Abstract
Pharmaceuticals and personal care products (PPCP) have been found in surface waters worldwide, but little is understood of their effects on the wildlife that inhabit these waters. Fluoxetine (Prozac; Eli Lilly), a highly prescribed selective serotonin reuptake inhibitor (SSRI), is a commonly found PPCP in surface water. The purpose of this project was to determine if environmentally relevant concentrations of fluoxetine impact behavior that is important for population survival in native fish species, including reproduction, feeding and predator avoidance. Chronic 4-week exposures were conducted with doses ranging from 100 ng/L to 100 μg/L to cover a range of environmentally relevant concentrations up to higher concentrations comparable to other published studies with the same drug that have documented various physiological impacts. Pimephales promelas (fathead minnow), a species native to North America, was used as it conducts a range of specific mating behaviors and therefore serves as an excellent model of specific impacts on brain function. Fluoxetine concentrations as low as 1 μg/L, a concentration that has been found in many freshwater environments, were found to significantly impact mating behavior, specifically nest building and defending in male fish. Males were also found to display aggression, isolation, and repetitive behaviors at higher concentrations. Female mating behavior was largely unaffected. In addition, predator avoidance behaviors in males and females were also impacted at 1 μg/L. Feeding was impacted at 10 μg/L and in the highest exposure (100 μg/L), egg production was limited by deaths of females due to significant male aggressive behaviors in first two weeks of exposure. Specific behavioral changes occurred at each concentration (most noticeably 1 μg/L and 100 μg/L) indicating a dose dependent effect that triggered different responses at lower exposures versus higher exposures or differential impacts of dose depending on brain region. Length of exposure also had an impact on aggressive behavior. Changes in hormone levels, indicating significant neuroendocrine changes, suggested as a mechanism of response in higher dose and acute studies, were not linked to changes in behaviors at the doses used in this study. This research provides detailed data on how exposures to fluoxetine impact specific fish behaviors and reproduction and that the effects are dose dependent.
Keywords: neuroendocrine, Prozac, fecundity, mating, SSRI, pharmaceuticals
1. Introduction
Widespread occurrences of pharmaceuticals and personal care products (PPCP) have been found in the surface waters of the United States and Canada including the Great Lakes (Blair et al. In press, Boyd et al., 2003; Brun et al., 2006; Kolpin et al., 2002; Metcalfe et al., 2003). Wastewater treatment plants are implicated as the main routes of entrance of PPCPs into freshwater systems as a large portion of PPCPs that are ingested are excreted by the human body and ultimately end up in sewage treatment systems. Some of these compounds are not completely eliminated by wastewater treatment plants and they are expelled with the effluent into surface waters (e.g. Heberer 2002; Blair et al. 2012). There is little known about the effects of a large number these PPCPs on aquatic organisms that inhabit these areas, specifically at the concentrations they are found in the environment. Overall, there is not enough experimental data to determine which PPCPs may be of most concern (Brooks et al., 2003). Several PPCPs that act on the central nervous system, such as selective serotonin re-uptake inhibitors (SSRIs), have been identified in the list of chemicals of concern as they have been suggested to have the potential to impact wildlife populations (Kostich and Lazorchak, 2008). SSRIs block the reuptake of serotonin at the pre-synaptic nerve cleft and enhance the effect of serotonin on the post-synaptic nerve cleft (Winder et al., 2009). Serotonin has been shown to also regulate physiological systems in aquatic vertebrates such as teleost fish (Fong 2001) including reproduction (Khan and Thomas, 1992), territorial behavior (Perreault et al., 2003), feeding (de Pedro et al., 1998), nitrogen waste excretion (Morando et al., 2009), and immune response (Ferrier et al., 1996). Therefore the SSRIs have the potential to have a significant impact on aquatic vertebrate populations.
Fluoxetine, the active ingredient in Prozac (Eli Lilly), is a commonly prescribed SSRI that has been found in final effluents from sewage treatment plants. Average surface water concentrations globally have been found from 0.012–1.4 μg/l (Christensen et al., 2009; Kolpin et al., 2002; Webb 2001). Fluoxetine is used primarily to treat depression but also aids in the treatment of obsessive compulsive disorder, bulimia nervosa, and panic disorder and is one of the most prescribed medications in the United States (Burt et al., 2007). Fluoxetine has been found to accumulate in the tissues of fish including the brain, liver, and muscle to the same levels that occur in the environment (Brooks et al., 2005; Chu and Metcalfe, 2007; Ramirez et al., 2009; Schultz et al., 2010).
Several authors have called for more studies on the importance of fluoxetine and SSRI’s specifically on behavior because there is not enough information available regarding their impacts from chronic low-level exposures (Brooks and Brain 2010; Brooks et al., 2003; Winder et al., 2009). With the exception of reduced locomotor activity in juvenile fish (Painter et al., 2009; Winder et al., 2011), most previous studies have found that at concentrations a minimum of ten times higher than what has been documented in the aquatic environment caused increased (McDonald et al., 2010) or decreased (Kohlert et al., 2012; Barry, 2013) aggression, disruption in the endocrine axis (Lister et al. 2009; Fernandes et al. 2011; Mennigen et al., 2010a,b), and declines in feeding behavior (Gaworecki and Klaine, 2008) and general movement (Beulig and Fowler 2008), but no impact on reproductive output (Foran et al., 2004). In some cases lower exposure levels were not tested and in the cases where lower exposures were actually tested, these studies have not shown an impact of fluoxetine. This may be due to a lack of true impact at lower concentrations but may also be due to the nature of the behavior and species tested, the length of exposure, differences in enantiomers of fluoxetine used (Brooks et al., 2003; Stanley et al., 2007).
In this study we examined the effects of chronic sub-lethal and realistic environmental exposures of fluoxetine on specific behaviors associated with reproduction, feeding and predator avoidance in the fathead minnow. Fathead minnows were chosen for this study as they are a native species to North America, a toxicological model for the U.S. Environmental Protection Agency and most importantly, exhibit a complicated series of mating behaviors that can be a sensitive indicator of problems with reproduction. Behavior is a sensitive endpoint that provides an early indication of the impact of a toxin (Jones et al. 1991; Lovern et al. 2007) and in the case of a chemical that specifically impacts the nervous system, behavior is an important endpoint to monitor (Brooks et al. 2003). Exposure concentrations used in this experiment were based on the range of concentrations documented in freshwater systems and included some higher concentrations used in other published experiments as a comparative reference. As it has been shown that response to fluoxetine is time dependent (Pérez et al., 2001) experiments were carried out over a four-week exposure. Hormone levels were also measured as changes in the endocrine system have been indicated as a potential mechanism for reproductive effects seen at higher exposure concentrations (Lister et al. 2009).
