Abstract
Changes in dendritic spines structure and function play a critical role in a number of physiological processes, including synaptic transmission and plasticity, and are intimately linked to cognitive function. Alterations in dendritic spine morphogenesis occur in a number of neuropsychiatric disorders and likely underlie the cognitive and behavioral changes associated with these disorders. The neuronal guanine nucleotide exchange factor (GEF) kalirin is emerging as a key regulator of structural and functional plasticity at dendritic spines. Moreover, a series of recent studies have genetically and functionally linked kalirin signaling to several disorders, including schizophrenia and Alzheimer’s disease. Kalirin signaling may thus represent a disease mechanism and provide a novel therapeutic target.
Keywords: schizophrenia, Alzheimer’s disease, mental disorder, genetic, glutamatergic, postmortem
Introduction
Most excitatory synapses in the mammalian forebrain are located on small protrusions from dendrites called dendritic spines. Actin dynamics can rapidly induce changes in spine morphology, which modulates synaptic properties and the potential for plasticity (Carlisle and Kennedy, 2005; Alvarez and Sabatini, 2007). Spine dynamics are tightly regulated throughout life. During development, spine dynamics play a critical role in neural circuit formation. In mature neurons, synaptic activity drives spine dynamics, contributing to remodeling of neural circuits and experience-dependent plasticity (Carlisle and Kennedy, 2005; Alvarez and Sabatini, 2007). Several decades of research have documented changes in spine size and number associated with a number of physiological, behavioral, and pathological conditions. Importantly, circuit-level analysis indicates that relatively small changes in synapse strength or number may have a much greater effect on the overall function of circuits (Chklovskii et al., 2004; Chen and Nedivi, 2010).
Postmortem neuropathological studies have revealed that dendritic spine morphology and number are altered in a number of disorders of the central nervous system (Penzes et al., 2011). These alterations have been well characterized in intellectual disability, Down’s syndrome (Kaufmann and Moser, 2000), Rett syndrome (Zhou et al., 2006), Fragile X syndrome (Bagni and Greenough, 2005), autism spectrum disorders (ASD) (Hutsler and Zhang, 2010), schizophrenia (Glantz and Lewis, 2000; Sweet et al., 2009) and addiction (Robinson and Kolb, 1997, 2004; Zhou et al., 2007). Furthermore, synaptic pathology has been associated with neurodegenerative disorders including Alzheimer’s (Knobloch and Mansuy, 2008), Parkinson’s (Day et al., 2006), and Huntington’s disease (Spires et al., 2004).
Spine morphogenesis is driven by actin remodeling and membrane trafficking events, which are regulated by small GTPase signaling (Tolias et al., 2011). When they are in the active, GTP-bound state small GTPases promote actin remodeling and trafficking; they become inactive by hydrolyzing GTP to GDP. Abnormal small GTPase signaling has been associated with several human pathologies. Signaling pathways involving Rac and Ras are particularly important since a large proportion of genes causing intellectual disability encode proteins in the small GTPase pathway (Ramakers, 2002; Newey et al., 2005). GTPases are modulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). GEFs and GAPs are complex, multidomain signaling proteins that may serve as signaling hubs by integrating multiple signals and have cell- and tissue-specific functions (Carlisle and Kennedy, 2005). Among these, the Rac/Rho-GEF kalirin has been associated with a range of psychiatric and neurodegenerative disorders.
Kalirin expression is highly enriched in the forebrain. The most abundant isoform of the KALRN gene, kalirin-7, is localized to dendritic spines on cortical pyramidal neurons (Figure 1a–b) where it plays a key role in structural and functional plasticity at excitatory synapses (Penzes and Jones, 2008). Kalirin facilitates remodeling of the actin cytoskeleton, leading to changes in spine size and density by activating Rac1 and its downstream effector, p21-activate kinase (PAK) (Penzes et al., 2001; Penzes et al., 2003). Kalirin-7 has also been shown to mediate activity-dependent plasticity in dendritic spines. Xie and colleagues (2007) found that NMDAR activation-induced spine enlargement and increases in synaptic expression of AMPARs were kalirin-7-dependent. Given these well-characterized effects of kalirin on synaptic plasticity at dendritic spines, it seems likely that changes in expression of kalirin, mutations, or alterations of its upstream or downstream signaling partners that occur in human disorders would lead to aberrant dendritic spine number and morphology. Consistent with this, kalirin has been functionally and genetically implicated in the pathogenesis of several human disorders, most of which are associated with changes in cognitive function and present with dendritic spine pathology (Table 1). Here we will discuss recent studies linking aberrant regulation of dendritic spine plasticity by altered kalirin signaling with several psychiatric and neurological disorders.
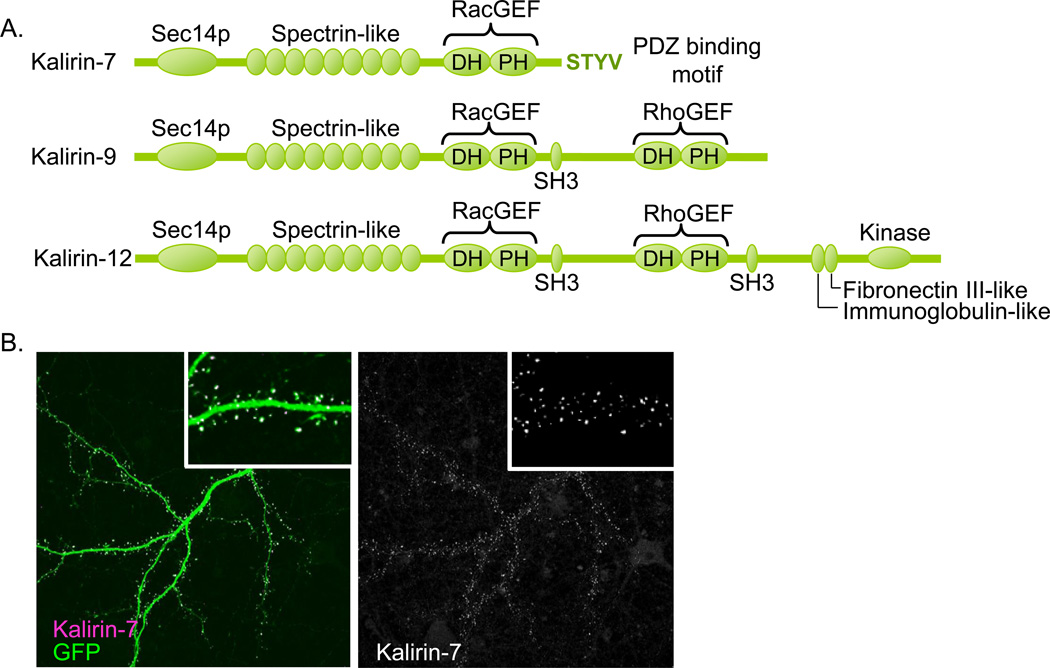
Figure 1. Kalirin-7 is localized to dendritic spines and modulates dendritic spine morphogenesis.
(A) This schematic of kalirin isoforms and domains shows the N-terminal Sec14p domain, followed by nine spectrin-like repeats common to all kalirin isoforms. The Dbl homology (DH) and pleckstrin homology (PH) domains confer kalirin’s GEF activity. The first GEF domain activates Rac1 and is present in kalirin-7, -9 and -12. Kalirin-7 also has a unique C-terminal PDZ-binding domain. Kalirin-9 and -12 have an SH3 domain and a second GEF domain that activates RhoA. Kalirin-12 has C-terminal fibronectin III-like and immunoglobulin-like domains as well as a kinase domain. (B) Kalirin-7 is localized to dendritic spines of cortical pyramidal neurons.
Table 1.
