Abstract
Background
Drugs commonly used to sedate nonhuman primates for physiological sample collection can affect the metabolic system and alter rates of glucose metabolism. This study was designed to compare the physiological and metabolic effects of ketamine/diazepam, telazol, and ketamine/dexmedetomidine.
Methods
Seven female rhesus monkeys underwent intravenous glucose tolerance testing under each of three anesthesia conditions. Blood glucose, insulin, physiological parameters, and sedation characteristics were measured and recorded.
Results
Glucose and insulin values were both significantly impacted by ketamine/dexmedetomidine sedation while remaining consistent during ketamine and telazol sedation. Heart rate was also significantly lowered during ketamine/dexmedetomidine anesthesia. Though, ketamine/dexmedetomidine resulted in a longer time between induction of anesthesia and need for a supplemental dose of anesthesia drug.
Conclusions
Telazol and ketamine have minimal cardiorespiratory and metabolic effects compared to ketamine/dexmedetomidine. Though practicably interchangeable, telazol appears to be the most efficient for IVGTTs with NHPs.
Keywords: nonhuman primate, rhesus macaque, IVGTT, glucose regulation, pharmacology
Introduction
Hyperglycemia describes the elevated blood glucose levels associated with type 2 diabetes mellitus. Complications associated with hyperglycemia include an increased risk of cardiovascular disease, coronary artery disease, stroke, atherosclerosis, nephropathy, neuropathy, and decreased muscle mass and strength [3, 8]. In addition, an increase in blood glucose concentrations among the critically ill is an indicator of morbidity and is associated with an increased risk of mortality [8, 15]. Thus, insulin response and blood glucose concentration are valuable markers of physiological health and well-being. Glucose regulation, measured with oral glucose tolerance testing or intravenous glucose tolerance testing (IVGTT), provides additional information about how the body breaks down sugar and is an important marker of health for diet and aging studies in both humans and nonhuman primates (NHPs) [6, 12].
Previous studies have demonstrated that anesthesia medications can alter blood glucose levels, either 1) through increased stress and subsequent release of catecholamines and cortisol, or 2) by directly affecting pancreatic insulin release [9, 14]. Ironically, anesthesia medications are often used to avoid the confounding effects that psychological stressors have on NHPs. For example, using a chair-restraint device to conduct oral glucose tolerance tests in NHPs with >4 years of chair experience was shown to cause activation of the HPA axis and resulting metabolic disturbance [11]. Thus, without specialized restraint caging a standardized sedation protocol is necessary for obtaining valid and reliable rates of glucose metabolism in primate research.
Our standard anesthesia protocol for IVGTT consists of induction of anesthesia through an intramuscular (IM) injection of ketamine hydrochloride [Ketaved®, St. Joseph, MO] followed by an IM injection of diazepam for muscle relaxation. The selection of this anesthesia protocol was based on previous research indicating that blood glucose values were not significantly affected by ketamine anesthesia [5]. However, there are several limiting factors relating to the use of ketamine as the sole sedative for a one-hour IVGTT. First, ketamine is short acting and results in unpredictable recovery times. Therefore, repeated injections are often required to sustain anesthesia for procedures requiring more than 20 minutes of sedation. In addition, ketamine is a highly acidic solution and repeated injection can cause tissue necrosis at the injection site [13]. Muscle tremors and rigidity are common responses to ketamine anesthesia and, in our experience, will often impede blood collection attempts. Thus, supplemental treatment is required to facilitate relaxation and ease blood sampling efforts.
For these reasons, alternative anesthesia protocols were explored. Specifically, Telazol® (Tel) [Wyeth, Philadelphia, PA] was examined because of its common use in animal research and ketamine with dexmedetomidine (Dex) (an α2-adrenoceptor agonist) [Dexdomitor®, Pfizer, New York, NY] was included because of its appealing quality of reversibility using atipamezole [Antisedan, Pfizer, New York, NY], a synthetic alpha2-adrenergic antagonist. Our aim was to determine if, compared to our current ketamine/diazepam protocol (Ket), Dex or Tel are more efficient at inducing a prolonged, relaxed anesthetic state with comparable or reduced effects on blood glucose for IVGTT in rhesus monkeys. Accordingly, our research objectives were to, 1) compare the effects of Ket, Dex, and Tel on blood glucose values obtained during a one-hour IVGTT, 2) to characterize differences in induction, duration, and depth of anesthesia, and need for supplemental doses, and 3) to determine the effects of these drugs on cardiorespiratory parameters including respiratory rate, heart rate, and blood pressure.
