Abstract
Background
Platelet abnormalities are well-recognized complications of diabetes mellitus (DM). Mitochondria play a central role in platelet metabolism and activation. Mitochondrial dysfunction is evident in DM. The molecular pathway for hyperglycemia-induced mitochondrial dysfunction in DM platelets is unknown.
Methods and Results
Using both human and humanized mouse models, we report that hyperglycemia-induced aldose reductase (AR) activation, and subsequent reactive oxygen species (ROS) production, leads to increased p53 phosphorylation (Ser15), which promotes mitochondrial dysfunction, damage and rupture by sequestration of the anti-apoptotic protein Bcl-xL. In a glucose dose dependent manner, severe mitochondrial damage leads to loss of mitochondrial membrane potential and platelet apoptosis (cytochrome c release, caspase 3 activation and phosphatidylserine exposure). Although platelet hyperactivation, mitochondrial dysfunction, AR activation, ROS production and p53 phosphorylation are all induced by hyperglycemia, we demonstrate that platelet apoptosis and hyperactivation are two distinct states, dependent upon the severity of the hyperglycemia and mitochondrial damage. Combined, both lead to increased thrombus formation in a mouse blood stasis model.
Conclusions
AR contributes to diabetes-mediated mitochondrial dysfunction and damage through the activation of p53. The degree of mitochondrial dysfunction and damage determines whether hyperactivity (mild damage) or apoptosis (severe damage) will ensue. These signaling components provide novel therapeutic targets for DM thrombotic complications.
Keywords: aldose reductase, p53, platelet, diabetes mellitus, mitochondria, apoptosis
Introduction
Diabetes mellitus (DM) is a complex disease characterized by absolute insulin deficiency or resistance to insulin, leading to hyperglycemia1. The altered glucose metabolism can lead to many organ complications including nephropathy, neuropathy and retinopathy2. Platelet abnormalities are one of the hallmarks of DM, contributing to the pathogenesis of many disease processes, including atherosclerosis and thrombosis3. Several mechanisms have been proposed to explain the platelet abnormalities in DM, among which metabolic alterations and oxidative stress appear to play pivotal roles 4.
Mitochondrial alterations contribute to the pathogenesis of DM5. Given the central role of mitochondria in platelet metabolism and activation, mitochondrial alteration may contribute to platelet abnormalities in DM. Indeed, previous studies using isolated platelets from type 2 DM patients have demonstrated low mitochondrial membrane potential compared to control subjects6 and upregulation of antioxidant enzymes (SOD2 and PRDX3), indicating increased oxidative stress7. Therefore, it is plausible that hyperglycemia-induced oxidative stress impairs mitochondrial function in DM platelets. Oxidative stress plays an important role in the development of microvascular and cardiovascular diabetic complications8. In platelets, hyperglycemia-induced oxidative stress has been shown to contribute to platelet hyperreactivity9, 10. However, the role of hyperglycemia-induced oxidative stress in platelet mitochondrial function is unknown.
Platelets have also been demonstrated to possess critical signaling proteins (Bcl family proteins) known to regulate mitochondria-dependent apoptosis11. Pharmacological inhibition of Bcl-xL with BH3 mimetic compounds ABT-737 or ABT-263 triggers platelet apoptosis in vitro, and acute thrombocytopenia in vivo12–14, suggesting that Bcl-xL plays a crucial role in regulating the mitochondria-dependent apoptosis in platelets. ABT-737 treatment causes platelet mitochondrial damage with concomitant activation of caspase-3, consequently leading to externalization of phosphatidylserine (PS)15. Despite these elegant studies demonstrating the presence of many stress and apoptosis signaling components, the pathway connecting these signaling components to hyperglycemia and mitochondrial damage, and their consequences in DM platelets, is not known.
We hypothesized that aldose reductase (AR) plays a key role in the pathway leading from hyperglycemia to platelet mitochondrial damage. AR, the first key enzyme in the polyol pathway, is well known to contribute to oxidative stress in DM16. AR contributes to platelet activation as well as to hyperactivation in platelets under hyperglycemia9, 17. In the present study, we aim to investigate the role of AR in diabetes-mediated mitochondrial dysfunction, and the signaling pathways involved in this process. We demonstrate that AR contributes to diabetes-mediated mitochondrial dysfunction and damage through the activation of p53. We report that the degree of mitochondrial dysfunction and damage determines whether hyperactivity (mild damage) or apoptosis (severe damage) will ensue.
Methods
More detailed description of the Methods can be found in Supplemental Materials.
Patient recruitment and preparation of human platelet samples
Venous blood was drawn from consenting volunteers (healthy and DM subjects) at Yale University School of Medicine (HIC# 1005006865). Sixty-six type 2 DM subjects (American Diabetic Association definition) and eight healthy controls (HS) were recruited for the studies (Supp. Table 1). The platelet-rich plasma (PRP) and washed platelets were collected by centrifugation.
Development of mouse model for DM with human AR expression
Mice were injected with STZ (50 mg/ml) intraperitoneally for 5 consecutive days to induce DM. Four weeks after STZ administration, mice were maintained on high cholesterol diet (HCD) for 12 weeks. Platelets were collected by centrifugation.
Ligation of mouse carotid artery
The carotid artery ligation model was performed as described previously18. Briefly, the left common carotid artery was ligated near the carotid bifurcation. After 7 days, mice were euthanized. The left and right arteries were fixed in 4 % paraformaldehyde, embedded in OCT compound, and sectioned for analysis.
Confocal microscopy
The washed platelets were settled on glass-bottom dishes for 30 min, fixed with 4% PFA, and stained with specific antibodies. The stained platelets were observed using a Nikon Eclipse-Ti confocal microscope with 100x oil immersion lens.
Determination of mitochondrial membrane potential (ΔΨm), phosphatidylserine (PS) externalization and mitochondrial superoxide production
To assess the changes in mitochondrial membrane potential (ΔΨm) and PS externalization using flow cytometry, platelets were stained with 40 nM Tetramethylrhodamine methyl ester (TMRM), followed by staining with 4 μg/ml annexin V. To measure mitochondrial superoxide production, platelets were stained with 5 μM MitoSOX™ Red.
