Abstract
Cardiac conduction is the process by which electrical excitation is communicated from cell to cell within the heart, triggering synchronous contraction of the myocardium. The role of conduction defects in precipitating life-threatening arrhythmias in various disease states has spurred scientific interest in the phenomenon. While the understanding of conduction has evolved greatly over the last century, the process has largely been thought to occur via movement of charge between cells via gap junctions. However, it has long been hypothesized that electrical coupling between cardiac myocytes could also occur ephaptically, without direct transfer of ions between cells. This review will focus on recent insights into cardiac myocyte intercalated disk ultrastructure and their implications for conduction research, particularly the ephaptic coupling hypothesis.
Keywords: Perinexus, Gap Junction, Connexin, Sodium Channel, Nav1.5, Ephaptic, Intercalated Disk, Conduction
Cardiac conduction is the process by which electrical excitation is communicated from cell to cell within the heart, triggering the synchronous contraction of the myocardium. Since being first demonstrated by Engelmann in 1874,(20) conduction has been the subject of intense scientific inquiry. Interest in the phenomenon stems mainly from the link between aberrant conduction and potentially lethal arrhythmias in a variety of pathologies.
Historical background
The current understanding of conduction is based largely upon the core conductor model.(45) The roots of this theoretical paradigm can be traced back to the application of continuous cable theory to cardiac conduction by Silvio Weidmann in the 1950s.(75) Subsequent experimental results, while numerous, have largely fit into the framework of this model, which envisions conduction as having two functional components: Membrane excitability and intercellular coupling. Membrane excitability, or the ability of an excitable membrane to depolarize in response to a given stimulus, is thought to be the province of membrane ion channels, particularly voltage-gated sodium channels. Intercellular coupling is seen as occurring via the passive, electrotonic flow of positive charge between cells via low resistance pathways afforded by gap junction (Gj) channels. However, emerging experimental evidence suggests that this view, while perhaps tidy, may not offer a complete and accurate description of cardiac conduction. For a detailed discussion of the electrotonic model of cardiac conduction, the reader is referred to the previous reviews by Spach et al.(58) and Kleber & Rudy(29).
The challenge to the electrotonic model of cardiac conduction comes in the form of ephaptic coupling, a process by which electrical excitation is communicated between cells via an extracellular electric field or ion accumulation/depletion without involvement of Gjs.(61, 65) This mechanism, known to occur in other excitable tissues such as the brain, the retina and the uterine myometrium,(28, 74, 76) has long been hypothesized to play a role in cardiac conduction by Dr. Nicholas Sperelakis and others.(12, 33, 40, 42, 63, 64) However, the lack of direct experimental evidence and a well-defined functional unit, i.e. an ephapse, has meant that the investigation of ephaptic coupling has remained almost exclusively the province of mathematical models. In this article, we will focus emerging evidence for ephaptic coupling in the heart and their theoretical implications - in particular, new functions for Gjs and voltage-gated sodium channels, blurring the boundary between excitability and intercellular coupling.
Intercellular coupling: Gap junctions and beyond