2. Materials and Methods
Adult fathead minnows (Pimephales promelas), approximately eight months post-hatch, were selected from a stock culture in the Klaper Lab at the Great Lakes WATER Institute (Milwaukee, WI). The original fish culture was obtained from the Environmental Protection Agency (Duluth, MN). Fish were fed Tetramin Tropical Flakes twice a day. Morning feedings occurred after filming of reproductive behavior. A 16:8 hour light: dark cycle was used to simulate the photoperiod present during mating season for the fish and tanks were kept at 23° C.
2.1 Exposures
Static exposures were conducted in standard 37.8 litre (25.5 cm × 25.5 cm × 30.5 cm) glass tanks, which were individually aerated. Twenty-four liters of dechlorinated water was then added to the tanks either with or without a chemical exposure. Each tank was dosed to concentration using a stock solution of 300 mg/l Fluoxetine in ultrapure water. Doses were chosen to mimic environmental concentrations and higher doses that mimicked those of other published studies and included 0 μg/L (control), 0.1 μg/L, 1 μg/L, 10 μg/L, and 100 μg/L. Fluoxetine HCl was purchased from Sigma-Aldrich (St. Louis, MI). Exposures were static-renewal with 100% water replacement every three days. Fish were exposed to a treatment for a total of 4 weeks. Fluoxetine concentrations were quantified immediately after tanks were dosed and at two days by passing 0.5 L of tank water through an SPE column and extracting and analyzing for fluoxetine per EPA Method 1694. Concentrations were found to be an average (± SE) 0.02 ± 0.007 μg/l for the 0 μg/l treatment, 0.087 ± 0.017 μg/l for the 0.1 μg/l treatment, 1.62 ± 0.42 μg/l for the 1.0 μg/l treatment, 10.45 ± 2.83 μg/l for the 10 μg/l treatment, and 94.85 ± 16.25 μg/l for the 100 μg/l treatment.
2.2 Mating Behavior Measures
Specific mating behaviors were monitored starting 2 weeks after first exposure to provide time for acclimation to the treatment. The mating procedure for fathead minnows is a complex series of events. The male first creates a suitable nesting place, generally on the underside of a rock or log. He scrubs the nest using his fat pad on his head and picks off debris with his mouth to prepare the nest for eggs, and then attracts the female into the nest through a series of chasing movements. The male then proceeds through a series of movements to turn the female on her side and pushes her to lay eggs on the underside of the top of the nest. The male protects the nest until the eggs are hatched and regularly cleans the eggs using his fat pad and mouth. We provided a nesting tile created from a 15 cm solid plastic core PVC pipe that was cut in half length-wise. This tile was then placed at the bottom of the tank so that the tile formed a nesting site that the fish could swim in and out of from both ends of the tile. One male and one female were placed with each mating tile. There were three pairs in each tank as these fish are group spawners. The fish could smell each other but did not interact across sections of the tank due to the dividers. Various mating behaviors quantified are described in Table 1.
Table 1.
Description of Observed Mating Behaviors
| Behaviors Observed | Description of Behaviors |
|---|---|
| Male visits mating tile (nest) | Male swims under mating tile to clean nest to prepare for eggs, engage in mating behavior with female, and/or guard tile from intruders. |
| Female swims under mating tile | Female swims under mating tile to engage in mating behavior with male or to eat eggs she had previously laid. |
| Mating behavior under mating tile | Both male and female are under the tile engaging in mating behavior and/or egg laying. |
| Male cleans mating tile (nest) | Male swims back and forth under mating tile using his fat pad to scrub the underside of the mating tile to provide a surface for eggs to adhere. |
| Male attacks female | Male attacks female with quick strikes and physically makes contact with female. |
| Male chases female | Male chases female around tank. |
| Male chases female under mating tile | Male successfully chases female under the mating tile to engage in mating behavior. |
Egg production was monitored from the beginning of the experiment. Egg production and mating behaviors were monitored one to three hours after lights were activated in the room. If fish laid eggs on the tile, the tile was removed from the section, eggs were counted, and a new tile was placed in the section. If eggs were laid on the bottom of the tank, the eggs were counted and then removed. Fish behavior was recorded for ten-minute intervals every other day during natural spawning times (one hour after lights turned on). Mating behavior was filmed using a Panasonic SDR-H200 digital video recorder.
2.3 Hormone Analysis
Previous studies have suggested neuroendocrine control may be responsible for reproductive changes in fish associated with SSRI exposures, so we measured hormone concentrations in males and females at the end of the exposure period. Plasma hormones, testosterone and 17β-estradiol were measured using competitive enzyme immunoassay (EIA) kits specific to each hormone per manufacturers instructions (Cayman Chemical, Ann Arbor, MI). Briefly, hormones were extracted using diethyl ether and then the ether was allowed to evaporate under nitrogen in a 30°C water bath. The remaining extract was dissolved in 0.5mL of assay buffer solution provided with EIA kit and placed into ELISA wells. Testosterone and 17β-estradiol calibration standards were prepared according to the kit. Absorbance readings were performed on the Wallac 1420 Explorer (Perkin Elmer, Irvine, CA) at 405nm. Concentrations were calculated using comparison to known standards.
2.4. Feeding and Predator Avoidance Behaviors
2.4.1 Feeding Behavior
The impact of a chronic low-dose exposure on feeding behavior was assessed at the end of the exposure trials for reproduction (after 4 weeks). Individual female and male fathead minnows were placed in a 2 L beaker with 1 L of untreated dechlorinated water and the amount of time it took each fish to eat 10 Daphnia magna was measured. Daphnia were selected randomly from a stock culture that originated from Aquatic Biosystems (Fort Collins, Co). Fish were allowed to acclimate in the beaker for 5 minutes and then 10 daphnids were placed in the beaker. If fish took longer than 45 minutes to eat all 10 daphnia, time was recorded as 2700 seconds. Fish were not fed 14 hours prior to the start of the feeding experiment. Fish were also placed back into untreated dechlorinated water for two weeks after the initial experiment to determine recovery time after month-long exposures. Fish were measured again for feeding behavior one week and two weeks after the experiment (at weeks 5 and 6 of the experiment where no exposures occurred).
