Summary
Background
Safety of individual probiotic strains approved under Investigational New Drug (IND) policies in cirrhosis with minimal hepatic encephalopathy (MHE) is not clear.
Aim
The primary aim of this phase I study was to evaluate the safety, tolerability of probiotic Lactobacillus GG (LGG) compared to placebo while secondary ones were to explore its mechanism of action using cognitive, microbiome, metabolome and endotoxin analysis in MHE patients.
Methods
Cirrhotic patients with MHE patients were randomized 1:1 into LGG or placebo BID after being prescribed a standard diet and multi-vitamin regimen and were followed for 8 weeks. Serum, urine and stool samples were collected at baseline and study-end. Safety was assessed at weeks 4 and 8. Endotoxin and systemic inflammation, microbiome using multi-tagged pyrosequencing, serum/urine metabolome were analyzed between groups using correlation networks.
Results
30 MHE patients (14 LGG and 16 placebo) completed the study without any differences in serious adverse events. However, self-limited diarrhea was more frequent in LGG patients. A standard diet was maintained and LGG batches were comparable throughout. Only in the LGG-randomized group, endotoxemia and TNF-α decreased, microbiome changed (reduced Enterobacteriaceae and increased Clostridiales Incertae Sedis XIV and Lachnospiraceae relative abundance) with changes in metabolite/microbiome correlations pertaining to amino acid, vitamin and secondary BA metabolism. No change in cognition was found.
Conclusions
In this phase I study, LGG is safe and well-tolerated in cirrhosis and is associated with a reduction in endotoxemia and dysbiosis.
Introduction
Patients with cirrhosis have alteration in their gut microbiota that can influence their cognition and systemic inflammatory profile1–3. Minimal hepatic encephalopathy (MHE), is associated with increased progression to overt HE, poor health-related quality of life (HRQOL) and alteration in systemic inflammation4–6. The manipulation of the gut microbiota is the mainstay of treatment for MHE, however the impact of probiotic administration on the systems biology of patients with MHE using standardized probiotic strains is not clear.
Prior trials with probiotics in general and in cirrhosis specifically have been limited by a lack of stability of the product as a drug, control of diet or detailed retrieval of the probiotic from stools7. Therefore, the results have been heterogeneous with regard to the duration, type of organism or combination of organisms and outcomes with mixed results being achieved in several studies8. This is also complicated by lack of uniformity in batch-to-batch variability and by not performing these studies under an investigational new drug (IND) regulatory procedure. Therefore the aim of this phase I study was to evaluate the safety and tolerability of a well-studied probiotic with a published history of safety and efficacy in humans, lactobacillus GG AT strain 53103 (LGG), in patients with cirrhosis and MHE in a randomized, placebo-controlled, double-blind trial9–11. The secondary aims were to explore the mechanism of LGG’s action by studying changes in the microbiome, endotoxin, inflammatory cytokines and metabolomics and effects on cognition and HRQOL.
Materials and methods
This study was carried out under the IND mechanism of Center for Biologics Evaluation and Research (CBER) of the Food and Drug Administration (FDA) (IND number BB13870) which involved demonstration of the safety, batch-to-batch variability and stability of the LGG as a drug. The LGG used was manufactured by Chr Hansen, Inc., (Milwaukee, WI, USA) and packaged and distributed by Amerifit, Inc (Cromwell, CT, USA). The study protocol was approved by the Institutional Review Board at Virginia Commonwealth University. Monitoring was conducted by an independent Data Safety Monitoring Board (DSMB), and an external NIH/NCCAM Clinical Research Organization [CRO] external monitoring oversight that adhered to FDA protocol for IND regulatory procedures for establishing safety. Monitoring visits were conducted at baseline, interim, and end of study.
Patients with cirrhosis defined as having histological evidence or evidence with radiology and endoscopy of cirrhosis whose disease had been stable for 6 months without specific treatment changes and were between the ages of 18 through 65 years of age were included. We excluded patients with an unclear diagnosis of cirrhosis, those who had consumed alcohol within 6 months, those with an upper gastrointestinal bleeding episode or need to be on systemic antibiotics within 6 weeks, those on current or past specific treatment for HE, with hepatocellular cancer, with yogurt/probiotic consumption within 2 weeks, those with inflammatory bowel disease, history of pancreatitis, psychoactive medication use (apart from chronic anti-depressants)12, with a recent absolute neutrophil count <500/mm3 and those with liver transplant.
The medical records of eligible patients were reviewed and potentially eligible patients were approached directly in the outpatient clinic setting for screening. At that visit, a detailed dietary inquiry, physical examination, inquiry into complications of cirrhosis and current use of psychoactive medications and illicit substances were performed. Patients who met the inclusion criteria were then administered the validated battery of cognitive tests; number connection test-A (NCT-A), number connection test-B (NCT-B), digit symbol (DST) and block design tests (BDT)13. NCT-A evaluates psychomotor speed while connecting dots from 1 through 25 while during NCT-B, that tests set-shifting also, subjects need to connect alternating numbers and letters. Time required to complete these correctly is the outcome for both NCT-A/B. During DST, subjects have to transcribe nonsense symbols into corresponding spaces over two minutes. This test evaluates psychomotor speed and working memory and the number successfully transcribed is the score. The subjects have to assemble blocks according to pre-specified patterns of increasing difficulty on time on the BDT. A high score on DST and BDT and a low score on NCT-A and B indicates good cognitive performance. The diagnosis of MHE was made based on criteria developed from our healthy, age-matched controls which required patients to have at least two of the four of NCT-A, NCT-B, DST or BDT abnormal (NCT-A>35 seconds, NCT-B>99 seconds, DST<72 or BDT<31 raw score)14. Screening laboratories were performed for amylase, lipase and neutrophil count per FDA recommendations and those with <500/ml neutrophils or a high amylase/lipase were excluded.