Materials and methods
Humane Care Guidelines
All procedures were approved by the NIA Intramural Research Program Institutional Animal Care and Use Committee.
Animals
Subjects were seven female rhesus monkeys (Macaca mulatta) housed at the NIH Animal Center. Females ranged from 13 – 21 years of age (16.04 ± 3.390) with baseline weights between 6 and 10 (9.03 ± 1.310) kilograms. All animals were housed in standard primate caging with controlled temperature and humidity and a 12-hour light cycle. Commercially prepared monkey chow was distributed twice per day along with daily food enrichment and water was available ad libitum.
Anesthesia procedures
IVGTTs were performed for all animals under each of three anesthesia protocols presented in a counterbalanced Latin squares design and with a minimum of two weeks between each testing episode. Order of anesthesia protocol was randomized and doses were as follows: ketamine (10mg/kg, IM) & diazepam (1 mg/kg, IM), ketamine (5mg/kg, IM) & dexmedetomidine (0.02mg/kg, IM) with atipamezole reversal (0.02mg/kg, split IM/IV), and Telazol (5mg/kg). At each testing period, animals were anesthetized using pre-calculated drug doses based on recent weights, removed from their home cage immediately at anesthesia induction, and transported to a procedure room. Once in the procedure room, a current weight was obtained and the animal was placed on a heated table where a patient monitoring system was attached, and a patent catheter was placed in the saphenous vein. In addition, each animal received a 0.015mg/kg IM dose of glycopyrrolate prophylactically to reduce excessive salivation and to stabilize the physiological effects of prolonged anesthesia episodes (e.g. decreased heart rate). On test days when animals received Dex anesthesia, atipamezole reversal was given at the completion of the IVGTT, immediately prior to returning the animal to their home cage.
Glucose and insulin measures
During each anesthesia episode, intravenous (IV) blood samples were collected at baseline and at minutes 1, 5, 10, 20, 30, 40, 50, and 60 following a dose of 300mg/kg of 50% dextrose administered IV through the saphenous vein. Glucose values were promptly measured in whole blood using an Ascensia® Breeze 2 blood glucose monitoring system (Bayer HealthCare LLC., Mishawaka, IN). Samples were then centrifuged and serum samples were aliquoted and stored at −80°C for subsequent analysis using an Insulin ELISA (Mercodia, Uppsala, Sweden).
Induction, duration, and depth of anesthesia
Induction of anesthesia was defined as the length of elapsed time from drug administration to time that the animal could be safely removed from its cage. Duration of anesthesia was defined as the time the animal was removed from their cage until they were able to sit upwards in their cage unassisted. Depth of anesthesia was assessed by a single-blinded technician using parameters adapted from those described in previous studies [7, 13] (Table 1). Parameters included spontaneous movement, toe pinch reflex, jaw tone, and limb manipulations. At specific time points throughout the test, animals were assessed for each parameter and given a score of 1 to 5; receiving a score of 1 if they were too awake for the parameter to be safely measured.
Table 1.
Scoring system for measuring depth of anesthesia parameters
| Score | Spontaneous Movement | Toe Pinch Reflex | Jaw Tone | Limb Manipulation |
|---|---|---|---|---|
| 1 | Whole body | Strongly pulls away | Increased tone | Animal controlled movement |
| 2 | Limb and hand/foot movement | Pulls away immediately | Normal tone | Withdrawn immediately when handled |
| 3 | Mouth or facial movements | Delayed pulling away | Decreased tone | Weakly withdrawn |
| 4 | Twitching | Flexes or extends digits | Minimal tone | Flexes or extends digits |
| 5 | No movement | No movement | No tone | No movement |
Spontaneous movement was assessed and recorded if it occurred at any point during the time that the other parameters were being measured. Toe pinch reflex was assessed by applying firm pressure to the digit of a hind limb with a hemostat. Jaw tone was determined by manually assessing the tensing of the jaw muscle. And, limb manipulation was assessed by lifting the animal’s arm with one hand and then releasing it, allowing it to fall onto the technician’s hand. Each parameter was assessed at baseline and again at minutes 10, 20, 30, 40, 50, and 60. Need for supplemental dosing was continuously evaluated throughout the test. If at any point animals began to move or were assessed with a score of 3 across all depth of anesthesia parameters, a supplemental dose of 5mg/kg of ketamine was administered and recorded, regardless of anesthesia protocol.
