Abstract
More than 60% of patients who are diagnosed with epithelial ovarian cancer (EOC) present with extensive peritoneal carcinomatosis. EOC cells typically disseminate by shedding into the peritoneal fluid where they survive as multi-cellular aggregates and then implant onto peritoneal surfaces. However, the mechanism that facilitates aggregation and implantation of EOC cells is poorly understood. The cell adhesion molecule P-cadherin (CDH3) has been reported to be induced during early progression of EOC and to promote tumor cell migration. In this study, P-cadherin not only promoted migration of EOC cells, but also facilitated the assembly of floating EOC cells into multi-cellular aggregates and inhibited anoikis in vitro. Furthermore, inhibiting P-cadherin by shRNAs or a neutralizing antibody prevented EOC cells from attaching to peritoneal mesothelial cells in vitro. In mouse intraperitoneal xenograft models of EOC, inhibition of P-cadherin decreased the aggregation and survival of floating tumor cells in ascites and reduced the number of tumor implants on peritoneal surfaces. These findings indicate that P-cadherin promotes intraperitoneal dissemination of EOC by facilitating tumor cell aggregation and tumor-peritoneum interactions in addition to promoting tumor cell migration.
Implications:
Inhibiting P-cadherin blocks multiple, key steps of EOC progression and has therapeutic potential.
Keywords: ovarian cancer, cell adhesion, anoikis, mesothelium, P-cadherin
INTRODUCTION
The lethality of epithelial ovarian cancer (EOC) stems from its propensity for intraperitoneal dissemination. More than 60% of EOC patients present with advanced-stage disease that has disseminated throughout the peritoneal cavity [1]. These patients are treated with limited success by surgery and conventional chemotherapy and have a 5-year survival rate of less than 30% [1]. Unlike many other types of solid tumors, EOC rarely spreads via hematogenous routes. The most widely recognized pattern of EOC dissemination is intraperitoneal ‘seeding’ whereby tumor cells passively shed into, and are transported by, the circulating peritoneal fluid [2-4]. EOC cells are frequently present in ascites either as floating multi-cellular aggregates or as single cells. It is thought that aggregation of floating EOC cells prevents anoikis and also facilitates the implantation of tumor cells on to peritoneal surfaces such as the cavity wall, bowel serosa and the omentum [3-5]. These surfaces are lined by a monolayer of mesothelial cells that acts as a protective barrier against tissue injury and infection [6]. Growth of tumor implants on peritoneal surfaces often causes substantial pain and leads to bowel obstruction. Targeting cell surface molecules that promote both the aggregation of EOC cells and their implantation therefore represents an attractive therapeutic approach to improve outcomes.
It is widely recognized that most types of epithelial tumors initially progress by undergoing epithelial-to-mesenchymal transition (EMT), a process that loosens adhesion between epithelial cells and eases their attachment to the basement membrane [7,8]. A hallmark of EMT is ‘cadherin-switching’, whereby expression of the cell adhesion molecule E-cadherin decreases and expression of N-cadherin concomitantly increases [7,8]. EOC cells are thought to undergo an EMT-like process during early stages of disease progression. Clustering of collagen-binding integrins induces matrix metalloproteinase-9 that cleaves the E-cadherin ectodomain, thereby loosening adhesion between EOC cells and enabling tumor cells to shed [9]. E-cadherin levels are often down-regulated in floating ascitic EOC cells as compared to matching primary solid tumors [10]. However, the mechanisms that mediate adhesion between floating EOC cells are poorly understood. Two independent studies have found a striking induction in levels of P-cadherin during early stages of EOC progression [11,12]. Induction of P-cadherin has been reported to increase migratory potential of EOC cells [13]. P-cadherin has also been found to be the most predominant type of cadherin expressed in malignant peritoneal effusions of EOC patients and in normal peritoneal tissues [11,14]. These findings raise the possibility that P-cadherin might facilitate the assembly of floating EOC cells into multi-cellular aggregates and also mediate tumor-peritoneum interactions. In this study, we identified that inhibition of P-cadherin not only decreases EOC cell migration, but also decreases the aggregation of floating EOC cells, increases anoikis and prevents EOC cells from implanting on to peritoneal surfaces. Targeting P-cadherin might therefore be a potential strategy to block multiple rate-limiting steps in the progression of EOC.
MATERIALS AND METHODS
Antibodies (Abs) and plasmids
Abs to human P-cadherin were purchased from BD Biosciences (for Western blot) and Abcam (for neutralization). Ab to mouse P-cadherin was purchased from Invitrogen. Other sources of Abs were as follows: E-cadherin (Invitrogen), N-cadherin, Cdc42, Rac1, active caspase-3 (BD Biosciences), actin, secondary Abs (Sigma-Aldrich). pGIPZ lentiviral plasmids containing non-targeting shRNA and shRNAs targeting CDH3 (encoding P-cadherin) were purchased from Thermo Scientific. Myc-tagged dominant-negative mutant forms of Rac1 (T17N) and Cdc42 (T17N) [15] were provided by Gary Bokoch (Scripps Research Institute) (Addgene plasmids 12984, 12973).
Cell culture and transfection
SKOV3ip and OVCA429 cell lines were provided by Gordon Mills (MD Anderson Cancer Center) and cultured in McCoys 5A and MEM media, respectively (Invitrogen). Cell lines were authenticated by STR analysis performed by the MD Anderson Cancer Center Characterized Cell Line Core Facility. The 293 cell line was purchased from American Type Culture Collection and cultured in DMEM medium (Invitrogen). All media were supplemented with 10% FBS and penicillin-streptomycin. 293 cells were transfected with pGIPZ plasmids by using Lipofectamine 2000 reagent (Invitrogen). At 2 days thereafter, culture supernatants were harvested and used to infect SKOV3ip and OVCA429 cells. Infected tumor cell lines were selected with puromycin (0.5 μg/ml). Primary cultures of normal human omental mesothelial cells have been previously described [16] and were provided by Ernst Lengyel (University of Chicago).