Western blotting, immunoprecipitation and subcellular fractionation
The protein samples were subjected to SDS-PAGE, and the proteins were transferred to nitrocellulose membrane and probed with the indicated antibodies. For immunoprecipitation, platelet lysates were incubated with agarose conjugated p53 antibody, and the immunoprecipitated proteins were eluted in SDS loading buffer for Western analysis. Subcellular fractionation was performed using a Mitochondria Isolation Kit (Pierce Biotechnology, Inc., Pierce, Rockford, IL, USA).
Statistical Analysis
All data were expressed as mean ± standard deviation. The parametric t-test was performed in comparing two groups. The parametric One-way ANOVA followed by Tukey's multiple comparison test, was used to assess difference between more than two groups within the same study. Analysis was performed using Prism software (GraphPad). A difference of P<0.05 was considered significant.
Results
Severe mitochondrial damage occurs in DM platelets
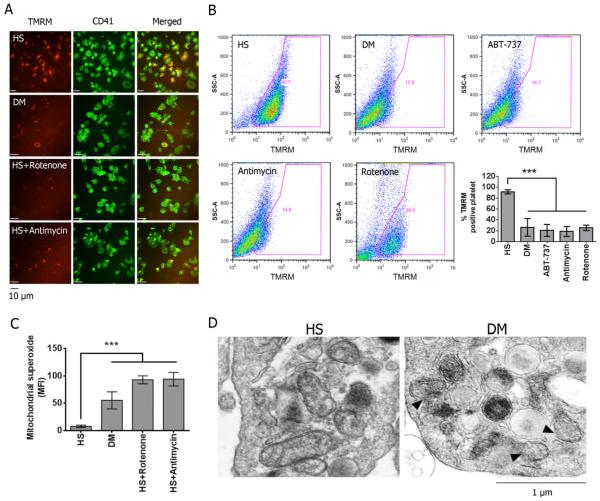
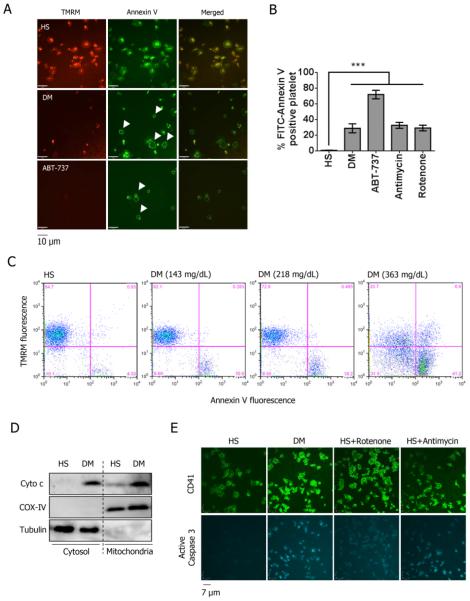
To initially assess mitochondrial function in DM platelets in comparison to platelets from control subjects, we measured the ΔΨm by TMRM staining. TMRM is readily sequestered by the mitochondria, but its fluorescence is rapidly lost when ΔΨm is dissipated. Compared to healthy platelets, DM platelets exhibited reduced ΔΨm (Fig 1A). Using FACS analysis, only 10% of platelets were TMRM-negative in healthy subjects (HS). In contrast, about 80% of DM and ABT-737 (Bcl-2 inhibitor) treated platelets were TMRM-negative (Fig 1B). As positive controls, healthy platelets treated with antimycin A and rotenone (inhibitors for mitochondrial complex I and III respectively) also demonstrated greater a TMRM-negative population (Fig 1B). In addition to ΔΨm dissipation, mitochondrial superoxide production was significantly increased, with rotenone and antimycin A (positive controls) (Fig 1C). Further supporting the severe mitochondrial damage, the mitochondrial permeability transition pore (MPTP) was also increased as demonstrated by the loss of the retained calcein fluorescence in the mitochondria using a cobalt chloride quenching assay (Suppl. Fig 1). Ionomycin was used as a control to trigger complete MPTP permeability. Using electron microscopy (EM), we also observed direct evidence of mitochondrial damage. The mitochondria from DM platelets exhibited varying degrees of damage and rupture of the outer mitochondrial membrane (OMM), compared to HS platelets, which predominantly showed the classic double-membrane mitochondria without OMM damage (Fig 1D). The complete EM field is provided in Supp Fig 2A and a lower power field showing distinct morphologic differences in HS versus DM platelets in Supp Fig 2B. Collectively, these results demonstrate that platelet mitochondrial dysfunction and damage is characteristic of DM platelets.
Figure 1.
Alteration of mitochondrial function in platelet isolated from diabetic (DM) subjects. (A) Mitochondrial membrane potential (ΔΨm) was measured by staining with 40 nM TMRM in platelets isolated from HS and DM, and the platelets were visualized by staining with FITC-CD 41. Healthy platelets treated with either 2 μM rotenone-treated or 20 μM antimycin were used as controls to induce the reduction of ΔΨm. (B) ΔΨm was also measured by FACS. Representative plots were shown. Data was presented as percentage of TMRM positive cells ± SD, n=5, ***P<0.001 compared with HS. (C) Mitochondrial superoxide production was labeled with 5 μM MitoSOX™ Red (mean fluorescence ± SD, n=4, ***P<0.001 compared with HS). (D) Electron microscopy images of HS and DM platelets. Arrows indicate rupture of the mitochondrial outer membrane in DM sample.
DM platelets exhibit increased p53 phosphorylation
In order to identify the key components leading to mitochondrial dysfunction in DM platelets, we utilized a proteome profiler array (R&D Systems), comparing platelets from HS (n=8 pooled) and type 2 DM patients (n=8 pooled). The most consistent observation was reduced expression of Bcl-xL by 2.5 fold in DM, and interestingly, increased phosphorylation of p53 at serine 15 by 2-fold (Supp Fig 3A & 3B). No change was found in the expression of the other Bcl-2 family proteins (Supp Fig 3C).