If the above criteria were fulfilled, the patient was sent home with a food recall diary and collection kit for stool. They were then seen for a baseline visit within two weeks at which point they were evaluated by a physician for a physical examination including questioning to confirm the continued eligibility and for health-related quality of life (HRQOL) using the Sickness Impact Profile (SIP)15. They were subsequently evaluated by the dietician who performed a resting energy expenditure (REE), evaluated their food diary and designed a diet providing 1.2g/kg protein and 35 Kcal/kg (or 1.2 times the REE) specifically educating patients using a pamphlet to avoid all probiotic containing foods. Stool, urine for metabolomics and blood (for MELD score, serum albumin/pre-albumin and metabolomics) were collected. Subjects were then randomized into placebo or LGG for 4 weeks using blocks of 4 created by the VCU Investigational Pharmacy using a random sequence generator. Subjects were also dispensed a daily multi-vitamin after ensuring that their prior MVI was stopped and were instructed to bring in capsules that were not used in the interim visit 4 weeks later.
The interim visit was carried out 4 weeks after the baseline visit where patients were examined by study investigators, adverse events and new diagnoses were evaluated, remaining study drug and adherence was queried and continued eligibility established. Blood was collected for MELD score, ammonia, serum albumin and pre-albumin and the dietician met with them to confirm continued adherence on the prescribed diet. If there were no adverse events requiring discontinuation, the subjects were re-prescribed their medication for another 4 weeks.
The end-of-drug visit was carried out 4 weeks later (8 weeks after drug initiation) where all procedures including physical examination, cognitive testing, HRQOL evaluation, dietary assessment, sample (blood, urine, stool) collection and evaluation of adherence and adverse events were performed.
Sample analysis
Serum inflammatory cytokines (IL-6, TNF-α, IL-1β, IL-2, IL-10 and IL-17) and endotoxin were analyzed using published techniques (Assaygate Inc, Ijamsville MD)2. Fresh stool was used to extract DNA2. This was used for microbiome analysis using published multi-tagged pyro-sequencing techniques and percentage relative abundances at the taxon level were expressed16. The Cirrhosis Dysbiosis ratio which is the ratio of autochthonous or beneficial taxa (Lachnospiraceae, Clostridiales XIV, Ruminococcaceae and Veillonellaceae) to other taxa (Enterobacteriaceae and Bacteroidaceae) was also analyzed; a low ratio indicates dysbiosis17. In addition the qualitative presence of LGG in the stool of patients at baseline and at week 8 were assessed (supplementary data)18. A portion of the stool was also stored to analyze for fecal bile acids using published HPLC techniques19. Serum and urine samples were analyzed for metabolomics using published techniques of GC and LC/MS (at the West Coast Metabolomics Laboratory of University of California, Davis)20.
Statistical Analysis
Basic analysis
Analysis of changes in cognition, quality of life, safety and tolerability were performed using paired t-tests and chi-square/Fisher exact tests where appropriate. We also used paired t-tests and non-parametric paired tests to assess serum changes in endotoxin, inflammatory cytokines compared to baseline in both groups. Unpaired t-tests were used to compare between groups at baseline. Given the exploratory, phase I nature of this study, multiple-comparison adjustments were not performed for the variables above.
Metabolomics analysis
These were analyzed using univariate statistics and multivariate modeling. All analyses were implemented in the R statistical programming environment. Statistical differences between the two cohorts were independently evaluated for known and unknown metabolites and separately in urine and serum matrices. A one-way analysis of variance (ANOVA) was used to compare the difference (delta) between baseline and week 8 to find out which metabolites changed in opposite directions in the LGG group compared to the placebo group. Metabolites displaying significantly altered delta values (p<0.05) were used for unsupervised [principal components analysis (PCA)] and supervised [orthogonal partial least squares projection to latent structures (OPLS)] analyses. These were done for serum, urine and the combined serum/urine delta values subjects between the two biofluids while accounting for a significant false discovery rate (p<0.05) 21.
Microbiota analysis: was performed using Metastats between groups and compared to baseline, which control for multiple comparisons22.
Systems Biology analysis
Correlation networks were then created between differences between baseline and week 8 for LGG and placebo groups with phenome (cirrhosis severity, cognitive tests, inflammatory markers, endotoxin), microbiome (stool MTPS findings at the taxon level) and metabolome (serum and urine GC/LC-MS) with cut-off of both p<0.001 and r >0.6 or <−0.6 and visualized in Cytoscape23, 24. Sub-networks of bacterial taxa deemed to be differently affected by LGG compared to placebo were created and evaluated from this overall network.
Sample size
Based on our prior study of yogurt with probiotics similar to LGG in which yogurt and no therapy were given in a 2:1 ratio, we found that 25% of patients randomized to no treatment had a serious adverse event compared to none in the probiotic yogurt group in the study period25. Using Fisher’s Exact test and assuming that the placebo group, which in this study will be equal to the LGG group in number, we expect a higher chance of serious events at 35%, compared to none in the LGG with 15 subjects per group and an alpha of 0.05, results in a power of 82%.
Results
We examined 150 charts of patients with cirrhosis; 73 were not eligible (56% were not eligible due to prior overt HE on treatment, 67% were on concurrent psychoactive medications) and ten patients refused.
Patient course
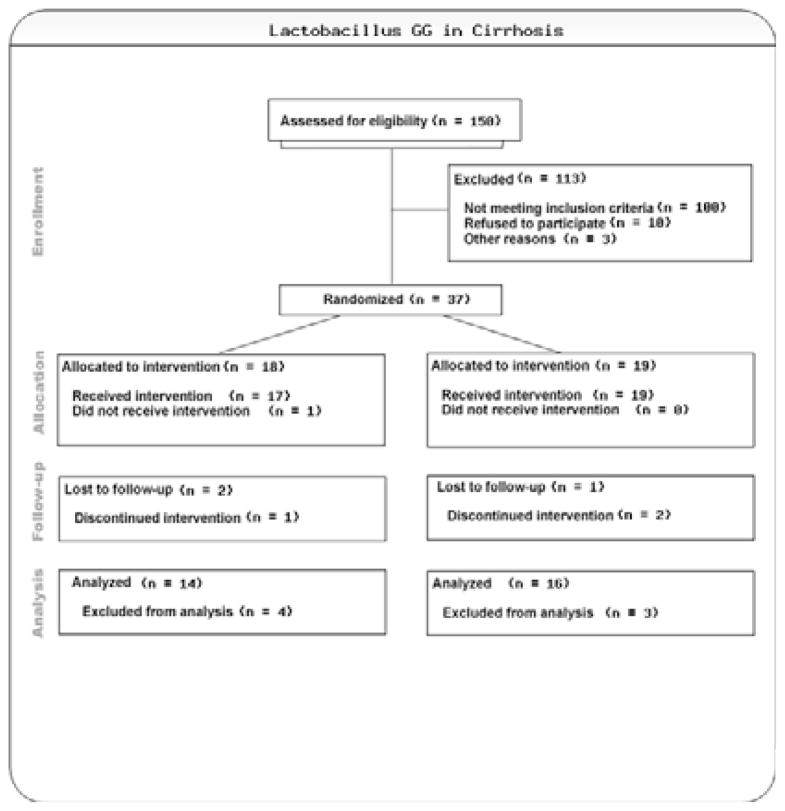
The remaining 67 patients signed the consent form for screening; three were excluded from further participation (one admitted to alcohol use within a week and two patients were scheduled for elective dental or interventional radiological procedures in the coming month that would require the use of antibiotics). The rest of the 64 were given the cognitive testing for MHE and 27 were not found to have MHE and were not considered further. Thirty-seven patients were randomized. Two patients withdrew consent within the first month due to logistic reasons without any adverse events (both LGG group). One additional patient had to be scheduled for a splenic arterial embolization for which he would need antibiotics and narcotics (LGG group) and was withdrawn before receiving medication. Four patients withdrew due to infections or other contra-indications to continuation of the study [one broke her wrist and needed antibiotics (placebo), one had an asymptomatic urinary tract infection based on urine collected before randomization with methicillin-sensitive Staphylococcus aureus (placebo), two were found to have dental issues within a week of randomization that needed antibiotics(one placebo and one LGG)].