Immunoprecipitation and Western blot analysis
Cell lysates were prepared by using M-PER buffer (Pierce Biotechnology), separated by SDS-PAGE and transferred to PVDF membranes (GE Healthcare). Active forms of Rac1 and Cdc42 were detected in cell lysates by immunoprecipitation using GST-tagged protein containing the PAK1 protein binding domain (Cytoskeleton, Inc.)
qRT-PCR
Transcripts of EMT-associated genes were analyzed by using SYBR®Green qPCR Master Mix (SABiosciences) and primers described in our previous work [17]. RPL32 transcript levels were used as controls for normalization.
Cell migration assays
Tumor cells were seeded in the upper chamber in 24-well transwell chambers (BD Biosciences) that were coated with Matrigel or left uncoated (5×104 cells per uncoated well, 1×105 cells per coated well). Migrating cells were assayed at 6 h (for uncoated wells) and at 16 h (for coated wells). Migrating cells were stained with Giemsa solution and counted in five random 100x microscopic fields per well. Three independent experiments were performed for each assay.
Cell viability and cell death assays
Cell viability was measured by the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche). Tumor cells were seeded in 96-well plates (1×104 cells per well) that were coated with poly(2-hydroxyethyl methacrylate) (polyHEMA) (Sigma-Aldrich) to block cell attachment to substratum as previously described [18]. Cell death was measured by assaying mono- and oligo- nucleosomes in cell lysates by using the Cell Death Detection ELISA kit (Roche). Three independent experiments were performed for each assay. Tumor cells were also assayed for cell death by staining with 7-amino actinomycin (7AAD) (Sigma-Aldrich) and with Ab to active caspase-3. Cells were stained with Hoechst dye (Sigma-Aldrich) to visualize nuclei and viewed by immunofluorescence microscopy. Staining was also evaluated by flow cytometry (FACS Calibur, BD Biosciences).
In vitro cell attachment assays
GFP-expressing tumor cells (1.5×104 per well) were seeded in 96-well plates containing confluent monolayers of omental mesothelial cells as previously described [18]. Where indicated, mesothelial cell monolayers were pre-incubated with neutralizing P-cadherin Ab or with control IgG at a final concentration of 10 μg/ml prior to seeding of tumor cells. At 1 hr after seeding of tumor cells, wells were washed with PBS to remove unattached tumor cells. Attached tumor cells were viewed by immunofluorescence microscopy and counted in three random 200x microscopic fields per well. Three independent experiments were performed for each assay.
Mouse i.p. xenograft studies
Four-week-old female nude mice were purchased from the National Cancer Institute and were inoculated i.p. with 2×106 cells of GFP-expressing SKOV3ip lines (n=5 mice per group). Mice were euthanized by CO2 asphyxiation at 3 weeks thereafter. In other sets of experiments, SKOV3ip cells were pre-incubated for 1 h with neutralizing Ab to human P-cadherin (50 μg), with control IgG or with no Ab, and then injected into mice. Mice were sacrificed at 3 days thereafter. Mice were also inoculated with SKOV3ip cells together with neutralizing Ab to mouse P-cadherin (50 μg), with control IgG or with no Ab, and sacrificed at 10 days thereafter. GFP-expressing tumor cells were visualized under a Leica MZML III stereomicroscope equipped with a mercury lamp power supply and GFP filter set. I.p. tumor burden was quantified by measuring areas of fluorescence signals within the abdominal cavity in captured images by using Image Pro Plus 5.0 software (Media Cybernetics) as previously described [19]. Cells were collected from peritoneal fluid and stained with Hoechst dye, 7AAD and phycoerythrin-conjugated anti-active caspase-3 Ab. Cell clusters were gently disaggregated by passing through 35 μm nylon mesh. Immediately thereafter, 7AAD and active caspase-3 staining was analyzed by flow cytometry within the gated population of GFP+ tumor cells. Sections of formalin-fixed, paraffin-embedded solid tumor tissues were stained with hematoxylin-eosin (HE). TUNEL-staining of tissue sections was performed by using the in situ Cell Death Detection kit (Roche). Evaluation of staining is described in the figure legends.
Statistical analysis
Statistical analysis was performed by using STATISTICA6 software (StatSoft Inc.). Values of statistical significance of data were assessed by unpaired two-tailed Student’s t-test. Data represent mean ± s.d. P values of < 0.05 were considered significant.
