Abstract
We examined whether age alters the emergence of high-affinity germinal center B (GCB) cells and switched memory B cells (swBmem) during a primary immune response to a thymus-dependent antigen, using a novel flow cytometric assay to distinguish relative BCR affinity. In young mice, high-affinity B cells predominate in the GCB pool and comprise a smaller proportion of the nascent swBmem pool two weeks after immunization. In aged mice, we observe significant reductions of high-affinity clones among GCB cells, but not nascent swBmem cells. The defect in GC affinity maturation was not overcome by providing excess carrier-specific T cells from young mice, as these cells still displayed compromised effector TFH differentiation in the aged animals. Our results suggest that B cells in aged animals have a reduced ability to prompt effector TFH differentiation, leading to a compromised GC response that results in reduced generation of high-affinity GCB and plasma cells; despite normal production of early swBmem cells.
Keywords: B cell, germinal center, affinity, age
INTRODUCTION
Aging is associated with a reduced ability to generate protective antibody responses. In T cell-dependent (TD) immune responses, high-affinity plasma cells and memory B cells are derived from the germinal center (GC). Following antigen encounter and cognate T cell help, B cells enter a GC reaction wherein immunoglobulin genes undergo somatic hypermutation (SHM) and selection for improved BCR antigen affinity, as well as isotype class switching (reviewed in (Shlomchik and Weisel, 2012; Victora and Nussenzweig, 2012)). Some of these cells ultimately exit the GC reaction and adopt either a plasma cell (PC) or memory B cell (Bmem) fate (Benson et al., 2007; Zotos and Tarlinton, 2012). Despite evidence that B cells in aged mice retain the ability to be activated by antigenic stimulation, GC B cells are reduced (Han et al., 2003; Zharhary and Klinman, 1983, 1986; Zheng et al., 1997), and anamnestic responses are likewise diminished (Han et al., 2003; Lu and Cerny, 2002; Miller and Kelsoe, 1995). Nevertheless, there is evidence that primary and secondary humoral responses are merely delayed in older individuals (Kosco et al., 1989; Roukens et al., 2011), raising questions about potential deficiencies in the initial generation of antigen-activated B cell subsets.
The response of C57BL/6 mice to carrier-conjugated 4-hydroxy3-nitrophenylacetyl (NP) hapten is dominated by immunoglobulin heavy-chain V segments of the Vh186.2 and V3 gene families, paired with lambda light chain (Lu and Cerny, 2002; Yang et al., 1996). In aged mice, or in young hosts reconstituted with either B cells or T cells from aged animals, shifts in Vh186.2 gene use as well as reduced somatic hypermutation in splenic GCs are observed (Miller and Kelsoe, 1995; Yang et al., 1996). This is associated with significantly reduced average affinity of serum anti-NP antibody (Han et al., 2003; Miller and Kelsoe, 1995). Further, both AID expression and class switch recombination are significantly reduced in aged mice and humans (Frasca et al., 2011; Frasca et al., 2004), and this is associated with affinity maturation and vaccine responses in elderly humans (Ademokun et al., 2011; Frasca et al., 2011; Frasca et al., 2010; Khurana et al., 2012). Age-associated changes in T cell function also contribute to changes in the antibody repertoire (Song. H., 1997; Zheng et al., 1997). Splenic or peripheral lymph node CD4 T cells from aged mice show decreased cognate helper function, leading to significant reductions in somatic hypermutation, NP+ GC B cell expansion, and NP-specific IgG antibody (Eaton et al., 2004; Maue et al., 2009; Nicoletti et al., 1991). There is additional evidence that T follicular helper cell (TFH) function is altered in aged mice and humans (Agrawal A., 2012; Lefebvre J.S., 2012). Furthermore, there is recent evidence that antigen presentation by B cells is required for TFH differentiation (Goenka et al., 2011). All of these observations led us to ask how age may influence the overall GC reaction, including TFH differentiation and function, affinity maturation among GC B cells per se, and development of the Bmem pool.
Here, we varied the ratio of NP directly conjugated to fluorophores in order to track high- and low-affinity GCB cells and early swBmem during a primary immune response to NP-OVA. Despite the presence of comparable numbers of TFH between aged and young hosts, we observed significant reductions in GCB cells of all affinities in aged animals, but no difference in the swBmem subset. In addition, TFH in aged hosts were compromised in their ability to express IL-4 and IL-21. Taken together, our results suggest that “aged” B cells contribute to GC maturation and outcome defects in aged individuals, including reduced generation of high-affinity GCB and plasma cells; nevertheless, early swBmem cells are produced normally.
MATERIALS & METHODS
Mice and immunization
Mice were maintained and used in accordance with the University of Pennsylvania Animal Care and Use Guidelines. 8–14 week old C57BL/6J were purchased from Jackson Laboratory. Young (3–4 mo.) or aged (22–30 mo.) mice were purchased from the NIA Aged Rodent Colony. CD45.1+ OTII mice were kindly provided by T. Kambayashi’s laboratory (Univ. of Pennsylvania). Mice were immunized intra-peritoneally (i.p.) with 50 µg NP15-OVA or NP77-Ficoll (Biosearch Technologies). For adoptive transfer experiments, we depleted splenocytes from OTII Tg mice of B220+, CD8+ and CD11b+ cells using a MACS™ magnetic bead depletion system (Miltenyi), then transferred 105 enriched CD4 T cells per mouse intravenously (i.v.).