Physiological parameters
A Carescape V100 [GE Healthcare, Little Chalfont, Buckinghamshire, UK] vital signs monitor was used to monitor rectal temperature, SpO2, and systolic and diastolic blood pressure. In addition, heart rate and respiratory rate were monitored by technicians. Each physiological parameter was measured and recorded at baseline and again at minutes 10, 30, and 60.
Statistical analysis
Statistical analyses were performed by using the SPSS 19.0 software package (SPSS Inc., Chicago, IL). Area under the curve (AUC) was calculated for glucose and insulin values assessed during each one-hour intravenous glucose tolerance test (IVGTT) and statistical significance was determined based on repeated-measures analysis of variance (ANOVA). Nonparametric Kruskal-Wallis ANOVA were employed to statistically compare need for supplemental doses of anesthesia during each anesthesia episode and also to assess depth of anesthesia measurements for each time point across anesthesia conditions. A one-way within-subject ANOVA was conducted to determine if the time until the first boost of anesthesia was needed during IVGTT testing significantly differed between the three anesthesia drugs and also to compare time to induction. Finally, repeated-measures ANOVA were used to compare cardiorespiratory parameters for each time point across anesthesia conditions.
Results
Variations in blood glucose and insulin
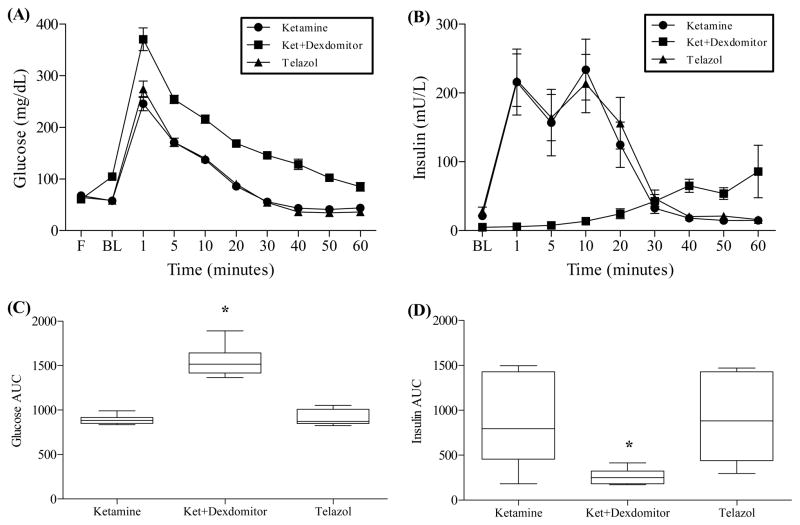
Significant differences in the mean AUC across the three anesthesia conditions were found for glucose values: F(1.311, 7.867) = 69.92, p < .001; the corresponding effect size was a partial η2 of 0.921. This indicates that after stable individual differences in glucose regulation are taken into account, about 92% of the variance in glucose regulation was related to anesthesia condition. Similarly, overall differences in mean AUC for insulin were statistically significant: F(2, 12) = 10.572, p = .002. However, this difference was not quite as strong as the corresponding effect size was a partial η2 of 0.638. Glucose and insulin curves are shown in Figs. 1A and 1B.
Fig. 1.
Changes in glucose and insulin concentrations after glucose challenge. (A) Glucose curve. (B) Insulin curve. (C) Glucose AUC. (D) Insulin AUC. IVGTT was performed under three anesthesia conditions: ketamine/diazepam, ketamine/Dexdomitor, and Telazol (n = 7 animals received all three drugs). Animals were administered glucose intravenously and blood samples were collected at baseline, and minutes 1, 5, 10, 20, 30, 40, 50, and 60. Ketamine/Dexdomitor anesthesia resulted in a significantly higher glucose curve (p < .001) and a significantly lower insulin curve (p < .01) compared to Telazol or ketamine/diazepam. Data represent mean ± SD (A & B) and mean AUC with 95% CI (C & D).