RESULTS
Knockdown of P-cadherin inhibits migration of EOC cells
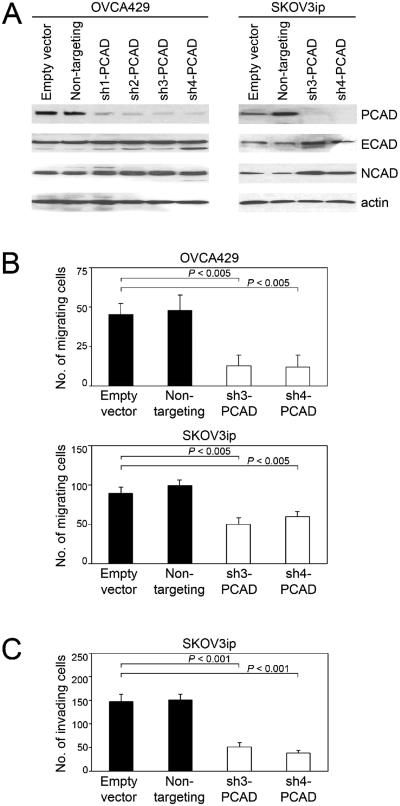
We evaluated several shRNAs that targeted different regions of the CDH3 gene for their ability to specifically down-regulate levels of P-cadherin, but not E- and N- cadherin levels, in the OVCA429 and SKOV3ip EOC cell lines [Figure 1A]. The two most effective shRNAs (sh3-PCAD, sh4-PCAD) were used for further study. As compared to control OVCA429 cells that expressed empty vector or non-targeting shRNA, OVCA429 cells in which P-cadherin was knocked-down showed significantly reduced motility (P < 0.005) [Figure 1B]. Identical results were obtained using SKOV3ip cells [Figure 1B]. Knockdown of P-cadherin also significantly reduced tumor cell invasiveness (P < 0.001) [Figure 1C]. These observations are consistent with studies using other EOC cell lines and cell lines derived from pancreatic and bladder cancers in which overexpression of P-cadherin was found to increase cell motility and invasion [13,20,21]. Because EMT is associated with alterations in cadherin expression [7,8], we evaluated the possibility that knockdown of P-cadherin reduces cell migration by inhibiting EMT. Knockdown of P-cadherin did not alter the expression of genes encoding transcription factors that orchestrate EMT such as Snail, Slug, Zeb1, Zeb2 and Twist [Supplemental Figure S1]. It has been reported that P-cadherin promotes cell motility and invasion by activating the Rho GTPases Rac1 and Cdc42 [13,20]. Consistent with the observed decreases in EOC cell motility and invasion, levels of the active forms of Rac1 and Cdc42 were reduced when P-cadherin was knocked-down [Supplemental Figure S2A]. Dominant-negative mutant forms of Rac1 and Cdc42 that block activation of these Rho GTPases [15] significantly inhibited tumor cell motility and invasion (P < 0.005), and this inhibition was as effective as that observed when P-cadherin was knocked-down [Supplemental Figures S2B-D].
Figure 1.
Knockdown of P-cadherin inhibits motility and invasiveness of EOC cells. (A) Western blot of cadherin levels in OVCA429 and SKOV3ip cells stably expressing empty pGIPZ vector, non-targeting shRNA and shRNAs that targeted different sites of CDH3 (sh-PCAD). (B) Tumor cell motility was assayed at 6 h after seeding in uncoated transwell chambers. (C) Tumor cell invasion was assayed at 16 h after seeding in Matrigel-coated transwell chambers. Shown in B and C are average results of 3 independent experiments.
P-cadherin promotes aggregation of floating EOC cells and inhibits anoikis
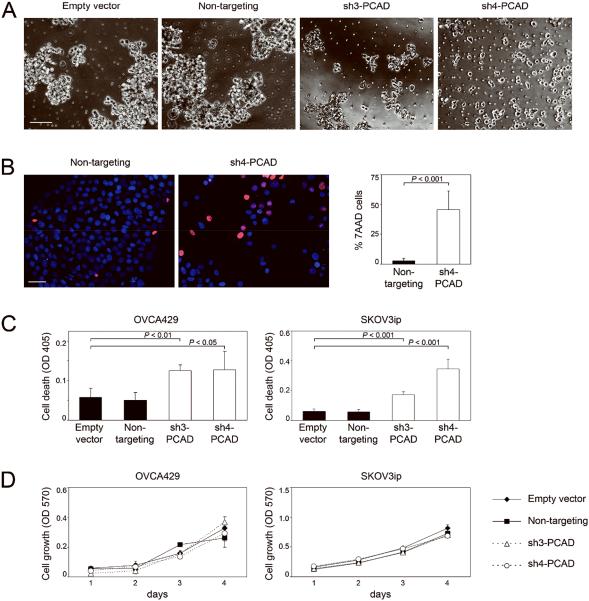
It is widely thought that the assembly of floating EOC cells into multi-cellular aggregates enables these cells to escape anoikis [3,4]. To evaluate whether P-cadherin promotes aggregation of floating EOC cells, we incubated cells of control and sh-PCAD EOC lines as suspension cultures in plates coated with polyHEMA, an inert polymer that prevents cells from adhering to substratum. Whereas control cells formed large aggregates, suspension cultures of sh-PCAD cells comprised of small aggregates and single cells [Figure 2A]. Cell death was observed in sh-PCAD cells as detected by staining with 7AAD [Figure 2B]. In contrast, control cells were mostly viable [Figure 2B]. The increased levels of cell death in suspension cultures of sh-PCAD cells were confirmed by assaying mono- and oligo- nucleosomes in cell lysates [Figure 2C]. Identical results were obtained using OVCA429 and SKOV3ip cells [Figure 2C]. Whereas knockdown of P-cadherin reduced cell viability in suspension cultures, knockdown of P-cadherin did not affect the viability of cells when cultured as adherent monolayers on uncoated plates [Figure 2D]. Together, these observations indicate that P-cadherin promotes the assembly of floating tumor cells into aggregates and inhibits anoikis.
Figure 2.
Knockdown of P-cadherin inhibits aggregation of floating EOC cells and increases anoikis. (A-C) Cells of control and sh-PCAD EOC lines were incubated as suspension cultures in polyHEMA-coated plates for 3 days. (A) Morphology of control and sh-PCAD SKOV3ip cells viewed under phase-contrast microscopy. Bar, 100 μm. (B) SKOV3ip cells were stained with Hoechst dye to visualize nuclei and with 7AAD to detect cell death, and viewed under immunofluorescence microscopy. Bar, 50 μm. The percent of 7AAD+ cells was calculated from the number of 7AAD+ cells and total number of cells within a 100x microscopic field. Five random fields were scored per assay. The average total number of cells in one field was 80. (C) Cell death was measured by assaying mono- and oligo- nucleosomes in cell lysates by ELISA. (D) Cell lines were incubated as adherent monolayers on uncoated plates. Cell viability was measured daily by MTT assay. Shown in B, C and D are average results of 3 independent experiments.