2.4.2 Predator Evasion
Individual fathead minnows were placed in a 30 L tank with 10 L of dechlorinated water. This limited fish movement to the horizontal plane of the tank. One fish was placed in the tank and let to acclimate to the water for at least 5 minutes so that routine swimming behavior could be observed before introduction to mock predator. A black circle (4.5cm in diameter) on a white background (21 cm × 14 cm) was used to mimic a predator. The mock predator was projected towards the side of the tank to startle the fish and the total distance the fish swam in a line from the start of the circle movement until it began meandering again was recorded. If the fish ignored the stimulus and continued to swim normally this was recorded as a null value. A total of five replications were conducted for each fish, where the circle was removed immediately after each approach and the subsequent test was done after the fish resumed routine swimming behavior before another introduction to the mock predator was conducted. Each trial was video recorded using a Panasonic SDR-H200 digital video recorder. Video was viewed by individual frames to determine the fish’s startle distance using MPlayer GUI for Windows.
2.4 Data Analysis
A one-way ANOVA was conducted to determine differences among behaviors and hormones in each treatment using SPSS 13.0 for Windows. Data was transformed where necessary to create a normal distribution. Tukey’s HSD was used to identify differences among specific treatments for each behavior and hormone analysis.
3. Results
3.1 Changes in mating behavior
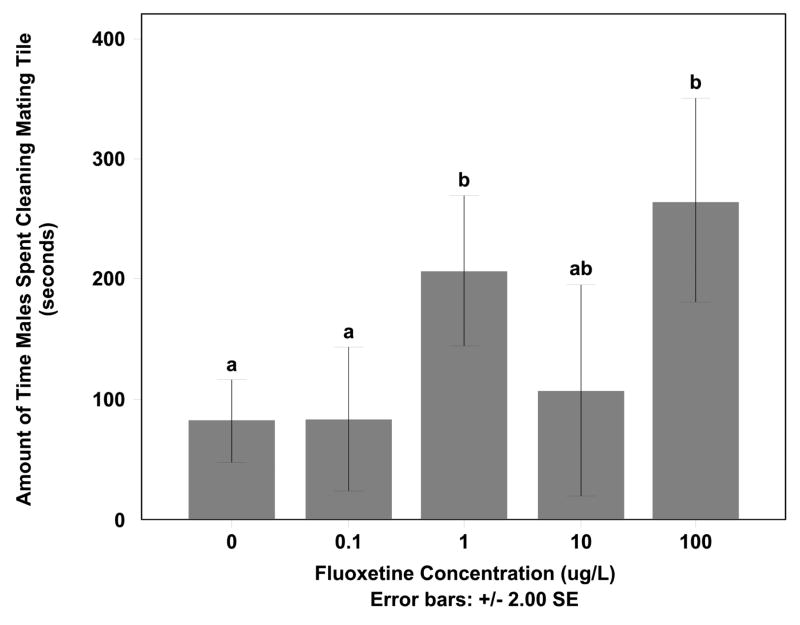
Fluoxetine exposure as low as 1 μg/L significantly impacted mating behaviors of fathead minnows and specifically those associated with male nest preparation, maintenance, and defense. Males spent more time cleaning their mating tile or nest with the increase in fluoxetine concentration (F=5.762, df=28, p < 0.05) (Fig. 1). The males in the 1.0 μg/L and 100 μg/L treatments spent significantly more time cleaning the nest than control and 0.1 μg/L (Tukey HSD p < 0.05). The males in the 100 μg/L exposures were also observed to have a different cleaning behavior than the other exposed males. The males in the 100 μg/L treatment would aggressively use their fat pad to scrub the underside of the tile and did this repetitively while the males in other treatments would graze by the tile and lightly scrub the tile with their fat pad. In the 1.0 and 100 μg/L exposures, males would clean also their mating tile for extended periods of time. As a contrast, the males in the 100 μg/L exposure spent 75.8% of their time observed under the tile cleaning it, where as males in the control exposures spent 22.6% of the time filmed cleaning the nest. The males treated with 1.0 μg/L fluoxetine had light scrubbing cleaning behavior, however they would frequent the tile more often and therefore their total amount of time spent cleaning increased. In the control and 0.10 μg/L exposures, the male would swim through one opening in the tile and out the other side without stopping to clean the tile.
Figure 1. Average amount of time male spent cleaning mating tile.
The average amount of time males spent cleaning mating tile in 0, 0.1, 1, 10, or 100 μg/l fluoxetine exposures. Time spent cleaning was significantly different between control, 0.1 μg/l fluoxetine doses and 1 or 100 μg/l fluoxetine doses (Tukey HSD p < 0.05).
