Abstract
IMPORTANCE
An increasingly varied clinical spectrum of cases with amyotrophic lateral sclerosis (ALS) has been identified, and objective criteria for clinical trial eligibility is necessary.
OBJECTIVE
We sought to develop a cerebrospinal fluid (CSF) biomarker sensitive and specific for the diagnosis of ALS.
DESIGN
Case-control study.
SETTING
Academic medical center.
PARTICIPANTS
51 individuals with ALS and 23 individuals with a disorder associated with a four-repeat tauopathy (4R-tau).
MAIN OUTCOME MEASURE
CSF level of tau phosophorylated at threonine 181 (ptau), and ratio of ptau to total tau (ttau).
RESULTS
Using a cross-validation prediction procedure, we found significantly reduced CSF levels of ptau and ptau:ttau in ALS relative to 4R-tau and to controls. In the validation cohort, the receiver operating characteristic area under the curve for the ptau:ttau ratio was 0.916, and the comparison of ALS to 4R-tau showed sensitivity=92% and specificity=91.7%. Correct classification based on low CSF ptau:ttau was confirmed in 18 (85.7%) of 21 cases with autopsy-proven or genetically-determined disease. In patients with available measures, ptau:ttau in ALS correlated with clinical measures of disease severity such as Mini Mental State Exam (n=51) and ALS Functional Rating Scale-Revised (n=42), and regression analyses related ptau:ttau to MRI (n=10) evidence of disease in the corticospinal tract and white matter projections involving prefrontal cortex.
CONCLUSIONS AND RELEVANCE
CSF ptau:ttau may be a candidate biomarker to provide objective support for the diagnosis of ALS.
Keywords: amyotrophic lateral sclerosis, cerebrospinal fluid, phosphorylated tau, biomarker
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative condition with upper motor neuron (UMN) and lower motor neuron (LMN) motor deficits. ALS patients experience a rapid rate of decline over 3–5 years1. Diagnostic evaluation of ALS is aimed typically at exclusion of other disorders. Phenotypic variability has resulted in controversy about clinical stratification strategies2: Patients may show strictly LMN or UMN disease, may have disease restricted to a particular segment (e.g., bulbar) or region (e.g., flail arm), and may be strongly lateralized3,4. ALS may exhibit non-pyramidal motor system involvement, including cognitive difficulty in 33%–50%5,6 that extends to frontotemporal degeneration (FTD)7. Clinically presymptomatic ALS may exist in carriers of genetic mutations such as TARDBP8 and C9orf72 hexanucleotide repeat expansion9,10. Given these challenges in an era of disease-modifying therapies, it is critical to identify objective biomarkers of ALS during life.
ALS is considered part of the frontotemporal lobar degeneration (FTLD) spectrum of disorders. Approximately 95% of individuals with ALS have transactive DNA binding protein of ~43 kDa (TDP-43) at autopsy, and TDP-43 is also the histopathologic feature in half of FTLD11. Most of the remaining FTLD patients have hyperphosphorylated tau12. Perhaps the most common tauopathy is associated with four-repeat tau (4R-tau) in progressive supranuclear palsy (PSP). Deposition of pathologic tau is negligible in ALS, except for individuals with Guam ALS/parkinsonism which is predominantly a tauopathy13.
Tau can be assayed in cerebrospinal fluid (CSF). Studies of CSF total tau (ttau) levels in FTLD have been mixed14–22. Since CSF ttau may be elevated following any neuronal injury, assays for tau phosphorylated at threonine 181 (ptau) attempt to improve specificity. Elevated CSF ptau is found in several conditions involving tau pathology, including AD23, pathologically-confirmed FTLD-tau such as PSP24–26, and FTLD due to a genetically-determined tauopathy27. By comparison, patients with FTLD-TDP pathology as in ALS may have low CSF ptau because tau pathology is rare in these patients24.
The present study evaluated the possibility that CSF ptau is reduced in ALS, while PSP individuals likely to have tau pathology were expected to have higher CSF ptau levels. We used a cross-validation prediction procedure to assess CSF ptau as a candidate ALS biomarker. We also assessed the relationship between CSF ptau and clinical markers of disease burden such as the Functional Rating Scale-revised28. White matter (WM) neuroimaging may be valuable diagnostically in ALS29–31 and PSP32–34. To evaluate the extent of WM disease and relate this to CSF ptau, we obtained fractional anisotropy (FA) measures of WM disease in ALS.