Summary of evidence for kalirin association with disease.
| Type of evidence | ||||
|---|---|---|---|---|
| Disease | Genetic association | Postmortem evidence | Molecular association | Animal models |
| Schizophrenia | GWAS (St. Jean, 2008; Ikeda et al., 2011) rare missense mutations (Kushima et al., 2010) |
↓ mRNA (Hill et al., 2006) ↓ kalirin-7 protein (Rubio et al., 2012) ↑ kalirin-9 (Deo et al., 2012). |
DISC1 (Millar et al., 2003; Hayashi-Takagi et al., 2010;) NRG1 /ErbB4 (Cahill et al., 2011; Cahill et al. 2013) 5-HT2A (Jones et al., 2009) NMDAR (Hayashi-Takagi et al., 2010) PSD-95 (Hayashi-Takagi et al., 2010;) PAK |
KALRN KO (Cahill et al., 2009) Kal7 KO (Ma et al., 2008b) |
| Alzheimer’s disease | -- | ↓ mRNA (Youn et al., 2007b) ↓ protein (Youn et al., 2007b; Murray et al., 2012) |
iNOS (Ratovitski et al., 1999) PAK (Zhao et al., 2006) EphB2 (Penzes et al., 2003) |
-- |
| ADHD | GWAS (Lesch et al., 2008) | -- | Cadherins (Xie et al., 2008) | -- |
| Addiction | -- | -- | -- | Kal7 KO, addiction in mouse model (Mains et al., 2011; Kiraly et al., 2010) |
| Huntington’s disease | -- | -- | HAP1 (Colomer et al., 1997) | -- |
| Parkinson’s disease | Synphillin-1 (Tsai et al., 2012) | |||
| Stress | -- | -- | -- | Chronic restraint stress in mice (Li et al., 2010) |
| Ischemic stroke | Case-control (Krug et al., 2010) | -- | -- | Mouse model of ischemia (Beresewicz et al., 2008) |
| Coronary artery disease | GWAS (Ikram et al., 2009) association linkage (Wang et al., 2007) |
-- | -- | -- |
Kalirin and schizophrenia
Schizophrenia is a psychiatric disorder that affects cognition and perception of reality that impacts 0.5–1% of the population. Symptoms emerge during late adolescence or early adulthood. The cause of this disease is unknown and there are no effective treatments for the negative and cognitive symptoms. One of the most consistent neuropathological findings in postmortem studies of schizophrenia patients is reduced spine density in forebrain regions. Spine loss in the dorsolateral prefrontal cortex (DLPFC) (Glantz and Lewis, 2000) and auditory cortex has been observed in postmortem studies in schizophrenic patients (Sweet et al., 2009). Loss of dendritic spines has also been reported in the subiculum and CA3 (Kolomeets et al., 2005; Steen et al., 2006). A number of classical regulators of spine plasticity have been genetically and functionally linked to schizophrenia; conversely, the protein products of a number of schizophrenia risk genes have been shown to modulate spine morphology (Penzes et al., 2011). Further investigation of these proteins might shed light on the molecular mechanisms underlying spine pathology in schizophrenia. Interestingly, several lines of evidence link altered kalirin signaling with schizophrenia.
In a postmortem study, kalirin mRNA was found to be reduced in the DLPFC of schizophrenia patients, irrespective of antipsychotic treatment (Hill et al., 2006). Interestingly, kalirin loss correlated with spine loss on layer 3 PFC neurons (Hill et al., 2006). Another postmortem study reported changes in protein levels of kalirin-7 (duo), and other proteins in the kalirin signaling pathway, in the DLPFC and ACC in schizophrenia patients (Rubio et al., 2012). Regional differences are also becoming apparent in kalirin expression in schizophrenia. In a recent study, Deo and colleagues demonstrated that kalirin-9 is upregulated, while other kalirin forms are not altered, in auditory cortex in schizophrenia (Deo et al., 2012). This upregulated kalirin-9 might contribute to reduced dendritic branching in the auditory cortex in schizophrenia, as overexpression of the kalirin-9 isoform in cultured neurons reduced dendritic arborization (Deo et al., 2012).
The above studies implicate kalirin in the pathophysiology of schizophrenia. But what about etiology, does kalirin play a role? A recent genome-wide association study (GWAS) in a Japanese population (Ikeda et al., 2011) detected association signals with schizophrenia at the region of the KALRN gene. Although single locus analysis did not reach genome-wide significance, the study confirmed a shared polygenic risk of schizophrenia between the Japanese and the Caucasian samples. An independent study also supported this association (St. Jean, 2008). Following up on these findings, Kushima and colleagues (2010) re-sequenced all exons of the KALRN gene and identified several rare missense mutations enriched in patients with schizophrenia as compared to non-psychiatric controls. A number of these sequence alterations are predicted to have functional and damaging consequences. The authors detected a significant association of the P2255T mutation (OR = 2.09) as well as combined association of all mutations (OR = 2.07) with schizophrenia. One of these mutaions, T1207M, occurs in the spectrin repeat region, proximal to the RacGEF domain that is present in all isoforms of kalirin. Interestingly, several of these mutations are in exons encoding protein domains present in kalirin-9 or -12 but not kalirin-7, including the R2049K mutation in the RhoGEF domain and the P2255T, P2265S and G2296C mutations that are just downstream of the RhoGEF domain. Thus, multiple rare missense mutations in KALRN may contribute to genetic risk in schizophrenia.
Kalirin-interacting proteins and schizophrenia
Recent studies also demonstrate that kalirin-7 physically and functionally interacts with several proteins previously implicated in schizophrenia. Kalirin-7 directly interacts with DISC1 (disrupted in schizophrenia) (Millar et al., 2003), the protein product of a leading schizophrenia susceptibility gene. DISC1 functions as a scaffold for kalirin-7, and modulates the access of kalirin-7 to Rac1, controlling the duration and intensity of Rac1 activation in response to NMDAR activation (Hayashi-Takagi et al., 2010). In this context, DISC1 functions as a scaffold that enhances the kalirin-7/PSD-95 interaction. Kalirin’s release from DISC1 enhances its GEF activity, thus leading to changes in spine structure. Knockdown of DISC1 in cultured neurons for extended periods of time causes a reduction in spine area (Hayashi-Takagi et al., 2010). If schizophrenia-associated mutations disrupt DISC1’s scaffolding function, they would be expected to have deleterious consequences on spine morphogenesis through altered kalirin signaling (Figure 2).
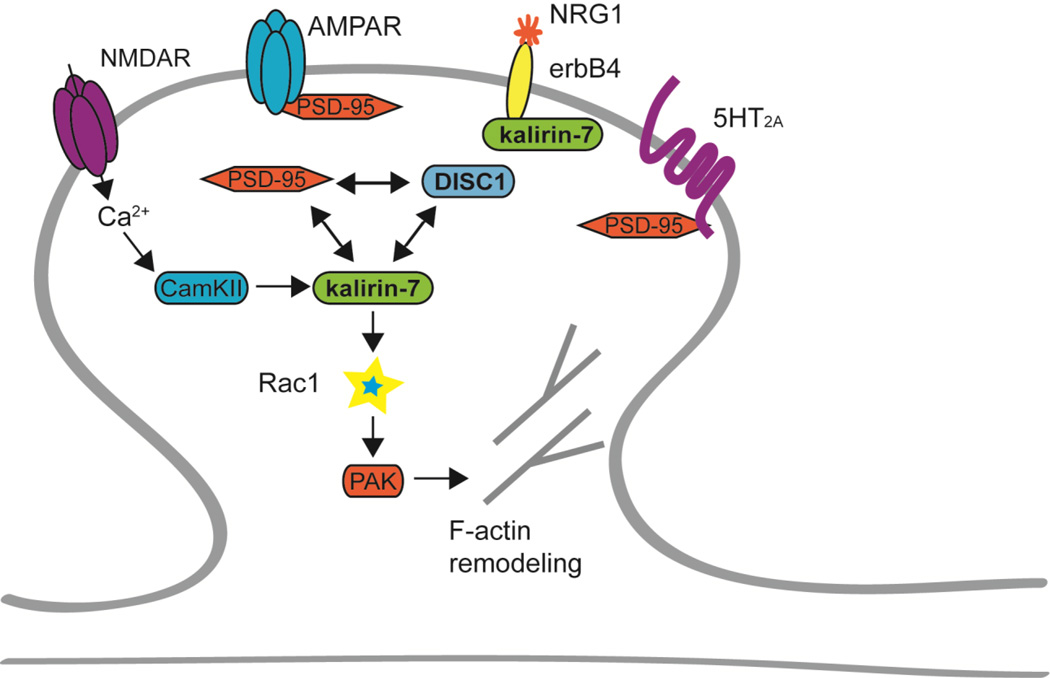
Figure 2. Kalirin-7 interacts with schizophrenia-associated proteins.