Over the last century, our understanding of cardiac intercellular coupling has gone through a series of revisions. In the early days of conduction research, the cytoplasms of cardiac myocytes were thought to be contiguous, thus accounting for electrical coupling. However, with the identification of high resistance membrane bounding each myocyte,(56) it was postulated that there had to exist low resistance pathways coupling neighboring myocytes.(13) Using electron microscopy to study the intercalated disk at high resolution, Dewey, Sjostrand and Andersson suggested that it may constitute a connecting surface between myocytes.(55) Subsequently, in the early 1960's, using electron microscopy, Lloyd Barr and colleagues identified 'fused membrane' structures connecting adjacent myocytes, which they dubbed the nexus.(18) Around the same time, Van der Kloot and Dane proposed the intercalated disk as the likely site of low resistance electrical contact between myocytes(72); shortly thereafter, Barr, Dewey and Berger provided direct evidence of the nexus’s involvement in conduction(5). In 1967, Revel and Karnovsky demonstrated the nexus to be membranes separated by a gap rather than fused and coined the term ‘gap junctions’.(46) The resistance of Gj was initially considered to be low enough to render coupled myocytes electrically continuous, thus conferring a syncitial nature upon the myocardium. However, experimental studies of action potential propagation at high temporal resolution revealed Gj resistance to be high enough to render cardiac conduction discontinuous at the cellular level.(60)
Cardiac Gjs have long been recognized to undergo remodeling in developmental(3, 23) and disease scenarios(36, 43, 57). In this regard, one key question has been the precise relationship between the degree of Gj uncoupling and the resulting level of conduction slowing. While conduction slowing in response to pharmacological uncoupling has been well characterized,(4, 7, 15, 17, 26, 30, 52) the electrophysiological impact of pathophysiologic Gj remodeling is less clear.(2, 8) Experiments in transgenic mice with 50% reduced expression of connexin43 (Cx43), the principal ventricular Gj protein, have yielded mixed results: Some studies reported slower conduction compared to wild-type (WT) littermates(19, 24) while others found no difference.(6, 41, 67, 68, 70, 71) Even more perplexingly, conduction, albeit slowed and susceptible to failure, still occurs in mice with a cardiac-specific conditional knockout of Cx43 resulting in a severe (>80%) loss of Cx43.(14)
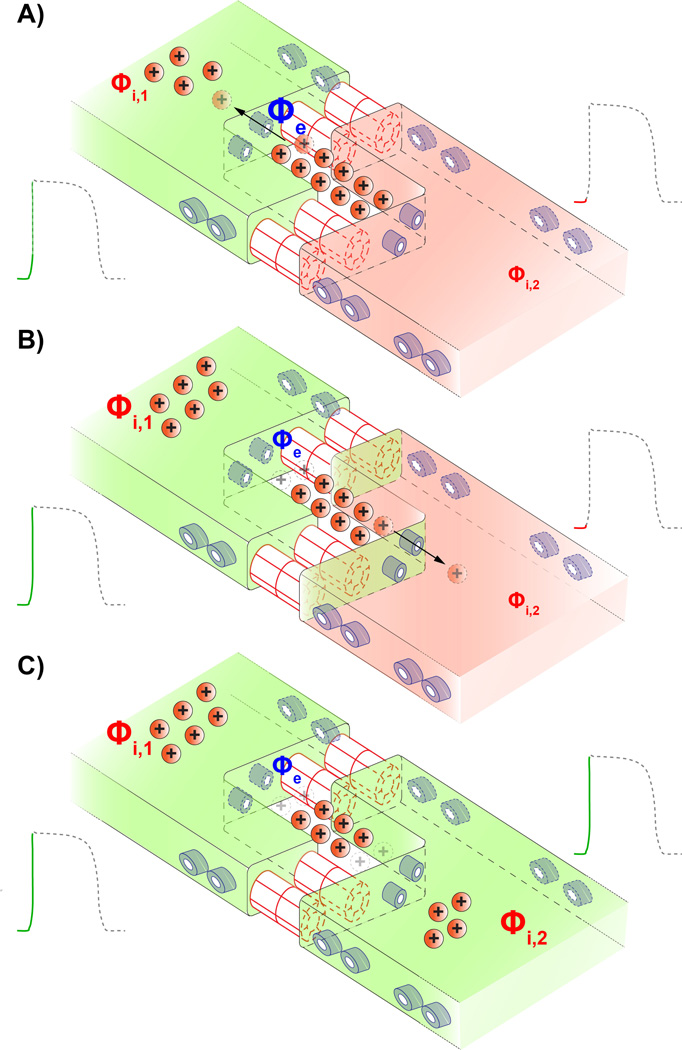
All these findings point back to a question first posed by Dr. Sperelakis during the 1960's(62): Can ephaptic coupling sustain cardiac conduction in the absence of Gjs? While initially viewed as an alternative to Gj coupling, more recent in silico studies have suggested the possibility of so-called mixed-mode coupling involving both mechanisms.(31–33, 40) These models envision intercellular coupling as occurring as follows: A depolarized myocyte withdraws sodium ions from the restricted junctional cleft via its intercalated disk-localized Nav1.5 channels (figure 1A). The resulting depletion of positive charge from the junctional cleft would render the local extracellular electrical potential more negative. Consequently, the transmembrane potential across the apposed membrane of the neighboring myocyte becomes more positive, causing the activation of Nav1.5 channels (figure 1B). Thus electrical activation is communicated from myocyte to myocyte without direct transfer of ions between them (figure 1C). Based on this view, the models almost unanimously predict that ephaptic coupling would require that:
the membranes of adjacent myocytes are closely apposed (< 10 nm apart) and,
the closely apposed membranes are rich in cardiac sodium channels (Nav1.5).(12, 31– 33, 40, 63, 64, 66)
Figure 1.