Pairwise comparisons were obtained using Bonferroni corrections to compare mean AUC for glucose across each of the anesthesia conditions. For glucose, the mean AUC during Ket anesthesia (MKet = 893.5) did not differ significantly from the mean during Tel anesthesia (MTel = 908.857). However, Fig. 1C shows means for the Ket and Tel conditions both were significantly lower compared to the Dex condition (MDex = 1564.29). Likewise, pairwise comparisons for insulin revealed that mean AUC were not significantly different for the Ket and Tel conditions but, as shown in Fig. 1D, means for the Ket (MKet = 835.17) and Tel (MTel = 891.99) conditions were both significantly higher compared to the Dex condition (MDex = 263.10).
Anesthesia induction, depth, and supplementation
No significant differences in time to induction were found between Ket, Dex, and Tel. Similarly, there were no differences in total duration of anesthesia between the three drug conditions even with the use of the reversal agent for the Dex condition. Overall differences in the length of time in minutes from anesthesia induction to the first required supplemental dose of ketamine was statistically significant across the three anesthesia conditions: F(2, 12) = 10.584, p = .002; with a corresponding effect size of partial η2 of 0.637 (Fig. 2A). Planned contrasts were obtained to compare time to first boost during Ket anesthesia to Tel and Dex. Here, the mean time in minutes until first supplemental treatment during ketamine anesthesia (MKet = 32.00) did not differ significantly from the time to first supplemental treatment during Tel anesthesia (MTel = 47.143). However, mean time to first supplemental treatment during Ket, but not Tel anesthesia, was significantly shorter compared to mean time to first supplemental treatment during Dex anesthesia (MDex = 64.857): F(1, 6) = 17.235, p = 0.006, signifying that animals remained in a relaxed sedation for longer periods of time under Dex anesthesia compared to Ket anesthesia (Fig. 2A). Finally, the number of supplemental doses of ketamine administered during testing did not statistically differ between the three drug conditions (Fig. 2B). Here, the mean (±SEM) number of additional doses of ketamine were MKet = 2.43 (±0.53), MDex = 1.0 (±0.38), and MTel = 1.71 (±0.29).
Fig. 2.
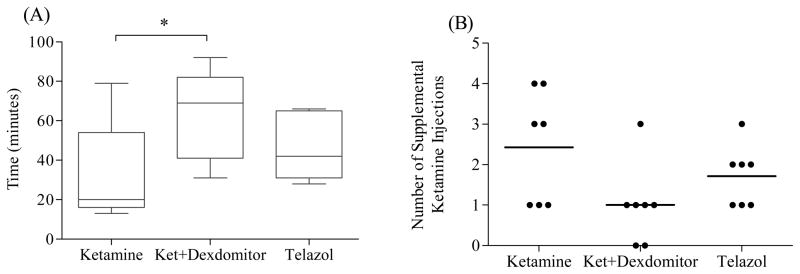
(A) The mean ± SD length of time from induction of anesthesia until first supplemental dose, or boost, of ketamine (5mg/kg) was administered to maintain sedation (n = 7 animals in each condition) and (B) the number of supplemental ketamine doses required during one-hour IVGTT. The mean time until first boost was significantly longer for animals sedated with ketamine/Dexdomitor compared to animals sedated with ketamine/diazepam (p < .001), while the Telazol conditions did not differ from ketamine/diazepam or ketamine/Dexdomitor. The number of supplemental doses of ketamine administered during testing did differ between the three drug conditions but this difference was not robust enough to be statistically significant.
To evaluate depth of anesthesia measurements, nonparametric Kruskal-Wallis ANOVA was performed for each time point across anesthesia conditions. No between group differences were found at any of the study time points for spontaneous movement or for limb manipulation measures. However, toe pinch at minute 10 differed across groups (p = 0.05), though none remained significant with posthoc tests: Ket (MKet = 1.57), Dex (MDex = 2.21), Tel (MTel = 2.21). Similarly, toe pinch at minute 60 differed across groups (p = 0.015) with no differences seen in the posthoc test: Ket (MKet = 1.29), Dex (MDex = 2.43), Tel (MTel = 2.29). Finally, jaw tone at minute 60 differed across groups (p = 0.005) – where Ket (MKet = 1.29) significantly differed from Dex (MDex = 2.79) at posthoc test (p = 0.015) but did not differ from Tel (MTel = 1.93).