Under non-pathological conditions, kalirin interacts with a number of schizophrenia-associated proteins. Activation of postsynaptic NMDARs leads to increased trafficking of AMPARs to the post-synaptic density. NMDAR activation also leads to dissociation of the PDS-95/DISC1/kalirin-7 complex, allowing kalirin-7 to activate Rac1. Post-synaptic erbB4 receptors are activated by NRG1 and modulate dendritic spines in a kalirin-dependent manner. 5-HT2A receptor activation also regulates spine density through kalirin. Kalirin-7 activates Rac1 by exchanging GDP for GTP. Rac1 in turn activates PAK, which then initiates actin remodeling. Kalirin-7, DISC1, erbB4, NRG1, 5-HT2A, PAK2 and PAK3 have been genetically associated with schizophrenia.
Kalirin also interacts with and is modulated by the 5-HT2A serotonin receptor (Figure 2), a target of atypical antipsychotics which has also been genetically linked to schizophrenia (Golimbet et al., 2007). Jones and colleagues (2009) demonstrated that 5-HT2A modulates spine morphogenesis in a kalirin-dependent manner. 5-HT2A receptors colocalize with PSD-95 and kalirin-7 in spines of mature cortical neurons. Treatment of cultured neurons with the 5-HT2A receptor agonist DOI rapidly induced an increase in spine size and PAK activation, and both of these effects were dependent on kalirin-7 targeting to the PSD (Jones et al., 2009). Thus, 5-HT2A and kalirin-7 are functionally linked, and 5-HT signaling may modulate kalirin-7 activity and synapse size at mature cortical synapses. Based on these studies, 5-HT receptors may act as upstream regulators of kalirin-7 signaling and thus may contribute a neuromodulatory element to kalirin-7 signal integration in spines.
Kalirin-7 also participates in a common pathway with neuregulin 1 (NRG1) and erbB4, two prominent schizophrenia susceptibility molecules, to regulate the morphology of interneurons and pyramidal neurons (Cahill et al., 2011; Cahill et al., 2013). The tyrosine kinase erbB4 is thought to be the principal receptor for NRG1. Polymorphisms in NRG1 have been identified in patients with schizophrenia (Mei and Xiong, 2008). A rare copy number variant (CNV) for erbB4 has also been associated with schizophrenia; this mutation results in a protein lacking most of its intracellular kinase domain that has the effect of a dominant negative protein (Walsh et al., 2008). ErbB4 is highly expressed in interneurons, and to a lesser extent, in cortical pyramidal cells and dendritic spines (Barros et al., 2009). This suggested that NRG1/erbB4/kalirin signaling may play a role in interneurons. Indeed, while kalirin is robustly expressed in pyramidal neurons, it is also expressed in interneurons (Ma et al., 2001). Interneuronal pathology is thought to play an important role in schizophrenia (Lewis et al., 2005). Ma and colleagues (2008a) demonstrated that endogenous kalirin-7 is localized to the postsynaptic side of excitatory synapses onto hippocampal interneurons. Consistent with this, Cahill and colleagues found that kalirin regulates interneuron dendritic growth downstream of NRG1 in mature cortical interneurons. Treatment of cortical interneurons with NRG1 increases dendritic length in a kalirin-dependent manner (Cahill et al., 2011). Conversely, cortical interneurons from erbB2/B4 conditional knockout mice have decreased dendritic length (Cahill et al., 2011). In cultured cortical interneurons, endogenous kalirin-7 colocalized with erbB4 and erbB4 co-immunoprecipitated with kalirin-7, indicating that kalirin-7 and erbB4 interact (Cahill et al., 2011). Kalirin may thus play a role in the normal development and function of inhibitory circuits, and its functional loss may contribute to the dysfunction of these circuits.
In addition, NRG1 and erbB4 have been shown to affect spine morphology (Li et al., 2007; Barros et al., 2009), thus kalirin may also function downstream of these molecules in spines (Figure 2). Indeed, Cahill and colleagues recently describe a role for NRG1/erbB4 signaling in regulation of dendritic spines (Cahill et al., 2013). These studies found that long-term incubation with NRG1 led to increased spine size and number, as well as increased AMPA receptor expression in spines in cortical pyramidal neurons. Loss of ErbB4 by shRNA-mediated knockdown prevented the effects of NRG1 on spine size, but not on spine density. Interestingly, the effects of effects of NRG1 and erbB4 on spines were mediated by kalirin.
P21-activiated kinase (PAK) is a key regulator of actin remodeling, and a downstream effector of kalirin-7 and Rac1 (Penzes et al., 2003). Interestingly, missense mutations in PAK3 have been associated with psychotic disorders, primarily schizophrenia with premorbid mental retardation (Morrow et al., 2008), and microdeletions that encompass PAK2 (3q29) have been found in schizophrenia patients (Mulle et al., 2010). These findings further implicate kalirin signaling in schizophrenia, as mutations in kalirin’s downstream effectors are genetically associated with the disorder.
In addition changes in expression and activation of PAK may occur in specific brain regions in schizophrenia patients. PAK1 expression was found to be increased in the DLPFC of schizophrenia patients and decreased phosphorylation of the catalytic domain of PAK1 was seen in both the DLPFC and ACC in schizophrenia patients (Rubio et al., 2012). However, in another study Deo and colleagues did not observe changes in PAK1 expression levels in the auditory cortex of schizophrenia patients (Deo et al., 2012).
Taken together, these findings support a model whereby proteins associated with schizophrenia, NRG1, erbB4, DISC1 and 5HT2A, function in the same pathway with kalirin-7 and PAK to control spine morphogenesis (Figure 2). These proteins might even form a “signalosome” (Hayashi-Takagi et al., 2010), a multiprotein signaling complex in dendrites or spines. If the functional output of this pathway is the control of spine plasticity, alterations in any of the molecules in the pathway/complex might impair the output of the entire pathway, leading to spine pathology.
Kalirin and Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by loss of memory, impairments of other cognitive function, and alterations in behavior that affects 11% of Americans over the age of 65 (www.alz.org). Neuropathologically, AD is characterized by the hallmark lesions of plaques (extracellular aggregates of amyloid β protein) and neurofibrillary tangles (intraneuronal aggregates of hyperphosphorylated microtubule associated protein tau). However, the strongest correlate of cognitive impairment in AD is not plaque or tangle burden, but loss of neocortical synapses, (DeKosky and Scheff, 1990; Selkoe, 2002)(Terry et al., 1991; Scheff and Price, 2003) with excitatory synapses onto dendritic spines particularly affected (DeKosky and Scheff, 1990; Baloyannis et al., 2007; Grutzendler et al., 2007). Postmortem analysis has also indicated that synapse loss occurs in mild cognitive impairment and worsens in AD, suggesting that synapse loss precedes the onset of AD (Arendt, 2009). Compensatory synaptic changes may counteract the effects of synapse loss in AD (Fiala et al., 2002). A better understanding of the early stages of the disease and the compensatory mechanisms that are acting at this stage would profoundly impact the direction of future investigation of therapeutic interventions in AD.
