SUMMARY
We conducted a retrospective cohort study assessing the association between diabetes mellitus (DM) and immune recovery in HIV-infected adults. Immune reconstitution after initiating antiretroviral therapy was more rapid in DM patients (120.4 cells/year) compared to non-DM patients (94.2 cells/year, p < 0.023). Metformin use was associated with improved CD4 recovery (p= 0.034).
INTRODUCTION
The scale up of antiretroviral therapy (ART) across sub Saharan Africa has led to remarkable reductions in AIDS-related morbidity and mortality rates. Although there is an emerging diabetes epidemic in Africa[1], immunologic response to ART in HIV-infected Africans with diabetes mellitus (DM) has not been described [2]. We used existing data on HIV-infected adults living in Botswana [3] to explore whether CD4 lymphocyte count recovery after initiation of ART was influenced by the presence of type 2 diabetes and/or diabetes medications.
METHODS
We conducted a retrospective cohort study at four sites between February 2011 and November 2012. Both DM patients (N= 48) and non-DM (N=108) patients were drawn from two semi-urban facilities (Orapa and Kanye) and two urban facilities (Gaborone and Francistown). DM cases were defined according to the World Health Organization (WHO) criteria for the diagnosis of diabetes[4]. In total, there were 48 patients with diabetes (28 females, 20 males, mean age 46.4 [SD=9.9]) and 108 without diabetes (57 females, 51 males, mean age 43.6 [SD=8.0]).
The primary outcome measure was the CD4 count at ART initiation and post initiation. Patient weight was included as a secondary outcome. The primary analyses used random intercept and random slope models, We also performed logistic regression to determine whether ART regimen and diabetes treatment influenced immune recovery.
RESULTS
In the group without diabetes, mean follow up period was 5.8 years (SD=1.9), compared to 3.8 years (SD=2.1) for those persons with diabetes (p < 0.001, Wilcoxon ranksum test). For the CD4 outcome variable, there were 156 patients with a total of 1369 values (mean = 8.8 per patient). For patient weight, the sample was 106 patients with a total of 485 observations (mean = 4.6 per patient).
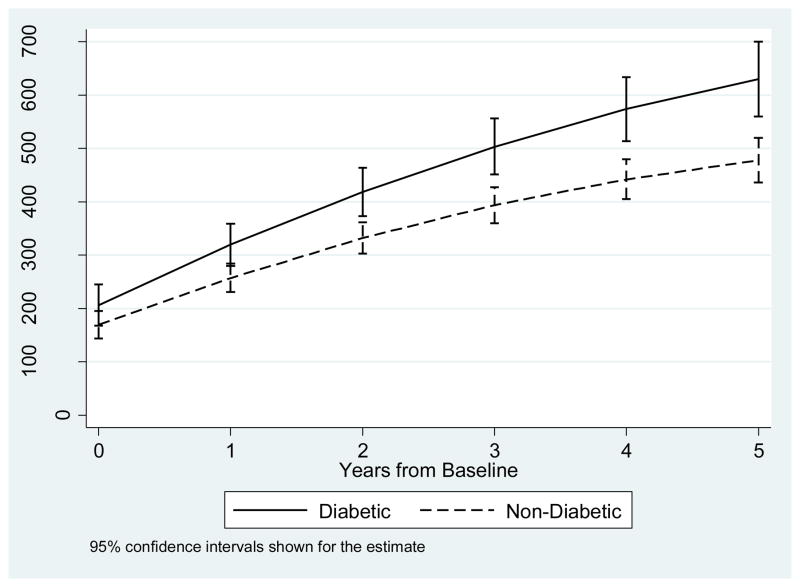
Table 1 shows the regression results for CD4 recovery and log of CD4. Both analyses produced similar results. At baseline, there was no difference in CD4 counts (p = 0.195) between those with diabetes and those without diabetes. After initiation of ART, CD4 counts increased by 94.2 cells/mL per year for those without diabetes, with an additional 26.4 cells/mL for those with diabetes, as shown in the diabetes by years interaction (p = 0.023). Figure 1 shows the modeled CD4 counts for males with diabetes and males without diabetes over time, demonstrating the enhanced recovery among those with diabetes compared to those without diabetes.
Table 1.
Results of the random intercept and random slope model predicting primary variables (CD4. log of CD4) and secondary variable (weight)
| No. of patients (Total no. of obs) | CD4 N=156 (Obs=1369) |
Log CD4 N=156 (Obs=1369) |
Weight (kg) N=106 (Obs=485) |
|||
|---|---|---|---|---|---|---|
| Coefficient (SE) | P-Value | Coefficient (SE) | P-Value | Coefficient (SE) | P-Value | |
| Constant (baseline) | 149.3 (54.2) | 0.006 | 5.05 (.20) | <0.001 | 65.9 (6.1) | <0.001 |
| Female Gender | −41.8 (20.5) | 0.041 | −.20 (0.07) | 0.007 | −1.5 (2.2) | 0.510 |
| Age (years) | 0.9 (1.2) | 0.440 | −0.002 (0.004) | 0.615 | −0.10 (.14) | 0.466 |
| Diabetic | 31.0 (24.0) | 0.195 | 0.14 (0.12) | 0.232 | 10.3 (2.6) | <0.001 |
| Years | 94.2 (5.5) | <0.001 | 0.45 (0.02) | <0.001 | 1.2 (0.3) | <0.001 |
| Years-squared | −6.5 (.7) | <0.001 | −0.04 (0.003) | <0.001 | −0.1 (0.04) | 0.001 |
| Diabetic x Years | 26.4 (11.6) | 0.023 | 0.10 (0.05) | 0.029 | −0.3 (0.7) | 0.673 |
| Diabetic x Years-squared | −0.74 (1.8) | 0.684 | −0.014 (0.007) | 0.042 | 0.1 (0.1) | 0.429 |
Figure 1.