Cardiorespiratory parameters
Repeated-measures ANOVA revealed no difference in respiratory rate, systolic blood pressure, or diastolic blood pressure across the three anesthesia conditions. Though, an overall main effect was found for mean heart rate between the three anesthesia conditions across seven time points of IVGTT (F(2,12) = 90.863, p < 0.001). As seen in Fig. 3, posthoc comparisons adjusted using Bonferroni indicate that heart rate during Dex anesthesia (MDex = 98.582) was significantly lower compared to Ket (MKet = 159.612) and Tel (MTel = 158.714). No statistical analyses were conducted on the body temperature data as all animals were kept on a heated table to prevent temperature decreases.
Fig. 3.
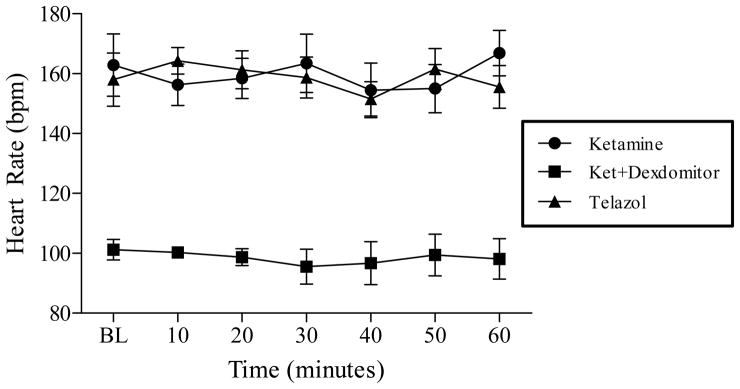
Mean ± SD heart rate during three anesthesia conditions across seven time points during one-hour IVGTT testing (n = 7 animals in each condition). Repeated-measures ANOVA revealed heart rate during sedation with ketamine/Dexdomitor was significantly lowered compared to sedation with Telazol or ketamine/diazepam (p < .001).
Discussion
Procedures for collecting blood or other physiological samples from NHPs often include sedation; providing safety for animals and technical staff and ameliorating environmental stressors as a potential confounding factor in sample analyses. Similarly, it is essential to ensure that the anesthesia drug used for such procedures does not alter the outcome of the intended assay or test. A potent indicator of health, glucose metabolism, can be markedly affected by environmental and/or pharmacological influences. Thus, it is central to characterize the physiological influence of anesthesia drugs commonly used in NHP studies on glucose metabolism.
Ketamine and telazol are two such drugs that are typically used for sedation protocols in primate research. Dexmedetomidine is a less traditional alternative anesthetic and has a reputation for providing a deeper, more relaxed sedative state with predictable and rapid recovery using atipamezole reversal [1, 4]. In this study, Dex, compared to Ket, was beneficial for extending the time from anesthesia induction to the time that an animal needed additional anesthetic administered. However, the number of supplemental doses administered and the total length of time an animal was anesthetized was not statistically improved with the use of Dex. Even so, the ability to reverse Dex anesthesia with atipamezole makes this a more desirable alternative in instances where shorter periods of relaxed anesthesia are needed and glucoregulatory processes are not of interest.
As predicted, blood glucose and insulin values were affected by anesthesia protocol. More specifically, dexmedetomidine appears to reduce insulin levels and elevate blood glucose values. This finding is consistent with other studies that suggest α2-agonists may induce dose-dependent hyperglycemia by stimulating receptors in the pancreas to decrease insulin release [4, 9, 14]. However, none of these reports were from NHPs. Nonetheless, because blood glucose and insulin are central measures of interest for IVGTT, dexmedetomidine as an addition to ketamine is not a suitable anesthetic for this purpose.
Dexmedetomidine is a potent α2-adrenoceptor agonist that has been shown to produce significant effects on rectal temperature, respiratory rate, blood pressure, and heart rate, causing bradycardia through a combination of vasoconstriction and decreased cardiac output [1, 10]. Our findings were not consistent here. In fact, the respiratory rate and blood pressure effects were not significantly different across anesthesia drugs. However, we used prophylactic glycopyrrolate and kept animals on a heated table to prevent decreases in body temperature. Additionally, the dexmedetomidine dose we used was low compared to doses reported by other labs [2, 10], which could have guarded against any dose-dependent cardiorespiratory effects.