Substantial evidence now indicates that aggregation of Aβ into soluble oligomers (dimers→protofibrils) is a primary source of synaptotoxicity in AD, even in subjects with early disease (Lue et al., 1999; Naslund et al., 2000; Selkoe, 2002; Walsh and Selkoe, 2007; Selkoe, 2008; Koffie et al., 2011; Penzes and Vanleeuwen, 2011). Studies of the synaptic effects of Aβ are further elucidating how it acts to eliminate dendritic spines, and identifying many potential points at which alterations in kalirin may interact with this process (Hsieh et al., 2006; Selkoe, 2008; Koffie et al., 2011). Other studies clearly indicate microtubule associated protein tau is a necessary downstream mediator of some of Aβ’s synaptotoxic effects (Shipton et al., 2011). However, tau’s dendritic mechanisms are just emerging (Zempel et al., 2010), leaving unclear how it may interact with other mediators of dendritic spine loss engaged by soluble Aβ. Similarly, whether tau interacts directly with kalirin is unknown, although kalirin-9 and kalirin-12 contain RhoA GEF domains, and RhoA can modify tau phosphorylation and its interactions with the cytoskeleton (Amano et al., 2003).
For example, several recent studies have identified other mechanisms by which soluble Aβ may engage kalirin, along with its upstream regulators and downstream targets, in the pathology of AD (Figure 3) (Sweet et al., 2002; Scarmeas et al., 2005; Emanuel et al., 2011; Penzes and Vanleeuwen, 2011; Murray et al., 2012). EphB2 receptors are upstream regulators of kalirin signaling (Penzes et al., 2003) that have been implicated in synaptic degeneration induced by Aβ. Postmortem studies of AD patient brains and studies using hAPP transgenic mice have found that EphB2 receptors are reduced in the hippocampus in AD (Simon et al., 2009). Furthermore, treating cultured hippocampal neurons with Aβ oligomers leads to decreased surface expression of NMDARs and EphB2 receptors (Lacor et al., 2007). In addition, EphB2 and Aβ oligomers may interact directly as they can be co-immunoprecipitated in a cell-free system as well as from primary neuron homogenates (Cisse et al., 2011). A critical downstream effector of EphB2 and kalirin-7 signaling, PAK, has also been associated with AD. Postmortem analysis of AD patient brains found that PAK expression and activation are markedly reduced and that phosphoPAK was mislocalized in the hippocampus of AD patients as well as in AD transgenic mice (Zhao et al., 2006).
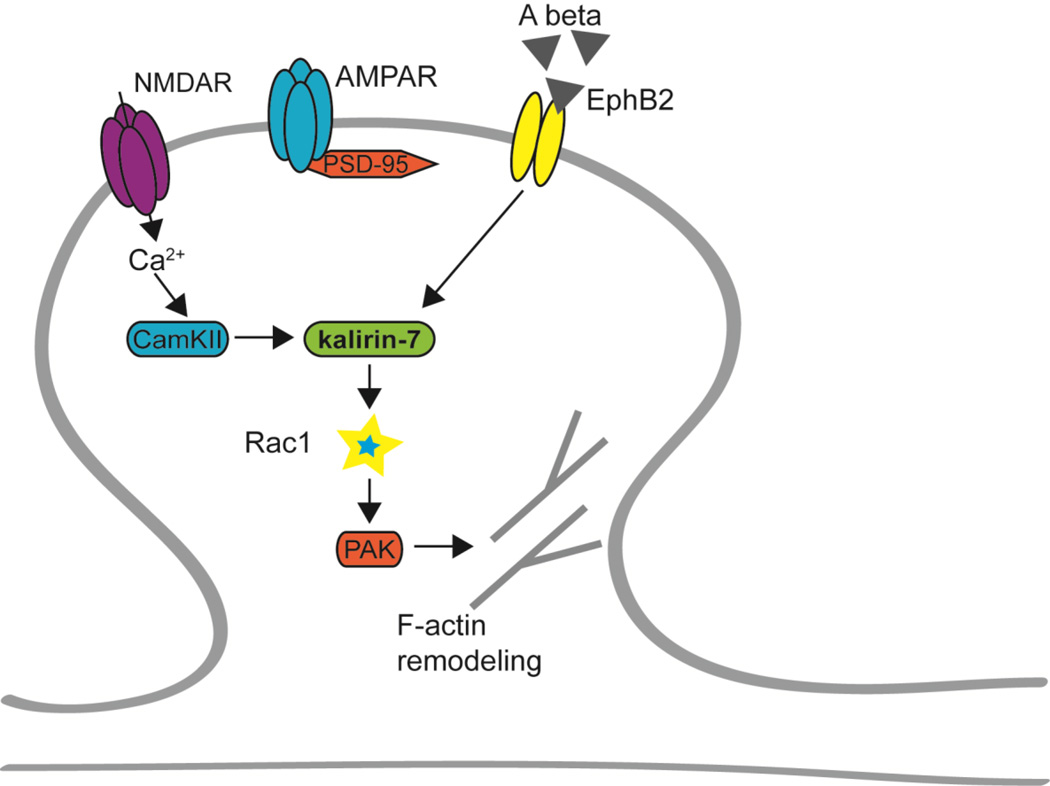
Figure 3. Kalirin-7 interacts with Alzheimer’s disease-associated proteins.
Amyloid β oligomers bind to dendritic spines in AD, leading to decreased surface expression of NMDARs and EphB2 receptors. This likely causes decreased kalirin/Rac/PAK signaling, leading to synapse loss and cognitive impairment. Aβ oligomers, kalirin-7, and PAK have been associated with AD.
Interestingly, kalirin-7 interacts with inducible nitric oxide synthase (iNOS) (Ratovitski et al., 1999), which has been shown to be neurotoxic in vitro (Mitrovic et al., 1995). Kalirin inhibits iNOS activity by preventing formation of active homodimers in a heterologous system (Ratovitski et al., 1999). Postmortem studies found that iNOS activity was significantly increased in AD hippocampus (Youn et al., 2007a). Co-immunoprecipitation from human brain lysates confirmed that kalirin-7 and iNOS interact in the human brain (Youn et al., 2007a). Furthermore, over-expression of kalirin-7 in cultured cells reduces iNOS activity (Youn et al., 2007a). Thus, it has been hypothesized that reduced kalirin-7 expression in the hippocampus of AD patients could account for increased iNOS activity and possibly cell death (Youn et al., 2007a).
Reduced function or loss of kalirin proteins in spines could precipitate spine collapse, leading to synapse loss and cognitive decline. There is some evidence to support this hypothesis. Postmortem studies have found that kalirin mRNA and kalirin-7 protein are specifically and significantly reduced in the hippocampus of AD patients compared to controls (Youn et al., 2007b; Youn et al., 2007a). Similarly, protein levels of kalirin-7, -9, and -12 are lower in prefrontal cortex in AD than in controls (Murray et al., 2012). Given the role of kalirin in schizophrenia, and the observations that AD patients with psychosis have greater impairment of cortical synapses than AD patients without psychosis (Sweet et al., 2002; Murray et al., In press), Murray and colleagues (Murray et al., 2012) further evaluated kalirin protein levels in prefrontal cortex in AD subjects with and without psychosis. They found kalirin-7, -9 and -12 were significantly reduced in the psychotic subjects in comparison to individuals without psychosis. These reductions occurred concurrently with increases in the ratio of soluble 42 amino acid Aβ peptide to 40 amino acid Aβ peptide (Aβ1–42:Aβ1–40), leaving open the question whether the greater kalirin reductions in psychotic individuals were due to greater pathologic drive from soluble Aβ (Murray et al., 2012).