Therefore in the end we enrolled the 30 patients needed for the sample size who completed the trial (table 1); 14 were randomized to the LGG group and 16 to the placebo group. There was no significant difference in the baseline demographic, anti-depressant use (25% vs. 30% placebo, p=0.4), cognitive performance or cirrhosis characteristics between the groups. Overall adherence was good with 95% of the assigned medication as well the MVI consumed.
Table 1.
Demographics of patients included in the trial
| Type | Intention to treat | Per protocol | ||
|---|---|---|---|---|
| LGG (n=18) | Placebo (n=19) | LGG (n=14) | Placebo (n=16) | |
| Age | 56.3±9.0 | 58.4±4.3 | 58.4±3.8 | 58.5±4.5 |
| Gender (Men / Women) | 12/6 | 13/6 | 10/4 | 12/4 |
| Education (years) | 13.8±2.3 | 13.6±2.1 | 13/9±2.3 | 13.5±2.3 |
| Etiology (HCV, HCV+alcohol, Alcohol, NASH, others) | 8/3/1/3/3 | 9/1/0/6/3 | 7/2/1/2/2 | 8/1/0/5/2 |
| MELD score | 8.6±2.2 | 8.3±2.0 | 7.9±1.8 | 8.6±2.0 |
| Number Connection Test-A | 38.9±9.5 | 39.3±14.0 | 38.4±10.6 | 36.6±8.0 |
| Number Connection Test-B | 91.6±27.7 | 100.9±31.4 | 85.8±24.6 | 100.6±30.9 |
| Digit Symbol test | 58.3±8.8 | 56.4±12.1 | 57.4±8.2 | 56.3±11.9 |
| Block Design test | 25.8±12.3 | 29.9±13.5 | 24.9±11.0 | 31.6±13.9 |
| Total SIP | 10.1±8.7 | 8.5±9.4 | 9.6±9.1 | 6.9±9.1 |
| Psychosocial SIP | 11.7±11.6 | 9.8±11.9 | 11.4±11.8 | 8.3±12.1 |
| Physical SIP | 6.5±7.1 | 5.4±6.9 | 6.5±7.1 | 4.4±6.8 |
No significant differences between any parameters were seen at baseline between the two groups. SIP: sickness impact profile
Three batches of LGG and placebo were used. Each LGG batch had >50 billion CFU/gm (51, 61 and 53 respectively), without any other organisms. No live organisms were detected in the placebo batches. The ideal diet prescription was 2033±333 Kcal/day and 1958±326 Kcal/day for placebo and LGG group respectively; recommended protein intake was 108±23 gm/day for placebo and 112±35 gm/day for LGG. The diet was re-assessed using 3-day recall and the average caloric intake between groups was statistically similar (placebo: 1975 Kcal/day or 97.1% of recommended, LGG: 1934Kcal/day or 98.8% of recommended) over the study duration. Protein intake was also statistically similar (placebo: 80g/day or 74% of recommended, LGG: 98g/day or 87% of recommended). There was no probiotic, alcohol intake or no use of psychoactive medications during the trial in any patient on multiple inquires by the study staff and the dieticians.
Safety and tolerability
There was a significantly higher incidence of diarrhea in the LGG group compared to the placebo but no difference in the bloating, abdominal pain, nausea, vomiting or other adverse events between groups. Investigation of diarrhea did not show stool WBCs and none of the affected patients had fever or needed specialized therapy for it. None of the patients needed to stop study medication due to the adverse events and none developed overt HE, or required hospitalization during the trial (Table 2).
Table 2.
Safety and Tolerability of LGG compared to Placebo:
| Type | Intention to treat analysis | Per protocol analysis | ||||
|---|---|---|---|---|---|---|
| LGG (n=18) | Placebo (n=19) | p-value | LGG (n=14) | Placebo (n=16) | p-value | |
| Adverse events | ||||||
| Abdominal Pain | 22.2% (4/18) | 21.1% (4/19) | 0.99 | 28.57% (4/14) | 25.00% (4/16) | 0.99 |
| Bloating | 27.8% (5/18) | 15.7% (3/19) | 0.45 | 35.71% (5/14) | 18.75% (3/16) | 0.42 |
| Cramping | 22.2% (4/18) | 21.1% (4/19) | 0.99 | 28.57% (4/14) | 25.00% (4/16) | 0.99 |
| Self-limited diarrhea | 33.3% (6/18) | 5.26% (1/19) | 0.04 | 42.86% (6/14) | 6.25% (1/16) | 0.03 |
| Fever | 5.5% (1/18) | 5.2% (1/19) | 0.99 | 7.14% (1/14) | 6.25% (1/16) | 0.99 |
| Throat Swelling | 5.2% (1/18) | 0.0% (0/19) | 0.72 | 7.14% (1/14) | 0.00% (0/16) | 0.47 |
| Skin Rash | 11% (2/18) | 5.2% (1/19) | 0.60 | 14.29% (2/14) | 6.25% (1/16) | 0.59 |
| Serious adverse events(requiring emergency room or clinic visits) | ||||||
| Infections | 5.5% (1/18) | 15.7% (3/19) | 0.60 | 7.14% (1/14) | 6.25% (1/16) | 0.99 |
| Unrelated ER visits* | 0.00% (0/18) | 5.2% (1/19) | 0.99 | 0.00% (0/14) | 0.00% (0/16) | _ |
| Overt HE | 0.00% (0/18) | 0.00% (0/19) | _ | 0.00% (0/14) | 0.00% (0/16) | _ |
| Hospitalizations | 0.00% (0/18) | 0.00% (0/19) | _ | 0.00% (0/14) | 0.00% (0/16) | _ |
one patient in the placebo group broke her wrist and needed emergency attention. Serious adverse events required specific attention between study visits. Other adverse events were only brought to the study staff’s attention on specific questioning on designated visits. None of the diarrheal episodes were accompanied by fever, leukocytosis or fecal WBCs and all were self-limited without need for antibiotic therapy. No overall differences in adverse events were observed between groups.