METHODS
Clinical Evaluation
Participants
We studied 51 patients with ALS and 23 patients likely to have 4R-Tau pathology recruited from the ALS Center and the Penn FTD Center at the University of Pennsylvania. Experienced neurologists diagnosed ALS according to El Escorial-revised criteria35, with initial evaluation showing definite=7, probable=18, possible=18, and suspected=5. Three additional individuals had ALS-FTD, with co-occurring FTD diagnosed according to published clinical criteria36. Onset was bulbar=10, cervical=15, thoracic=1, and lumbosacral=22 (onset was unknown in those with FTD). Five patients had autopsy confirmation, 5 had a C9orf72 expansion, and 1 had a pathogenic TARDBP mutation (p.N390S), consistent with TDP-43 pathology. The 4R-tau cohort was comprised of patients clinically diagnosed with PSP (n=15), which is highly associated with 4R-tau pathology at autopsy37, autopsy-confirmed four-repeat tauopathy (CBD=3, PSP=2), and pathogenic mutations consistent with 4R-tau (MAPT E10+16=2 with one autopsy-confirmed, and MAPT p301.L=1). A subset of 43 ALS and PSP patients participated in another CSF study38. We excluded patients with a three-repeat tauopathy to define a homogeneous contrast group. CSF was also available in 23 healthy seniors screened for dementia using a mini-mental state exam (MMSE)39 score >28/30, were screened for AD pathology using an autopsy-validated t-tau to beta-amyloid ratio (<0.34)40 and no neurological or psychiatric history. Table 1 summarizes demographic features (all p>0.05). Another cohort of 28 demographically-matched healthy seniors (age, education and gender p-values>0.1) with no neurological or psychiatric history were recruited as neuroimaging controls.
TABLE 1.
MEAN (±S.D.) DEMOGRAPHIC & FUNCTIONAL MEASURES FOR AMYOTROPHIC LATERAL SCLEROSIS (ALS), FOUR-REPEAT TAUOPATHIES (4R-TAU), AND HEALTHY SENIORS
| FUNCTIONAL MEASURE | ALS (N=51) |
4R-TAU (N=23) |
HEALTHY SENIORS (N=23) |
|---|---|---|---|
| Age | 54.88 (10.6) | 65.4 (9.4)* | 59.9 (6.0) |
| Education | 14.86 (3.9) | 14.55 (3.4) | 15.9 (4.3) |
| Gender (M / F) | 35 / 16 | 14 / 9 | 12 / 11 |
| MMSE (adjusted) | 27.53 (3.3) | 24.45 (5.13)^# | 29.39 (0.7) |
| Disease Duration (months) | 24.72 (14.1) | 54.87 (23.4)* | -- |
| ALSFRS-R (n=42) | 37.74 (7.9) | -- | -- |
| Progression Rate (n=42) | 0.46 (0.4) | -- | -- |
NOTE:
4R-TAU differs significantly from ALS at p<0.001;
4R-TAU differs significantly from ALS at p<0.01;
4R-TAU differs significantly from healthy seniors at p<0.001.
All individuals participated in a written informed consent procedure with their caregivers, when appropriate, that was approved by the Institutional Review Board at the University of Pennsylvania.
Functional measures
As summarized in Table 1, ALS patients, 4R-tau patients, and Seniors were evaluated clinically with the MMSE (n=78). A subset of ALS patients (n=42) were additionally evaluated on the Functional Rating Scale (ALSFRS-R)28.
Lumbar Puncture Procedure and Analysis
Lumbar puncture (LP)
CSF samples were obtained during routine diagnostic LP, as described40. Briefly, LP was performed at the L3/L4 lumbar space using a 20-gauge needle to collect 15 ml of CSF in polypropylene tubes (Corning Life Sciences, Lowell, MA). Samples were aliquotted and immediately stored at −80°C until analysis. CSF sample collection, storage, and analysis were performed according to published standard operating procedures41.
CSF analysis
Samples were analyzed using a Luminex xMAP platform (INNO-BIA AlzBio3™, Innogenetics, Ghent, Belgium) (n=52) or an ELISA assay (INNOTEST®, Innogenetics, Ghent, Belgium) (n=13), as described41. Briefly, the xMAP platform utilized capture monoclonal antibodies (MAbs) 4D7A3 (Aβ1–42), AT120 (ttau), and AT270 (ptau) bound to color-specific beads. We used an assay sensitive to phosphorylation at threonine-181 since this is the Alzheimer’s Disease Neuroimaging Initiative standard for which the highly reliable Luminex method is available41. Biomarker analytes were detected using reporting MAbs 3D6 (Aβ1–42) and HT7 (ttau, ptau). Some older samples were analyzed with an ELISA method, where MAbs for capturing and reporting ttau and ptau were AT120/HT7 and BT2, HT7/AT270, respectively. As described previously20, ELISA values for Aβ also were measured using an “in house” method with the capturing MAb BAN-50, and the reporting MAb BC-05. Using an autopsy-validated formula40, a linear regression model converted natural-log transformed raw CSF values from ELISA to an xMAP equivalent.