Taken together, postmortem neuropathological and functional data suggest that kalirin reductions, through interactions with EphB and iNOS and its downstream effector PAK may contribute to the pathogenesis of AD, and to the additional impairments found in AD subjects with psychosis. However, currently there is no published genetic evidence implicating kalirin or these signaling partners in the etiology of AD, thus the postmortem data indicating kalirin reductions could equally arise as a consequence of dendritic spine loss. While ultimately disentangling these mechanisms will require direct mechanistic tests in model systems, a potential causal role of kalirin would be suggested if kalirin reductions were already present in the earliest stages of AD, or with regard to AD and psychosis, in the stages of AD preceding onset of this behavioral symptom. The most rapid rise in the prevalence of psychosis in AD occurs in early to middle stages of cognitive impairment, (Lopez et al., 2003; Ropacki and Jeste, 2005) and the more rapid cognitive decline associated with psychosis in AD is detectable even before psychosis onset (Paulsen et al., 2000; Emanuel et al., 2011). Neuropathologically, Braak stages 3–5 correspond roughly to the early to middle stages of cognitive impairment, i.e. those associated with earliest cognitive symptoms and increasing frequency of psychosis onset (Nelson et al., 2009). In contrast, Braak stage 6 is associated with end-stage cognitive impairment (e.g. see Fig 2B in (Nelson et al., 2009)), a stage after most psychosis onset has occurred. We therefore re-analyzed the data reported in Murray et al, focusing on Braak stage 3–5 subjects (Murray et al., 2012).
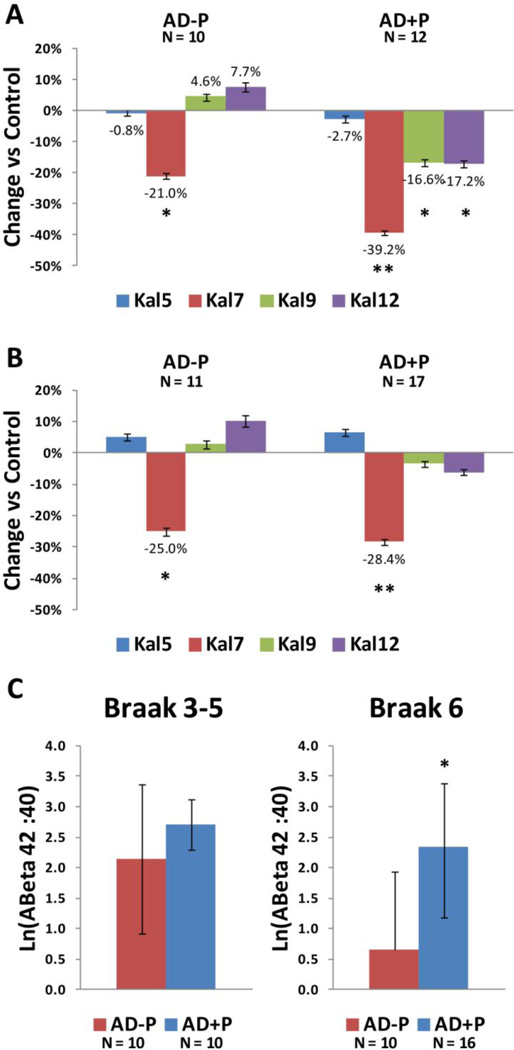
In Braak stage 3–5 cases, the previously identified reductions in kalirin-9, and kalirin-12 isoforms were selective for AD cases with associated psychosis (Fig 4A). Reductions in kalirin-7 were markedly accelerated in AD cases with psychosis. Kalirin-5 was not reduced in AD with or without psychosis. These patterns contrasted with that for Aβ1–42:Aβ1–40 ratio. The previously reported elevation in Aβ1–42:Aβ1–40 ratio in AD with psychosis was only seen in Braak stage 6, (due largely to a terminal drop in the non-psychotic group, rather than to further elevation in psychotic subjects).
Figure 4. Reductions in kalirin isoforms relative to control in Braak stages 3–5.
(A) and Braak stage 6 (B) subjects. (C) Aβ ratio by Braak stage. AD−P: AD without psychosis; AD+P: AD with psychosis; * p < 0.05, ** p<0.001.
This reanalysis suggests several tentative conclusions. First, the selectivity of reductions in kalirin-9 and kalirin-12 in AD subjects with psychosis (and possibly the substantially greater reductions in kalirin-7) indicate that this molecular mechanism may be unique to the roughly 50% of AD patients at risk for psychotic behaviors, rather than being common to all AD cases. Second, the kalirin reductions noted above precede any differentiation of soluble Aβ1–42:Aβ1–40 ratio between AD subjects with and without psychosis, indicating that this unique mechanism is not just secondary to an increased tempo of Aβ drive. Third, the lack of reduction in kalirin-5 in both groups, and the lack of reductions of kalirin-9 and kalirin-12 in the AD group, does not support the detected reductions of kalirin being subsequent to synapse loss, which should deplete all four isoforms. Rather, as reviewed above, studies of kalirin outside the context of Aβ overproduction indicate that reduced kalirin induces spine loss and cognitive impairment. Clearly these conclusions require validation in model systems in which kalirin levels can be manipulated independently of Aβ overproduction.
Kalirin and other disorders
Kalirin has been genetically and functionally associated with several other human disorders. Parkinson’s disease (PD) is a common neurodegenerative disorder that is characterized by accumulation of cytoplasmic inclusions of α-synuclein, called Lewy bodies, and degeneration of dopaminergic neurons in the substantia nigra (Tsai et al., 2012). Synphilin-1 is an interacting partner of α-synuclein and promotes formation of cytoplasmic inclusions. A recent study identified kalirin-7 as a novel interacting partner for synphilin-1 (Tsai et al., 2012). They found that kalirin-7 enhanced recruitment of synphilin-1 inclusions into aggresomes and promoted the degradation of these aggresomes via an HDAC6-mediated mechanism (Tsai et al., 2012).
Huntington’s disease (HD) is a genetic neurodegenerative disorder that is caused by expanded trinucleotide repeats in the widely expressed huntingtin protein (Group, 1993; Li et al., 1993). Huntingtin-associated protein 1 (HAP1) is one of only a few known proteins that interact with huntingtin in a repeat length-dependent manner (Li et al., 1995). Kalirin has been shown to interact with HAP1 (Colomer et al., 1997). While this interaction requires further examinations, it is of interest as it may provide insight into the functional significance of the interaction between huntingtin and HAP1 and also suggests a potential role for Rac signaling in HD.
The KALRN gene has been genetically linked to adult ADHD. In a recent study, Lesch and colleagues (2008) identified 15 SNPs in the KALRN open reading frame that associated with ADHD. In the same study, SNPs associated with ADHD were also identified in CDH13, the gene encoding cadherin 13 and CTNNA2, the gene encoding αN-catenin (Lesch et al., 2008). Cadherins have been shown to regulate spine morphology in a kalirin-dependent manner, thus aberrant function of one or both of these proteins may have detrimental effects on spine remodeling (Xie et al., 2008).
Kalirin has also been identified as a key factor in regulation of dendritic spines in a mouse model of addiction. Addiction is a compulsive need for drug use and it has been hypothesized that drug addiction is the result of aberrant learning mechanisms, which could be explained by abnormal regulation of plasticity at dendritic spines (Hyman et al., 2006). Since kalirin plays a key role in regulating structural and functional plasticity at dendritic spines, it is possible that kalirin may play a role in addiction.
Kiraly and colleagues used Kal7 KO mice to demonstrate that kalirin-7 is required for normal morphological and behavioral responses to chronic cocaine administration (Kiraly et al., 2010). In wild-type mice, chronic cocaine treatment induced an increase in kalirin-7 protein and mRNA in the nucleus accumbens (NAc) as well as an increase in dendritic spine size and density. Interestingly, chronic cocaine treatment in Kal7 KO mice did not alter spine density and induced a decrease in spine size. Moreover, Kal7 KO mice showed increased locomotor sensitization to cocaine following chronic treatment and maintained this higher sensitization for at least one week following the last dose of cocaine. In addition, Kal7 KO mice showed significantly diminished conditioned place preference for cocaine. Importantly, Kal7 KO mice showed no difference from wild-type mice in place preference for food, indicating a cocaine-specific deficit in place conditioning. These studies indicate that kalirin-7 plays a key role in formation of dendritic spines following cocaine treatment.