We did not find any change in the complete blood count, MELD score or venous ammonia in the groups before and after the medications (Table 3). There was no change in any cognitive test but there was a worsening of the psycho-social aspect of the Sickness Impact Profile in patients assigned to placebo whereas no change was seen in those in the LGG group (Table 4). The only other notable change was a significant decrease in serum endotoxin and TNF-α only in the LGG group, not placebo (Table 5). No change in other inflammatory cytokines was observed.
Table 3.
Change in Lab Values, Cognition and Quality of Life (Per-protocol analysis)
| LGG | Placebo | |||
|---|---|---|---|---|
| Mean Change | Std. Dev. Change | Mean Change | Std. Dev. Change | |
| BMI | −0.28 | 0.67 | −0.01 | 0.42 |
| WBC count | −0.44 | 0.78 | 0.11 | 1.52 |
| Hemoglobin | 0.24 | 1.08 | 0.17 | 0.80 |
| INR | −0.01 | 0.06 | −0.03 | 0.10 |
| Creatinine | −1.36 | 5.12 | −0.00 | 0.09 |
| Bilirubin | −0.11 | 0.32 | −0.14 | 0.48 |
| Ammonia | −3.08 | 10.23 | −2.7 | 4.22 |
| Albumin | 0.01 | 0.16 | 0.04 | 0.24 |
| Cognition and QOL | ||||
| Number Connection Test-A | 1.29 | 12.25 | 2.13 | 6.62 |
| Number Connection Test-B | −6.71 | 35.58 | 10.33 | 42.01 |
| Digit Symbol test | −0.29 | 8.08 | −0.67 | 11.29 |
| Block Design test | −0.14 | 7.16 | −0.20 | 4.90 |
| Total SIP Score | 0.37 | 7.01 | 1.18 | 6.70 |
| SIP Psychosocial Score | 1.11 | 9.54 | 4.18 | 8.62* |
| SIP Physical Score | 0.02 | 4.99 | 1.31 | 5.26 |
No significant change in routine laboratory values was seen before or after LGG or placebo; There was no significant change in cognitive tests but a significant worsening (increase in score) was seen on the SIP psychosocial domain in patients randomized to placebo, a high SIP score indicates poor quality of life
p<0.05, SIP: sickness impact profile.
Table 4.
Endotoxin and inflammatory cytokine change in patients who completed the study
| LGG (n=14) | Placebo (n=16) | |||
|---|---|---|---|---|
| Baseline | Week 8 | Baseline | Week 8 | |
| Endotoxin (EU/ml) | 0.4±0.5 | 0.1±0.1* | 0.2±0.2 | 0.3±0.5 |
| TNF-α (pg/ml) | 10.8±5.6 | 9.0±4.0* | 12.2±8.1 | 11.2±7.8 |
| IL-6 (pg/ml) | 1.5±4.8 | 1.7±5.2 | 1.1±2.9 | 0.7±2.1 |
| IL-2 (pg/ml) | 0.7±2.0 | 0.8±1.6 | 1.2±3.5 | 0.6±1.1 |
| IL-1β (pg/ml) | 2.7±8.4 | 3.8±8.4 | 1.7±4.3 | 1.4±4.3 |
| IL-10 (pg/ml) | 1.1±2.8 | 1.2±2.3 | 0.4±0.7 | 0.5±0.9 |
| IL-17 (pg/ml) | 0.7±1.3 | 0.9±2.1 | 0.4±0.4 | 0.5±0.4 |
Data are presented as mean ± standard deviation. There was a significant reduction in endotoxemia and TNF-α levels after LGG use but no change was seen in the placebo group.
p<0.05
Table 5.
Relative abundance of bacterial taxa before and after the trial
| LGG (n=14) | Placebo (n=16) | |||
|---|---|---|---|---|
| Phylum_Taxon | Baseline | Week 8 | Baseline | Week 8 |
| Bacteroides_Bacteroidaceae | 40.6 | 40.6 | 42.7 | 41.2 |
| Bacteroides_Prevotellaceae | 12.3 | 10.3 | 1.9 | 4.2 |
| Bacteroides_Rikenellaceae | 8.8 | 6.9 | 3.7 | 2.0* |
| Bacteroides_Porphyromonadaceae | 6.4 | 4.4* | 5.2 | 4.9 |
| Firmicutes_Lachnospiraceae | 8.5 | 12.5** | 11.0 | 11.6 |
| Firmicutes_Ruminococcaceae | 8.8 | 11.6 | 10.6 | 10.5 |
| Firmicutes_Incertae sedis Clostridia XIV | 1.4 | 2.2* | 2.6 | 1.8 |
| Firmicutes_Veillonellaceae | 1.9 | 2.1 | 4.1 | 5.8 |
| Proteobacteria_Enterobacteriaceae | 4.7 | 1.8* | 2.7 | 2.6 |
| Cirrhosis Dysbiosis Ratio | 0.66 | 0.94* | 0.92 | 1.0 |
Data are presented as mean abundances as percentage of the total abundance. No significant difference in microbiome composition was seen at baseline between groups using unpaired t-tests but a significant improvement in dysbiosis (increase in autochthonous and reduction in potentially pathogenic taxa) were seen in the LGG group; there was significant reduction in Rikenellaceae in the placebo group on Metastats;
p<0.05,
≤p=0.01
Detection of LGG from stool DNA
LGG was qualitatively detectable in stool in ten of the 14 patients randomized to the LGG group at week 8 (71%) but no one in the placebo group had the presence of LGG in their stool throughout the study.