To confirm our findings, we evaluated the effects of a P-cadherin neutralizing Ab on cell aggregation and anoikis. Suspension cultures of EOC cells that were treated with P-cadherin neutralizing Ab comprised of small aggregates and single cells, whereas cells that were treated with control IgG or with no Ab formed large aggregates [Supplemental Figure S3A]. As compared to control cells, cells that were treated with P-cadherin Ab exhibited significantly higher levels of cell death (P < 0.01) [Supplemental Figure S3B]. Whereas P-cadherin Ab blocked cell aggregation, cell aggregation was not markedly affected by dominant-negative mutant forms of Rac1 and Cdc42 [Supplemental Figure S3C]. This suggests that the Rho GTPase pathway downstream of P-cadherin is alone insufficient to facilitate intercellular interactions. Inactivation of Rac1 and Cdc42 increased anoikis, but not as effectively as P-cadherin inhibition [Supplemental Figure S3D].
P-cadherin facilitates EOC-mesothelial cell interactions
P-cadherin is the most predominant type of cadherin expressed in normal peritoneal tissues as well as in malignant peritoneal effusions [11,14]. We therefore investigated the possibility that P-cadherin facilitates interactions between EOC cells and mesothelial cells that line the peritoneal surfaces. To evaluate the effect of inhibiting P-cadherin in EOC cells, attachment assays were performed by seeding equivalent numbers of control and sh-PCAD EOC cells onto confluent monolayers of mesothelial cells isolated from normal human omentum. The numbers of sh-PCAD EOC cells that bound to mesothelial cells were significantly lower than the numbers of bound control EOC cells (P < 0.01) [Figures 3A,3B]. In reciprocal experiments, we evaluated the effect of inhibiting P-cadherin in mesothelial cells by pre-incubating mesothelial monolayers with neutralizing P-cadherin Ab prior to seeding EOC cells. Pre-incubation of mesothelial cells with P-cadherin Ab significantly reduced the number of bound EOC cells, as compared to mesothelial cells that were pre-incubated with control IgG or with no Ab (P < 0.005) [Figures 3C,3D]. Together, these findings indicate that P-cadherin facilitates interactions between EOC cells and peritoneal mesothelial cells.
Figure 3.
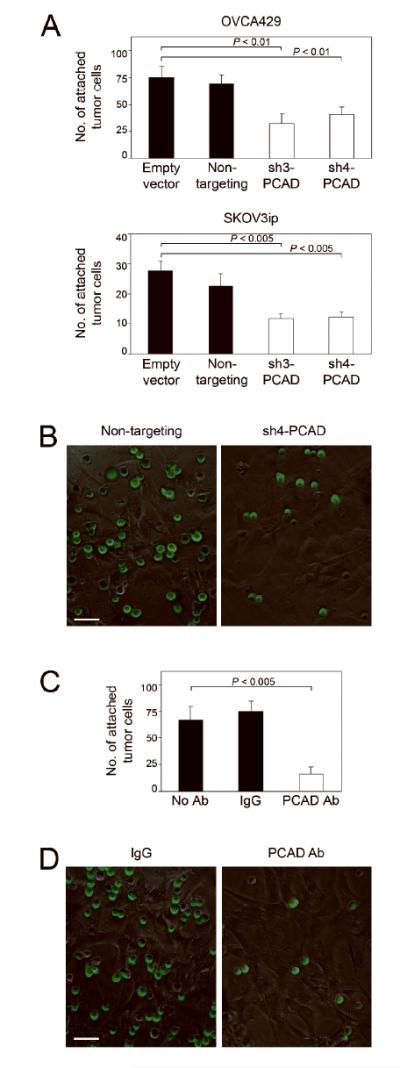
Inhibiting P-cadherin prevents EOC-mesothelial cell interactions. (A) Equivalent numbers of control and sh-PCAD EOC cells that stably expressed GFP were seeded onto confluent monolayers of normal human omental mesothelial cells. At 1 h thereafter, attached EOC cells were viewed under immunofluorescence microscopy and counted in three random 200x microscopic fields per well. Three independent experiments were performed for each assay. (B) Representative examples of attachment assays using control and sh-PCAD OVCA429 cells. Bar, 50 μm. (C) Attachment of OVCA429 control (non-targeting) cells to mesothelial cell monolayers, where mesothelial cell monolayers were pre-incubated with neutralizing P-cadherin Ab, mouse IgG or no Ab prior to seeding of tumor cells. (D) Representative examples of attachment assays where mesothelial cells were pre-incubated with IgG or with P-cadherin Ab. Bar, 50 μm.
Blocking P-cadherin decreases viability of floating tumor cells and prevents tumor cell attachment in vivo
To confirm the findings of our in vitro studies, we evaluated the effects of blocking P-cadherin on peritoneal attachment of EOC cells in vivo. Based on our prior experience using mouse i.p. xenograft models derived from SKOV3ip cells [17], the earliest time-point at which peritoneal tumor implants can be detected in mice is at 10 days following tumor cell inoculation. To evaluate the effect of blocking P-cadherin on peritoneal attachment of human EOC cells, mice were injected i.p. with GFP-expressing SKOV3ip cells together with neutralizing Ab to mouse P-cadherin, control IgG or no Ab. Mice were sacrificed at 10 days thereafter. Tumor implants that were attached to the peritoneal cavity wall were viewed under a fluorescence stereoscope and counted [Figure 4A]. The numbers of tumor nodules in mice that were treated with P-cadherin Ab were significantly fewer than the numbers of tumor nodules in mice that treated with control IgG or with no Ab (P < 0.02) [Figure 4B]. These findings indicate that P-cadherin facilitates interactions between peritoneal mesothelial cells and EOC cells.