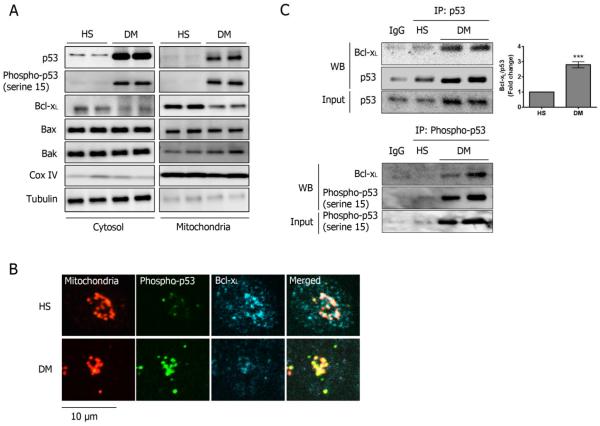
In both the cytosolic and mitochondrial fractions of platelets, the expression of p53 and its phosphorylation at serine 15 were markedly upregulated in DM compared to HS subjects, but no detectable changes in Bax and Bak were observed (Fig 2A). Utilizing high power confocal microscopy, we demonstrate that in DM platelets, the level of p53 phosphorylated at serine 15 was increased, and much of it localized to the mitochondria, whereas Bcl-xL was diminished (Fig 2B). The complete field where these platelets were selected is shown in Supp Fig 4A. In addition to the phosphorylation, we also found that glutathionylation and acetylation of p53 was increased in DM platelets (Supp Fig 4B).
Figure 2.
Upregulation of p53 and its binding to Bcl-xL in DM platelets. (A) Subcellular fractionation was performed to harvest the cytosolic and mitochondrial fractions. Representative western blots are shown (n=5). (B) The expression and localization of Bcl-xL (Cy5), p53 and its phosphorylation at serine 15 (green) were assessed by immunostaining in HS & DM platelets (n=5). Mitochondria were visualized by staining with anti-OxPhos Complex V (red). The bar represents 10 μm. (C) The binding of Bcl-xL to p53 was assessed by immunoprecipitation in HS & DM platelets (mean ± SD, n=4 ***P<0.001 compared with HS). The binding of Bcl-xL to phosphorylated p53 was also assessed by immunoprecipitation in HS & DM platelets.
Previous studies have suggested that p53 can induce mitochondrial opening by interacting with Bcl-xL19. Bcl-xL co-immunoprecipitated with p53 markedly increased in DM platelets by 3-fold compared with HS (Fig 2B) and can be attributed to phosphorylated p53. The detection was limited in healthy platelets due to low levels of phosphorylated p53 (Fig 2C). Together with the colocalization studies from confocal microscopy, our results showed that Bcl-xL is sequestered by the increased phosphorylated p53. In platelets, Bcl-xL exerts its pro-survival reaction by binding to the pro-apoptotic mediators Bax and Bak at the mitochondria20. Therefore, as Bcl-xL is sequestered by phosphorylated p53, Bax and Bak would mediate the outer membrane permeabilization, leading to the release of cytochrome c from the intermembrane space21. The loss of cytochrome c in the intermembrane space can no longer inhibit ROS formation in the electron transport, leading to increased oxidative stress, and further mitochondrial permeability22.
In addition to the interaction between p53 and Bcl-xL, recent data has demonstrated that Bcl-xL alone can stabilize the Δψm by interacting with ATP synthase23. ATP synthase activity was not changed in the diabetic platelets (Supp Fig 1A). P53 has been associated with regulation of the mitochondrial respiration24. We found that mitochondrial ATP level were reduced in diabetic platelets, suggesting that the ATP generation from oxidative phosphorylation was impaired in DM platelets. Interestingly, cytosolic ATP was increased in diabetic platelets, suggesting that increased glycolytic flux may ensue in DM platelets (Supp Fig 1B). These findings are supported by a previous study showing that platelets from type 2 DM are characterized by high level of total ATP but low Δψm25.
AR contributes to hyperglycemia-induced p53 phosphorylation in human platelets
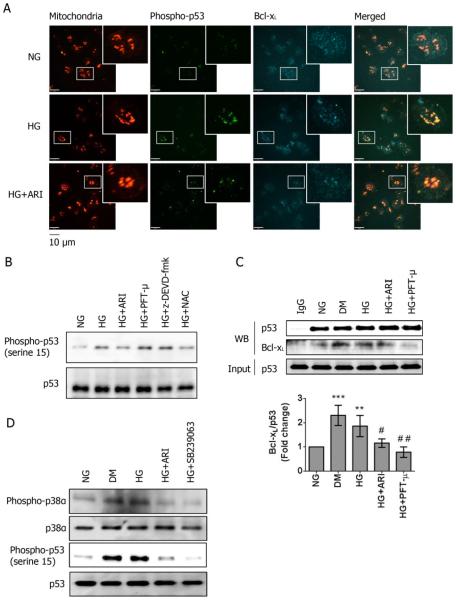
To understand whether acute hyperglycemia contributes to p53 phosphorylation human platelets isolated from healthy subjects (HS) were incubated with 5 mM glucose (NG) or 25 mM glucose (HG) for 2 hrs. Platelets incubated with HG showed increased p53 phosphorylation localized to mitochondria (Fig 3A). However, in contrast to DM platelets, no reduction in Bcl-xL expression was observed in normal platelets treated with HG (Fig 3A). The increased phosphorylation of p53 at serine 15 was further confirmed by Western blot (Fig 3B). In contrast to DM platelets, total p53 expression was not changed after incubation of healthy platelets with HG.
Figure 3.