Microbiome changes: there was no significant difference in microbiome composition between groups at baseline. However when compared to baseline, there was a significant increase in autochthonous taxa (Lachnospiraceae and Clostridiales XIV with a trend towards increase in Ruminococcaceae) in LGG-assigned patients but not in placebo. Correspondingly there was also a decrease in taxa associated with worse disease or cognition (Enterobacteriaceae and Porphyromonadaceae) in the LGG group but not in the placebo group. Rikenellaceae were decreased significantly in the placebo group only. As a result, there was a significant reduction in dysbiosis in LGG group displayed by the increased Cirrhosis Dysbiosis ratio (Table 5).
Fecal BAs
There was no significant change in total BAs, primary or secondary BAs or their ratios between baseline to week 8 using HPLC in the LGG groups but there was a significant increase in the secondary BA, deoxycholic acid, in the placebo group compared to baseline (Table S1).
Metabolomics
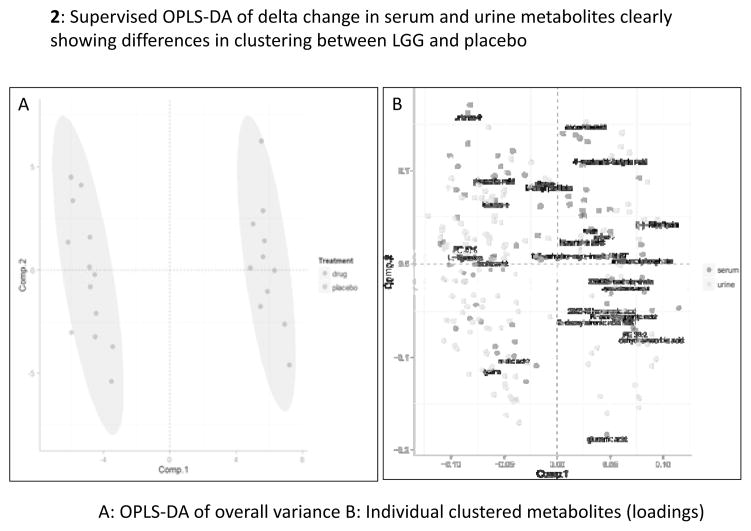
Serum (183 named and 1527 total) and urine (195 named and 1210 total) metabolites were studied and metabolites changing in opposite directions compared between LGG and placebo groups. Serum metabolite changes: there was a significant reduction in amino acids (isoleucine, threonine, methionine) and increase in hydroxylamine and benzoic acid in LGG-randomized compared to an increase in the placebo group over 8 weeks. Urine metabolite changes: A significant reduction in phosphatidylcholine species, the secondary bile acid glycodeoxycholic acid, vitamin intermediates (vitamin C and riboflavin metabolites; (Table S2) were seen. There was a significant separation in urine and serum metabolites individually and together using principal component and OPLS-DA analysis (supplementary figures 1–4 and figure 2).
Figure 2.
Supervised OPLS-DA of delta change in urine and serum metabolites clearly showing differences in clustering between LGG and placebo; the closer the dots, the more related the subjects are in their change of urine and serum metabolites. 2A: overall variances in differences between groups, 2B: individual metabolites that were significantly different between groups.
Correlation network analysis
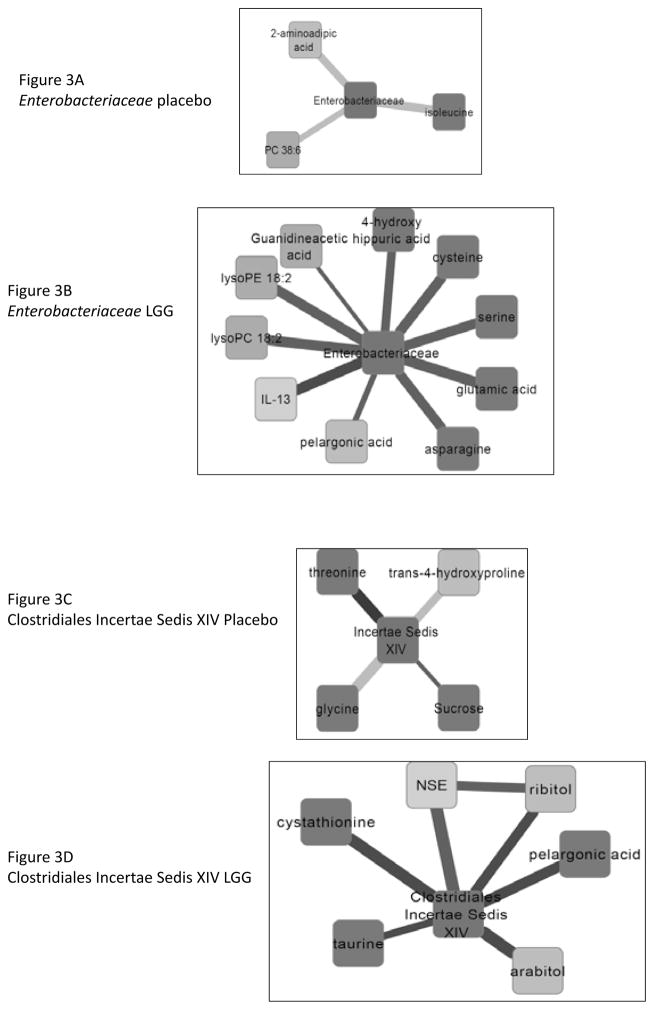
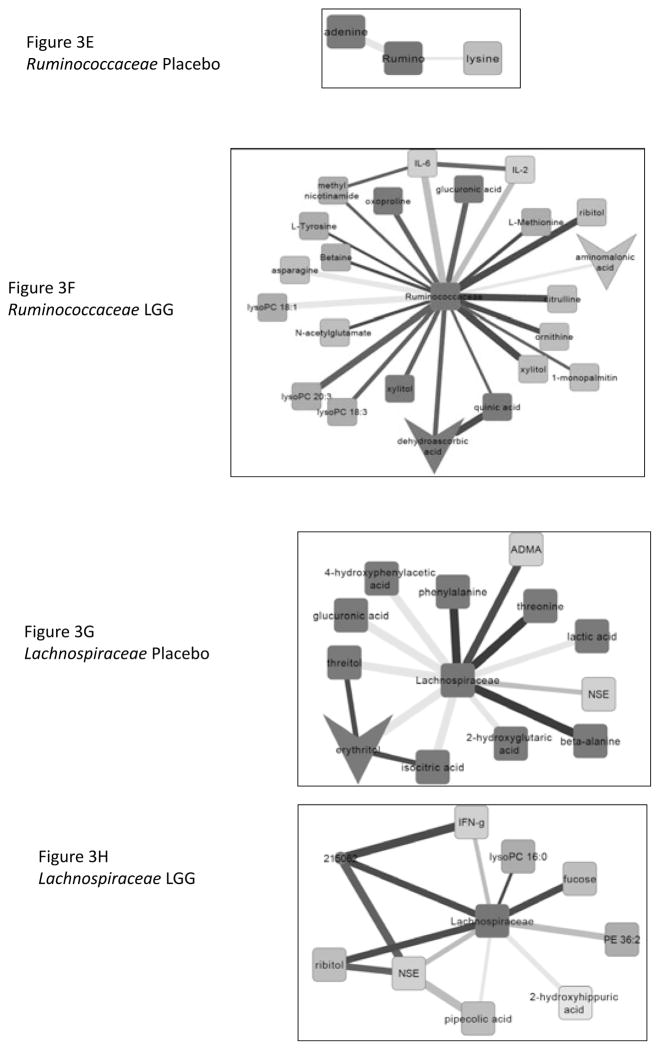
At week 8, in the LGG group compared to baseline there were 525 nodes (named and unnamed) with 825 edges or lines joining them. In contrast the change in the correlation networks of those randomized to placebo were considerably less with 426 nodes and 580 edges joining them. We specifically noted sub-networks of differences in correlations between baseline and week 8 in placebo and LGG subjects centered on Lachnospiraceae, Ruminococcaceae, Clostridiales Incertae Sedis XIV and Enterobacteriaceae (Figure 3A–H). We found significant changes in correlation differences in the LGG group compared to the placebo group based on linkages of microbiota with endotoxin, cytokines and vitamin metabolites.
Figure 3.
Sub-networks showing correlation network differences from baseline to week 8 in placebo and in Lactobacillus GG (LGG) groups separately centered on selected bacterial taxa. The following color scheme is applicable to all sub-networks Color of nodes: Blue: Inflammatory cytokine, Light green: serum metabolites, Dark green: urine metabolites. Color of Edges: Pink: negative remained negative but there is a net loss of negative correlation, Dark Blue: negative changed to positive, Yellow: positive remained positive but there is a net loss of positive correlation, Red: positive to negative, Dark green: shift negative to positive completely, Military green: shift positive to negative completely