Statistical analysis
We evaluated overall group-level differences for raw ptau, ttau, and ptau:ttau ratio with non-parametric Kruskal-Wallis and post hoc Mann-Whitney U tests for descriptive purposes. We also confirmed that a potential covariate for ALS progression rate, defined using previously published criteria (48 - ALSFRS-R)/Disease Duration in Months)28 did not contribute to group differences. We used a cross-validation procedure to evaluate ptau, ttau, and ptau:ttau ratio as candidate biomarkers for individual patient screening. We randomly divided ALS and 4R-tau cohorts into comparably sized training (ALS n=26; 4R-Tau n=11) and validation (ALS n=25; 4R-Tau n=12) cohorts. eTable 1 summarizes the training and validation cohorts. Since ALS and 4R-tau differ in age and disease duration and these factors may influence CSF analyte levels42, we performed a logistic regression for each CSF analyte that included age and disease duration nuisance covariates. These logistic regressions were completed in the training cohort to generate a probabilistic likelihood of ALS, and then these probabilities were entered into receiver operating characteristic (ROC) curves. We defined the optimal cutoff to assess sensitivity and specificity at a probability ≥0.703, equivalent to the proportion of ALS patients in the training cohort (26 out of 37) and then applied this logistic regression model to the independent validation cohort. We report screening accuracy using a chi-squared (χ2) test: Patients in the validation cohort whose ALS probability exceeded the 0.703 threshold were predicted as having ALS and otherwise assigned to the predicted 4R-tau group. We performed Pearson’s correlations between each analyte (ptau:ttau ratio, ptau, and ttau) and functional measures, summarized in Table 1. For each correlation, we used the predicted probability of ALS as an age- and disease duration-adjusted proxy for each CSF analyte.
Imaging Procedure and Analysis
Acquisition
Diffusion-weighted MRIs were available for 10 ALS patients (1 with ALS-FTD) from a SIEMENS 3.0T Trio scanner using an 8-channel coil. Diffusion-weighted images (DWI) were acquired with a 30-directional sequence involving single-shot, spin-echo, diffusion-weighted echo planar imaging (FOV=245mm; matrix size=128×128; number of slices=57; voxel size=2.2mm isotropic; TR=6700msec; TE=85msec; fat saturation). We acquired 30 volumes with diffusion weighting (b=1000 s/mm2) along 30 non-collinear directions per subject, and either one (n=2) or four (n=17) without diffusion weighting (b=0 s/mm2). When four volumes were collected without diffusion weighting, these volumes were averaged to increase signal-to-noise ratio. Reasons for exclusion included health and safety (e.g., difficulty breathing while supine, metallic implants, shrapnel, claustrophobia) and lack of interest in an imaging study. DWI were also available for 9 4R-tau patients. T1-weighted MRI volumes were also acquired in the same scanning session with MPRAGE acquisition parameters: repetition time=1620msec; echo time=3msec; slice thickness=0.9mm; flip angle=15°; matrix=192×256, and in-plane resolution=0.9×0.9mm.
Preprocessing
Whole-brain MRI volumes were preprocessed using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTs, http://www.picsl.upenn.edu/ANTS/), as described43. Briefly, PipeDream deformed each individual dataset into local template space in a canonical stereotactic coordinate system. Each participant’s T1 image was warped to the template via the symmetric diffeomorphic procedure in ANTs. For DWI, motion and distortion artifacts were removed by affine co-registration of each image with diffusion weighting to the unweighted (b=0) image. Diffusion tensors (DTs) were computed using a linear least-CSF biomarker for ALS Grossman et al 8 squares algorithm44 implemented in Camino45, and tensors were reoriented using the preservation-of-principal-directions algorithm46. Fractional anisotropy (FA) was computed from the DT image for each subject. Distortion between T1 and DT images was corrected by registering the FA image to the T1 image. The DT image was then warped to template space by applying both the FA-to-T1 and T1-to-template warps for each subject. FA images were smoothed using a 4mm full-width half-maximum isotropic Gaussian kernel.
Statistical Analysis
Analyses of FA were performed in SPM8 using the two-samples t-test module. FA volumes were analyzed using an explicit mask (FA≥0.25) to constrain comparisons to WM regions. Comparisons of ALS patients to healthy seniors used a height threshold of q<0.05 with false discovery rate (FDR) correction for multiple comparisons, and comparisons of 4R-tau to seniors used a height threshold of q<0.005 with FDR correction. Both comparisons used an extent threshold of 200 voxels. Regression analyses related FA to the adjusted ptau:ttau ratio at p<0.05 (uncorrected) with a 50-voxel extent. Regression analyses were constrained to WM fibers with reduced FA using explicit masks generated from the results of the direct comparisons with healthy seniors; different thresholds were used for group comparisons to create disease masks of comparable size. Using a deterministic tractography procedure in Camino, WM fibers were tracked in a healthy elderly template using the DTI sequence described above. Fiber tracts that passed through voxels of reduced FA were retained to define the masks for regression analyses. This was done to limit the interpretation of a correlation between WM and CSF to WM fibers with disease.