Kiraly and colleagues went on to show that expression of larger kalirin splice variants, kalirin-9 and -12, is upregulated in the Kal7 KO mice following cocaine administration (Kiraly et al., 2013). They also found that expression of the NMDAR subunit NR2B is upregulated following cocaine administration in wild-type, but not Kal7 KO mice (Kiraly et al., 2013). Furthermore, chronic treatment with cocaine has been shown to alter the expression profile of specific kalirin isoforms by altering usage of isoform-specific promoters. Chronic cocaine exposure increases the usage of the promoter and the 3’ exon that are specific to kalirin-7 and Δ-kalirin-7, while usage of the kalirin-9 specific 3’ exon was reduced (Mains et al., 2011).
In another recent study, Wang and colleagues assessed changes in kalirin signaling and AMPAR localization following cocaine sensitization (Wang et al., 2013). They found that the kalirin-Rac-PAK signaling cascade was activated following cocaine sensitization and that this signaling is necessary for sensitization-induced upregulation of AMPARs and dendritic spine density (Wang et al., 2013). These findings provide evidence that couples AMPARs to spine plasticity following cocaine exposure.
Kalirin signaling may also be involved in depression and chronic stress. Chronic restraint stress (CRS) causes a depression-like behavioral and physiological response in mice, including decreased time to immobility on a forced-swim test and reduced body weight (Li et al., 2010). Interestingly, recent studies have demonstrated that kalirin-7 expression is significantly reduced in the mouse hippocampus following CRS (Li et al., 2010). Furthermore, administration of estrogen during CRS attenuated the behavioral and physiological responses and increased kalirin-7 expression (Li et al., 2010).
Genetic studies have identified KALRN as a risk factor for coronary artery disease (Wang et al., 2007; Ikram et al., 2009). Based on this genetic evidence and the fact that kalirin has been implicated in nitric oxide (NOS) signaling, recent genetic studies have sought to determine if KALRN is genetically linked to ischemic stroke. In a case-control study, Krug and colleagues identified two SNPs in the 5’ region of KALRN gene that associated with increased risk for ischemic stroke (Krug et al., 2010). However, the link between SNPs in the KALRN gene and risk for ischemic stroke requires further validation, as another study was unable to replicate the linkage of all three SNPs in two of the three sample populations used (Olsson et al., 2011). Another recent study characterized changes in kalirin-7 expression following ischemic stroke in the gerbil hippocampus. Beresewicz and colleagues (2008) found that under control conditions, kalirin-7 levels were significantly higher in the ischemia-resistant CA2/3 and DG regions than in the ischemia-vulnerable CA1 region of the hippocampus. Interestingly, after 5 minutes of ischemia and 1 hour of reperfusion, kalirin-7 was significantly reduced in CA2/3 and DG and was significantly elevated in CA1. In another study, Jourdain and colleagues (2002) observed cytoskeletal rearrangements in organotypic hippocampal cultures following oxygen and glucose deprivation, these were found to be Ca2+ and NMDA receptor-dependent. Thus, changes in kalirin-7 levels may act downstream of NMDA receptors in ischemia to induce cytoskeletal rearrangements.
In sum, accumulating evidence implicates kalirin dysfunction in a broad range of neurological and psychiatric disorders, in addition to schizophrenia and AD. As all of these disorders are associated with abnormal spine plasticity and neuronal connectivity, kalirin may be part of the disease pathway, and may provide a therapeutic target for reversing these deficits.
Disease-related phenotypes in kalirin knockout mice
Given the role of kalirin in dendritic spine morphogenesis and its association with human disorders, it seems plausible that alterations in kalirin signaling may contribute to behavioral alterations relevant for brain disorders. To investigate the effects of kalirin loss in vivo, Cahill and colleagues generated a mouse line carrying a full knockout of the KALRN gene (Cahill et al., 2009). Analysis of these mice revealed an age-dependent reduction of spine density in the cortex and hippocampus (Cahill et al., 2009; Vanleeuwen and Penzes, 2012). Cortical spine loss is not observed at 3 weeks of age, but is evident at 3 months while hippocampal spine deficits are not observed until 11 months.
Behavioral analysis of KALRN KO mice shows that these mice have impaired working memory but do not show deficits in reference memory, indicating that kalirin may play a specific role in modulating working memory. Importantly, working memory deficits are a core endophenotype of schizophrenia. Recurrent excitation in microcircuits in the prefrontal cortex has been hypothesized to underlie working memory (Durstewitz et al., 2000). Therefore, decreased dendritic spine density could lead to reduced output of these circuits in KALRN KO mice and schizophrenia patients. In addition to working memory deficits, KALRN KO mice exhibit other schizophrenia-related behavioral phenotypes such as impaired prepulse inhibition, decreased social interaction and locomotor hyperactivity (Cahill et al., 2009).
The age-dependent onset of cortical morphological and behavioral phenotypes in these mice parallels the onset of schizophrenia in humans, which also occurs during adolescence or young adulthood (Lewis and Gonzalez-Burgos, 2008). This could be useful in understanding the age-dependence of schizophrenia emergence in humans. The molecular and cellular mechanisms underlying this delayed phenotype emergence are not known, and warrant more investigation. One contributor could be “phenotype unmasking”, whereby natural changes in expression levels of similar proteins might uncover the effects of a genetic defect later in life. While multiple synaptic Rac1-GEFs (kalirin-7, Tiam1, βPIX) are present in both the hippocampus and cortex in young animals, the expression levels of Tiam1 and βPIX decrease significantly in the adult cortex, but not hippocampus (Penzes et al., 2008). Thus by adulthood, kalirin-7 is the predominant synaptic Rac1-GEF in the cortex. Reduction in kalirin expression may thus lead to enhanced spine elimination or impaired spine stabilization. Alternatively, kalirin may play a unique role in the prefrontal cortex, which would account for regional differences in spines. Interestingly, prenatal knockdown of DISC1 also results in delayed onset dysfunction of cortical circuits (Niwa et al., 2010), suggesting that the kalirin/DISC1 interaction may play a role in periadolescent spine stabilization. An important future direction for these studies is to characterize the development of spine morphological and behavioral phenotypes that correspond to presymptomatic and symptomatic stages of the disease.
Ma and colleagues generated another kalirin mouse model, Kal7 KO, in which the exon unique to kalirin-7 has been deleted (Ma et al., 2008b). While kalirin-7 and Δkalirin-7, a truncated isoform of kalirin-7 that is also expressed in the brain, are absent in the brains of Kal7 KO mice, kalirin-9 and kalirin-12 are upregulated by approximately 50% (Ma et al., 2008b). Contrary to the KALRN KO mice, Kal7 KO mice have significantly reduced spine size and density in the hippocampus (Ma et al., 2008b). As loss of the entire KALRN gene does not affect hippocampal spine density (Cahill et al., 2009), upregulation of other kalirin isoforms may account for this, as both kalirin-9 and -12 (but not kalirin-7) contain RhoA GEF domains (Rabiner et al., 2005), which have been shown to play a role in spine elimination (Tashiro et al., 2000). Although Deo and colleagues did not observe spine loss after isolated kalirin-9 upregulation in vitro, whether the sustained exposure to upregulated kalirin-9 obtainable in an in vivo model (alone or in combination with kalirin-12 and kalirin-7 changes), might be a pathological mechanism in schizophrenia is worth further investigation.
Kal7 KO mice have impaired contextual fear learning (Ma et al., 2008b). This learning deficit seems specific to fear associations, as Kal7 KO mice performed well in radial arm maze and novel object recognition tasks (Ma et al., 2008b). Consistent with this, in KALRN KO mice both context and cue-dependent fear conditioning are impaired, suggesting an amygdala deficit (Xie et al., 2011). Both of these mouse models have deficits in contextual learning; however, the relevance of these deficits for schizophrenia is unclear. It seems plausible that behaviors in mouse models do not closely mimic behaviors observed in human disease because kalirin loss is global in the mouse, whereas it is likely cell population or region-specific in humans.