Flow cytometry
Monoclonal antibodies reactive to the following antigens were used: IgD (clone 11–26), CD4 (clone GK1.5), B220 (clone RA3-6B2), CD11b (M1/70), all from eBioscience; F4/80 (clone BM8), Fas/CD95 (clone Jo2), CD19 (clone 1D3), Ly-6G/GR1 (clone RB6-8C5), Ki67 all from Pharmingen. NP was conjugated to APC or PE (ProZyme) in house. The conjugation ratio of NP-APC ranged from 27 to 30, whereas the conjugation ratio of NP-PE ranged from 5–6. Cells were first stained with NP5–6-PE along with antibodies against other surface antigens, washed, and then stained with NP27–30-APC as well as secondary stain if applicable. DAPI (Invitrogen) or Live/Dead Aqua (Invitrogen) was used to identify live cells, and doublets were excluded by forward and side scatter height × width analysis. Cells were analyzed on a LSRII™ cytometer (BD Biosciences), and data were analyzed using FlowJo software (TreeStar). FACS sorts were performed on a FACS Aria II™ (BD).
Somatic hypermutation analysis
RNA was extracted from FACS-sorted NP27+NP6+ or NP27+NP6− GCB cells and reverse transcribed to cDNA using RTCγ primer as described previously (Rohatgi et al., 2008), to enrich for IgG transcripts. Amplicons were purified using the PureLink ™ gel extraction kit (Invitrogen) and cloned using the TOPO TA Cloning Kit (Invitrogen). Plasmids containing a Vh insert were isolated (GenElute HP Plasmid Miniprep Kit, Sigma) and sequenced at the Univ. of Pennsylvania DNA Sequencing Facility. Sequences were identified and aligned with IgBLAST.
ELISPot
Splenocyte suspensions were incubated on Immunosorb™ plates (Nunc) coated with 10 ug/ml of NP4-BSA or NP26−-BSA (Biosearch Technologies) and subsequently blocked with 1% BSA. After 3–4 hours, plates were developed with anti-IgM or anti-IgG1 as previously described (Goenka et al., 2011).
Quantitative polymerase chain reaction (qPCR) analysis
RNA was extracted with the RNeasy kit (Qiagen) and reverse-transcribed to cDNA using SuperScript II Reverse Transcriptase (Invitrogen) following manufacturer’s protocols. QPCR was conducted using Taqman probes and Master Mix in an ABI 7300 machine (Applied Biosystems). 18S ribosomal subunit was used as a housekeeping gene, and samples were normalized to universal total RNA (Zyagen).
Statistical Analysis
Data were subjected to a two-tailed t test at α=0.05 using Graph Pad Prizm.
RESULTS
Flow cytometric resolution of B cells bearing high- and low-affinity BCRs
The relative affinities of class-switched antibodies or antibody secreting cells (ASCs) can be determined based on the conjugation ratio of haptens in solid phase assays (Han et al., 2003; Herzenberg et al., 1980; Oropallo et al., 2012; Scholz et al., 2008; Shimizu et al., 2003). Similarly, the relative affinity of murine BCRs has been measured flow cytometrically using doubly-conjugated BSA carrier proteins (Shimizu et al., 2003). Here, we asked whether a similar approach could be adapted to flow cytometry by varying the number of hapten groups attached per molecule of fluorophore, rather than a carrier protein. Accordingly, we prepared low or high conjugation ratios of NP covalently bound to two different flourophores: PE-NP5–6 (phycoerythrin) and APC-NP27–30 (allophycocyanin). We sequentially stained splenocytes from C57BL/6 mice, first with PE-NP5–6 along with antibodies to cell surface antigens, then with the APC-NP27–30 reagent, at various time points after immunization with TD or T cell-independent (TI) antigen (gating strategies and typical results are shown in Figures 1 and 2 and discussed further below). Our rationale for the staining order was that labeling with APC-NP27–30 first would hinder subsequent labeling with PE-NP5–6, since APC-NP27–30 should bind to BCRs with higher avidity. Conversely, staining with PE-NP5–6 first would allow identification of cells with the highest-affinity BCRs, which should be PE+ APC+ “double positives” and represent a subset of all antigen-specific (APC+) B cells.
