn.2009.05.002. [DOI] [PubMed] [Google Scholar]