RESULTS
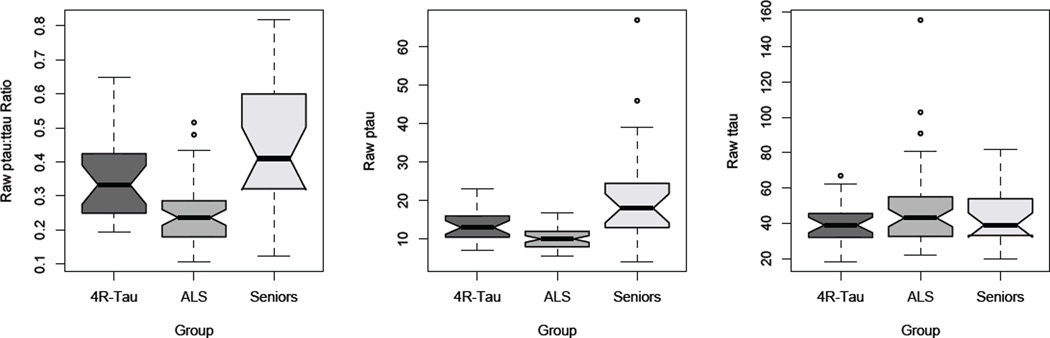
Median raw CSF analyte values for ALS, 4R-tau, and Seniors are illustrated in Figure 1. Kruskal-Wallis tests revealed group differences for ptau:ttau ratio [χ2=30.55; p<0.001] and for ptau ng/ml [χ2=22.80; p<0.001]. Planned post hoc Mann-Whitney tests revealed that, relative to 4R-tau, ALS has reduced ptau:ttau ratio [Z=3.74; p<0.001] and reduced ptau levels [Z=2.82; p=0.005]. ALS also had reduced ptau:ttau ratio [Z=4.92; p<0.001] and ptau levels [Z=4.36; p<0.001] relative to Seniors. There was no group effect for ttau ng/ml [χ2=1.73; p>0.1]. By comparison, 4R-tau had only marginally reduced ptau [Z=2.27; p=0.05] relative to Seniors.
FIGURE 1.
BOXPLOTS OF CSF ANALYTES PTAU NG/ML, TTAU NG/ML, AND PTAU:TTAU RATIO IN AMYOTROPHIC LATERAL SCLEROSIS, FOUR-REPEAT TAUOPATHY, AND HEALTHY SENIORS. NOTE. Dark lines in boxplots illustrate median CSF value, notches illustrate interquartile range (non-overlapping notches are significantly different), and error bars represent full range of data.
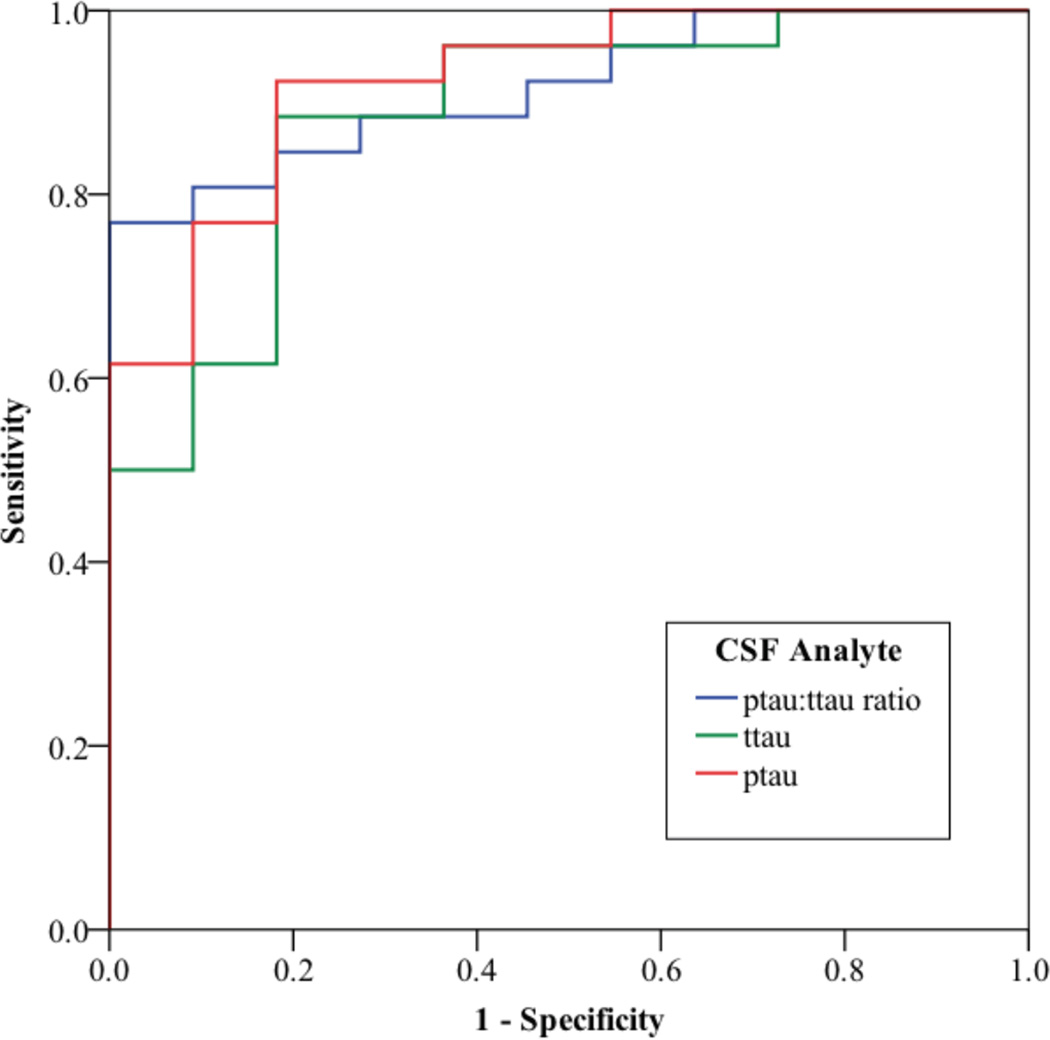
ROC analyses illustrated in Figure 2 showed an area under the curve (AUC) for ptau:ttau ratio of 0.916 (p<0.001). In the training cohort, the probabilistic-ALS cutoff achieved 80.8% sensitivity and 90.9% specificity. A cross-validation analysis using the same cutoff in the validation cohort revealed 92% sensitivity and 91.7% specificity [χ2=24.90; p<0.001]. An analysis of ptau alone also was robust (AUC=0.923; p<0.001). We found 80.8% sensitivity and 81.8% specificity in the training cohort, but the validation cohort achieved high sensitivity (88%) with only modest specificity (75%) [χ2=17.42; p<0.001]. The ttau analyte also achieved a significant AUC (AUC=0.885; p<0.001), with 84.6% sensitivity and 81.8% specificity in the training cohort, and high sensitivity (92%) with modest specificity (75%) in the validation cohort [χ2=14.69; p<0.001].