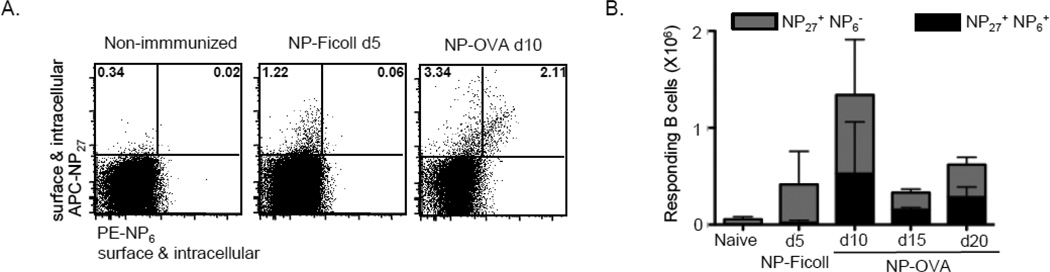
Figure 1. NP6+ B cells emerge in TD, but not TI, responses.
Mice were immunized with NP-OVA in alum and assessed at 10 days, or NP-Ficoll in saline and assessed at 5 days. Non-immunized (naïve) mice were left untreated. Splenocytes were harvested at the peak of each response and labeled with NP6-PE first and then NP27APC during the surface (shown) or intracellular staining. (A) Representative FACS gating strategy for identification of responding B cells. Live, singlet, dump− (CD4− CD8− F4/80− Gr1− CD11b−) IgD− IgM− CD19+ cells were sub-divided into NP27+ NP6− or NP27+ NP6+ “double-positive” cells. (B) Number of NP27+NP6− and NP27+NP6+ B cells per spleen (n of 4–5 mice per group). Data represented as mean ± SD and representative of 3 independent experiments.
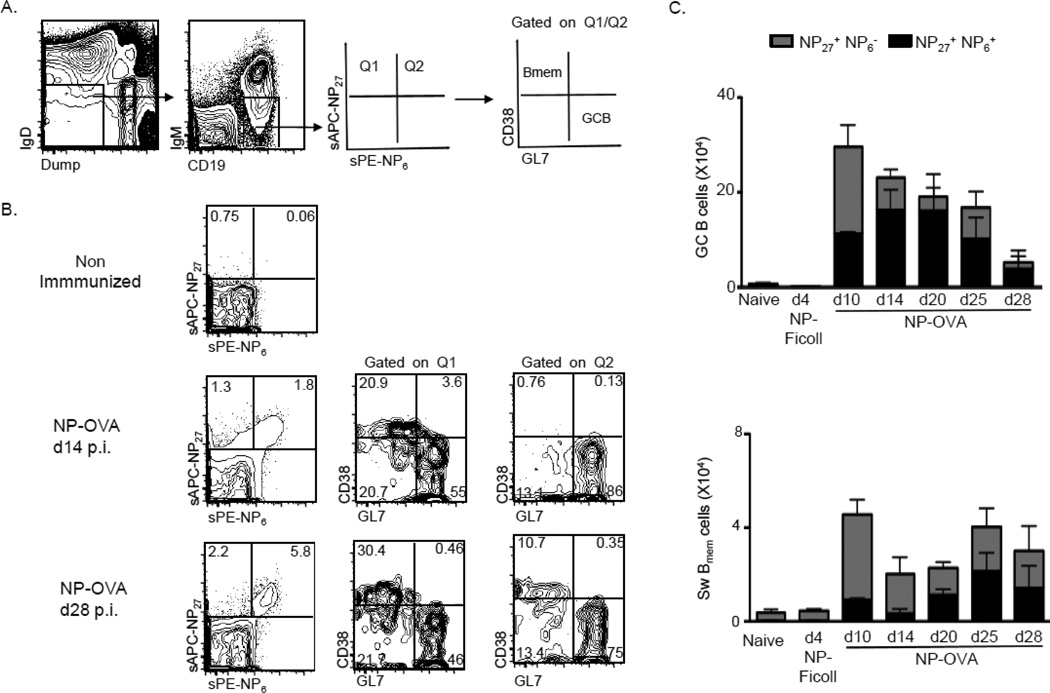
Figure 2. Kinetics of NP6−/NP6+ cells within GCB or Bmem subsets during the primary immune response.
Splenocytes were harvested and stained with NP6-PE first and then NP27-APC at various times after immunization with NP-OVA. NP27+NP6− (Gate Q1) and NP27+NP6+ (Gate Q2) were further subsetted into GL7+CD38− (GCB) or GL7− CD38+ (Bmem) cells. (A) Schematic representation of the gating strategy. (B) Representative FACS gating strategy observed in mice that were either naïve or immunized with NP-OVA for 14 or 28 days. (C) Number of splenic NP27+NP6− and NP27+NP6+ GCB or switched (sw) Bmem cells. Data represented as mean ± SD with n of 3–5 mice per group.
Affinity maturation occurs efficiently in response to TD, but not TI, antigens (Benson et al., 2007; Victora and Nussenzweig, 2012). Thus, we predicted that PE-NP5–6 binders would emerge only in TD responses, with the TI response providing a negative control. Thus, we immunized 3–5 month old mice with either the TI-2 antigen NP-Ficoll or the TD antigen NP-OVA, then analyzed the emergence of B cells that bound both conjugates (NP27+NP6+) or only the APC-NP27 conjugate (NP27+NP6−) (Figure 1). Similar results were obtained for surface and intracellular staining, so results for surface staining are shown in all remaining figures. As expected, double-stained cells (NP27+NP6+) were absent at the peak of the NP-Ficoll response (day 5), while NP27+NP6− B cells were common (Fig. 1A). In contrast, 10 days after NP-OVA immunization, we detected both double-stained (NP27+NP6+) and single-stained (NP27+NP6−) B cells. Further, NP27+ NP6+ B cells comprised approximately half of the total NP-binding B cells at various time points post-NP-OVA-immunization (Fig. 1B).