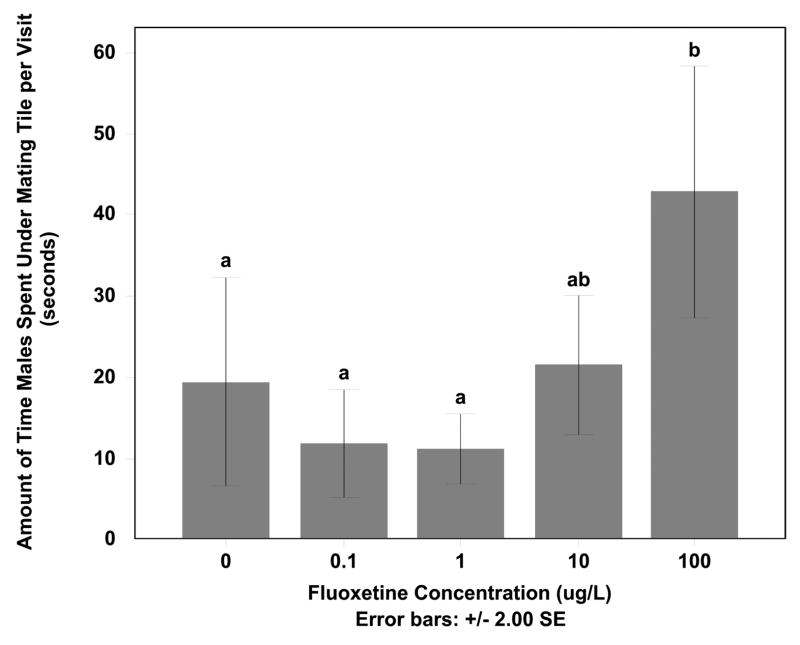
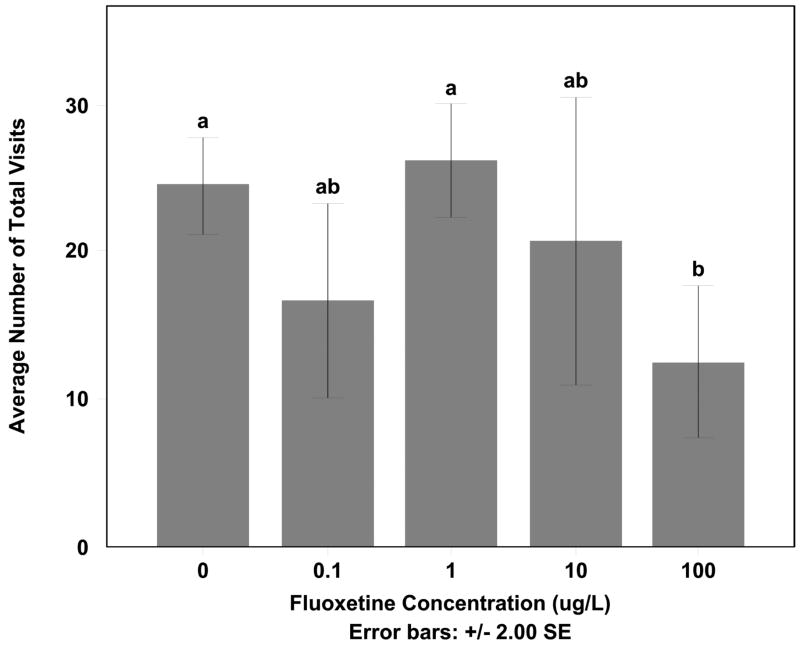
The differences in cleaning behavior, and specifically the difference in the types of cleaning being done by the males exposed to the highest concentrations, led to an increase in amount of time the male spent per visit under the tile (F=6.098, df=25, p < 0.05) (Fig. 2). Males exposed to 100 μg/L fluoxetine concentration spent more time for each visit under the tile compared to the control, 0.10 μg/L, and 1 μg/L males (Tukey HSD p < 0.05). There was also a decrease in the number of total visits to the tile by the male with increasing fluoxetine concentration (F=4.364, df=28, p < 0.05) (Fig. 3). The 100 μg/L exposed males visited the tile significantly less than both control and 1 μg/L males (Tukey HSD p < 0.05) but for each visit spent significantly more time under the tile when compared to all other groups.
Figure 2. Average amount of time males spent under mating tile for each visit.
The average amount of time males spent under mating tile for each visit during the observation period of fathead minnows while exposed to 0, 0.1, 1, 10, or 100 μg/l of fluoxetine are shown. The amount of time males spent under the tile for each visit was significantly different between 100 μg/l fluoxetine and other doses (Tukey HSD p < 0.05).
Figure 3. Average amount male visited mating mating tile.
The average amount males visited the mating tile to clean, mate with female, or swim under tile during observations while exposed to 0, 0.1, 1, 10, or 100 μg/l fluoxetine. Number of exits was significantly different between control and 1 μg/l fluoxetine doses and the 100 μg/l fluoxetine dose (Tukey HSD p<0.05).
There was also a decline in mating behavior by males as the concentration of fluoxetine increased. Males in the 100 μg/L exposure did not spend time chasing females to get them under the tile and instead attacked the females in this treatment seen as chasing and biting the female (F=4.606, df=29, p < 0.05) (Tukey HSD p < 0.05). Although not measured this was particularly evident in the first two weeks of exposure where this led to some mortality in the females. More aggressive mating behaviors (nips or attacks) also led to mortality in females. The males in the 100 μg/L treatment often killed females and did so more than any of the other exposures (and therefore twice as many exposures were conducted for this treatment to have enough pairs to examine behavioral changes). Female survivorship in this treatment was only 33 % compared to the other exposures that had a survivorship of 77–87.5% (Table 2.). The females that died had visible bruising and tissue damage. Fluoxetine did not affect frequency of males chasing the females around the tank but changed the level of aggressiveness once a female approached the nest for mating.
Table 2.
Female Survivorship
| Fluoxetine Concentration (μg/l) | # of Female Fish | # of Female Deaths | % Female Survivorship |
|---|---|---|---|
| 0 (control) | 9 | 2 | 77.8 |
| 0.10 | 7 | 1 | 85.7 |
| 1 | 8 | 1 | 87.5 |
| 10 | 5 | 1 | 80 |
| 100 | 15 | 10 | 33.3 |
None of the female behaviors measured varied significantly among treatments. Neither the amount of time females spent under the mating tile or the amount of time spent engaged in mating behavior were found to vary significantly between concentrations (p > 0.05).
3.2 Changes in reproduction
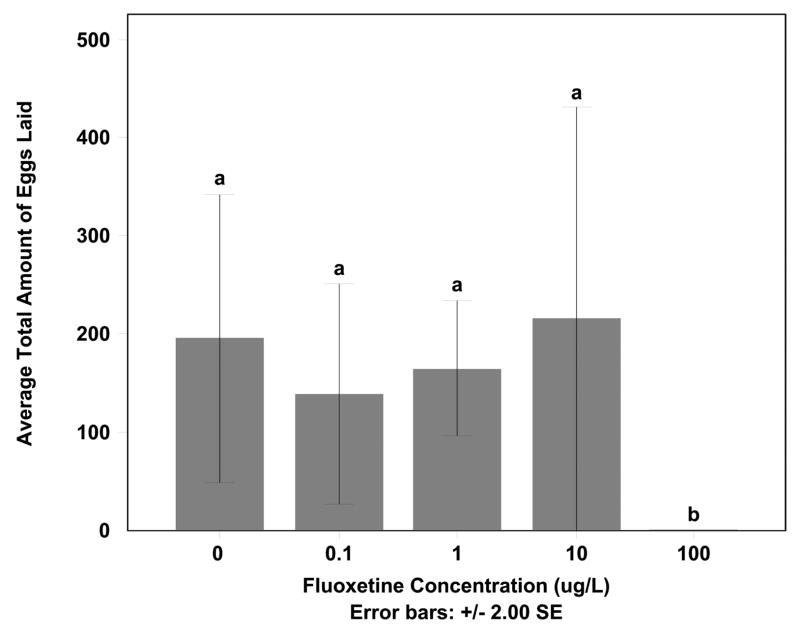
There was a significant difference in the amount of eggs laid among control and fluoxetine exposures (F=8.700, df=40, p < 0.05) (Fig. 4). This level of significance was due to differences between the 100 μg/L exposures and all other treatments. In this highest exposure, fish had a significant decline in total egg production compared to all other concentrations (Tukey HSD p < 0.05) due to the aggression in the male fish mentioned above leading to unsuccessful mating and in some cases death of the females.