Schematic cartoon illustrating the mechanism of ephaptic coupling. A) Sodium channels (shown in blue) on the depolarized myocyte's membrane activate, withdrawing positively charged sodium ions (Na+) from the restricted extracellular cleft at the intercalated disk. This raises the intracellular potential (Φi,1) of the first myocyte. B) Concomitantly, the depletion of positive charge from the restricted extracellular cleft lowers the local extracellular potential (Φe). There is a resultant increase in the transmembrane potential across the membrane of the second myocyte which is defined as the difference between its intracellular potential (Φi,2) and the extracellular potential (Φe). In turn sodium channels located at or near the intercalated disk of the second myocyte activate. C) Entry of sodium ions into the second myocyte via its sodium channels further depolarize it, triggering an action potential. Thus activation is communicated 'ephaptically' from cell-to-cell without the direct transfer of ions between them.
Ion Channels at the Intercalated Disk: Functional Implications
Recent insights into the ultrastructural organization of ion channels within cardiac myocytes have sparked interest in the ephaptic coupling hypothesis, particularly when interpreted in the context of the aforementioned model predictions. The first evidence that cardiac sodium channels are preferentially localized at the intercalated disks of cardiac myocytes came in 1996, when Dr. Sidney Cohen published immunofluorescence images of rat TTX-resistant sodium channels (rH1)(11). Since then there has been mounting evidence for the intercalated disk localization of ion channels, long predicted by mathematical models as a requirement for ephaptic coupling.(12, 31, 39, 40, 61, 65, 77) Since then, other studies have recapitulated the preferential localization of cardiac sodium channels (Nav1.5) to the intercalated disk.(31, 37) More importantly, Dr. Kucera and colleagues demonstrated in a 1D strand model of cardiac conduction that the high density of sodium channels at the intercalated disk could impact cardiac conduction in previously un-appreciated ways: Specifically, they concluded that Gj are still likely the principal mechanism of electrical transmission between cells, however, sodium channels at the intercalated disk could couple myocytes ephaptically, particularly when Gj coupling is compromised.
Since then a more detailed picture of proteins at the intercalate disk has emerged, revealing the existence of a macromolecular complex containing Cx43, cardiac sodium channels (Nav1.5) and various cytoskeletal proteins. Cx43 and Nav1.5 have been demonstrated to co-immunoprecipitate from mouse heart lysates(38) and to colocalize at the intercalated disk(44). Dr. Mario Delmar and colleagues have provided evidence suggesting that Cx43 and Nav1.5 participate in a macromolecular complex at the intercalated disk which includes the desmosomal protein plakophilin-2 (PKP2) as well as ankyrin-G, a sub-membrane adapter protein involved in localizing cardiac sodium channels (Nav1.5) in the membrane. In primary cultures of neonatal rat ventricular myocytes, they found that Cx43 gap junctions and ankyrin-G (AnkG) are recruited to sites of cell-to-cell contact following the localization of mechanical adhesion proteins(22). Subsequently, they demonstrated regulation of the sodium current by both PKP2 and AnkG(10, 53, 54) and mutations in both proteins have been associated with Brugada syndrome, an inherited arrhythmia syndrome characterized by decreased sodium current density.(9, 25) Recently, Dr. Delmar and colleagues reported loss of Nav1.5 from the membrane in conditional Cx43 knockout mice(27) and suggested that Cx43 play a role in the recruitment of Nav1.5 channels into the intercalated disk membrane.(1, 16) Further support for this hypothesis comes from their observation of decreased sodium and potassium current levels as well as loss of Nav1.5 from the intercalated disk without any concomitant loss of Gj coupling in mice lacking the last 5 C-terminal amino acids of Cx43.(35) In all, emerging evidence both structural(44) and functional(34) suggests the existence of two distinct pools of Nav1.5 located at the intercalated disk and at the lateral membrane.