Figure 3A and B: Sub-networks of correlation changes from baseline to week 8 centered around Enterobacteriaceae in the placebo and LGG group respectively. In patients randomized to LGG, there were significant compositional changes from a direct correlation at baseline to inverse correlations after LGG supplementation. Several urinary ammoniagenic amino acids, products of nitrogen metabolism were positively linked with anti-inflammatory cytokine IL13. In placebo subjects there were no notable changes and inverse correlations.
Figure 3C and D: Sub-networks of correlation changes from baseline to week 8 centered around Clostridiales Incertae Sedis XIV in the placebo and LGG group respectively. There were inverse correlation changes with taurine, arabitol, ribitol (intermediates with riboflavin and ascorbate metabolism) and the anti-oxidant cystathionine changed to positive after LGG supplementation. NSE, a marker of neuronal inflammation changed from positive to negative after LGG supplementation. By contrast, in the placebo group there only a minor loss of inverse correlation of Clostridiales Incertae Sedis XIV with three amino-acids, threonine, glycine and proline.
Figure 3E and F: Sub-networks of correlation changes from baseline to week 8 centered around Ruminococcaceae in the placebo and LGG group respectively. Intermediates of urea cycle were positively correlated after LGG supplementation while there were consistent negative correlations with IL-6 and IL-2. There was lower urinary dehydro-ascorbate with lower intermediates in urine such as xylitol and increased serum vitamin C intermediates such as ribitol and xylitol. There was a decrease in correlation with aminomalonic acid, a marker of oxidative stress. In contrast, after placebo, there were little changes centered on adenine and lysine.
Figure 3G and H: Sub-networks of correlation changes from baseline to week 8 centered around Lachnospiraceae in the placebo and LGG group respectively. In LGG-randomized patients, there were consistent negative associations between IFN-gamma and neuron-specific enolase (NSE) and change from negative to positive with fucose and ribitol. There was also a reduction in the extent of positive correlations with bacterial urinary nitrogen metabolism (hippuric acid) and pipecolic acid, a bacterial product of lysine. In placebo randomized patients there was a shift from negative to positive with urinary threonine, phenylalanine and alanine and shift completely from negative to positive with ADMA indicating continued endothelial dysfunction. There was a continued negative correlation with NSE and positive with lactate that reduced and with intermediates of riboflavin and ascorbate metabolism; threitol and erythritol.
Discussion
The current study shows that using an IND-protocol that the probiotic Lactobacillus GG is well-tolerated, despite a higher incidence of self-limited diarrhea in patients with cirrhosis. Our data also showed beneficial changes in the stool microbial profile with reduction in TNF-α and endotoxemia with change in serum and urine metabolomics compared to MHE patients randomized to placebo. LGG was also recovered at the end of the study from the majority of LGG-randomized patients.
Alteration in gut microbiota in cirrhosis, especially in HE, is an important determinant of the prognosis and modulates treatment in patients26. However, given the immunosuppressed nature of patients with cirrhosis and recent reports of probiotic-associated negative consequences in pancreatitis patients, this study was mandated as a Phase I study by the FDA to assess the safety and tolerability in these patients27, 28. The probiotic was associated with a significantly higher rate of diarrhea that was indeed self-limiting and not associated with any changes in stool WBC. The incidence of diarrhea with probiotics has varied in prior studies in cirrhotic and non-cirrhotic patients but it appears to be non-infectious or non-inflammatory in origin and could represent the entry of the large batch of these microbes into the colonic microbiome8, 29. Infections were similarly distributed among groups and were not related to LGG. Patients randomized to LGG were able to complete the study without any further issues related to the product.