Figure 4.
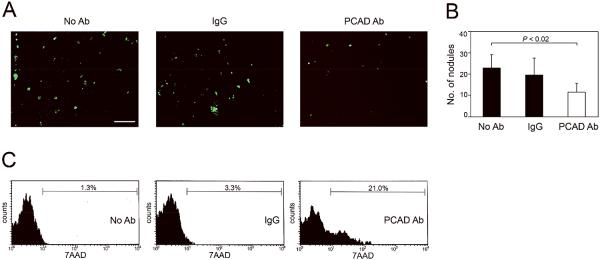
Blocking P-cadherin increases anoikis and decreases EOC cell attachment in vivo. (A,B) Female nude mice were injected i.p. with equivalent numbers of GFP-expressing control (non-targeting) SKOV3ip cells together with neutralizing Ab to mouse P-cadherin, control IgG or with no Ab. Mice were sacrificed at 10 days thereafter. (A) Tumor implants that were attached to the peritoneal cavity wall were viewed under a fluorescence stereoscope. Bar, 1 mm. (B) Numbers of tumor implants on the peritoneal cavity wall were counted in five random 25mm2 fields per mouse. (C) GFP-expressing control SKOV3ip cells were pre-incubated with neutralizing Ab to human P-cadherin, control IgG or with no Ab, and then injected i.p. into mice. Mice were sacrificed at 3 days thereafter. Floating cells in the peritoneal cavity were collected and stained with 7AAD. Cell death within the gated population of GFP+ tumor cells was evaluated by flow cytometric analysis of 7AAD staining.
We also evaluated the effect of blocking P-cadherin on the viability of floating EOC cells. Mice were inoculated i.p. with GFP-expressing SKOV3ip cells that had been pre-incubated with neutralizing Ab to human P-cadherin, control IgG or no Ab, and were sacrificed at 3 days thereafter. Cell death was evaluated within the population of GFP+ tumor cells in the peritoneal fluid by flow cytometric analysis of 7AAD staining. As shown in Figure 4C, the proportion of floating tumor cells that exhibited cell death was substantially higher in the P-cadherin Ab-treated model than in the control models. These observations are highly consistent with our findings in suspension cultures of SKOV3ip cells [Figures 2B,2C; Supplemental Figure S3B], and support the notion that P-cadherin prevents anoikis in EOC cells.
Knockdown of P-cadherin prevents EOC progression in i.p. xenograft models
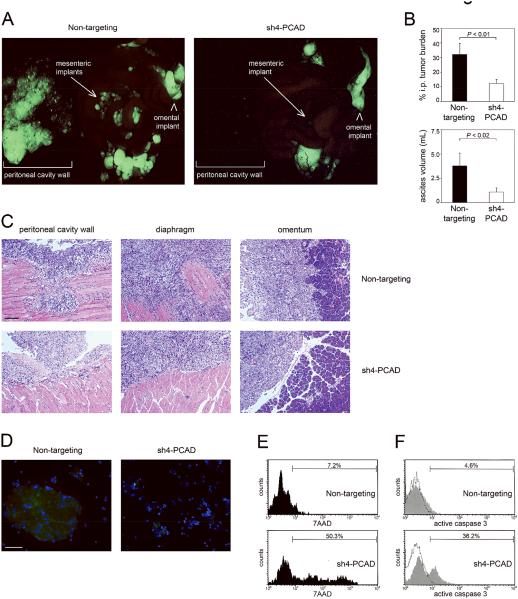
In subsequent experiments, we evaluated the overall effects of inhibiting P-cadherin on EOC progression in i.p. xenograft models. Mice were inoculated i.p. with equivalent numbers of control and sh-PCAD SKOV3ip cells that expressed GFP and were sacrificed at 3 weeks thereafter. Mice that were inoculated with control SKOV3ip cells developed numerous tumor implants, particularly on the mesentery and peritoneal cavity wall [Figure 5A]. By contrast, mice that were inoculated with sh-PCAD SKOV3ip cells developed fewer discrete peritoneal nodules [Figure 5A]. Consistent with these observations, the overall tumor burden in mice with sh-PCAD tumors as compared to the control group was significantly reduced (P < 0.01) [Figure 5B]. The volume of ascites was also significantly reduced (P < 0.02) [Figure 5B]. In addition, tumor implants derived from control SKOV3ip cells were locally invasive, whereas invasion of adjacent tissues was not observed for tumors derived from sh-PCAD SKOV3ip cells [Figure 5C].
Figure 5.
Knockdown of P-cadherin prevents EOC progression in i.p. xenograft models. Female nude mice were inoculated i.p. with equivalent numbers of GFP-expressing control and sh-PCAD SKOV3ip cells and were sacrificed at 3 weeks thereafter (n=5 mice per group). (A) Tumor implants were viewed under a fluorescence stereoscope. (B) I.p. tumor burden was quantified by measuring areas of fluorescence signals in captured images and is expressed as the average percent of area of fluorescence within the abdominal cavity. Fluorescence signals on the peritoneal cavity wall were excluded from analysis. Also shown are volumes of ascites in mice. (C) Representative examples of HE-stained tumor implants on the peritoneal cavity wall and diaphragm. Also shown are omental implants and the adjacent pancreas. Bar, 100 μm. (D) Cells were collected from ascites, stained with Hoechst dye to visualize nuclei (blue) and viewed under immunofluorescence microscopy. GFP-expressing ascitic tumor cells were visualized using a fluorescein filter. Bar, 50 μm. (E,F) Evaluation of cell death within the gated population of GFP+ tumor cells in ascites by flow cytometric analysis of (E) 7AAD staining and (F) staining of active caspase-3.