Prior sequence analyses have revealed that GC B cells are enriched for high-affinity variants during early stages of the immune response. The early Bmem pool is largely comprised of non-mutated clones, but becomes enriched with mutated clones as the response progresses and resolves (reviewed in (Shlomchik and Weisel, 2012; Victora and Nussenzweig, 2012)). Thus, we next asked how NP-binding cells were distributed between GCB or nascent Bmem subsets by conducting a kinetic analysis of total and high-affinity NP-specific B cells during a primary TD response in young mice (Figure 2). To identify surface Ig+ cells, we conducted labeling of NP conjugates sequentially, as described above. The gating strategy to phenotype GCB and Bmem subsets (Fig. 2A) was applied to splenocytes from non-immunized animals and to NP-OVA-immunized mice at various time points to 28 days post-immunization (Fig. 2B). Double-positive NP27+NP6+ cells predominate in the GC pool from day 14 onward, although the total number of GCB gradually wanes as expected (Fig. 2C, upper panel). As further proof of concept, we FACS sorted GL7+CD38− GC B cells that were either NP27+NP6− or NP27+NP6+ at d14 post-immunization (with the gating strategy shown in Fig. 2A and B), then cloned and sequenced VH186.2 segments (Table 1). Both subsets had comparable numbers of mutations per sequence, including the number of Replacement (R) or Silent (S) mutations in the Framework Regions (FWRs) or Complementarity Determining Regions (CDRs). However, NP27+NP6+ GC B cells were enriched for the canonical mutation that improves affinity in the NP-response, a tryptophan to leucine exchange at position 33 (W33L) in VH186.2, consistent with previous results (Lu et al., 2001). Taken together, these results indicate that the NP5–6 reagent identifies B cells bearing BCRs that have improved affinity for the NP antigen.
Table 1.
Summary of B cell selection within the NP-specific GCB cells at day 14 post-immunizationa
| Populationsa | n | Average # mutations/ sequence |
FWR (1+2+3) | CDR (1+2) | #W33L | ||
|---|---|---|---|---|---|---|---|
| # Rb | # Sb | #R | #S | ||||
| NP27+ NP6− | 28c | 6.9 ± 3.0e | 2.0 ± 1.9 | 1.4 ± 1.8 | 2.9 ±1.5 | 0.6 ± 0.8 | 8 |
| NP27+ NP6+ | 36d | 6.9 ± 2.3 | 1.8 ± 1.1 | 0.4 ± 0.9 | 3.3 ± 1.2 | 0.2 ± 0.4 | 35 |
GCB cells (Dump− IgD−CD19+GL7+CD38−) were FACS sorted at d14 after immunization with NP-OVA.
R denotes Replacement and S denotes silent mutations
Number of V186.2 sequences: 23 out of 28 Sequences
Number of V186.2 sequences: 33 out of 36 Sequences
Values represent Mean ± S.D.
In the developing Bmem pool, double-positive NP27+NP6+ cells represent only one-fifth of the swBmem subset between days 10 and 14, but increased to about half by day 20 (Fig. 2C, lower panel). Concurrently, the proportion and number of NP27+NP6− Bmem decreased sharply between days 10 and 14, as the GC reaction had peaked and started to wane. At d28, a majority of high-affinity Bmem cells were CD73+, consistent with GC derivation (not shown; (Taylor et al., 2012; Tomayko et al., 2010)). Taken together, these results validate our novel method for flow cytometric tracking of total and high-affinity B cells. We conclude that PE-NP6 detects high-affinity B cells, whereas APC-NP27 detects BCRs ranging from low to high affinity for the NP antigen.