These observations resonate with computer models which suggest that intercalated disk localized sodium channels may be involved in ephaptic coupling(31–33), while those on the lateral membrane are important for maintaining the stability of conduction(69), particularly when Gj coupling is reduced. In all there is mounting support for the hypothesis that microdomains could exist within the intercalated disk that have the necessary density of sodium channels for ephaptic coupling to occur. Close apposition between cell membranes, the other criterion for ephaptic coupling predicted by models, could help identify specific structures that might function as a cardiac ephapse.
Ephaptic coupling: experimental traces
The functional observations suggesting a role for ephaptic coupling in the heart come from investigation of conduction dependence on interstitial volume. Under the electrotonic view of conduction, the interstitial space provides the return path for electrical current flowing between myocytes, thus completing the circuit. Based on this notion, conduction velocity should be directly proportional to interstitial volume,(45, 59) and observations in cable-like papillary muscle have been consistent with this notion.(21) However, we recently demonstrated that increasing interstitial volume in a heart slows conduction and vice versa, i.e. an inverse relationship between conduction velocity and interstitial volume(73): These observations are inconsistent with a purely electrotonic view of cardiac conduction. Additionally, we found that levels of Gj uncoupling too small to alter conduction normally, significantly slowed conduction when the interstitial volume was increased. These findings are consistent with the hypothesis that increased interstitial volume impairs ephaptic coupling, thus slowing conduction and increasing its dependence on Gj coupling. These results underscore the importance of interstitial volume, i.e. the spacing between membranes of adjacent myocytes, as a determinant of conduction and offer further impetus for a critical reassessment of the role ephaptic coupling may play in cardiac conduction. Additionally, they dovetail with the predictions made by computer models of ephaptic coupling.(31, 32, 40) However, as previously stated, any attempt to directly assess whether ephaptic coupling plays a role in cardiac conduction must first contend with the question of its structural underpinnings. In other words, a functional unit of ephaptic coupling, an ephapse, will need to be identified.
Ultrastructural breadcrumbs leading to the ephapse?
Taking together the aforementioned structural and functional insights in the context of the in silico predictions, it could be hypothesized that the cardiac ephapse is likely to be a microdomain within the intercalated disk with a high density of cardiac sodium channels (Nav1.5) and close apposition between the membranes of adjacent myocytes. One structure that emerges as a promising candidate for the ephapse is the perinexus - a specialized membrane microdomain surrounding Gj plaques and rich in undocked connexon hemichannels.(47, 48) While previous studies identified the interaction between Cx43 and Nav1.5 at the intercalated disk(35, 37, 38, 53), proximity ligation assays (PLA - e.g., Duolink) of protein-protein association enabled imaging of Cx43-Nav1.5 interaction at the perinexus.(49, 50) By virtue of its location at the periphery of Gj plaques, the perinexus features close apposition between the membranes of adjacent myocytes.(51) This feature, together with the focal concentrations of sodium channels generated by a scaffold that includes Cx43 hemichannels, indicates that the perinexus potentially meets both criteria identified by mathematical models to function as an ephapse between cardiac myocytes.
Further studies utilizing emerging modalities such as superresolution microscopy and the PLA interaction assay, combined with the high resolution of electron microscopy will be critical to fleshing out the structure of the macromolecular complex located at the intercalated disk. As the constituents of this complex are identified, functional experiments in intact myocardium will be needed in order to elucidate their roles in forming and maintaining the machinery of cardiac conduction. And, identifying the ephapse will only be the first step in formulating a new theory of cardiac conduction. Fuller appreciation of the role of ephaptic coupling, will require a two-pronged approach: a) Further experiments to investigate the relationship between the molecular ultrastructure of the intercalated disk and the electrophysiology of the heart.; and b) Structurally detailed, multi-dimensional mathematical models which incorporate ephaptic coupling to probe the mechanisms underlying the new experimental observations.