Although the overall tumor burden was reduced in mice with sh-PCAD tumors, there was no significant difference in the mitotic activity of solid i.p. tumors derived from control and sh-PCAD tumor cells [Supplemental Figures S4A,S4B]. These observations are consistent with the observed lack of effect of P-cadherin on EOC cell proliferation per se [Figure 2D]. There was also no significant difference in the level of cell death in solid i.p. tumors derived from control and sh-PCAD tumor cells [Supplemental Figures S4C,S4D]. Floating tumor cells in ascites that were collected from the control models were present as large aggregates, whereas ascites collected from sh-PCAD models contained smaller aggregates or single floating tumor cells [Figure 5D]. Cell death was evaluated within the population of GFP+ ascitic tumor cells by flow cytometric analysis of 7AAD. As shown in Figure 5E, the proportion of ascitic tumor cells that exhibited cell death was substantially higher in the sh-PCAD model than in the control model. This finding was confirmed by evaluating the level of active caspase 3 in ascitic tumor cells [Figure 5F]. Collectively, these findings indicate that inhibiting P-cadherin prevents EOC progression via the combinatorial effects of inhibiting aggregation and survival of ascitic tumor cells, peritoneal attachment and invasiveness.
DISCUSSION
Intraperitoneal ‘seeding’ is a distinctive pattern of dissemination that is unique to EOC and markedly differs from the hematogenous or lymphatic metastasis found for many other types of tumors. A key and initial rate-limiting step in the ‘seeding’ of EOC is the survival of floating tumor cells in the peritoneal fluid. It is widely recognized that aggregation enables floating tumor cells to escape anoikis, although it is unclear whether tumor cells shed into the peritoneal fluid as aggregates or shed as single cells that then assemble into aggregates [3]. The mechanisms that mediate adhesion between floating EOC cells are poorly understood. EOC cells express several integrins and extracellular matrix (ECM) proteins, and interactions between α5β1 integrin and fibronectin have been reported to mediate assembly of EOC cells into spheroids [22]. Cadherins are transmembrane glycoproteins that have well-established functions in mediating homophilic cell adhesion [7]. The role of cadherins in regulating cell aggregation and anoikis varies depending on the type of cell. For example, treatment of Ewing sarcoma cells with E-cadherin neutralizing Ab increases anoikis sensitivity [23], whereas knockdown of E-cadherin in mammary epithelial cells confers anoikis resistance [23,24]. Inhibition of N-cadherin-mediated intercellular interactions increases apoptosis in melanoma cells [25]. Studies of clinical specimens of EOC have implicated the importance of P-cadherin, rather than of E- and N- cadherins, in facilitating aggregation of floating EOC cells. P-cadherin has been found to be the predominant type of cadherin expressed in tumor cells in the peritoneal fluid of EOC patients [11]. On the other hand, expression levels of E-cadherin and N-cadherin have been found to be markedly reduced in floating tumor cells as compared to solid tumors [10,11].
In this study, we provide evidence that P-cadherin facilitates the assembly of floating EOC cells into multi-cellular aggregates. Our studies support the notion that P-cadherin primarily inhibits anoikis by promoting tumor cell aggregation, but it is not possible to exclude the possibility that P-cadherin also promotes cell survival by a mechanism that is independent of cell aggregation. This possibility seems unlikely because inhibition of P-cadherin decreased the viability of tumor cells in suspension cultures but not when tumor cells were cultured as adherent monolayers. Furthermore, inhibition of P-cadherin increased cell death in ascitic tumor cells in xenograft models but did not increase cell death in solid tumors. Classical cadherins such as P-cadherin primarily promote homotypic interactions (i.e. between cadherins of the same type) [7]. Nevertheless, it is possible that P-cadherin might also promote aggregation of EOC cells by facilitating the clustering of other surface molecules. There is evidence in some cell types that EMT confers anoikis resistance [24]. The possibility that P-cadherin promotes anoikis resistance by inducing EMT is unlikely because knockdown of P-cadherin in EOC cells did not affect expression of genes that orchestrate EMT. P-cadherin is known to activate Rac1 and Cdc42 [20]. Consistent with prior reports that Rac1 and Cdc42 increase anoikis-resistance [26,27], we observed increased anoikis when Rac1 and Cdc42 were inhibited. However, inhibition of these Rho GTPases did not markedly disrupt tumor cell aggregation and was not as effective as P-cadherin inhibition in increasing anoikis. Although activation of Rac1 and Cdc42 could partially explain the ability of P-cadherin to confer anoikis resistance, our findings support the notion that P-cadherin primarily prevents anoikis by promoting tumor cell aggregation and indicate that downstream Rho GTPase signaling is alone insufficient to facilitate cell aggregation. This is not surprising as cell adhesion is achieved via interactions between the extracellular domains of two cadherin molecules of adjacent cells [7].