Compared to ketamine/dexmedetomidine, our present anesthesia protocol for IVGTT had no effect on blood glucose and insulin values and minimal effects on cardiorespiratory parameters. However, ketamine/diazepam anesthesia resulted in the need for earlier administration of supplemental anesthetics compared to ketamine/dexmedetomidine and caused the animals to be more reactive at minutes 10 and 60 based on the toe pinch and jaw tone depth parameters relative to Tel. Due to the effects that dexmedetomidine has on glucose and insulin values and the less efficient anesthesia produced by ketamine/diazepam, Telazol appears to be the best choice for anesthesia medications for one-hour IVGTT in rhesus monkeys and could feasibly be interchangeable with ketamine/diazepam anesthesia.
Acknowledgments
The authors would like to thank Joe Travis, Rob Young, and Ed Tilmont for their technical support. This project was supported entirely by the Intramural Research Program, National Institute on Aging, NIH.
References
- 1.Biermann K, Hungerbuhler S, Mischke R, Kastner SB. Sedative, cardiovascular, haematologic and biochemical effects of four different drug combinations administered intramuscularly in cats. Veterinary anaesthesia and analgesia. 2012;39:137–150. doi: 10.1111/j.1467-2995.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 2.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci U S A. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci. 2012;67:74–81. doi: 10.1093/gerona/glr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanda T, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared to xylazine in healthy cats. The Canadian Journal of Veterinary Research. 2008;72:278–286. [PMC free article] [PubMed] [Google Scholar]
- 5.Kemnitz JW, Kraemer GW. Assessment of glucoregulation in rhesus-monkeys sedated with ketamine. Am J Primatol. 1982;3:201–210. doi: 10.1002/ajp.1350030118. [DOI] [PubMed] [Google Scholar]
- 6.Lane MA, Tilmont EM, De Angelis H, Handy A, Ingram DK, Kemnitz JW, Roth GS. Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta) Mech Ageing Dev. 2000;112:185–196. doi: 10.1016/s0047-6374(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee VK, Flynt KS, Haag LM, Taylor DK. Comparison of the effects of ketamine, ketamine-medetomidine, and ketamine-midazolam on physiological parameters and anesthesia-induced stress in rhesus (macaca mulatta) and cynomologus (macaca fascicularis) macaques. J Am Assoc Lab Anim Sci. 2010;49:57–63. [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsberg PJ, Tuomi T, Kaste M. Oral glucose tolerance test should be performed after stroke and transient ischemic attack. Int J Stroke. 2011;6:317–320. doi: 10.1111/j.1747-4949.2011.00619.x. [DOI] [PubMed] [Google Scholar]
- 9.Saha JK, Xia J, Grondin JM, Engle SK, Jakubows JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: Mechanisms and implications for preclinical models. Exp Biol Med. 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- 10.Selmi AL, Mendes GM, Boere V, Cozer LA, Filho ES, Silva CA. Assessment of dexmedetomidine/ketamine anesthesia in golden-headed lion tamarins (Leontopithecus chrysomelas) Veterinary Anesthesia and Analgesia. 2004;31:138–145. doi: 10.1111/j.1467-2987.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirasaki Y, Yoshioka N, Kanazawa K, Maekawa T, Horikawa T, Hayashi T. Effect of physical restraint on glucose tolerance in cynomolgus monkeys. J Med Primatol. 2013;42:165–168. doi: 10.1111/jmp.12039. [DOI] [PubMed] [Google Scholar]
- 12.Stout RW. Glucose tolerance and ageing. J R Soc Med. 1994;87:608–609. doi: 10.1177/014107689408701015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun FJ, Wright DE, Pinson DM. Comparison of ketamine versus combination of ketamine and medetomidine in injectable anesthetic protocols: chemical immobilization in macaques and tissue reaction in rats. Contemp Top Lab Anim. 2003;42:32–37. [PubMed] [Google Scholar]
- 14.Tanaka K, Kawano T, Tsutsumi YM, Kinoshita M, Kakuta N, Hirose K, Kimura M, Oshita S. Differential effects of propofol and isoflurane on glucose utilization and insulin secretion. Life Sci. 2011;88:96–103. doi: 10.1016/j.lfs.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]