Figure 4. Average total amount of eggs laid impacted by highest fluoxetine exposure.
The average total amount of eggs laid by all pairs during exposure to 0, 0.1, 1, 10, or 100 μg/L fluoxetine. Fluoxetine dose of 100 μg/l was significant to all other doses (Tukey HSD p < 0.05).
3.3 Changes in testosterone and estrogen
Testosterone was measured in all males from this experiment after the 4-week exposure period and estrogen concentrations were measured in females. There were no significant differences found in hormone concentrations across any of the treatments indicating no association with hormones and the behaviors at these exposure concentrations of fluoxetine.
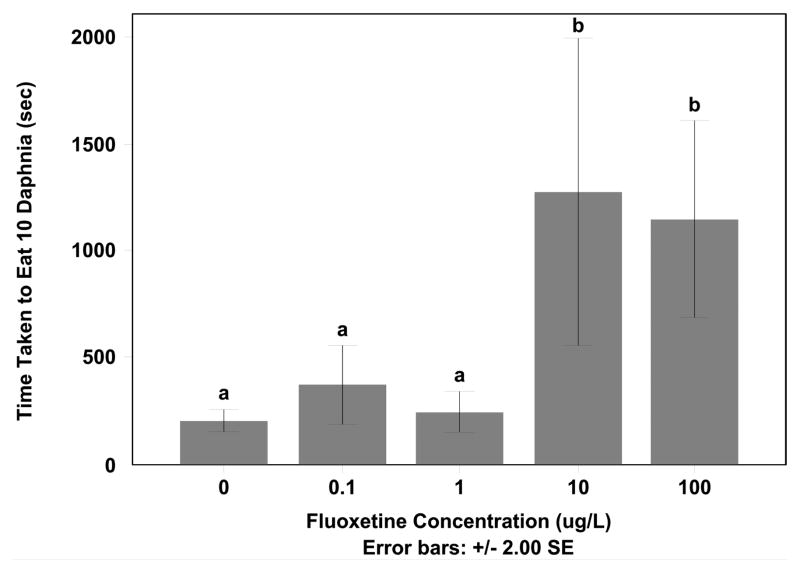
3.4 Changes in feeding behavior
Fluoxetine exposure increased the time it took the fathead minnows to eat 10 daphnids. The 10 and 100 μg/L fluoxetine exposed groups took longer to find and eat all of the daphnids in their feeding arena compared to that of the control, 0.10 μg/L and 1.0 μg/L exposures (Figure 5) (F=7.033, df=60, p < 0.05; Tukey HSD p < 0.05). Several of the fish in the 10 μg/L fluoxetine exposure and 100 μg/l fluoxetine exposures did not eat any of the daphnia during the allotted time of 2700 seconds.
Figure 5. Average Time To Eat Prey Items Increases with Fluoxetine Exposure.
Fish exposed to 1, 10 and 100 μg/L took longer to eat prey when presented 10 Daphnia than 0 and 0.1 μg/L treatments (Tukey HSD p < 0.05).
Fish were removed from fluoxetine exposure and placed in a control environment to see if the removal of fluoxetine would return fish to a control like state. Experiments were repeated one week and two weeks after being placed in control settings. Fish that were initially exposed to 10 μg/L fluoxetine and had a significant increase in the time to find prey after a 4-week exposure were not statistically different than the controls after 2 weeks (p > 0.05). However fish in the 100 μg/L did not recover after the 2-week recovery period.
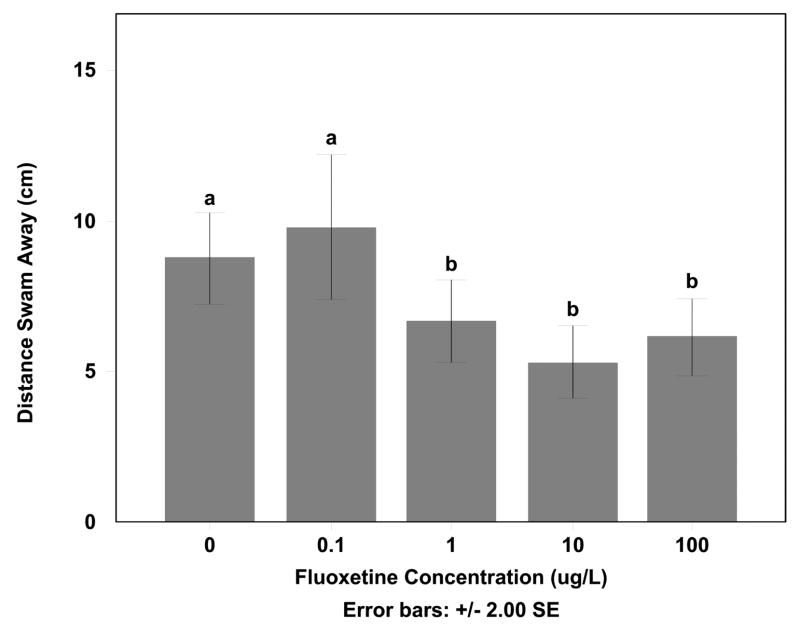
3.5 Changes in predator avoidance
Fluoxetine significantly decreased the startle reaction after the introduction of a mock predator in exposures as low as 1 μg/L fluoxetine (F=6.462, df=75, p < 0.05), decreasing the distance in half compared to that of the control (Fig 6). In the most extreme cases individual fish in both the 10 and 100 μg/L exposures did not respond to the mock predator.