We found a significant reduction in dysbiosis suggested by the reduction in relative abundance of Enterobacteriaceae and Porphyromonadaceae and a relative increase in beneficial autochthonous taxa of Lachnospiraceae and Clostridiales Incertae Sedis XIV with a trend towards increased Ruminococcaceae only in the LGG-randomized group at the end of trial30. This change in bacterial flora composition is intriguing because there was no specific change in Lactobacillaceae since we did not analyze taxa <1% in abundance. This may be due to the ability of LGG to promote the development of other beneficial microbiota, which we speculate could be through quorum sensing31. Alternatively since LGG has pili that allow it to attach to the intestinal mucosa and promote epithelial function, displace pathogens and stimulate the host immune system through soluble molecule cross-talk, it could be that the majority of microbial abundance change could be mucosal, which has differing microbial populations than the stool1, 32.
Interestingly, we found changes in bacterial relative abundance as well as association with metabolites that were beneficial in the LGG-randomized group. The reduction in Enterobacteriaceae was associated with a change in its linkage with anti-inflammatory cytokine IL-13 and ammoniagenic amino acids that was not seen in the non-LGG group. There was a consistent reduction in potentially ammoniagenic amino acids with a contrasting increase in “fixed” forms of ammonia such as benzoic acid and hydroxylamine in the serum in LGG patients compared to placebo while there was a corresponding decrease in a product of oxidative stress, aminomalonate33. With LGG there was also a lower urinary excretion of phosphatidylcholines and a reduction in the positive associations of urinary phosphatidylcholine moieties in the sub-networks with bacteria only in the LGG group. Phosphatidylcholines are a major dietary source of choline and phospholipids, which are usually consumed in patients with active gut microbiota and the change in microbiota functional capacity could alter their systemic concentration34. Interestingly there was a significant reduction in the urinary secondary bile acid, the glycine conjugate of deoxycholic acid in LGG group and increase in the deoxycholic acid levels in feces in the placebo group. With gut flora manipulation using rifaximin, there was also a reduction in conversion of the primary to secondary bile acids19. This is a complementary reduction in secondary BA in the systemic circulation with LGG and increase without LGG over time as occurred in placebo-randomized patients. These changes are intriguing because in studies by our group in cirrhotics of similar severity, there was no difference in the microbiota composition or systemic inflammation with a non-absorbable antibiotic, rifaximin35. However rifaximin was associated with reduction in secondary BAs unlike LGG19. Both treatments were associated with changes in bacterial function and endotoxemia, similar to this experience with LGG. There has been keen interest in studying prebiotics, probiotics and antibiotics in hepatic encephalopathy and this differing mode of action may increase the rationale for combining therapies for synergy36.
We found that despite being on a stable multi-vitamin dose, there was a reduction in vitamin C and riboflavin excretion in the LGG-group and several intermediates of vitamin C and riboflavin metabolism were correlated with bacteria differentially after LGG. This could indicate a higher ability to consume riboflavin and vitamin C in the LGG group. Studies in animals with burn injury and in those with vitamin deficiency have shown a defect in gut barrier that can potentially be ameliorated with the addition of these vitamins37, 38. Cirrhotic patients often have vitamin deficiencies even in the earlier stages which could worsen this barrier function 39. The lower urinary excretion and higher serum levels of the vitamin C and riboflavin intermediates (galactonate, xylulose, ribitol) after LGG compared to placebo could be indicative of a synergy between LGG and these vitamins in improving this barrier function, however detailed mechanistic studies are required.
With advancing cirrhosis, with and without alcoholic liver disease, there is expansion of Proteobacteria, the phylum containing gram-negative families such as Enterobacteriaceae2, 3. LGG supplementation in rodent model of alcoholic liver disease prevented this increase in Proteobacteria and also increased the gram-positive autochthonous components, Firmicutes. This was associated with a reduction in endotoxemia as would be expected with reduced gram-negative abundance but also a reduction in TNF-α levels. The mechanism of action of LGG is largely unknown but of late, the role of its soluble factors such as p40 protein has been shown to reduce colonic disruption, maintain intestinal integrity as well as reduce specifically macrophage innate immune response that produces cytokines such as TNF-α40. The reduction in endotoxemia is significant in cirrhosis, since it represents bacterial product translocation and is associated with worsening disease in patients over time41.
The current study also shows that the focus of microbiota studies should shift from mere microbiota identification to “functional” inter-relationships based on metagenomic analyses related to metabolic consequences in dysbiosis. Such information is valuable in diagnosis and prognostics because it would allow targeting a particular bacterium or cluster, enzymes and host-microbial pathways, proteins and cell-based processes involved in inflammatory and metabolic responses driving disease etiologies.
The use of drug-quality probiotics in our study is a first in patients with cirrhosis. This was accompanied by strict diet control, careful monitoring of the batch-to-batch variability of the drug and successful retrieval from stools. This issue is critical because in prior reports, the over-the-counter probiotics have had significantly lower proportion of claimed colony forming units of the claimed bacteria42. This creates a serious issue in the interpretation of non-IND probiotic studies. Another advantage is the use of a single, well-characterized strain of the probiotic LGG instead of a multi-species probiotic in which the contribution of individual strains is not clear. Also the intense scrutiny and oversight assured the objective risk assessment of adverse events was less likely to be under-reported as has been a continuous subject of debate and concern in the literature previously. The value of the DSMB, and the FDA regulatory level of the CRO monitoring contracted by NIH/NCCAM for assuring there was no reason to stop the study or withdraw subjects, given that all adverse events monitored were identified and quality assured as being considered unrelated to study treatment determined by independent internal and external monitoring protocols.