Conduction: A new multi-factorial understanding
Continuing the trend of the last 130 years, our understanding of cardiac conduction appears to be on the verge of yet another revision – and perhaps a shift in paradigm. The primary driver of this change is the new picture that we are obtaining of the intercalated disk - the prime locus of the machinery of cardiac conduction. With respect to its role in cellular contact and communication, the intercalated disk is an intricate, dynamically regulated machine rather than a simple, naïve structure. Continuing a trend that began a decade or so ago, we are beginning to see connexins not just as channels, but as multi-functioned constituents of macromolecular complexes, heralding a new chapter in the biology of these molecules. As we understand myocyte structure at finer and finer resolution, so are we also are beginning to appreciate the importance of biophysical phenomena that occur at the scale of nanometers. The erosion of existing conceptual boundaries, such as that between tissue excitability and intercellular coupling, is leading to a new, multi-factorial view of conduction where the same molecules appear to play several roles and multiple mechanisms work in tandem to achieve a single function. This deeper understanding could help explain the processes underlying pathological conduction defects and open up novel avenues for therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agullo-Pascual E, Lin X, Pfenniger A, Lubkemeier I, Willecke K, Rothenberg E, Delmar M. A novel noncanonical role of cx43 in the heart: ensuring the arrival of nav1.5 to the intercalated disk. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:1742. [Google Scholar]
- 2.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circulation research. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 3.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circulation research. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- 4.Balke CW, Lesh MD, Spear JF, Kadish A, Levine JH, Moore EN. Effects of cellular uncoupling on conduction in anisotropic canine ventricular myocardium. Circulation research. 1988;63:879–892. doi: 10.1161/01.res.63.5.879. [DOI] [PubMed] [Google Scholar]
- 5.Barr L, Dewey MM, Berger W. Propagation of Action Potentials and the Structure of the Nexus in Cardiac Muscle. The Journal of general physiology. 1965;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circulation research. 2004;95:170–178. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 7.Callans DJ, Moore EN, Spear JF. Effect of coronary perfusion of heptanol on conduction and ventricular arrhythmias in infarcted canine myocardium. Journal of cardiovascular electrophysiology. 1996;7:1159–1171. doi: 10.1111/j.1540-8167.1996.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Cascio WE, Yang H, Muller-Borer BJ, Johnson TA. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. Journal of electrocardiology. 2005;38:55–59. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko-Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, Rothenberg E, Chen HS, Napolitano C, Priori S, Delmar M. Missense Mutations in Plakophilin-2 Cause Sodium Current Deficit and Associate with a Brugada Syndrome Phenotype. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, van Rijen HV, Delmar M. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovascular research. 2012;95:460–468. doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. doi: 10.1161/01.cir.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 12.Copene ED, Keener JP. Ephaptic coupling of cardiac cells through the junctional electric potential. Journal of mathematical biology. 2008;57:265–284. doi: 10.1007/s00285-008-0157-3. [DOI] [PubMed] [Google Scholar]
- 13.Crill WE, Rumery RE, Woodbury JW. Effects of membrane current on transmembrane potentials of cultured chick embryo heart cells. The American journal of physiology. 1959;197:733–735. doi: 10.1152/ajplegacy.1959.197.4.733. [DOI] [PubMed] [Google Scholar]
- 14.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circulation research. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JP, Wilms-Schopman FJ, Wiegerinck RF, Bourier J, Belterman CN, Coronel R, Verheijck EE. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovascular research. 2003;60:288–297. doi: 10.1016/j.cardiores.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Delmar M. Connexin43 regulates sodium current; ankyrin-G modulates gap junctions: the intercalated disc exchanger. Cardiovascular research. 2012;93:220–222. doi: 10.1093/cvr/cvr343. [DOI] [PubMed] [Google Scholar]
- 17.Delmar M, Michaels DC, Johnson T, Jalife J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circulation research. 1987;60:780–785. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- 18.Dewey MM, Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962;137:670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- 19.Eloff BC, Gilat E, Wan X, Rosenbaum DS. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- 20.Engelmann TW. Ueber die Leitung der Erregung im Herzmuskel. Pflüger, Arch. 1875;11:465–480. [Google Scholar]