Figure 6. Distance Minnow Swam When Presented With a Model Predator Fish exposed to.
Distance fathead minnows swam away when presented with a large spot representing the approach of a predator. Fish exposed to 10 and 100 μg/L fluoxetine moved a smaller distance when compared with other treatments (Tukey HSD p < 0.05).
4. Discussion
Our results show that fluoxetine at concentrations in the range of what has been documented in freshwater systems and wastewater effluent, pose a threat to the normal mating and predator avoidance behavior of the fathead minnow. Behaviors specifically related to nest building and care were affected by concentrations of fluoxetine as low as 1 μg/L. Males in this species are responsible for providing a safe nesting site, cleaning the nest, and then cleaning and protecting the eggs laid until they hatch. Any disturbance to these tasks could alter offspring success and have population consequences. We saw an increase in time the male spent under the tile in the nest, for each visit, a decrease in frequency male visited the nest, an increase in time male spent cleaning the tile to provide a suitable nesting site, and a decrease in the frequency of the male attempting to mate with the female with various concentrations of fluoxetine.
We also found an influence of fluoxetine exposure on aggressive behavior in that males were so aggressive in the 100 μg/L treatment that 67% of the females were killed (Table 2). This aggressive reaction to females rather than males is unusual but similar to other studies that find aggression towards other males increases with serotonin level changes as well as fluoxetine exposures (Barry 2013; Dzieweczynski and Herbert 2012; Lynn et al. 2007). This aggression towards the females and an increase in male isolation time under the tile with the increase in fluoxetine concentration could be a model for other behavioral problems. The complex suicidal traits of humans (aggression, impulsivity, irritability and hopelessness) are currently being modeled in rodents to better understand if SSRIs might be linked to suicidal thoughts and behaviors (Malkesman et al., 2009). Male fish exposed to the concentrations of fluoxetine of 10 and 100 μg/L displayed some of these traits that are modeled in rats (isolation and aggression) and could make fathead minnows a model organism for understanding the behavior effects of exposure dose, length of exposure to SSRIs and aggression and suicide and the potential side effects of these medications in humans.
Changes related to a given exposure concentration were not uniform across all behaviors and we also saw a difference from the initial week of exposure to the time period where filming began, indicating a particular dose or differential brain responses at different exposure concentrations. Studies in mammals point toward differential impacts of SSRIs in different brain regions, which could cause a differential response for each behavior (Griffin and Mellon, 1999; Muneoka et al., 2009). In addition, research in fish has demonstrated that behaviors such as aggression and feeding are decoupled and associated with serotonin mechanisms in different regions of the brain (Elipot et al. 2013) and exposures to fluoxetine impact the brain stem over other brain regions (Airhart et al. 2007) supporting the idea that behavioral responses to fluoxetine may differ within the same exposure.
Other studies have suggested that SSRIs may have a significant neuroendocrine effect, which would have an impact on behavior, hormones and potential the number of eggs laid (Lister et al., 2009; Fernandes et al., 2011; Menningen et al., 2011). This may be true at higher exposure concentrations, however in this study behavioral changes were not related to hormonal changes in either male or female fish. In addition, testosterone (males) and estrogen (females) concentrations did not differ with treatment.
Although we did not find a difference overall in the number of eggs laid among treatments, male nest behavior can have a significant impact on fry surviving to adulthood as they are responsible for cleaning and taking care of the eggs. This study did not measure changes in the survival of offspring but this type of measure would be of value to determine if changes in male behavior had a larger consequence. In addition, other studies have found that fluoxetine exposed fish may have a greater frequency in developmental abnormalities and behavioral issues (Foran et al., 2004). These abnormalities were not measured or readily apparent in this study but if present could also mean a less viable egg population despite the similar numbers of eggs laid.
Adult fathead minnow behaviors associated with predator evasion were also impacted by the exposure of environmentally relevant concentrations of fluoxetine. Their swim distance, which is important in normal predator avoidance behavior across fish species (Eaton et al., 2001; Eaton and Hackett, 1984; Domenici and Batty, 1997), was significant shorter than the control fish. Fish exposed to the highest concentration of fluoxetine that we tested (100 μg/l) often did not react to the mock predator being introduced to them. This negative effect on predator evasion indicates a slower response or a reduced mobility in these fish and can lead to increased predation. This is supported by other research indicating fluoxetine can have an impact on fish movement (Maximino et al., 2011) and reaction to predation cues (Barbosa Júnior et al., 2012; Gaworecki and Klaine, 2008).
Fluoxetine at concentrations 10 times what has been documented in the environment (10 μg/L) also caused a change in feeding behavior as minnows were slower at capturing their prey. The results in this study were similar to what has been found with other fish species at similar or higher concentrations (Gaworecki and Klaine, 2008; Mennigen et al., 2010b; Thomas et al. 2011). As there are many serotonin receptors in the gut as well as central nervous system it may also act as an appetite suppressor rather than directly affecting behavior through brain function (de Pedro et al., 1998). Fluoxetine has also been shown reduce food intake and body weight in mammals (Uphouse et al., 2006). These impacts were seen at concentrations higher than what might be expected in the environment.
One of the promising findings of this research was the return to control like feeding and predator avoidance behavior with removal of even the greatest exposures. This recovery time was only two weeks for all but the highest exposure conditions, indicating that although the presence of SSRIs in aquatic systems may be pervasive, the impacts on behavior seen in this study may be reversible with improved removal from the waste stream.
5. Conclusion
The research presented here demonstrates that environmentally relevant concentrations of fluoxetine, an SSRI antidepressant, have an impact on specific behaviors important to reproduction and predator avoidance. This research also indicates that the impacts of fluoxetine are dose dependent and are more pronounced in males. They may also be brain region specific as not all behaviors are impacted at the same exposure dose and males and females do not demonstrate the same effects. Removal of fluoxetine exposure largely results in a return to normal behavior indicating environmental remediation of any damaging effects to populations is possible.