The current study, being a phase I study, is aimed towards safety, is exploratory for mechanisms of action under IND and was not powered for cognitive or HRQOL improvement. These results in an IND-quality trial should encourage further therapy with single-strain probiotics for judging efficacy. This trial also excluded several patients with poor HRQOL due to their use of forbidden medications, which could also explain the lack of major change. However there was a worsening of the psycho-social score of the SIP over the trial in the group randomized to placebo, which was not seen in LGG-randomized patients. While this per se does not imply that probiotics improved HRQOL, it may add insight into the progression of cirrhosis-related worsening of HRQOL without therapy and may add impetus to study symptom-based changes with probiotics in cirrhosis. Given the phase I nature of this study, several comparisons were not subjected to the multiple-comparison corrections in the data regarding cognitive, HRQOL and adverse events but the microbiome and metabolome comparisons indeed were corrected for false discovery rates.
We conclude that LGG use in patients with cirrhosis and MHE is well-tolerated using an IND protocol. LGG-randomized patients have a reduction in endotoxemia and a reduction in gut dysbiosis with improved gut microbiome-metabolome linkages. Further larger studies to evaluate efficacy of probiotics in MHE using IND protocols are warranted.
Supplementary Material
Figure 1.
CONSORT diagram: The arm on the left is LGG while placebo is on the right
Acknowledgments
We would like to acknowledge Dr Linda Duffy, Program Official at NCCAM for her invaluable support and advice, the West Coast Metabolomics Center at University of California, Davis, Robin Sculthorpe at the Investigational Pharmacy at VCU Medical Center, Dr David Snydman and Ian Mahoney at Tufts Medical Center and the members of the Data Safety Monitoring Board from Virginia Commonwealth University and McGuire VA Medical Center (Mitchell Schubert, MD, Chairperson, Bimaljit S Sandhu, MD and Marjolein de Wit, MD, all three from department of Medicine, Roy Sabo, PhD, Biostatistics and Mary Ellen Olbrisch, PhD, Psychiatry).
Financial Support
Declaration of funding interests:
This study was funded fully by grant UO1AT004428 from the NIH National Center for Complementary and Alternative Medicine awarded to JSB. All writing and analyses were performed by the authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ declaration of personal interests:
no personal conflicts of interest
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Registration www.clinicaltrials.gov NCT00992290
References
- 1.Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G168–75. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45–53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22(1):125–38. doi: 10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31(5):537–47. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman FA, Heimbach JT, Sanders ME, Hibberd PL. Executive summary: scientific and regulatory challenges of development of probiotics as foods and drugs. Clin Infect Dis. 2008;46 (Suppl 2):S53–7. doi: 10.1086/523342. [DOI] [PubMed] [Google Scholar]
- 8.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;(11):CD008716. doi: 10.1002/14651858.CD008716.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Szajewska H, Skorka A, Ruszczynski M, Gieruszczak-Bialek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38(5):467–76. doi: 10.1111/apt.12403. [DOI] [PubMed] [Google Scholar]
- 10.Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31(8):1231–3. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zocco MA, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23(11):1567–74. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj JS, Thacker LR, Heuman DM, et al. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transpl. 2012;18(10):1179–87. doi: 10.1002/lt.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Hafeezullah M, Franco J, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135(5):1591–1600. e1. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19(8):787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7(5):1065–75. doi: 10.1002/cbdv.200900322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajaj JS, Hylemon PB, Heuman DM, et al. The Cirrhosis Dysbiosis Ratio defines Changes in the Gut Microbiome Associated with Cirrhosis and its Complications. Journal of Hepatology. 2014 doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlroos T, Tynkkynen S. Quantitative strain-specific detection of Lactobacillus rhamnosus GG in human faecal samples by real-time PCR. J Appl Microbiol. 2009;106(2):506–14. doi: 10.1111/j.1365-2672.2008.04018.x. [DOI] [PubMed] [Google Scholar]
- 19.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58(5):949–55. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiehn O, Barupal DK, Kind T. Extending biochemical databases by metabolomic surveys. J Biol Chem. 2011;286(27):23637–43. doi: 10.1074/jbc.R110.173617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wold S, Sjostrom M, Eriksson L. PLS-regression: A basic tool for chemometrics. Chemometrics and Intelligent Laboratory Systems. 2001;58(2):109–130. [Google Scholar]
- 22.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi A, Rangwala H, Keshavarzian A, Gillevet P. Network-based modeling of the human gut microbiome. Chem Biodivers. 2010;7(5):1040–50. doi: 10.1002/cbdv.200900324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103(7):1707–15. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 26.Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58(5):1020–7. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 28.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune Dysfunction and Infections in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Mangalat N, Liu Y, Fatheree NY, et al. Safety and tolerability of Lactobacillus reuteri DSM 17938 and effects on biomarkers in healthy adults: results from a randomized masked trial. PLoS One. 2012;7(9):e43910. doi: 10.1371/journal.pone.0043910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2(2) doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebeer S, De Keersmaecker SC, Verhoeven TL, Fadda AA, Marchal K, Vanderleyden J. Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. J Bacteriol. 2007;189(3):860–71. doi: 10.1128/JB.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douillard FP, Ribbera A, Kant R, et al. Comparative Genomic and Functional Analysis of 100 Lactobacillus rhamnosus Strains and Their Comparison with Strain GG. PLoS Genet. 2013;9(8):e1003683. doi: 10.1371/journal.pgen.1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copley SD, Frank E, Kirsch WM, Koch TH. Detection and possible origins of aminomalonic acid in protein hydrolysates. Anal Biochem. 1992;201(1):152–7. doi: 10.1016/0003-2697(92)90188-d. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–63. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 37.Bodiga VL, Boindala S, Putcha U, Subramaniam K, Manchala R. Chronic low intake of protein or vitamins increases the intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition. 2005;21(9):949–60. doi: 10.1016/j.nut.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang ZT, Yao YM, Xiao GX, Sheng ZY. The protective effect of supplementation of probiotics combined with riboflavin on the intestinal barrier of the rats after scald injury. Zhonghua Shao Shang Za Zhi. 2004;20(4):202–5. [PubMed] [Google Scholar]
- 39.Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325–36. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 40.Yan F, Polk DB. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes. 2012;3(1):25–8. doi: 10.4161/gmic.19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellot P, Frances R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2012;33(1):31–9. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 42.Canganella F, Paganini S, Ovidi M, et al. A microbiology investigation on probiotic pharmaceutical products used for human health. Microbiol Res. 1997;152(2):171–9. doi: 10.1016/s0944-5013(97)80009-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.