FIGURE 2.
RECEIVER OPERATING CHARACTERISTIC CURVE ILLUSTRATING THE SENSITIVITY AND SPECIFICITY OF CSF PTAU, TTAU, AND PTAU:TTAU RATIO IN AMYOTROPHIC LATERAL SCLEROSIS RELATIVE TO FOUR-REPEAT TAUOPATHIES.
Follow-up analyses of individuals with autopsy confirmation or a genetic mutation (n=21) showed correct classification in 18 (85.7%) of 21 patients using the most robust analyte, ptau:ttau ratio. The three misclassified cases included one C9orf72 expansion patient, one MAPT (E.10+16 C>T) mutation patient, and one autopsy-confirmed ALS patient.
Correlation analyses in ALS using age- and disease-duration-adjusted CSF levels revealed that MMSE is related to pttau:ttau ratio (r=0.342; p<0.05), ptau (r=0.354; p<0.05), but not to ttau; ALSFRS-R is related to ptau (r=0,448; <0.005) and ttau (r=0.406; p<0.01), but less to pttau:ttau ratio (r=0.263). CSF levels were not related to Progression Rate (r<0.25).
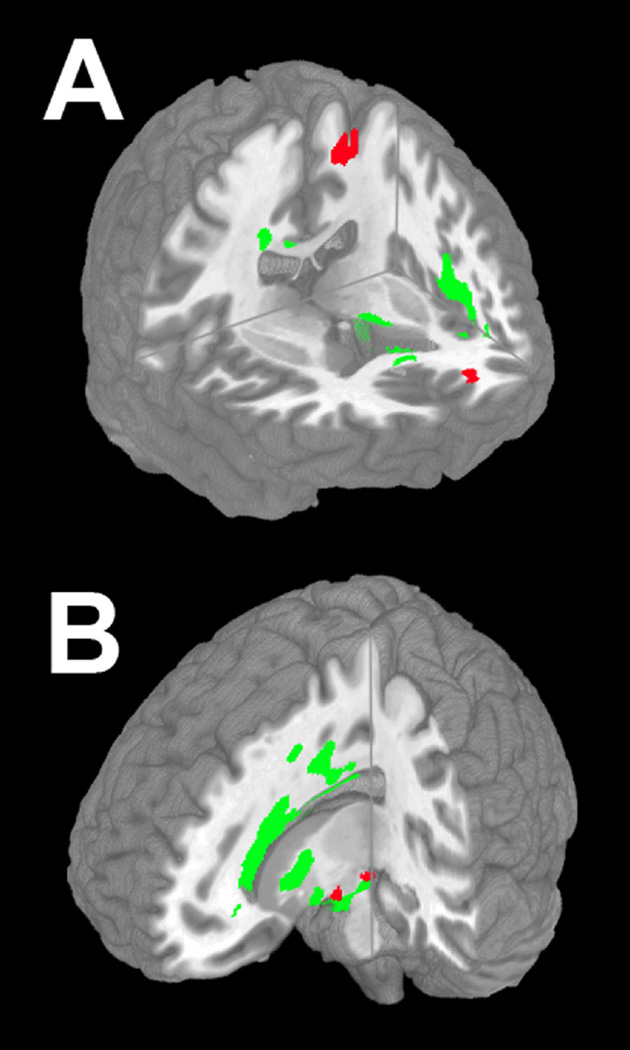
Figure 3 Panel A illustrates reduced WM FA in ALS that extends throughout frontal WM, the corpus callosum and the anterior limb of the internal capsule. Specific anatomic loci and WM tracts are summarized in eTable 2. Regression analysis related reduced ptau:ttau ratio to reduced WM FA in the corticospinal tract subjacent to primary motor cortex, prefrontal WM projections, and the corpus callosum (not shown). Figure 3 Panel B shows areas of reduced FA in PSP. Peak foci of reduced FA, summarized in eTable 2, were found in frontal, parietal, corpus callosum, internal capsule and brainstem regions. Regression analysis related reduced ptau:ttau ratio to reduced FA in the midbrain and uncinate fasciculus (not shown).