Aged mice show reduced high-affinity GC B cells, yet similar swBmem numbers
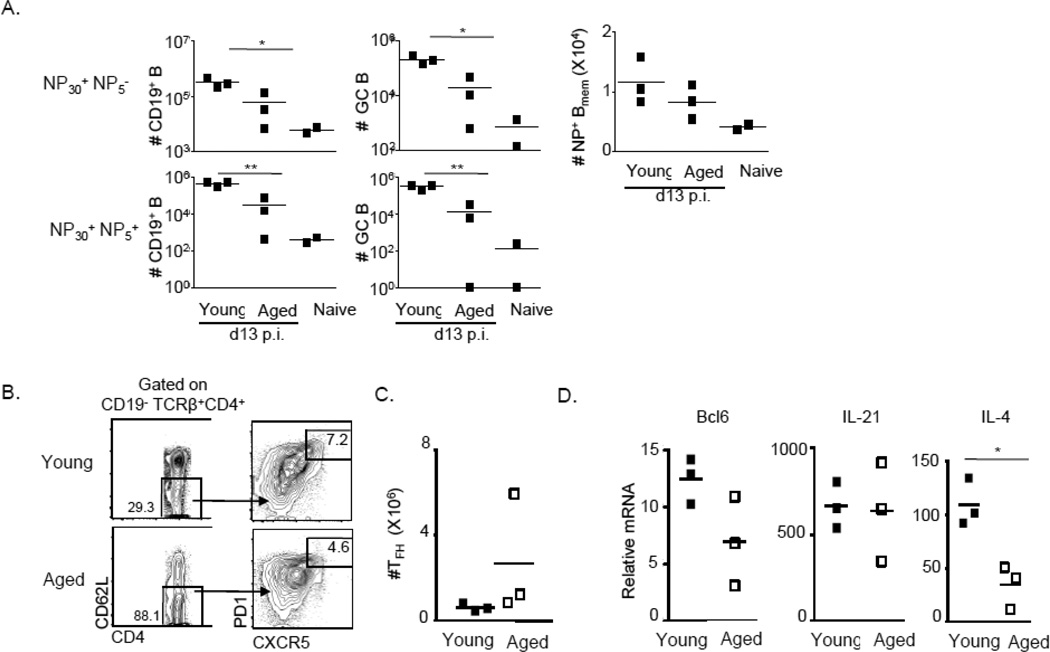
Although the contribution of aged B cells to GC maturation and antibody outcomes has been well studied, whether age affects the generation of Bmem cells early in a primary immune response is not yet known. To investigate this question, we first immunized young (3–4 mo.) or aged (22–30 mo.) mice with NP-OVA and assessed subsets of effector B cells approximately 2 weeks post-immunization (Figure 3). Consistent with previous reports (Han et al., 2003)), numbers of NP positive as well as GC B cells were significantly lower in aged mice (Fig. 3A, left and center panels). However, numbers of NP positive swBmem cells of all affinities were comparable (Fig. 3A, right panel), as were the proportion and number of high-affinity cells (not shown), suggesting that early events leading to B cell memory are intact.
Figure 3. Primary immune responses are blunted in aged mice.
Splenic NP-reactive B cell populations (as described above) as well as TFH effectors were analyzed in aged or young mice at d13 post-NP-OVA-immunization. (A) Numbers of NP27+NP6− and NP27+NP6+ total responders, GCB or Bmem cells. (B) Representative FACS gating strategy for identification of endogenous TFH cells as CD19−TCRβ+CD4+CD62L−PD1hiCXCR5+ cells. (C) Numbers of endogenous TFH present in immunized aged or young mice (D) Expression of transcript in FACS sorted endogenous TFH generated in young or aged mice at d13 post-immunization normalized to universal total mRNA controls.
Since T follicular helper cells (TFH) are important for the generation and maintenance of TD responses (reviewed in (Crotty, 2011; Johnston et al., 2009)), we also investigated the number and effector potential of endogenous TFH present in aged mice. Bcl6 and cytokines such as IL-4 and IL-21 are produced by TFH cells, these and are key for GC formation, somatic hypermutation, and IgG1 class-switching (reviewed in (Crotty, 2011)). Thus, we sorted endogenous TFH from young or aged mice using the phenotyping and gating strategy shown (Fig. 3B), enumerated TFH (Fig. 3C), and analyzed the expression of key genes by qPCR (Fig. 3D). There were similar numbers of TFH cells present in aged and young mice, and while their TFH cells expressed similar levels of Bcl6 and IL-21, there was a defect in IL-4 expression by TFH present in aged animals. We therefore conclude that effector TFH differentiation is perturbed in aged mice, which may in part explain the observed reductions in NP-specific total B cell and GCB subsets (Fig. 3A).