Because knockdown of P-cadherin did not decrease mitotic activity or cell death in solid i.p. tumors, the reduction in overall i.p. tumor burden in mice with sh-PCAD tumors as compared to control tumors is likely to be due to, or at least in part from, increased cell death in floating, ascitic tumor cells. This is supported by our short-term in vivo studies in which treatment with P-cadherin Ab increased cell death in floating tumor cells. Another important rate-limiting step in the progression of EOC is the implantation of floating tumor cells on to peritoneal surfaces. Our in vitro studies and short-term in vivo studies demonstrate that P-cadherin also facilitates interactions between EOC cells and peritoneal mesothelial cells. The observed reduction in overall i.p. tumor burden in mice with sh-PCAD tumors could therefore also be attributable to the decreased implantation capability of sh-PCAD tumor cells. Our findings support those of a recent study in which P-cadherin induction was implicated as the mechanism by which gonadotropin-releasing hormone receptor signaling stimulates attachment of EOC cells to mesothelial cells [28]. The glycoprotein CA125, the most-characterized EOC biomarker, has been also implicated in facilitating implantation by its ability to bind mesothelin, a glycoprotein that is expressed by mesothelial cells [29]. The glycoprotein CD44 is widely expressed in ovarian cancers and binds hyaluronic acid, a glycosaminoglycan that is synthesized by mesothelial cells [30]. Neutralizing Abs to CD44 have been found to prevent EOC cells from attaching to mesothelial cells in vitro and to reduce the number of tumor implants in xenograft models [30-32]. As observed for P-cadherin inhibition in our xenograft models, Strobel and colleagues found that treatment of mice with CD44 Ab did not inhibit tumor cell growth per se [31]. Although EOC-mesothelial cell interactions can be inhibited by Abs to CD44 and to β1 integrin, this inhibition has been found to be only partial [30,32,33]. We likewise observed that inhibition of P-cadherin did not completely block attachment of EOC cells to mesothelial monolayers in vitro or totally eliminate implantation in vivo. Because increasing evidence indicates that EOC-peritoneum interactions are facilitated by multiple cell surface proteins, targeting a single adhesion molecule might not to be therapeutically efficacious.
Although several studies have demonstrated that various integrin heterodimers mediate attachment of EOC cells to mesothelial cells, other studies indicate that integrins primarily facilitate tumor cell interactions with the submesothelial ECM [18,32-36]. Electron microscopy studies by Niedbala and colleagues have suggested that mesothelial cells retract after coming in contact with tumor cells [37]. Live cell imaging studies by Iwanicki and colleagues have revealed that spheroids of ovarian cancer cells physically displace mesothelial cells to gain access to the underlying stroma [33]. These authors identified that spheroids use myosin-generated mechanical force to clear mesothelial cells at the site of contact in a manner that is dependent on α5β1 integrin and talin I [33]. The role of P-cadherin in cell migration varies depending on the cell type. P-cadherin has been reported to inhibit migration of melanoma and oral squamous cell carcinoma cells [38,39], but promotes migration of pancreatic and bladder cancer cells [20,21]. Our findings that knockdown of P-cadherin inhibits migration of OVCA429 and SKOV3ip cells are consistent with findings by Cheung and colleagues that overexpressing P-cadherin in the EOC cell line Caov-3 promotes cell migration [13]. The notion that P-cadherin increases migration of EOC cells and also facilitates adhesion between floating EOC cells seems somewhat discordant. The ability of P-cadherin to promote both of these processes might stem from different mechanisms. Our observations that Rho GTPase inactivation and P-cadherin inhibition were almost equally effective in blocking EOC cell migration support prior reports that P-cadherin–mediated cell migration depends on Rho GTPase activation [20]. In contrast, our findings indicate that Rho GTPase signaling alone is insufficient to facilitate cell adhesion. Furthermore, the ability of P-cadherin to promote aggregation of floating EOC cells and also invasiveness could explain the reported invasiveness of ascitic EOC spheroids [40]. Indeed, EOC cells in ascites have been reported to be 4-fold more invasive than their solid tumor counterparts [10].
In summary, our study demonstrates that inhibiting P-cadherin blocks multiple and key steps of EOC progression. Together with prior reports that α5β1 integrin facilitates both the assembly of EOC cells into spheroids and breach of the mesothelial barrier [22,33], our findings support a model in which implantation of EOC cells is dynamically and mechanistically coupled to the aggregation of floating EOC cells. A human monoclonal Ab to P-cadherin has been reported to inhibit metastasis in xenograft models of breast, colon and prostate cancer and is undergoing evaluation in clinical trials [41]. Our findings that blocking P-cadherin inhibits the viability and peritoneal attachment of floating EOC cells suggest that P-cadherin targeted therapy might not be highly beneficial for patients who have already developed carcinomatosis. On the other hand, anti-P-cadherin therapy might be effective in preventing formation of new implants after tumor-debulking surgery. However, because our study indicates that inhibiting P-cadherin alone does not inhibit tumor cell growth per se, combining anti-P-cadherin therapy with other cytotoxic agents warrants further investigation.
Supplementary Material
Acknowledgements
Grant support: This work was supported by Cancer & Prevention Research Institute of Texas grant RP120390 (to H. Naora) and U.S. National Institutes of Health grant CA141078 (to H. Naora).
We thank members of the Naora laboratory for helpful discussions.