Acknowledgments
This work was funded by a pilot project grant to R. Klaper from the UWM Children’s Environmental Health Center, NIH NIEHS Grant P30ES004184-24. The authors wish to thank the anonymous reviewers for their feedback on this manuscript.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Aizenberg D, Naor S, Zemishlany Z, Weizman A. The serotonin antagonist mianserin for treatment of serotonin reuptake inhibitor-induced sexual dysfunction in women: An open-label add-on study. Clin Neuropharmacol. 1999;22:347–350. [PubMed] [Google Scholar]
- Barbosa A, Júnior, Alves FL, Pereira A, de S, Ide LM, Hoffmann A. Behavioral characterization of the alarm reaction and anxiolytic-like effect of acute treatment with fluoxetine in piauçu fish. Physiol Behav. 2012;105:784–90. doi: 10.1016/j.physbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Barry MJ. Effects of fluoxetine on the swimming and behavioral responses of the Arabian killifish. Ecotoxicol. 2013;22:425–32. doi: 10.1007/s10646-012-1036-7. [DOI] [PubMed] [Google Scholar]
- Beulig A, Fowler J. Fish on Prozac: effect of serotonin reuptake inhibitors on cognition in goldfish. Behav Neurosci. 2008 Apr;122(2):426–32. doi: 10.1037/0735-7044.122.2.426. [DOI] [PubMed] [Google Scholar]
- Blair BD, Crago JP, Hedman CJ, Klaper R. Pharmaceutical and Personal Care Products Found in the Great Lakes Above Concentrations of Environmental Concern. Chemosphere. 2013 doi: 10.1016/j.chemosphere.2013.07.057. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Blair BD, Crago JP, Hedman CJ, Treguer RJF, Magruder C, Royer LS, Klaper RD. Evaluation of a model for the removal of pharmaceuticals, personal care products, and hormones from wastewater. Sci Total Environ. 2013;444:515–521. doi: 10.1016/j.scitotenv.2012.11.103. [DOI] [PubMed] [Google Scholar]
- Boyd GR, Reemtsma H, Grimm DA, Mitra S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ. 2003;311:135–149. doi: 10.1016/S0048-9697(03)00138-4. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Brain RA. Introduction and Background for Alternative Nontraditional End Points in Aquatic Toxicology. Chem Res Toxicol. 2010;23:271–271. [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem. 2005;24:464–469. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Foran CM, Richards SM, Weston J, Turner PK, Stanley JK, Solomon KR, Slattery M, La Point TW. Aquatic ecotoxicology of fluoxetine. Toxicol Lett. 2003;142:169–183. doi: 10.1016/s0378-4274(03)00066-3. [DOI] [PubMed] [Google Scholar]
- Brun GL, Bernier M, Losier R, Doe K, Jackman P, Lee HB. Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem. 2006;25:2163–2176. doi: 10.1897/05-426r.1. [DOI] [PubMed] [Google Scholar]
- Burt CW, McCaig LF, Rechtsteiner EA. Advance data from vital and health statistics. 388. Hyattsville, MD: National Center for Health Statistics; 2007. Ambulatory medical care utilization estimates for 2005. [PubMed] [Google Scholar]
- Christensen AM, Markussen B, Baun A, Halling-Sørensen B. Probabilistic environmental risk characterization of pharmaceuticals in sewage treatment plant discharges. Chemosphere. 2009;77:351–358. doi: 10.1016/j.chemosphere.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Chu S, Metcalfe CD. Analysis of paroxetine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1163:112–118. doi: 10.1016/j.chroma.2007.06.014. [DOI] [PubMed] [Google Scholar]
- De Pedro N, Pinillos ML, Valenciano AI, Alonso-Bedate M, Delgado MJ. Inhibitory effect of serotonin on feeding behavior in goldfish: Involvement of CRF. Peptides. 1998;19:505–511. doi: 10.1016/s0196-9781(97)00469-5. [DOI] [PubMed] [Google Scholar]
- Domenici P, Blake R. The kinematics and performance of fish fast-start swimming. J Exp Biol. 1997;200:1165–1178. doi: 10.1242/jeb.200.8.1165. [DOI] [PubMed] [Google Scholar]
- Dzieweczynski TL, Herbert OL. Fluoxetine alters behavioral consistency of aggression and courtship in male Siamese fighting fish, Betta splendens. Physiol Behav. 2012;107:92–97. doi: 10.1016/j.physbeh.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Hackett JT. The role of the Mauthner cell in fast-starts involving escape in teleost fishes. Neural mechanisms of startle behavior. 1984:213–266. [Google Scholar]
- Eaton RC, Lee RKK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Progr Neurobiol. 2001;63:467–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Elipot Y, Hinaux H, Callebert J, Rétaux S. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Curr Biol. 2013;23:1–10. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Schnell S, Porte C. Can pharmaceuticals interfere with the synthesis of active androgens in male fish? An in vitro study. Mar Pollut Bull. 2011;62:2250–2253. doi: 10.1016/j.marpolbul.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Ferriere F, Khan NA, Troutaud D, Deschaux P. Serotonin modulation of lymphocyte proliferation via 5-HT1A receptors in rainbow trout (Oncorhynchus mykiss) Dev Comp Immunol. 1996;20:273–283. doi: 10.1016/0145-305x(96)00012-2. [DOI] [PubMed] [Google Scholar]
- Fong P. Antidepressants in aquatic organisms: A wide range of effects. In: Daughton CG, Jones-Lepp TL, editors. Pharmaceuticals and personal care products in the environment : scientific and regulatory issues. American Chemical Society; Washington, DC: 2001. p. 396. [Google Scholar]
- Foran CM, Weston J, Slattery M, Brooks BW, Huggett DB. Reproductive assessment of Japanese medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch Environ Contam Toxicol. 2004;46:511–517. doi: 10.1007/s00244-003-3042-5. [DOI] [PubMed] [Google Scholar]
- Gaworecki KM, Klaine SJ. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquatic Toxicology. 2008;88:207–213. doi: 10.1016/j.aquatox.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. PNAS. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett. 2002;131:5–17. doi: 10.1016/s0378-4274(02)00041-3. [DOI] [PubMed] [Google Scholar]
- Jones M, Folt C, Guarda S. Characterizing Individual, Population and Community Effects of Sublethal Levels of Aquatic Toxicants - an Experimental Case-Study Using Daphnia. Freshwater Biol. 1991;26:35–44. [Google Scholar]
- Kanaly KA, Berman JR. Sexual side effects of SSRI medications: potential treatment strategies for SSRI-induced female sexual dysfunction. Curr Womens Health Rep. 2002;2:409–416. [PubMed] [Google Scholar]