FIGURE 3.
REDUCED WHITE MATTER FRACTIONAL ANISOTROPY IN AMYOTROPHIC LATERAL SCLEROSIS AND 4R-TAU, AND REGRESSIONS RELATING ADJUSTED CSF PTAU:TTAU RATIO TO FRACTIONAL ANISOTROPY. NOTE. Panel A: Right anterior view of anatomic distribution of reduced fractional anisotropy in ALS (q<0.05, FDR-corrected; green). Red areas indicate anatomic distribution of regressions relating adjusted ptau:ttau ratio to fractional anisotropy in corticospinal tract, prefrontal centrum semiovale, and body of corpus callosum (not illustrated). Panel B: Left anterior view of anatomic distribution of reduced fractional anisotropy in 4R-tau (q<0.005, FDR-corrected; green). Red areas indicate anatomic distribution of regressions relating adjusted ptau:ttau ratio to fractional anisotropy in midbrain, right uncinate (not illustrated).
DISCUSSION
CSF levels of phosphorylated tau were very low in ALS. A cross-validation analysis revealed that ptau and ptau:ttau ratio appear to distinguish individuals with ALS from 4R-tau and from Seniors. This was confirmed in the subgroup of patients with known histopathology. Lower ptau and ptau:ttau ratio correlated with clinical measures of disease, and with MRI measures of reduced WM FA in the corticospinal tract and prefrontal cortex in ALS subgroups.
The histopathologic abnormality in sporadic ALS is TDP-43, and ALS patients (except for those with ALS/parkinsonism who are Chamorro from Guam) have negligible brain hyperphosphorylated tau at autopsy24. Thus, we predicted low CSF ptau levels in ALS. The ptau:ttau ratio was consistently sensitive and specific, generalizing from training to validation cohorts, and thus is a candidate biomarker for screening ALS. Another study of TDP-43 proteinopathies and 4R-tauopathies reported similar findings38. Additionally, almost all cases with known TDP-43 pathology had a low ptau:ttau ratio. Two of the 3 incorrectly classified cases had genetically-determined disease, and we cannot rule out that these patients may have additional pathology due to another condition47.
Some previous work described elevated CSF ttau in ALS48,49, while others reported normal ttau levels50. Interpretation of inconsistent results should be performed cautiously because of the substantial variability associated with the ELISA method used in those studies41. The present study used a more reliable Luminex method to assess most CSF analyte levels. Other studies reported significantly reduced CSF amyloid precursor protein levels in ALS, and elevated CSF Aβ levels were related to shorter survival51,52, possibly reflecting the small number of ALS patients who have concurrent Alzheimer’s pathology53. Some studies described abnormal axonal markers that were related to survival54 and abnormal glial markers that were related to progression rate49, although our observations of reduced ptau were unrelated to survival and progression rate. Two reports described CSF TDP-43 levels in ALS55,56, although the variability of results, including substantial overlap with control values, suggests that TDP-43 assays may be premature.
Lower CSF ptau and ptau:ttau in ALS correlated with clinical measures, and although there are many measures of clinical functioning, this suggests that ptau:ttau ratio may be a sensitive marker of disease. ALS is associated with cognitive difficulty in many individuals5,6, and we found that ptau and ptau:ttau ratio correlates with cognitive functioning. ALS-FRS-R is commonly used to reflect disease severity in ALS and this correlated with ptau. Additional converging evidence suggesting that ptau:ttau ratio may be biologically meaningful comes from WM neuroimaging in anatomic regions known to be compromised pathologically in ALS57. Since CSF ptau:ttau ratio appears to be related to both clinical and imaging measures, ptau:ttau ratio may be a candidate marker to assess eligibility in clinical trials for disease-modifying treatments of ALS.
Our findings also suggest that a low ptau:ttau ratio may be specific for ALS. We demonstrated this by contrasting ALS with individuals highly likely to have 4R-tau histopathology. Others also have reported comparative studies to demonstrate specificity52,54. While not unreasonable to expect elevated CSF ptau levels of in this cohort because tau is hyperphosphorylated in autopsy assessments of these conditions, some reports have described elevated levels14–17, some normal levels18,25,58, and some reduced levels19 relative to healthy controls. This variability may be due in part to mixed etiology in clinically-diagnosed groups and the less reliable ELISA method used in most prior studies. Regardless of the basis for previous findings, our observations suggest a reliable difference between individuals with ALS and those highly likely to have 4R-Tau pathology.
Several caveats should be kept in mind when evaluating our findings. Our cohort was relatively small. Tau is phosphorylated at several sites, and assaying other phosphorylation sites may be informative. Most participants were assessed soon after the onset of typical ALS, and it would be important for future work to assess ALS patients with other phenotypic presentations and lengths of disease, and to evaluate CSF ptau levels in these different presentations. The contrast group consisted of patients four-repeat forms of tau because of limited CSF available from individuals with three-repeat tau pathology and our desire to have a relatively homogeneous contrast group, and it would be important to evaluate CSF ptau levels in individuals with three-repeat tau pathology. Limited MRI assessments were available because of patient limitations, and verification of FTLD-TDP pathology was possible in only a subset of cases. With these caveats in mind, our cross-validation prediction design suggests that individual patients with ALS highly likely to be due to FTLD-TDP pathology are characterized by a low CSF ptau:ttau ratio relative to individuals highly likely to have FTLD-Tau pathology, and low CSF ptau:ttau is associated with several clinical and imaging measures of ALS.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by NIH (AG032953, AG017586, NS044266, AG038490, AG043503), the ALS Association, and the Wyncote Foundation. These funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data analysis was conducted by Murray Grossman, Corey T. McMillan, and John Powers.