Aldose reductase contributes to hyperglycemia-induced p53 activation in human platelets. (A) Platelets were incubated with either 5 mM (NG) or 25 mM (HG) glucose for 2 hours. The expression and localization of Bcl-xL and phosphorylated p53 (serine 15) were assessed by immunostaining in NG, HG, and HG platelets treated with 10 μM epalrestat (HG+ARI) (n=4). Platelets were stained with anti-phospho-p53 (green) and anti-Bcl-xL (Cy5). Mitochondria were visualized by staining with anti-OxPhos Complex V inhibitor protein (red). The bar represents 10 μm. (B) The phosphorylation of p53 was also assessed by Western blot in NG, HG, HG+ARI, HG+20 μM PFT-μ, HG+20 μM z-DEVD-fmk, and 1 mM antioxidant N-acetyl-cysteine (NAC), and normalized by total p53 expression. The representative blots are shown (n=4). (C) The binding of Bcl-xL to p53 was assessed by immunoprecipitation in NG, HG and DM platelets. The effect of ARI, PFT-μ and z-DEVD-fmk on the p53 and Bcl-xL interaction was also studied in the HG platelets. Representative blots are shown (mean ± SD, n=4 ***P<0.001 & **P<0.01 compared with NG; ##P<0.01 & #P<0.05 compared with HG). (D) The phosphorylation of p38α MAPK and p53 were assessed by Western blot in NG, DM, HG+ARI & HG+ 50 μM SB239063, and normalized by total p38α MAPK and p53 expression. The representative blots are shown (n=4).
It is well recognized that phosphorylation of p53 at serine 15 is mediated by oxidative stress26–28. Therefore, we hypothesized that hyperglycemia-induced oxidative stress (mediated by AR16) phosphorylated p53 in platelet, which translocates to the mitochondria with Bcl-xL29. Treatment with 10 μM epalrestat, an AR inhibitor, and the antioxidant N-acetyl-cysteine attenuated the HG-induced phosphorylation of p53 (Fig 3A–B). Treatment with Pifithrin-μ (PFT-μ), an inhibitor of p53 binding to mitochondrial Bcl-xL protein30, attenuated the interaction with Bcl-xL, but had no effect on the phosphorylation of p53 (Fig 3B–C). Treatment with z-DEVD-fmk (downstream caspase-3 inhibitor) also showed no effect on the phosphorylation of p53. The binding of Bcl-xL with immunoprecipitated p53 also increased in platelets incubated with HG, which is comparable to DM (Fig 3C), suggesting that hyperglycemia enhanced phosphorylation of p53 and protein-protein interaction with Bcl-xL. Treatment with ARI also attenuated the interaction between Bcl-xL and p53, although the effect was less compared to PTF-μ (Fig 3C).
We have previously shown in human platelets that high glucose can induce the phosphorylation of p38α MAPK through AR activity9, and it is known that p53 can be phosphorylated by the activation of p38α MAPK28, 31. In both DM and HG-treated platelets, the phosphorylation of p38α MAPK was increased, which was attenuated by ARI and SB239063 (p38α MAPK inhibitor). Inhibition of p38α MAPK also reduced the HG-induced p53 phosphorylation, suggesting that AR activity increased the phosphorylation of p38α MAPK, leading to p53 activation in human platelets under HG condition (Fig 3D). With regards to mitochondrial function, HG platelets exhibited mild mitochondrial alterations, including reduced Δψm and MPTP opening. Moreover, Annexin-V positive platelets were also modestly increased under HG condition. All these changes were attenuated by treatment with ARI or PFT-μ (Supp Fig 5A & 5B). Taken together, our results demonstrated that a 2 hour hyperglycemia treatment is sufficient to activate p53 and lead to mild mitochondrial dysfunction by increasing its phosphorylation and binding to Bcl-xL, and this is at least in part due to AR activation and ROS production in platelets.
Platelet mitochondrial dysfunction in the huAR transgenic DM mice
Wild-type mice have very little native AR (analogous to a functional knockout). The lack of AR may be a key reason why mice are resistant to the deleterious effect of hyperglycemia32. We therefore induced DM in a mouse model where huAR was expressed and had activity comparable to those found in human platelets (Supp Fig 6A). Blood glucose levels were significantly elevated in DM mice and no significant difference was found in body weight (Supp Fig 6B).
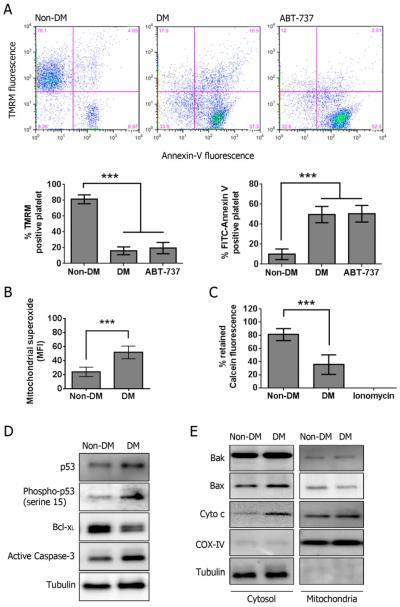
Consistent with the findings in DM patients, huAR transgenic murine platelets exhibit dissipation of Δψm and PS externalization (apoptosis) under DM conditions. A clear reduction in the TMRM positive population and significant increases in the Annexin-V positive population was also observed in mouse platelets treated with 10 μM ABT-737 (Fig 4A). Likewise, mitochondrial superoxide and MPTP opening were significantly increased in DM murine platelets (Fig 4B & 4C). All these results suggested that mitochondrial function is severely altered in DM murine platelets where cellular apoptosis occurs.
Figure 4.
Alteration of mitochondrial function in platelet isolated from huAR transgenic diabetic mouse. (A) Mitochondrial membrane potential (ΔΨm) was measured by staining with 40 nM TMRM, and PS externalization was assessed with FITC-Annexin V. Murine platelets were treated with 10 μM ABT-737 to serve as a positive control for apoptosis. Data was presented as percentage of TMRM or Annexin-V positive cells ± SD (n=6), ***P<0.001 compared with the corresponding non-DM. (B) Mitochondrial superoxide production was labeled with 5 μM MitoSOX™ Red. Data was presented as mean fluorescence ± SD (n=4), ***P<0.001 compared with non-DM. (C) Activation of MPTP was assessed by loss of retained calcein fluorescence. Data was presented as a percentage of retained calcein fluorescence ± SD (n=4). ***P<0.001 compared with non-DM. (D) The total expression of p53, Bcl-xL, phosphorylated p53 (serine 15) and active caspase-3 was determined by Western blot. Representative blots are shown (n=4). (E) The expression of Bax, Bak and cytochrome c (Cyto c) was determined by Western blot in cytosolic and mitochondrial fractions. Representative blots are shown (n=4).