Abbrevations
- 7AAD
7-amino actinomycin
- Ab
antibody
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- EOC
epithelial ovarian cancer
- HE
hematoxylin-eosin
- polyHEMA
poly(2-hydroxyethyl methacrylate)
- shRNAs
short hairpin RNAs
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Naora H, Montell DJ. Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 3.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodek KL, Murphy KJ, Brown TJ, Ringuette MJ. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31:397–414. doi: 10.1007/s10555-012-9351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegama TR, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–81. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Mutsaers SE. Mesothelial cells: Their structure, function and role in serosal repair. Respirology. 2002;7:171–91. doi: 10.1046/j.1440-1843.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Science. 2008;121:727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–9. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 10.Veatch AL, Carson LF, Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer. 1994;58:393–9. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- 11.Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. Cadherin switching in ovarian cancer progression. Int J Cancer. 2003;106:172–7. doi: 10.1002/ijc.11086. [DOI] [PubMed] [Google Scholar]
- 12.Quattrocchi L, Green AR, Martin S, Durrant L, Deen S. The cadherin switch in ovarian high-grade serous carcinoma is associated with disease progression. Virchows Arch. 2011;459:21–9. doi: 10.1007/s00428-011-1082-1. [DOI] [PubMed] [Google Scholar]
- 13.Cheung LW, Leung PC, Wong AS. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene. 2010;29:2427–40. doi: 10.1038/onc.2009.523. [DOI] [PubMed] [Google Scholar]
- 14.Chen GT, Tai CT, Yeh LS, Yang TC, Tsai HD. Identification of the cadherin subtypes present in the human peritoneum and endometriotic lesions: potential role for P-cadherin in the development of endometriosis. Mol Reprod Dev. 2002;62:289–94. doi: 10.1002/mrd.10121. [DOI] [PubMed] [Google Scholar]
- 15.Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Cell Biol. 1998;9:1863–71. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extracellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121:1463–72. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 17.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–17. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko SY, Lengyel E, Naora H. The Müllerian HOXA10 gene promotes growth of ovarian surface epithelial cells by stimulating epithelial-stromal interactions. Mol Cell Endocrinol. 2010;317:112–9. doi: 10.1016/j.mce.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko SY, Guo H, Barengo N, Naora H. Inhibition of ovarian cancer growth by a tumor-targeting peptide that binds eukaryotic translation initiation factor 4E. Clin Cancer Res. 2009;15:4336–47. doi: 10.1158/1078-0432.CCR-08-2924. [DOI] [PubMed] [Google Scholar]
- 20.Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–9. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- 21.Mandeville JA, Silva Neto B, Vanni AJ, Smith GL, Rieger-Christ KM, Zeheb R, Loda M, Libertino JA, Summerhayes IC. P-cadherin as a prognostic indicator and a modulator of migratory behavior in bladder carcinoma cells. BJU Int. 2008;102:1707–14. doi: 10.1111/j.1464-410X.2008.08115.x. [DOI] [PubMed] [Google Scholar]
- 22.Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegama TR, Ruff LE, Skubitz AP. β1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159:2071–80. doi: 10.1016/s0002-9440(10)63058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Park SH, Cieply B, Schupp J, Killiam E, Zhang F, Rimm DL, Frisch SM. A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol Cell Biol. 2011;31:4036–51. doi: 10.1128/MCB.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–25. [PubMed] [Google Scholar]
- 26.Zugasti O, Rul W, Roux P, Peyssonnaux C, Eychene A, Franke TF, Fort P, Hibner U. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol Cell Biol. 2001;21:6706–17. doi: 10.1128/MCB.21.19.6706-6717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coniglio SJ, Jou TS, Symons M. Rac1 protects epithelial cells from anoikis. J Biol Chem. 2001:28113–20. doi: 10.1074/jbc.M102299200. [DOI] [PubMed] [Google Scholar]
- 28.Cheung LWT, Yung S, Chan TM, Leung PCK, Wong AST. Targeting gonadotropin-releasing hormone receptor inhibits the early step of ovarian cancer metastasis by modulating tumor-mesothelial adhesion. Mol Ther. 2013;21:78–90. doi: 10.1038/mt.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B, Ho M. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–49. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53:3830–8. [PubMed] [Google Scholar]
- 31.Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228–32. [PubMed] [Google Scholar]
- 32.Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–37. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen R, Merritt M, Danuser G, Ince TA, Brugge JS. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discovery. 2011;1:144–57. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–25. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 35.Heyman L, Kellouche S, Fernandes J, Dutoit S, Poulain L, Carreiras F. Vitronectin and its receptors partly mediate adhesion of ovarian cancer cells to peritoneal mesothelium in vitro. Tumour Biol. 2008;29:231–44. doi: 10.1159/000152941. [DOI] [PubMed] [Google Scholar]
- 36.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69:1469–76. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 37.Niedbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160:499–513. doi: 10.1016/0014-4827(85)90197-1. [DOI] [PubMed] [Google Scholar]
- 38.Van Marck V, Stove C, Van Den Bossche K, Stove V, Paredes Y, Vander Haeghen Y, Bracke M. P-cadherin promotes cell-cell adhesion and counteracts invasion in melanoma. Cancer Res. 2005;65:8774–83. doi: 10.1158/0008-5472.CAN-04-4414. [DOI] [PubMed] [Google Scholar]
- 39.Bauer K, Dowejko A, Bosserhoff AK, Reichert TE, Bauer RJ. P-cadherin induces an epithelial-like phenotype in oral squamous cell carcinoma by GSK3beta-mediated Snail phosphorylation. Carcinogenesis. 2009;30:1781–8. doi: 10.1093/carcin/bgp175. [DOI] [PubMed] [Google Scholar]
- 40.Burleson KM, Hansen LK, Skubitz AP. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial monolayers. Clin Exp Metastasis. 2004;21:685–97. doi: 10.1007/s10585-004-5768-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang CC, Yan Z, Zhang Q, Kuszpit K, Zasadny K, Qiu M, Painter CL, Wong A, Kraynov E, Arango ME, Mehta PP, Popoff I, Casperson GF, Los G, Bender S, Anderes K, Christensen JG, VanArsdale T. PF-03732010: a fully human monoclonal antibody against P-cadherin with anti-tumor and antimetastatic activity. Clin Cancer Res. 2010;16:5177–88. doi: 10.1158/1078-0432.CCR-10-1343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.