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Hu declares that he has filed a provisional patent on phosphorylated-tau: total-tau ratio as a biomarker for FTLD-TDP and ALS through Emory University.
Footnotes
All other authors have nothing to declare.
REFERENCES
- 1.del Aguila MA, Longstreth WT, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: A population-based study. Neurology. 2003;60:813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 2.Turner MR, Hardiman O, Benatar M, et al. Controversies and priorities in amyotrophic lateral sclerosis. The Lancet Neurology. 2013;12(3):310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chio A, Calvo A, Moglia C, et al. Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. Journal of Neurology, Neurosurgery and Psychiatry. 2011;82(7):740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 4.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Archives of Neurology. 2000;57(8):1171–1176. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- 5.Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. Journal of Neurology, Neurosurgery & Psychiatry. 2012 Jan 1;83(1):102–108. doi: 10.1136/jnnp-2011-300188. 2012. [DOI] [PubMed] [Google Scholar]
- 6.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurology. 2007;6:994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- 7.Chawla S, Wang S, Moore P, et al. Quantitative proton magnetic resonance spectroscopy detects abnormalities in dorsolateral prefrontal cortex and motor cortex of patients with frontotemporal lobar degeneration. Journal of Neurology. 2010;257(1):114–121. doi: 10.1007/s00415-009-5283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Deerlin VM, Leverenz JB, Bekris LM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurology. 2008;7(5):409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renton AE, Majounie E, Waite A, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclereosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 12.Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathologica. 2011;122(2):137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geser F, Winton MJ, Kwong LK, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathologica. 2008;145:115–133. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 14.Arai H, Morikawa Y, Higuchi M, et al. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochemical and Biophysical Research Communications. 1997;236:262–264. doi: 10.1006/bbrc.1997.6908. [DOI] [PubMed] [Google Scholar]
- 15.Green AJ, Harvey RJ, Thompson EJ, Rossor MN. Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer's disease. Neuroscience Letters. 1999;259:133–135. doi: 10.1016/s0304-3940(98)00904-5. [DOI] [PubMed] [Google Scholar]
- 16.Riemenschneider M, Wagenpfeil S, Diehl J, et al. Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology. 2002;58:1622–1628. doi: 10.1212/wnl.58.11.1622. [DOI] [PubMed] [Google Scholar]
- 17.Pijnenburg YAL, Schoonenboom NS, Rosso SM, et al. CSF tau and Abeta42 are not useful in the diagnosis of frontotemporal lobar degeneration. Neurology. 2004;62:1649. doi: 10.1212/01.wnl.0000123014.03499.a7. [DOI] [PubMed] [Google Scholar]
- 18.Mecocci P, Cherubini A, Bregnocchi M, et al. Tau protein in cerebrospinal fluid: A new diagnostic and prognostic marker in Alzheimer disease? Alzheimer's Disease and Associated Disorders. 1998;12:211–214. doi: 10.1097/00002093-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile distinguishes frontotemporal dementia from Alzheimer's disease. Annals of Neurology. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 20.Bian H, van Sweiten JC, Leight S, et al. Cerebrospinal fluid biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Harten AC, Kester MI, Visser PJ, et al. Tau and p-tau as CSF biomarkers in dementia: A meta-analysis. Clinical and Chemical Laboratory Medicine. 2011;49(3):353–366. doi: 10.1515/CCLM.2011.086. [DOI] [PubMed] [Google Scholar]
- 22.Borroni B, Malinverno M, Gardoni F, et al. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology. 2008 Nov 25;71(22):1796–1803. doi: 10.1212/01.wnl.0000335941.68602.39. 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hampel H, Buerger K, Zinkowski R, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Archives of General Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathologica. 2012;124(1):23–35. doi: 10.1007/s00401-012-0983-7. 2012/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoonenboom NSM, Reesink FE, Verwey NA, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012 Jan 3;78(1):47–54. doi: 10.1212/WNL.0b013e31823ed0f0. 2012. [DOI] [PubMed] [Google Scholar]
- 26.Koopman K, Le Bastard N, Martin JJ, Nagels G, De Deyn PP, Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau(181P) Neurochemistry International. 2009;55:214–218. doi: 10.1016/j.neuint.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Rosso SM, van Herpen E, Pijnenburg YAL, et al. Total tau and phosphorylated tau 181 levels in the cerebrospinal fluid of patients with frontotemporal dementia due to P301L and G272V tau mutations. Archives of Neurology. 2003;60:1209–1213. doi: 10.1001/archneur.60.9.1209. [DOI] [PubMed] [Google Scholar]
- 28.Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006 Jan 24;66(2):265–267. doi: 10.1212/01.wnl.0000194316.91908.8a. 2006. [DOI] [PubMed] [Google Scholar]