Transfer of carrier-specific TCR transgenic helper T cells from young mice does not correct the GC defect in aged mice
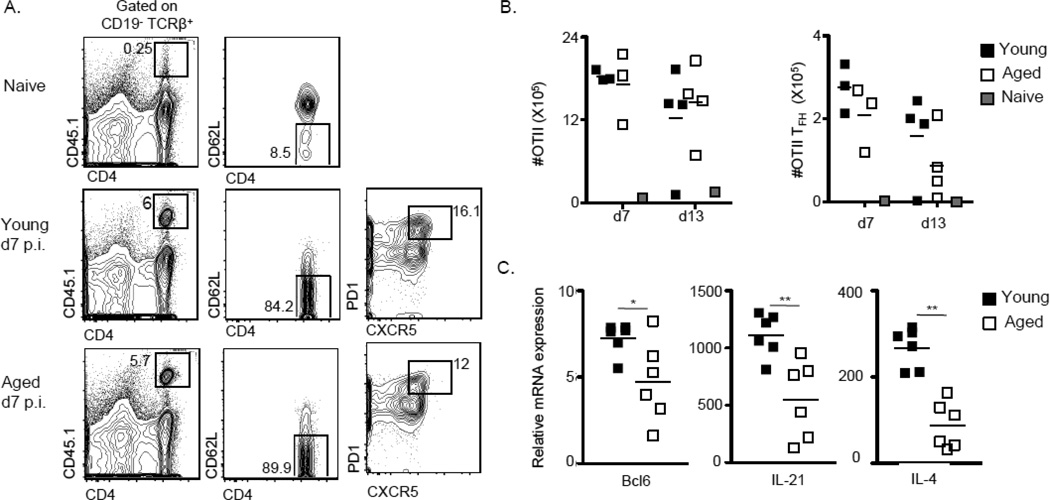
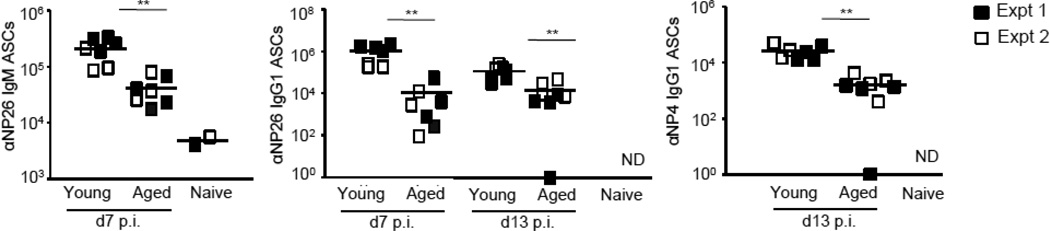
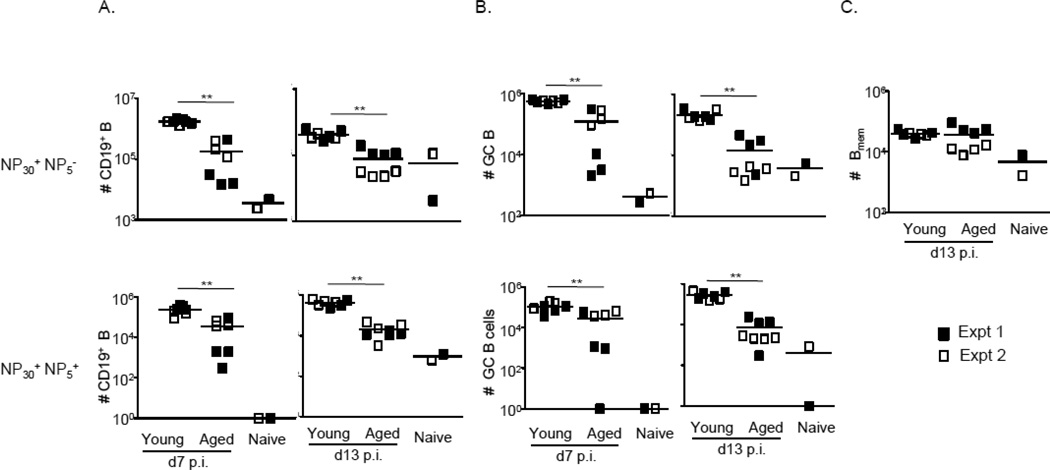
Since effector differentiation of TFH cells appeared to be perturbed in aged mice, we next asked whether the transfer of helper T cells from young mice would correct the observed defect in GC induction. To this end, we transferred 105 OVA-specific CD4 T cells from young OTII mice into each aged or young B6 animal 1–4 hours before immunizing with NP-OVA, and assayed the response at days 7 and 13 (Figures 4–6). As expected, OTIIs did not expand in naïve mice (Fig. 4A, 4B). However, despite similar degrees of expansion in young and aged immunized mice (Fig. 4B), OTII-derived TFH expressed significantly lower levels of Bcl6, IL-4, and IL-21 in aged animals (Fig. 4C). Consistent with defects in TFH differentiation, aged mice were unable to mount optimal primary immune responses. There were severe defects in total and high-affinity B cells, including GC B cells, as early as day 7 (Fig. 5A, 5B). In addition, the GC response waned earlier in the aged mice: there were approximately 4-fold fewer GC B cells at d7, and ~13 fold fewer GC B cells at d13, in aged animals (Fig. 5B). Moreover, despite the provision of “young” T cell help, aged mice were compromised in their ability to generate NP26-specific IgM and IgG1 ASCs at d7 post-immunization, and class switched, NP4-specific ASCs at d13 post-immunization (Figure 6). Of note, as was the case for young and aged mice that did not receive exogenous helper T cells (Fig. 3A), the number of swBmem did not differ at d13 (Fig. 5C).
Figure 4. Defects in effector differentiation of OTII cells in aged mice.
(A) Representative FACS gating strategy for identification of splenic OTII T cells (CD19−TCRβ+CD4+CD45.1+). OTII TFH cells were further identified as CD62L−PD1hiCXCR5+ cells. (B) Total number of splenic OTII and OTII TFH present in immunized aged or young mice at d7 and d13 p.i. with NP-OVA. The numbers present in non-immunized young mice were combined from d7 and d13 post-transfer. (C) Expression of transcript in FACS sorted OT-II TFH generated in young or aged mice at d7 post-immunization normalized to universal total mRNA controls.