We further studied whether phosphorylation of p53 at serine 15 was also found in DM murine platelets. Consistent with our findings in human platelets, the expression of p53 and its phosphorylation at serine 15 was increased in DM murine platelets (Fig 4D). Expression of Bcl-xL was reduced, and Caspase-3 activation and cytochrome c release were increased in DM murine platelets. Confirming our human data, the subcellular level of Bax and Bak was not changed in DM murine platelets compared to non-DM (Fig 4E). Combined, these mouse studies further validate our observations in human platelets where mitochondrial function is altered in DM platelets, likely from AR-dependent activation of p53. Moreover, platelet apoptosis may ensue.
AR contributes to acute hyperglycemia-induced mitochondrial dysfunction by increasing p53 phosphorylation in non-DM murine platelet
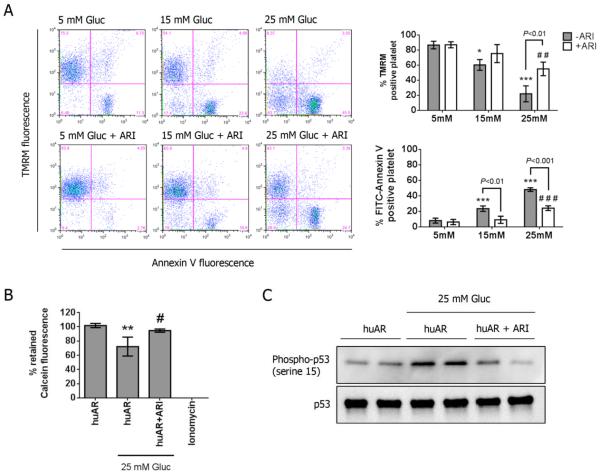
To further understand how AR contributes to hyperglycemia-induced mitochondrial alterations, huAR transgenic murine platelets (non-DM) were subjected to different concentrations of glucose for 2 hrs. For incubation with HG, Δψm was significantly dissipated with concomitant PS externalization in huAR transgenic murine platelets in dose-dependent manner, and the effect was markedly attenuated by the treatment with ARI (Fig 5A). The opening of MPTP also increased with treatment of 25 mM glucose, and was restored by treatment with ARI (Fig 5B). This further supports a role of AR as a key player in hyperglycemia-induced mitochondrial dysfunction. Consistent with mitochondrial dysfunction, phosphorylation of p53 at serine 15 was significantly increased in murine platelets incubated with 25 mM glucose, which was attenuated by AR inhibition (Fig 5C). Taken together, our results demonstrate that huAR expression in murine platelets exacerbates hyperglycemia-induced mitochondrial dysfunction through p53 activation.
Figure 5.
Requirement of aldose reductase for hyperglycemia-induced mitochondrial dysfunction in mouse platelet. (A) Mitochondrial membrane potential (ΔΨm) was measured by staining with 40 nM TMRM, and PS externalization was assessed by labeling with FITC-Annexin V. Platelets of huAR mice were incubated with different concentration of glucose (i.e. 5, 15 and 25 mM glucose), and the role of AR was determined by pre-treatment with 10 μM epalrestat (ARI). For TMRM staining, data was presented as mean percentage of TMRM positive platelets ± SD (n=4), ***P<0.001, *P<0.05 compared with 5 mM glucose group in the absence of ARI; ##P<0.01 compared with 5mM glucose group in the presence of ARI. For Annexin-V staining, data was presented as mean percentage of Annexin-V positive platelets ± SD (n=4). ***P<0.001, compared with 5 mM glucose group in the absence of ARI; ###P<0.001 compared with 5mM glucose group in the presence of ARI. (B) Activation of MPTP was assessed by loss of retained calcein fluorescence. Data was presented as mean percentage of retained calcein fluorescence ± SD (n=4). **P<0.01 compared with huAR; #P<0.05 compared with huAR incubated with 25 mM glucose. (C) The phosphorylation of p53 was assessed by Western blot in huAR platelets incubated with normal or 25 mM glucose. The level of phosphorylated p53 was normalized to total p53 expression. Representative blots are shown (n=4).
Platelet activation and apoptosis are distinct states in DM human platelets
In DM human platelets, the dissipation of Δψm is so severe that there is concomitant increase in PS externalization (Fig 6A). Such severe mitochondrial damage and apoptosis is visible as a paucity in red staining (dissipation of Δψm) inside the green rings (PS externalization). Treatment with 10 μM ABT-737 was also found to reduce Δψm and increase PS externalization (Fig 6A). Also, compared with HS, a larger population of Annexin-V positive platelets (apoptotic platelets) was found in both DM and ABT-737 (3% vs 30% and 70%, respectively) treated platelets (Fig 6B). Using FACS analysis, we also showed that DM platelets exhibited an increase in the apoptotic population compared with HS platelets in a glucose (in vivo) dose dependent manner (Fig 6C). Consistent with severe mitochondrial dysfunction and PS externalization, active caspase-3 and cytochrome c release were also increased in the DM platelets (Fig 6D & 6E).
Figure 6.
DM induces apoptotic features in human platelets. (A) Phosphatiydylserine (PS) externalization assessed by labeling with FITC-Annexin V in platelets isolated from healthy (HS) and diabetic (DM), and mitochondrial membrane potential (ΔΨm) was measured by staining with 40 nM TMRM. Healthy platelets treated with 10 μM ABT-737 were used as an apoptosis control. Apoptotic platelets were characterized by PS externalization (green ring) with low ΔΨm (see arrow). (B) PS externalization was presented as mean percentage of Annexin V positive platelets ± SD (n=5). ***P<0.001 compared with HS. (C) Representative dot blots showing TMRM and Annexin V staining in HS and DM subjects. Plasma blood glucose levels were measured. (D) Release of cytochrome c (Cyto c) into the cytosol was measured by Western blot in HS and DM platelets (n=3). (E) Caspase 3 activation was assessed by immunostaining in platelets (n=3).