- 29.Agosta F, Pagani E, Petrolini M, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. American Journal of Neuroradiology. 2010 Apr 15; doi: 10.3174/ajnr.A2105. 2010:ajnr.A2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thivard L, Pradat P-F, Lehericy S, et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry. 2007 Aug 1;78(8):889–892. doi: 10.1136/jnnp.2006.101758. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Poptani H, Bilello M, et al. Diffusion tensor imaging in amyotrophic lateral sclerosis: Volumetric analysis of the corticospinal tract. American Journal of Neuroradiology. 2006;27:1234–1238. [PMC free article] [PubMed] [Google Scholar]
- 32.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:457–463. doi: 10.1136/jnnp.2005.075713. 04/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitwell JL, Master AV, Avula R, et al. Clinical Correlates of White Matter Tract Degeneration in Progressive Supranuclear Palsy. Arch Neurol. 2011 Jun 1;68(6):753–760. doi: 10.1001/archneurol.2011.107. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 36.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011 Aug 2;134:2456–2477. doi: 10.1093/brain/awr179. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. 01/10/ [DOI] [PubMed] [Google Scholar]
- 38.Hu WT, Watts K, Grossman M, et al. Reduced CSF p-tau(181) to tau ratio is a biomarker for FTLD-TDP. Neurology. 2013 doi: 10.1212/01.wnl.0000436625.63650.27. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folstein MF, Folstein SF, McHugh PR. "Mini Mental State." A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 40.Irwin DJ, McMillan CTBTJ, et al. Comparison of cerebrospinal fluid levels of tau and aBeta 1–42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Archives of Neurology. 2012;69(8):1018–1025. doi: 10.1001/archneurol.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw LM, Vanderstichele H, Knapik-Csajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of Neurology. 2009;65 doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjogren M, Vanderstichele H, Agren H, et al. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21–93 years of age: Establishment of reference values. Clinical Chemistry. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 43.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2010;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvador R, Pena A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human brain mapping. 2005 Feb;24(2):144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook PA, Bai Y, Nadjati-Gilani S, et al. International Society for Magnetic Resonance in Medicine. Berkeley: ISMRM; 2006. Camino: Open-source diffusion-MRI reconstruction and processing; p. 2759. [Google Scholar]
- 46.Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001 Nov;20(11):1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- 47.Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012 Mar 1;135(3):693–708. doi: 10.1093/brain/awr355. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussmuth SD, Tumani H, Ecker D, Ludolph AC. Amyotrophic lateral sclerosis: disease stage related changes of tau protein and S100 beta in cerebrospinal fluid and creatine kinase in serum. Neuroscience Letters. 2003;353:57–60. doi: 10.1016/j.neulet.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Sussmuth SD, Sperfeld AD, Hinz A, et al. CSF glial markers correlate with survival in amyotrophic lateral sclerosis. Neurology. 2010 Mar 23;74(12):982–987. doi: 10.1212/WNL.0b013e3181d5dc3b. 2010. [DOI] [PubMed] [Google Scholar]
- 50.Jiménez-Jiménez FJ, Hernánz A, Medina-Acebrón S, et al. Tau protein concentrations in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neurologica Scandinavica. 2005;111(2):114–117. doi: 10.1111/j.1600-0404.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 51.Rusina R, Ridzoň P, Kulišt'ák P, et al. Relationship between ALS and the degree of cognitive impairment, markers of neurodegeneration and predictors for poor outcome. A prospective study. European Journal of Neurology. 2010;17(1):23–30. doi: 10.1111/j.1468-1331.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- 52.Steinacker P, Fang L, Kuhle J, et al. Soluble beta-amyloid precursor protein Is related to disease progression in amyotrophic lateral sclerosis. PLoS ONE. 2011;6(8):e23600. doi: 10.1371/journal.pone.0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brettschneider J, Libon DJ, Toledo JB, et al. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathologica. 2012;123(3):395–407. doi: 10.1007/s00401-011-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006 Mar 28;66(6):852–856. doi: 10.1212/01.wnl.0000203120.85850.54. 2006. [DOI] [PubMed] [Google Scholar]
- 55.Steinacker P, Hendrich C, Sperfeld AD, et al. TDP-43 in cerebrospinal fluid of patients With frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Archives of Neurology. 2008 Nov 1;65(11):1481–1487. doi: 10.1001/archneur.65.11.1481. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasai T, Tokuda T, Ishigami N, et al. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathologica. 2009;117(1):55–62. doi: 10.1007/s00401-008-0456-1. [DOI] [PubMed] [Google Scholar]
- 57.Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009 Feb 1;66(2):180–189. doi: 10.1001/archneurol.2008.558. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aerts MB, Esselink RAJ, Bloem BR, Verbeek MM. Cerebrospinal fluid tau and phosphorylated tau protein are elevated in corticobasal syndrome. Movement Disorders. 2011;26(1):169–173. doi: 10.1002/mds.23341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.