Mandela and colleagues recently developed another KALRN knockout model that was generated by inserting LoxP sites on either side of exon 13 of the spectrin region of KALRN; cre-mediated excision of this exon prevents production of a functional protein (Mandela et al., 2012). Global cre expression yields global KALRN knockout mice, KalSRKO/KO. Surprisingly, these mice do not display deficits in PPI, but they do show decreased anxiety-like behavior on an elevated zero maze task. The mice also exhibit hippocampal deficits, shown by a decrease in passive avoidance conditioning (Mandela et al., 2012). Pituitary cultures from these mice have reduced basal secretion of growth hormone and prolactin, indicating a deficit in secretory function. KalSRKO/KO. The authors went on to characterize the effects of kalirin loss on the neuromuscular junction and found that they have abnormal structure of the neuromuscular junction. KalSRKO/KO mice showed impaired neuromuscular function as evidenced by decreased ability to stay on a rotarod and decreased ability to perform the wire hang task (Mandela et al., 2012). These studies reveal and interesting novel role for kalirin outside of the brain. The described phenotypes in the neuromuscular junction are consistent with the role of kalirin signaling in NRG1-ErbB4 signaling, which is important at the neuromuscular junction.
All three of the kalirin mouse models discussed here exhibit disease-relevant phenotypes and will be powerful tools for future investigation of mechanisms underlying synaptic plasticity and pathology (Table 2). However, direct comparisons between these mutants are not ideal for a number of reasons. First, there is differential expression of other kalirin isoforms in the Kal7 KO. Furthermore, differences in methods and quality of testing could also account for some of the variability, since not all phenotypes were analyzed in the same way in all mutants.
Table 2.
Summary of mouse models
| Phenotypes | ||||
|---|---|---|---|---|
| Mouse model: |
Morphological | Behavioral impairments | Molecular | Other |
| KALRN KO | ↓ dendritic spine density in hippocampus and cortex (age-dependent) | Working memory Prepulse inhibition Social interaction Locomotor hyperactivity context and cue-dependent fear conditioning |
↓ Rac activation | -- |
| Kal7 KO | ↓ dendritic spine density in hippocampus and cortex | Contextual fear learning | ↑ expression of kalirin-9 and -12 | -- |
| KalSRKO/KO | -- | ↓ anxiety-like behavior ↓ passive avoidance conditioning |
-- | Impaired secretory and pituitary function |
Conclusions and future directions
Taken together, several lines of evidence support a role for kalirin in spine pathology in brain disorders. In many cases, postmortem studies find that kalirin expression is reduced in affected individuals, such as schizophrenia and AD. This reduction in kalirin levels could be caused by diverse mechanisms, including removal from spines and relocation to other regions (such as the dendrites), proteosomal degradation, reduced transcription and translation, and altered splicing. Further studies are required to determine whether kalirin alterations are a cause or consequence of spine pathology. However, in schizophrenia and AD, it seems that reduced kalirin levels are primary, because other synaptic proteins are not equally reduced.
Here we have discussed a series of genetic, morphological, and functional studies that implicate kalirin as a common mediator of synaptic pathology in a number of psychiatric and neurological disorders. The strongest evidence to date links kalirin to schizophrenia, but there is now evidence that suggests a possible role in AD, addiction, chronic stress, stroke, Huntington’s, and Parkinson’s. This is consistent with the fact that kalirin functions in common pathways with molecules previously associated with these disorders, to regulate normal spine formation, maintenance, and plasticity. Kalirin-mediated spine pathology might thus be a cellular phenotype of interest in these disorders. Therapeutically manipulating kalirin activity might provide an efficient way to reverse spine plasticity deficits in some psychiatric and neurological disorders.
Highlights.
We describe recent genetic and postmortem studies linking kalirin to schizophrenia.
We discuss evidence linking aberrant kalirin signaling to Alzheimer’s disease.
We also cite recent studies that implicate kalirin in ADHD, addiction, and stroke.
We provide a comprehensive review of animal models of kalirin loss.
Acknowledgements
This work was supported by a grant from NIH-NIMH to P.P. (R01MH071316), by Veterans Health Administration (BX000452) and NIH grants (MH071533 and AG005133) to R.A.S, and by National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) through Grant Number 8UL1TR000150 to C.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or, the National Institutes of Health, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Amano M, Kaneko T, Maeda A, Nakayama M, Ito M, Yamauchi T, Goto H, Fukata Y, Oshiro N, Shinohara A, Iwamatsu A, Kaibuchi K. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 2003;87:780–790. doi: 10.1046/j.1471-4159.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Costa V, Mauroudis I, Psaroulis D, Manolides SL, Manolides LS. Dendritic and spinal pathology in the acoustic cortex in Alzheimer's disease: morphological and morphometric estimation by Golgi technique and electron microscopy. Acta Otolaryngol. 2007;127:351–354. doi: 10.1080/00016480601126986. [DOI] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Muller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresewicz M, Kowalczyk JE, Zablocka B. Kalirin-7, a protein enriched in postsynaptic density, is involved in ischemic signal transduction. Neurochem Res. 2008;33:1789–1794. doi: 10.1007/s11064-008-9631-y. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Remmers C, Jones KA, Xie Z, Sweet RA, Penzes P. Neuregulin1 signaling promotes dendritic spine growth through kalirin. J Neurochem. 2013 doi: 10.1111/jnc.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Jones KA, Rafalovich I, Xie Z, Barros CS, Muller U, Penzes P. Control of interneuron dendritic growth through NRG1/erbB4-mediated kalirin-7 disinhibition. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF, Surmeier DJ, Penzes P. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Chen JL, Nedivi E. Neuronal structural remodeling: is it all about access? Curr Opin Neurobiol. 2010;20:557–562. doi: 10.1016/j.conb.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, Ho K, Yu GQ, Mucke L. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Engelender S, Sharp AH, Duan K, Cooper JK, Lanahan A, Lyford G, Worley P, Ross CA. Huntingtin-associated protein 1 (HAP1) binds to a Trio-like polypeptide, with a rac1 guanine nucleotide exchange factor domain. Hum Mol Genet. 1997;6:1519–1525. doi: 10.1093/hmg/6.9.1519. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Deo AJ, Cahill ME, Li S, Goldszer I, Henteleff R, Vanleeuwen JE, Rafalovich I, Gao R, Stachowski EK, Sampson AR, Lewis DA, Penzes P, Sweet RA. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: its role in dendritic pathology. Neurobiol Dis. 2012;45:796–803. doi: 10.1016/j.nbd.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Emanuel JE, Lopez OL, Houck PR, Becker JT, Weamer EA, Demichele-Sweet MA, Kuller L, Sweet RA. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am J Geriatr Psychiatry. 2011;19:160–168. doi: 10.1097/JGP.0b013e3181e446c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Golimbet VE, Lavrushina OM, Kaleda VG, Abramova LI, Lezheiko TV. Supportive evidence for the association between the T102C 5-HTR2A gene polymorphism and schizophrenia: a large-scale case-control and family-based study. Eur Psychiatry. 2007;22:167–170. doi: 10.1016/j.eurpsy.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Group THsDCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Helmin K, Tsai J, Gan WB. Various dendritic abnormalities are associated with fibrillar amyloid deposits in Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:30–39. doi: 10.1196/annals.1379.003. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010 doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikeda M, et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry. 2011;69:472–478. doi: 10.1016/j.biopsych.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Ikram MA, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Nikonenko I, Alberi S, Muller D. Remodeling of hippocampal synaptic networks by a brief anoxia-hypoglycemia. J Neurosci. 2002;22:3108–3116. doi: 10.1523/JNEUROSCI.22-08-03108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Nemirovsky NE, Larese TP, Tomek SE, Yahn SL, Olive MF, Eipper BA, Mains RE. Constitutive knockout of kalirin-7 leads to increased rates of cocaine self-administration. Molecular pharmacology. 2013;84:582–590. doi: 10.1124/mol.113.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Mansuy IM. Dendritic Spine Loss and Synaptic Alterations in Alzheimer's Disease. Mol Neurobiol. 2008 doi: 10.1007/s12035-008-8018-z. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Krug T, et al. Kalirin: a novel genetic risk factor for ischemic stroke. Hum Genet. 2010;127:513–523. doi: 10.1007/s00439-010-0790-y. [DOI] [PubMed] [Google Scholar]