Since both activation and apoptosis can ensue in DM platelets, we sought to understand the role of mitochondrial dysfunction in platelet activation. Platelets were divided into high Δψm (red population) and low Δψm (blue population) according to TMRM fluorescence using flow cytometry. Platelet activation, in response to 1 μM ADP, can be demonstrated by an increase in size and shape change of the cell population to a narrower elongated shape33. Platelet activation was observed in the red population of platelets incubated with either 5mM or 15 mM glucose and DM platelets, suggesting that platelet with high Δψm contributes to the platelet activation. However, ABT-737 treated platelets exhibited majority of blue population, so the platelet activation in the red population was not found (Supp Fig 7A). In addition, increased P-selectin expression as marker of platelet activation was found in platelets incubated with either 5mM or 15 mM glucose but not in ABT-737 treated platelets (Supp Fig 7B). These findings indicate distinct pools of platelets arising from hyperglycemia. Depending on the severity of the mitochondrial damage caused by hyperglycemia, platelets with a large decrease in Δψm does not respond to agonist stimulation (apoptotic platelets), but those with mild (active platelets) or no decrease in Δψm (normal platelets) were able to respond to ADP stimulation. Thus mitochondrial dysfunction appears to play a central role in determining platelet activation or apoptosis under conditions of hyperglycemia.
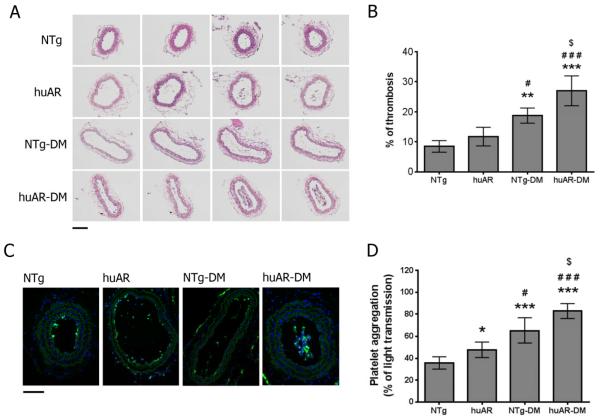
Diabetes-induced platelet hyperreactivity leads to arterial thrombosis
It is well established that DM platelets can be hyperactive, as demonstrated by our aggregation studies in human platelets (Supp Fig 8). Previous studies have shown that increased platelet apoptosis, as indicated by increased phosphatidylserine externalization, leads to increased procoagulant activity, which enhances thrombosis in rat34. Moreover apoptosis induces increased platelet turnover and the ensuing larger younger platelets are more active35, further contributing to increased thrombosis. To further study the role of AR in platelet function in vivo, the carotid artery ligation was performed in our mouse model, and the thrombus formation was assessed by H&E and immunofluorescent staining. After 7-day carotid artery ligation (blood stasis model), thrombus formation was increased under DM conditions, particularly in the presence of huAR (Figure 7A–B). Platelet deposition, as determined by platelet marker CD41 staining, was also increased in the huAR mice compared to NTg mice (Fig 7C). Under non-DM conditions, thrombus formation was not significantly increased in huAR mice compared to NTg mice. Platelets from DM mice showed increased aggregation in response to 10 μg/ml of collagen, and platelet aggregation was increased in huAR mice compared to NTg mice (Figure 7D). These results support that huAR expression contributes to DM-induced platelet hyperreactivity and apoptosis, leading to the increased arterial thrombosis.
Figure 7.
Aldose reductase exacerbates thrombus formation after carotid artery ligation in diabetic mice. (A) H&E staining of serial cross sections from a ligated carotid artery from non-transgenic (NTg) and huAR-transgenic (huAR) mice (with or without DM) after 7 days (upper panel; bar=10 μm; n=4). (B) The percentage of thrombosis in the ligated carotid artery was calculated by dividing the area of thrombus by the area of the vessel lumen (area of thrombus/area of lumen from multiple contiguous sections). Data was presented as mean ± SD (n=4 mice). ***P<0.001 & **P<0.01 compared with NTg; ###P<0.001 & #P<0.05 compared with huAR; $P<0.05 compared with NTg-DM. (C) Platelet deposition in thrombus after carotid artery ligation was assessed by the immunohistochemical detection of platelets using FITC-CD41 antibody in NTg and huAR mice. Nucleated cells were visualized by DAPI staining (lower panel; bar=10 μm; n=4). (D) Aggregation of mouse platelet was measured by light transmission aggregometer in response to 10 μg/ml collagen. Data was presented as mean ± SD (n=4). ***P<0.001 & *P<0.05 compared with NTg; ###P<0.001 & #P<0.05 compared with huAR; $P<0.05 compared with NTg-DM.
Discussion
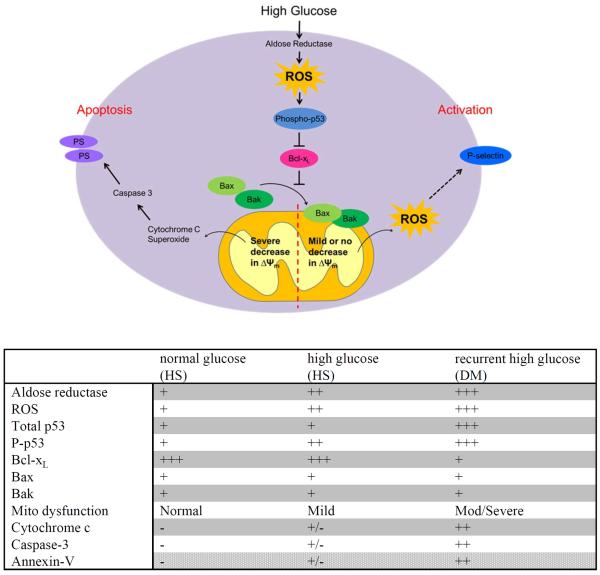
We demonstrate for the first time that AR-mediated p53 phosphorylation contributes to Δψm dissipation, MPTP activation, and mitochondrial dysfunction, damage and rupture in DM platelets (Fig 8). This can result in Caspase-3 activation and PS externalization in DM human platelets, features typical of apoptosis. These mechanistic studies provide new insights into platelet abnormalities and potential therapy (e.g. AR inhibitors) in DM to prevent cardiovascular complications.
Figure 8.