Figure 6. Transfer of helper T cells from young mice does not restore the defects in the immune response observed in the aged mice.
CD45.1+ OTII T cells (105 i.v.) were transferred into either young or aged mice and the mice were immunized with NP-OVA/alum.
NP-specific splenic antibody secreting cells (ASCs) using NP33-BSA or NP4-BSA coated plates to determine total NP-specific IgM and IgG1 ASCs as well as high affinity ASCs, respectively.
Figure 5. Transfer of helper T cells from young mice does not restore the defect in GC formation observed in the aged mice.
CD45.1+ OTII T cells (105 i.v.) were transferred into either young or aged mice and the mice were immunized with NP-OVA/alum. Number of splenic NP30+NP5− (top panel) and NP30+NP5+ (bottom panel) responding B (A), GC (B) or sw Bmem (C) cells at d7 or d13 post-OTII-transfer and post-NP-OVA immunization.
Taken together, these results suggest that in early primary immune responses in aged animals, B cell intrinsic defects may dampen TFH differentiation and function, leading to reduced GC B cell numbers and reduced generation of high-affinity GCB and plasma cells. However, despite a compromised GC response, swBmem cells are generated normally during the initial stages of GC maturation.
DISCUSSION
Here we describe and employ a novel flow cytometric approach for resolving high- and low relative affinity B cells. To validate the approach, we show that the low conjugation ratio PE-NP5–6 reagent binds to high-affinity B cells based on several lines of evidence. First, the enrichment of W33L mutations in the NP5+ fraction indicates that the NP5–6 reagent permits detection of B cells that express a high-affinity BCR. Further, NP5–6+ cells are virtually absent in TI-2 responses, which are known to yield smaller GCs that dissipate quickly and thus cannot generate high-affinity B cells (Garcia de Vinuesa C., 1999). In contrast, NP5–6+ cells emerge at the peak of a TD response in the GCB pool, and are subsequently detected in the swBmem pool. Preservation of NP5+ cells concomitant with the decline of NP5− GCB cells by 2–3 weeks is consistent with the observation that higher-affinity clones outcompete lower-affinity clones early in the response (Schwickert et al., 2011; Victora et al., 2010), leaving GCB that are mostly NP5–6+ as observed here. In contrast, only ~half of the swBmem pool was NP5+ at 2–3 weeks. Co-staining with an NP27–30 reagent reveals an additional population of responding B cells with Vh186.2 genes that are not enriched for the W33L mutation, indicating that most of these cells have BCRs with low-to-intermediate affinity for the NP epitope. These cells increased in both TI-2 and early TD responses, and predominated in the GCB but not swBmem subset, again consistent with prior sequencing results (Gonzalez-Fernandez et al., 1994; Lu et al., 2001; Yang et al., 1996). An important additional note is that an approach similar to ours has been validated using hybridomas that produce anti-NP antibody of known affinity (Shimizu et al., 2003).
We applied our methodology to track high-affinity B cells in GCB cell and nascent Bmem subsets during early TD responses in aged mice. We find that aged animals are compromised in their ability to induce potent GC responses, including both high- and low/intermediate-affinity GCB subtypes. This is consistent with prior reports based on analyses of aged mice responding to NP-carrier protein conjugates (for example, (Han et al., 2003; Yang et al., 1996)). Interestingly, numbers of switched NP+ Bmem cells of all affinities were similar between young and aged animals at ~ 2 weeks post-immunization, and in aged hosts that received “young” T cell help; suggesting that the memory B cell pool begins to fill normally in aged animals. Other explanations are possible for this observation, however, including an extrafollicular response to the NP antigen (MacLennan et al., 2003; Sweet et al., 2010) that is intact in aged animals.
Impairment in effector differentiation of TFH cells is a feature of immune senescence that contributes to defects in GC maturation (Eaton et al., 2004; Lefebvre J.S., 2012; Maue et al., 2009). Nevertheless, we found “endogenous” cells of the TFH phenotype in aged mice. Contrary to a previous report (Haynes et al., 2003), we also found that “young” transgenic helper T cells expanded and adopted a TFH fate in aged mice. However, in our experiments both endogenous TFH, as well as TFH derived from transferred T cells of aged donors, expressed reduced levels of effector cytokine transcripts, especially IL-4. This suggests that the transferred CD4 T cells were unable to complete differentiation into GC TFH, the predominant producers of IL-4 in germinal centers (Crotty, 2011; Kroenke et al., 2012). The intact IL-21 and Bcl6 expression we observed in endogenous, polyclonal TFH is perhaps not surprising, because this subset likely contains a very small number of TFH cells that are actively responding to OVA. Moreover, it may include memory TFH that have accumulated with age, some of which may have differentiated in a younger microenvironment and therefore retain robust function (Haynes et al., 2003; Lefebvre J.S., 2012). Since B-MHCII priming is required to upregulate effector cytokine production and Bcl6 message in pre-TFH (Goenka et al., 2011; Kerfoot et al., 2011; Poholek et al., 2010), it is tempting to speculate that B cells in aged individuals are inefficient at driving TFH differentiation. This might reflect accumulation of functionally distinct B cell subsets, or global dampening of B cell presenting activity. Alternatively, other elements of effective T cell priming, including dendritic cell function or the systemic regulatory cytokine milieu, may contribute. These possibilities are not mutually exclusive, and tools are available that should enable assessments of their relative contributions.