- 21.Fleischhauer J, Lehmann L, Kleber AG. Electrical resistances of interstitial and microvascular space as determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation. 1995;92:587–594. doi: 10.1161/01.cir.92.3.587. [DOI] [PubMed] [Google Scholar]
- 22.Geisler SB, Green KJ, Isom LL, Meshinchi S, Martens JR, Delmar M, Russell MW. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes. Journal of biomedicine & biotechnology. 2010;2010:624719. doi: 10.1155/2010/624719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gourdie RG, Green CR, Severs NJ, Thompson RP. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anatomy and embryology. 1992;185:363–378. doi: 10.1007/BF00188548. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. The Journal of clinical investigation. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. Journal of molecular and cellular cardiology. 2009;47:203–209. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalife J, Sicouri S, Delmar M, Michaels DC. Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. The American journal of physiology. 1989;257:H179–H189. doi: 10.1152/ajpheart.1989.257.1.H179. [DOI] [PubMed] [Google Scholar]
- 27.Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaassen LJ, Fahrenfort I, Kamermans M. Connexin hemichannel mediated ephaptic inhibition in the retina. Brain research. 2012;1487:25–38. doi: 10.1016/j.brainres.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 29.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiological reviews. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 30.Kojodjojo P, Kanagaratnam P, Segal OR, Hussain W, Peters NS. The effects of carbenoxolone on human myocardial conduction: a tool to investigate the role of gap junctional uncoupling in human arrhythmogenesis. Journal of the American College of Cardiology. 2006;48:1242–1249. doi: 10.1016/j.jacc.2006.04.093. [DOI] [PubMed] [Google Scholar]
- 31.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circulation research. 2002;91:1176–1182. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Keener JP. Ephaptic coupling in cardiac myocytes. IEEE transactions on bio-medical engineering. 2013;60:576–582. doi: 10.1109/TBME.2012.2226720. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Keener JP. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20935–20940. doi: 10.1073/pnas.1010154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1923–1930. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubkemeier I, Requardt RP, Lin X, Sasse P, Andrie R, Schrickel JW, Chkourko H, Bukauskas FF, Kim JS, Frank M, Malan D, Zhang J, Wirth A, Dobrowolski R, Mohler PJ, Offermanns S, Fleischmann BK, Delmar M, Willecke K. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic research in cardiology. 2013;108:348. doi: 10.1007/s00395-013-0348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luke RA, Saffitz JE. Remodeling of ventricular conduction pathways in healed canine infarct border zones. The Journal of clinical investigation. 1991;87:1594–1602. doi: 10.1172/JCI115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. The Journal of biological chemistry. 2004;279:40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 39.Medvinskii AB, Pertsov AM. Fiber interaction during impulse propagation in smooth muscle and myocardial tissues. Electrotonic interaction. Biofizika. 1979;24:135–140. [PubMed] [Google Scholar]
- 40.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. Journal of cardiovascular electrophysiology. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 42.Pertsov AM, Medvinskii AB. Effect of specialized contacts on fiber interaction during the spread of excitation in smooth muscle and myocardial tissues. Biofizika. 1979;24:293–298. [PubMed] [Google Scholar]
- 43.Peters NS, Rowland E, Bennett JG, Green CR, Anderson RH, Severs NJ. The Wolff-Parkinson-White syndrome: the cellular substrate for conduction in the accessory atrioventricular pathway. European heart journal. 1994;15:981–987. doi: 10.1093/oxfordjournals.eurheartj.a060619. [DOI] [PubMed] [Google Scholar]
- 44.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circulation research. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 45.Plonsey R, Barr RC. Bioelectricity: a quantitative approach. Springer; 2007. [Google Scholar]
- 46.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. The Journal of cell biology. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhett JM, Gourdie RG. The perinexus: a new feature of Cx43 gap junction organization. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:619–623. doi: 10.1016/j.hrthm.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Molecular biology of the cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 Associates with Na(v)1.5 in the Cardiomyocyte Perinexus. The Journal of membrane biology. 2012;245:411–422. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhett JM, Palatinus JA, Jourdan JA, Gourdie RG. Connexin43 Interacts with Voltage-Gated Sodium Channel 1.5 in the Perinexus. Circulation. 2011;124:A9561. [Google Scholar]