The proposed mechanism for the mitochondrial dysfunction in diabetic platelets. Hyperglycemia-induced oxidative stress via AR activation leads to phosphorylation of p53, inhibiting the anti-apoptotic function of Bcl-xL, thereby increasing the mitochondrial transition pore (MPTP) formation and dissipating membrane potential and mitochondrial dysfunction or damage. This will in turn activate caspase-3, leading to platelet apoptosis when severe, or platelet activation when mild. A table is presented which summarizes the signaling component changes under each condition; normal glucose in healthy subjects, high glucose (healthy subjects), and recurrent high glucose (DM subjects).
We were surprised to discover that p53 played an important role in hyperglycemia-mediated platelet mitochondria dysfunction. In addition to its role in transcription, p53 has been demonstrated to have a direct apoptogenic role at the mitochondria19. In the present study, hyperglycemia-induced phosphorylation of p53 at serine 15 was also found to contribute to mitochondrial dysfunction, which was significantly attenuated by treatment with ARI and antioxidant NAC. ROS is an early event in p53-induced mitochondrial damage36–38. AR activity can induce the phosphorylation of p38α MAPK by increasing oxidative stress under hyperglycemic condition9, 39, 40, leading to phosphorylation of p5328, 31. Consistent with these studies, our results showed that pharmacological inhibition of p38α MAPK attenuated the phosphorylation of p53 under hyperglycemia, indicating that AR activity contributes to phosphorylation of p53 through the oxidative stress and activation of p38α MAPK.
Bcl-xL plays a central role in regulating platelet survival13. Moreover, loss of Bcl-xL results in thrombocytopenia41. Consistent with the previous studies that Bcl-xL can be downregulated in various DM tissues42–44, we also found that the expression of Bcl-xL was reduced in DM platelets but not in acute hyperglycemia. Our study and another29 now demonstrate that p53 phosphorylated at serine 15 can translocate into mitochondria, and sequester Bcl-xL, leading to dissipation of Δψm. These results in human platelets showed that AR contributed to p53 phosphorylation and its binding to Bcl-xL through hyperglycemia-induced oxidative stress, consequently leading to mitochondrial damage (Fig 8).
It has been previously suggested that platelet activation and apoptosis are closely related, as Δψm dissipation, caspase-3 activation, and PS externalization were found with thrombin-stimulated platelets45. Previous studies showed that Bax activation (a pro-apoptotic protein that physically targets mitochondria)46 generates a subpopulation of highly activated platelets, suggesting that mitochondria and Bcl-2 family proteins participate in the process of platelet activation and mitochondrial damage and apoptosis. Therefore, it is plausible that mitochondrial alteration as well as the resultant apoptotic signaling (caspase-3 activation & PS externalization) is triggered in DM platelets, contributing to thrombus formation. Indeed, patients with type 2 DM exhibit platelet hyperreactivity both in vitro and in vivo, coupled with biochemical evidence of persistently increased thromboxane-dependent platelet activation47, accelerated turnover of platelets48, increased platelet apoptosis and excessive thrombus formation49. Consistent with such studies, we provide evidence that both platelet activation and apoptosis ensue in DM platelets, and these two distinct processes are dependent on the activity of AR as well as the resultant mitochondrial damage. Phosphatidylserine (PS) externalization promotes thrombin formation 50, a marker for procoagulant activity. Moreover, with increased apoptosis, increasing number of younger platelets is released and these larger younger platelets are more active35. Taken together, the present findings suggest that both platelet activation and apoptosis synergistically contributes to increased thrombosis in DM.
Currently, over 19.7 million adults (8.3%) in the USA have diagnosed DM and 38.2% of the USA adult population has prediabetes51. Sixty five percent of patients with DM will die from thrombotic cardiovascular events where platelets play key roles52. Of great concern is that 10 to 40% of DM patients are biochemically resistant to aspirin, the most commonly used drug to prevent and treat heart attacks and strokes52, 53. New therapies targeting the underlying mechanism for the platelet dysfunction are urgently warranted. Based upon our studies we now propose that drugs targeting platelet AR/ROS/p53 may be effective against hyperglycemic induced platelet dysfunction.
Study Limitations
The inherent fragile nature of platelets, particularly those from DM patients required that studies be performed within a few hours of blood acquisition and thus many DM patient recruits were required. However, the reproducibility and consistency from the use of 66 heterogeneous DM patient patients adds strength to our conclusions. Inherent differences are known to exist between mouse and human platelets, particularly as mouse lack AR. However, conferring p53 induction and mitochondrial sensitivity by overexpressing human AR supports the importance of this hyperglycemia-induced signaling mechanism.
Supplementary Material
Acknowledgements
We wish to thank Silvio Inzucchi M.D. and Robert Sherwin M.D. for assistance in patient recruitment.
Funding Sources: This study was supported in part by NIH grants to JH (HL074190, HL115247, HL117798), a T32 training fellowship to JS, Browne-Cox Fellowship to RL, and AHA Fellowships to WHT and SG.