- Kushima I, Nakamura Y, Aleksic B, Ikeda M, Ito Y, Shiino T, Okochi T, Fukuo Y, Ujike H, Suzuki M, Inada T, Hashimoto R, Takeda M, Kaibuchi K, Iwata N, Ozaki N. Resequencing and Association Analysis of the KALRN and EPHB1 Genes And Their Contribution to Schizophrenia Susceptibility. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT, Craig DW, Romanos J, Heine M, Meyer J, Freitag C, Warnke A, Romanos M, Schafer H, Walitza S, Reif A, Stephan DA, Jacob C. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Schilling G, Young WS, 3rd, Li XJ, Margolis RL, Stine OC, Wagster MV, Abbott MH, Franz ML, Ranen NG, et al. Huntington's disease gene (IT15) is widely expressed in human and rat tissues. Neuron. 1993;11:985–993. doi: 10.1016/0896-6273(93)90127-d. [DOI] [PubMed] [Google Scholar]
- Li W, Li QJ, An SC. Preventive effect of estrogen on depression-like behavior induced by chronic restraint stress. Neurosci Bull. 2010;26:140–146. doi: 10.1007/s12264-010-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, Habeych M, DeKosky ST. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol. 2001;429:388–402. doi: 10.1002/1096-9861(20010115)429:3<388::aid-cne3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008a;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Kiraly DD, Eipper-Mains JE, Ma XM, Eipper BA. Kalrn promoter usage and isoform expression respond to chronic cocaine exposure. BMC Neurosci. 2011;12:20. doi: 10.1186/1471-2202-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandela P, Yankova M, Conti LH, Ma XM, Grady J, Eipper BA, Mains RE. Kalrn plays key roles within and outside of the nervous system. BMC Neurosci. 2012;13:136. doi: 10.1186/1471-2202-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Christie S, Porteous DJ. Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochem Biophys Res Commun. 2003;311:1019–1025. doi: 10.1016/j.bbrc.2003.10.101. [DOI] [PubMed] [Google Scholar]
- Mitrovic B, Ignarro LJ, Vinters HV, Akers MA, Schmid I, Uittenbogaart C, Merrill JE. Nitric oxide induces necrotic but not apoptotic cell death in oligodendrocytes. Neuroscience. 1995;65:531–539. doi: 10.1016/0306-4522(94)00491-m. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Kane A, Goff DC, Walsh CA. Sequence analysis of P21-activated kinase 3 (PAK3) in chronic schizophrenia with cognitive impairment. Schizophr Res. 2008;106:265–267. doi: 10.1016/j.schres.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle JG, Dodd AF, McGrath JA, Wolyniec PS, Mitchell AA, Shetty AC, Sobreira NL, Valle D, Rudd MK, Satten G, Cutler DJ, Pulver AE, Warren ST. Microdeletions of 3q29 confer high risk for schizophrenia. Am J Hum Genet. 2010;87:229–236. doi: 10.1016/j.ajhg.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Kumar S, MA D-M-S, RA S. Psychosis in Alzheimer's Disease. Biol Psychiatry. doi: 10.1016/j.biopsych.2013.08.020. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Kirkwood CM, Gray MC, Ikonomovic MD, Paljug WR, Abrahamson EE, Henteleff RA, Hamilton RL, Kofler JK, Klunk WE, Lopez OL, Penzes P, Sweet RA. beta-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging. 2012;33:2807–2816. doi: 10.1016/j.neurobiolaging.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, Huang B, Kohda K, Noda Y, O'Donnell P, Nakajima K, Sawa A, Nabeshima T. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson S, Jood K, Melander O, Sjogren M, Norrving B, Nilsson M, Lindgren A, Jern C. Lack of association between genetic variations in the KALRN region and ischemic stroke. Clin Biochem. 2011;44:1018–1020. doi: 10.1016/j.clinbiochem.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Vanleeuwen JE. Impaired regulation of synaptic actin cytoskeleton in Alzheimer's disease. Brain Res Rev. 2011;67:184–192. doi: 10.1016/j.brainresrev.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Rabiner CA, Mains RE, Eipper BA. Kalirin: a dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist. 2005;11:148–160. doi: 10.1177/1073858404271250. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Ratovitski EA, Alam MR, Quick RA, McMillan A, Bao C, Kozlovsky C, Hand TA, Johnson RC, Mains RE, Eipper BA, Lowenstein CJ. Kalirin inhibition of inducible nitric-oxide synthase. J Biol Chem. 1999;274:993–999. doi: 10.1074/jbc.274.2.993. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer's disease: a review of 55 studies published from 1990 to 2005. Am J Psychiatry. 2003;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Rubio MD, Haroutunian V, Meador-Woodruff JH. Abnormalities of the Duo/Ras-related C3 botulinum toxin substrate 1/p21-activated kinase 1 pathway drive myosin light chain phosphorylation in frontal cortex in schizophrenia. Biol Psychiatry. 2012;71:906–914. doi: 10.1016/j.biopsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Devanand D, Honig L, Marder K, Bell K, Wegesin D, Blacker D, Stern Y. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, Dawson HN, Vitek MP, Wade-Martins R, Paulsen O, Vargas-Caballero M. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Perez-Mediavilla A, Avila J, Del Rio J, Frechilla D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer's disease. J Alzheimers Dis. 2009;17:773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Spires TL, Grote HE, Garry S, Cordery PM, Van Dellen A, Blakemore C, Hannan AJ. Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington's disease transgenic mice. Eur J Neurosci. 2004;19:2799–2807. doi: 10.1111/j.0953-816X.2004.03374.x. [DOI] [PubMed] [Google Scholar]
- St.Jean PMNC. GENES ASSOCIATED WITH SCHIZOPHRENIA IDENTIFIED USING A WHOLE GENOME SCAN. US. 2008 [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Riess O, Soehn AS, Nguyen HP. The Guanine nucleotide exchange factor kalirin-7 is a novel synphilin-1 interacting protein and modifies synphilin-1 aggregate transport and formation. PLoS One. 2012;7:e51999. doi: 10.1371/journal.pone.0051999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanleeuwen JE, Penzes P. Long-term perturbation of spine plasticity results in distinct impairments of cognitive function. J Neurochem. 2012;123:781–789. doi: 10.1111/j.1471-4159.2012.07899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang L, Hauser ER, Shah SH, Pericak-Vance MA, Haynes C, Crosslin D, Harris M, Nelson S, Hale AB, Granger CB, Haines JL, Jones CJ, Crossman D, Seo D, Gregory SG, Kraus WE, Goldschmidt-Clermont PJ, Vance JM. Peakwide mapping on chromosome 3q13 identifies the kalirin gene as a novel candidate gene for coronary artery disease. Am J Hum Genet. 2007;80:650–663. doi: 10.1086/512981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, Loweth JA, Marinelli M, Russo SJ, Penzes P, Wolf ME. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11012–11022. doi: 10.1523/JNEUROSCI.1097-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, Huganir RL, Penzes P. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Cahill ME, Radulovic J, Wang J, Campbell SL, Miller CA, Sweatt JD, Penzes P. Hippocampal phenotypes in kalirin-deficient mice. Mol Cell Neurosci. 2011;46:45–54. doi: 10.1016/j.mcn.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H, Ji I, Ji HP, Markesbery WR, Ji TH. Under-expression of Kalirin-7 Increases iNOS activity in cultured cells and correlates to elevated iNOS activity in Alzheimer's disease hippocampus. J Alzheimers Dis. 2007a;12:271–281. doi: 10.3233/jad-2007-12309. [DOI] [PubMed] [Google Scholar]
- Youn H, Jeoung M, Koo Y, Ji H, Markesbery WR, Ji I, Ji TH. Kalirin is under-expressed in Alzheimer's disease hippocampus. J Alzheimers Dis. 2007b;11:385–397. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]