- 51.Rhett JM, Veeraraghavan R, Poelzing S, Gourdie RG. The perinexus: Sign-post on the path to a new model of cardiac conduction? Trends in cardiovascular medicine. 2013;23:222–228. doi: 10.1016/j.tcm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circulation research. 1998;83:781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 53.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circulation research. 2011;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circulation research. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sjostrand FS, Andersson-Cedergren E, Dewey MM. The ultrastructure of the intercalated discs of frog, mouse and guinea pig cardiac muscle. Journal of ultrastructure research. 1958;1:271–287. doi: 10.1016/s0022-5320(58)80008-8. [DOI] [PubMed] [Google Scholar]
- 56.Sjostrand FS, Andersson E. Electron microscopy of the intercalated discs of cardiac muscle tissue. Experientia. 1954;10:369–370. doi: 10.1007/BF02160542. [DOI] [PubMed] [Google Scholar]
- 57.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. The American journal of pathology. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 58.Spach MS. Transition from a continuous to discontinuous understanding of cardiac conduction. Circulation research. 2003;92:125–126. doi: 10.1161/01.res.0000056973.54305.67. [DOI] [PubMed] [Google Scholar]
- 59.Spach MS, Heidlage JF, Dolber PC, Barr RC. Changes in anisotropic conduction caused by remodeling cell size and the cellular distribution of gap junctions and Na(+) channels. Journal of electrocardiology. 2001;34(Suppl):69–76. doi: 10.1054/jelc.2001.28833. [DOI] [PubMed] [Google Scholar]
- 60.Spach MS, Miller WTr, Geselowitz DB, Barr RC, Kootsey JM, Johnson EA. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circulation research. 1981;48:39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- 61.Sperelakis N. An electric field mechanism for transmission of excitation between myocardial cells. Circulation research. 2002;91:985–987. doi: 10.1161/01.res.0000045656.34731.6d. [DOI] [PubMed] [Google Scholar]
- 62.Sperelakis N. Lack of electrical coupling between contiguous myocardial cells in vertebrate hearts. Experientia Supplementum. 1969;15:135–165. doi: 10.1007/978-3-0348-6800-6_10. [DOI] [PubMed] [Google Scholar]
- 63.Sperelakis N, Mann JEJ. Evaluation of electric field changes in the cleft between excitable cells. J Theor Biol. 1977;64:71–96. doi: 10.1016/0022-5193(77)90114-x. [DOI] [PubMed] [Google Scholar]
- 64.Sperelakis N, Marschall RA, Mann JE. Propagation down a chain of excitable cells by electric field interactions in the junctional clefts: effect of variation in extracellular resistances, including a "sucrose gap" simulation. IEEE transactions on biomedical engineering. 1983;30:658–664. doi: 10.1109/tbme.1983.325068. [DOI] [PubMed] [Google Scholar]
- 65.Sperelakis N, McConnell K. Electric field interactions between closely abutting excitable cells. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2002;21:77–89. doi: 10.1109/51.993199. [DOI] [PubMed] [Google Scholar]
- 66.Sperelakis N, Ramasamy L. Modeling electric field transfer of excitation at cell junctions. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2002;21:130–143. doi: 10.1109/memb.2002.1175149. [DOI] [PubMed] [Google Scholar]
- 67.Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovascular research. 2009;83:52–60. doi: 10.1093/cvr/cvp124. [DOI] [PubMed] [Google Scholar]
- 68.Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kleber AG. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circulation research. 2003;92:1209–1216. doi: 10.1161/01.RES.0000074916.41221.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsumoto K, Ashihara T, Haraguchi R, Nakazawa K, Kurachi Y. Roles of subcellular Na+ channel distributions in the mechanism of cardiac conduction. Biophysical journal. 2011;100:554–563. doi: 10.1016/j.bpj.2010.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circulation research. 2001;88:1196–1202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- 71.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 72.Vanderkloot WG, Dane B. Conduction of the Action Potential in the Frog Ventricle. Science. 1964;146:74–75. doi: 10.1126/science.146.3640.74. [DOI] [PubMed] [Google Scholar]
- 73.Veeraraghavan R, Salama ME, Poelzing S. Interstitial Volume Modulates the Conduction Velocity-Gap Junction Relationship. American journal of physiology Heart and circulatory physiology. 2012 doi: 10.1152/ajpheart.00868.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vroman R, Klaassen LJ, Kamermans M. Ephaptic communication in the vertebrate retina. Frontiers in human neuroscience. 2013;7:612. doi: 10.3389/fnhum.2013.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weidmann S. The electrical constants of Purkinje fibres. The Journal of physiology. 1952;118:348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young RC. Myocytes, myometrium, and uterine contractions. Annals of the New York Academy of Sciences. 2007;1101:72–84. doi: 10.1196/annals.1389.038. [DOI] [PubMed] [Google Scholar]
- 77.Zemlin CW, Mironov S, Pertsov AM. Near-threshold field stimulation: intramural versus surface activation. Cardiovascular research. 2006;69:98–106. doi: 10.1016/j.cardiores.2005.08.012. [DOI] [PubMed] [Google Scholar]