Despite the GC defects we observed, aged mice were able to generate low/intermediate-affinity swBmem cells at levels comparable to young mice. Given the observation that some swBmem cells can be generated in a GC-independent manner (Taylor et al., 2012), we speculate that after receipt of help, perhaps at the T-B border, some switched low-affinity B cells seed the memory pool, where they subsequently persist as NP5− NP27+ Bmem cells. Alternatively, these could be low-affinity switched B cells that were outcompeted in the GC response and thus adopted the Bmem fate instead, consistent with studies using low-affinity BCR transgenics (Dal Porto et al., 2002; Dal Porto et al., 1998).
Highlights.
-
▪
B cells bearing high- or low-affinity receptors are resolved with flow cytometry
-
▪
Aged mice show reduced numbers of high-affinity germinal center B cells
-
▪
Transfer of T cell help does not correct the germinal center defect
-
▪
There is no defect in early, class-switched memory B cell numbers in aged mice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–280. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33:281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170:1267–1273. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- Zharhary D, Klinman NR. Antigen responsiveness of the mature and generative B cell populations of aged mice. J Exp Med. 1983;157:1300–1308. doi: 10.1084/jem.157.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharhary D, Klinman NR. The frequency and fine specificity of B cells responsive to (4-hydroxy-3-nitrophenyl)acetyl in aged mice. Cell Immunol. 1986;100:452–461. doi: 10.1016/0008-8749(86)90044-4. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Lu YF, Cerny J. Repertoire of antibody response in bone marrow and the memory response are differentially affected in aging mice. J Immunol. 2002;169:4920–4927. doi: 10.4049/jimmunol.169.9.4920. [DOI] [PubMed] [Google Scholar]
- Miller C, Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J Immunol. 1995;155:3377–3384. [PubMed] [Google Scholar]
- Kosco MH, Burton GF, Kapasi ZF, Szakal AK, Tew JG. Antibody-forming cell induction during an early phase of germinal centre development and its delay with ageing. Immunology. 1989;68:312–318. [PMC free article] [PubMed] [Google Scholar]
- Roukens AH, Soonawala D, Joosten SA, de Visser AW, Jiang X, Dirksen K, de Gruijter M, van Dissel JT, Bredenbeek PJ, Visser LG. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS One. 2011;6:e27753. doi: 10.1371/journal.pone.0027753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–970. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- Ademokun A, Wu YC, Martin V, Mitra R, Sack U, Baxendale H, Kipling D, Dunn-Walters DK. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell. 2011;10:922–930. doi: 10.1111/j.1474-9726.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, Ryan JG, Blomberg BB. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–8084. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 2012;8:e1002920. doi: 10.1371/journal.ppat.1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, P PW, Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- Agrawal A, S H, Chen J, Osann K, Agrawal S, Gupta S. Increased IL-21 secretion by aged CD4+ T cells is associated with prolonged STAT-4 activation and CMV seropositivity. Aging (Albany, NY) 2012;4:648–659. doi: 10.18632/aging.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JS, M AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell. 2012;11:732–740. doi: 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg LA, Black SJ, Tokuhisa T. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980;151:1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropallo MA, Held KS, Goenka R, Ahmad SA, O'Neill PJ, Steward O, Lane TE, Cancro MP. Chronic spinal cord injury impairs primary antibody responses but spares existing humoral immunity in mice. J Immunol. 2012;188:5257–5266. doi: 10.4049/jimmunol.1101934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JL, Crowley JE, Tomayko MM, Steinel N, O'Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Oda M, Azuma T. Estimation of the relative affinity of B cell receptor by flow cytometry. J Immunol Methods. 2003;276:33–44. doi: 10.1016/s0022-1759(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Lu YF, Singh M, Cerny J. Canonical germinal center B cells may not dominate the memory response to antigenic challenge. Int Immunol. 2001;13:643–655. doi: 10.1093/intimm/13.5.643. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012;33:590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Vinuesa C, O LP, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, Nussenzweig MC. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez A, Gilmore D, Milstein C. Age-related decrease in the proportion of germinal center B cells from mouse Peyer's patches is accompanied by an accumulation of somatic mutations in their immunoglobulin genes. Eur J Immunol. 1994;24:2918–2921. doi: 10.1002/eji.1830241151. [DOI] [PubMed] [Google Scholar]
- MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–618. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol. 1998;161:5373–5381. [PubMed] [Google Scholar]
- Rohatgi S, Ganju P, Sehgal D. Systematic design and testing of nested (RT-)PCR primers for specific amplification of mouse rearranged/expressed immunoglobulin variable region genes from small number of B cells. J Immunol Methods. 2008;339:205–219. doi: 10.1016/j.jim.2008.09.017. [DOI] [PubMed] [Google Scholar]