Footnotes
Conflict of Interest Disclosure: None.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997;46(Suppl 2):S101–103. doi: 10.2337/diab.46.2.s101. [DOI] [PubMed] [Google Scholar]
- 3.Winocour PD. Platelet abnormalities in diabetes mellitus. Diabetes. 1992;41(Suppl 2):26–31. doi: 10.2337/diab.41.2.s26. [DOI] [PubMed] [Google Scholar]
- 4.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 5.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high atp content and low mitochondrial membrane potential. Platelets. 2009;20:588–593. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 7.Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes. 2012;120:248–251. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WH, Stitham J, Gleim S, Di Febbo C, Porreca E, Fava C, Tacconelli S, Capone M, Evangelista V, Levantesi G, Wen L, Martin K, Minuz P, Rade J, Patrignani P, Hwa J. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest. 2011;121:4462–4476. doi: 10.1172/JCI59291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagishi S, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 11.Vanags DM, Orrenius S, Aguilar-Santelises M. Alterations in bcl-2/bax protein levels in platelets form part of an ionomycin-induced process that resembles apoptosis. Br J Haematol. 1997;99:824–831. doi: 10.1046/j.1365-2141.1997.4813284.x. [DOI] [PubMed] [Google Scholar]
- 12.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. Abt-263: A potent and orally bioavailable bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 13.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, Iciek LA, Morgan SJ, Nasarre MC, Nelson R, Preusser LC, Reinhart GA, Smith ML, Rosenberg SH, Elmore SW, Tse C. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 15.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O'Reilly LA, Henley KJ, Ono A, Hsiao S, Willcox A, Roberts AW, Huang DC, Salem HH, Kile BT, Jackson SP. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 16.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz C, Leuschen NV, Frohlich T, Lorenz M, Pfeiler S, Gleissner CA, Kremmer E, Kessler M, Khandoga AG, Engelmann B, Ley K, Massberg S, Arnold GJ. Identification of novel downstream targets of platelet glycoprotein vi activation by differential proteome analysis: Implications for thrombus formation. Blood. 2010;115:4102–4110. doi: 10.1182/blood-2009-07-230268. [DOI] [PubMed] [Google Scholar]
- 18.Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- 19.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. P53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 20.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DCS, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Leung AWC, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Bba-Bioenergetics. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 23.Chen YB, Aon MA, Hsu YT, Soane L, Teng XC, McCaffery JM, Cheng WC, Qi B, Li HM, Alavian KN, Dayhoff-Brannigan M, Zou SF, Pineda FJ, O'Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-x(l) regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lago CU, Sung HJ, Ma WZ, Wang PY, Hwang PM. P53, aerobic metabolism, and cancer. Antioxid Redox Sign. 2011;15:1739–1748. doi: 10.1089/ars.2010.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo XM, Wu JJ, Du JJ, Ran JM, Xu J. Platelets of type 2 diabetic patients are characterized by high atp content and low mitochondrial membrane potential (vol 20, pg 588, 2009) Platelets. 2010;21:84–84. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 26.Long X, Goldenthal MJ, Marin-Garcia J. Oxidative stress enhances phosphorylation of p53 in neonatal rat cardiomyocytes. Mol Cell Biochem. 2007;303:167–174. doi: 10.1007/s11010-007-9470-1. [DOI] [PubMed] [Google Scholar]
- 27.Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M, Dai W. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–36199. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 28.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 29.Park BS, Song YS, Yee SB, Lee BG, Seo SY, Park YC, Kim JM, Kim HM, Yoo YH. Phospho-ser 15-p53 translocates into mitochondria and interacts with bcl-2 and bcl-xl in eugenol-induced apoptosis. Apoptosis. 2005;10:193–200. doi: 10.1007/s10495-005-6074-7. [DOI] [PubMed] [Google Scholar]
- 30.Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 31.She QB, Chen NY, Dong ZG. Erks and p38 kinase phosphorylate p53 protein at serine 15 in response to uv radiation. J Biol Chem. 2000;275:20444–20449. doi: 10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- 32.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halene S, Gao YA, Hahn K, Massaro S, Italiano JE, Schulz V, Lin S, Kupfer GM, Krause DS. Serum response factor is an essential transcription factor in megakaryocytic maturation. Blood. 2010;116:1942–1950. doi: 10.1182/blood-2010-01-261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae ON, Lim KM, Noh JY, Chung SM, Kim SH, Chung JH. Trivalent methylated arsenical-induced phosphatidylserine exposure and apoptosis in platelets may lead to increased thrombus formation. Toxicol Appl Pharmacol. 2009;239:144–153. doi: 10.1016/j.taap.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong JF, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:743–749. doi: 10.1016/j.jacc.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Li PF, Dietz R, von Harsdorf R. P53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by bcl-2. Embo J. 1999;18:6027–6036. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 38.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 39.Price SA, Agthong S, Middlemas AB, Tomlinson DR. Mitogen-activated protein kinase p38 mediates reduced nerve conduction velocity in experimental diabetic neuropathy: Interactions with aldose reductase. Diabetes. 2004;53:1851–1856. doi: 10.2337/diabetes.53.7.1851. [DOI] [PubMed] [Google Scholar]
- 40.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase c and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 41.Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Li W, Miyagi T, Hosui A, Tatsumi T, Ishida H, Kanto T, Hiramatsu N, Yin XM, Hayashi N. Bh3-only activator proteins bid and bim are dispensable for bak/bax-dependent thrombocyte apoptosis induced by bcl-xl deficiency: Molecular requisites for the mitochondrial pathway to apoptosis in platelets. J Biol Chem. 2011;286:13905–13913. doi: 10.1074/jbc.M110.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of langerhans: A potential role for regulation of specific bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 43.Kern TS, Du Y, Miller CM, Hatala DA, Levin LA. Overexpression of bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol. 2010;176:2550–2558. doi: 10.2353/ajpath.2010.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: Influence of subcellular spatial location. Am J Physiol Heart Circ Physiol. 2010;298:H633–642. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006;4:2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- 46.Dale GL, Friese P. Bax activators potentiate coated-platelet formation. J Thromb Haemost. 2006;4:2664–2669. doi: 10.1111/j.1538-7836.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- 47.Davi G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, Patrono C. Thromboxane biosynthesis and platelet function in type ii diabetes mellitus. N Engl J Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 48.Tschoepe D, Roesen P, Esser J, Schwippert B, Nieuwenhuis HK, Kehrel B, Gries FA. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost. 1991;17:433–438. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 49.Zbidi H, Redondo PC, Lopez JJ, Bartegi A, Salido GM, Rosado JA. Homocysteine induces caspase activation by endoplasmic reticulum stress in platelets from type 2 diabetics and healthy donors. Thromb Haemostasis. 2010;103:1022–1032. doi: 10.1160/TH09-08-0552. [DOI] [PubMed] [Google Scholar]
- 50.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 51.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798–813. doi: 10.1161/CIRCULATIONAHA.109.913376. [DOI] [PubMed] [Google Scholar]
- 53.Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS. Aspirin for primary prevention of cardiovascular events in people with diabetes: A position statement of the american diabetes association, a scientific statement of the american heart association, and an expert consensus document of the american college of cardiology foundation. Circulation. 2010;121:2694–2701. doi: 10.1161/CIR.0b013e3181e3b133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.