- Khan IA, Thomas P. Stimulatory effects of serotonin on maturational gonadotropin release in the Atlantic croaker, Micropogonias undulatus. Gen Comp Endocrinol. 1992;88:388–396. doi: 10.1016/0016-6480(92)90233-a. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Mangan BP, Kodra C, Drako L, Long E, Simpson H. Decreased aggressive and locomotor behaviors in Betta splendens after exposure to fluoxetine. Psych Rep. 2012;110:51–62. doi: 10.2466/02.13.PR0.110.1.51-62. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in streams, 1999–2000: A national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Lazorchak JM. Risks to aquatic organisms posed by human pharmaceutical use. Sci Total Environ. 2008;389:329–339. doi: 10.1016/j.scitotenv.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lister A, Regan C, Van Zwol J, Van Der Kraak G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat Toxicol. 2009;95:320–329. doi: 10.1016/j.aquatox.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lorenzi V, Carpenter RE, Summers CH, Earley RL, Grober MS. Serotonin, social status and sex change in the bluebanded goby Lythrypnus dalli. Physiol Behav. 2009;97:476–83. doi: 10.1016/j.physbeh.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovern SB, Strickler JR, Klaper R. Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx) Environ Sci and Technol. 2007;41:4465–70. doi: 10.1021/es062146p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SE, Egar JM, Walker BG, Sperry TS, Ramenofsky M. Fish on Prozac: a simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv Physiol Educ. 2007;31:358–63. doi: 10.1152/advan.00024.2007. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Pine DS, Tragon T, Austin DR, Henter ID, Chen G, Manji HK. Animal models of suicide-trait-related behaviors. Trends Pharmacol Sci. 2009;30:165–173. doi: 10.1016/j.tips.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, da Silva AW, Gouveia A, Jr, Herculano AM. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:624–31. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- McDonald MD, Gonzalez A, Sloman KA. Higher levels of aggression are observed in socially dominant toadfish treated with the selective serotonin reuptake inhibitor, fluoxetine. Comp Biochem Physiol C Toxicol Pharmacol. 2010;153:107–12. doi: 10.1016/j.cbpc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Lado WE, Zamora JM, Duarte-Guterman P, Langlois VS, Metcalf CD, Change JP, Moon TW, Trudeau VL. Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus. Aquat Toxicol. 2010a;100:354–64. doi: 10.1016/j.aquatox.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Sassine J, Trudeau VL, Moon TW. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish, Carassius auratus. Aquat Toxicol. 2010b;100:128–37. doi: 10.1016/j.aquatox.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL. Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J Toxicol Environ Health B Crit Rev. 2011;14:387–412. doi: 10.1080/10937404.2011.578559. [DOI] [PubMed] [Google Scholar]
- Metcalfe CD, Miao XS, Koenig BG, Struger J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ Toxicol Chem. 2003;22:2881–2889. doi: 10.1897/02-627. [DOI] [PubMed] [Google Scholar]
- Morando MB, Medeiros LR, McDonald MD. Fluoxetine treatment affects nitrogen waste excretion and osmoregulation in a marine teleost fish. Aquat Toxicol. 2009;93:253–260. doi: 10.1016/j.aquatox.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Shirayama Y, Takigawa M, Shioda S. Brain Region-Specific Effects of Short-Term Treatment with Duloxetine, Venlafaxine, Milnacipran and Sertraline on Monoamine Metabolism in Rats. Neurochem Res. 2009;34:542–555. doi: 10.1007/s11064-008-9818-2. [DOI] [PubMed] [Google Scholar]
- Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior fo larval fathead minnows (Pimephales promelas) Environ Toxicol Chem. 2009;28:2677–84. doi: 10.1897/08-556.1. [DOI] [PubMed] [Google Scholar]
- Perez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F. Augmentation of fluoxetine’s antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors. J Clin Psychopharmacol. 2001;21:36–45. doi: 10.1097/00004714-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Perreault HAN, Semsar K, Godwin J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol Behav. 2003;79:719–724. doi: 10.1016/s0031-9384(03)00211-7. [DOI] [PubMed] [Google Scholar]
- Ramirez AJ, Mottaleb MA, Brooks BW, Chambliss CK. Analysis of pharmaceuticals in fish using liquid chromatography-tandem mass spectrometry. Anal Chem. 79:3155–3163. doi: 10.1021/ac062215i. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM. Antidepressant pharmaceuticals in two US effluent-impacted streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ Sci Technol. 44:1918–1925. doi: 10.1021/es9022706. [DOI] [PubMed] [Google Scholar]
- Stanley JK, Ramirez AJ, Chambliss CK, Brooks BW. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere. 2007;69:9–16. doi: 10.1016/j.chemosphere.2007.04.080. [DOI] [PubMed] [Google Scholar]
- Thomas M, Joshi PP, Klaper RD. Gene-class analysis of expression patterns induced by psychoactive pharmaceutical exposure in fathead minnow (Pimephales promelas) indicates induction of neuronal systems. Comp Biochem Physiol C. 2011;155:109–20. doi: 10.1016/j.cbpc.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphouse L, Hensler JG, Sarkar J, Grossie B. Fluoxetine disrupts food intake and estrous cyclicity in Fischer female rats. Brain Res. 2006;1072:79–90. doi: 10.1016/j.brainres.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Webb SF. A data based perspective on the environmental risk assessment of human pharmaceuticals II - aquatic risk characterization. In: Kümmerer K, editor. Pharmaceuticals in the environment : sources, fate, effects and risks. Springer; Berlin; New York: 2001. p. 265. [Google Scholar]
- Weston JJ, Huggett DB, Rimoldi J, Foran CM, Stattery M. Determination of fluoxetine (Prozac™) and norfluoxetine in the aquatic environment. Annual Meeting of the Society of Environmental Toxicology and Chemistry; Baltimore, MD. 2001. [Google Scholar]
- Winder VL, Sapozhnikova Y, Pennington PL, Wirth EF. Effects of fluoxetine exposure on serotonin-related activity in the sheepshead minnow (Cyprinodon variegatus) using LC/MS/MS detection and quantitation. Biochem Physiol C Toxicol Pharmacol. 2009;149:559–565. doi: 10.1016/j.cbpc.2008.12.008. [DOI] [PubMed] [Google Scholar]