Modeled CD4 count over time for diabetics and non-diabetics
In a sub-analysis of those with diabetes on diabetes treatment, we found that CD4 count increases in the first 12 months on ART were significantly higher for patients on metformin (n=17) compared to those on insulin-based regimens (n=5). CD4 counts increased by 99 cells/mL per year for those with diabetes on non-metformin based treatments, with an additional 46.5 cells/mL per year increase for those on metformin (interaction term, p=0.034).
Across groups, mean initial weight gain was 1.2 kg per year, with a slowing over time. At two years the rate of gain was 0.8 kg and at three years, 0.3 kg. Although those with diabetes were 10.3 kg heavier than those without diabetes at baseline, the interaction of weight over time and diabetes was not significant (p = 0.673).
DISCUSSION
To our knowledge, this is the first analysis comparing immune reconstitution in HIV-infected DM and non-DM patients after initiation of ART. Notably, we found that individuals with DM and HIV were more likely to have a greater increase in CD4 count after initiation of ART compared to those without diabetes We speculate that the differences in immune reconstitution between those with diabetes and the group without diabetes may be related in part to the independent influence of persistent hyperglycemia on humoral immunity and in part to the medications used to treat diabetes. Data from HIV-uninfected persons with type 2 diabetes, demonstrating higher CD4 cell counts in patients with higher glycated HbA1c levels and increased prevalence of advanced glycation end products, would support the hypothesis that hyperglycemia enhances CD4 cell counts [5]. Future studies need to explore whether the difference between DM and non-DM patients are the consequence of unaccounted for confounders, such as thymic dysfunction and/or the presence of other co-morbidities in less healthy persons without diabetes.
Subjects prescribed metformin concurrently with ART exhibiteda more robust immune recovery in the first 12 months of ART, compared to those individuals on insulin-containing regimens. While an association between metformin and improved immune reconstitution has not been previously described, there is data from HIV-uninfected patients demonstrating that metformin exerts anti-inflammatory properties, beyond its glucose properties. Metformin inhibits release of cytokines, such as interleukin-6 and interleukin-8, from coronary artery vasculature [6]. Whether this anti-inflammatory effect is at work to enhance immune recovery in HIV-infected patients is less clear. We cannot exclude that those with diabetes on metformin were healthier at baseline than those on insulin-based regimens. However, the potential association between metformin and accelerated immune recovery warrants further research, particularly given that the drug is inexpensive, is a key component in the management of DM in developing settings, is safe and has numerous other beneficial effects in HIV-infected patients [7, 8].
The study had several limitations. There were several potential confounders of immune recovery that were not recorded in our analysis. Although the sample size was small, the findings warrant exploration in a larger study including a more comprehensive assessment of the differential response rates of naïve and memory T cells, %CD4 T-cells and CD4/CD8 T-cell ratios, as well as analysis of levels of cytokines, such as IL-6 and IL-8.
CONCLUSION
In conclusion, our results suggest for the first time that both diabetes and metformin use may influence immune recovery in individuals initiating ART in southern Africa. Larger prospective studies are needed to evaluate if insulin resistance, hyperglycaemia and/or diabetes therapy are clinically important determinants of immune recovery. If our results are confirmed and clinically validated, discussions should follow about the potential role of metformin as neo-adjuvant treatment for HIV.
Acknowledgments
FUNDING
This publication was made possible through support provided by the Penn Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 045008).
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whiting DR, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Dave JA, et al. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. Journal of acquired immune deficiency syndromes. 2011;57(4):284–9. doi: 10.1097/QAI.0b013e318221863f. [DOI] [PubMed] [Google Scholar]
- 3.Moyo D, TG, Mushsisha O. Diabetes mellitus in HIV-infected patients on antiretroviral therapy. South African Medical Journal. 2013 doi: 10.7196/samj.6792. In Press. [DOI] [PubMed] [Google Scholar]
- 4.WHO, W.H.O. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. World Health Organization; 2006. [Google Scholar]
- 5.Giubilato S, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. European heart journal. 2011;32(10):1214–26. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- 6.Joven J, et al. Metformin: a cheap and well-tolerated drug that provides benefits for viral infections. HIV Med. 2013;14(4):233–40. doi: 10.1111/hiv.12000. [DOI] [PubMed] [Google Scholar]
- 7.Fitch K, et al. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS. 2012;26(5):587–97. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheth SH, Larson RJ. The efficacy and safety of insulin-sensitizing drugs in HIV-associated lipodystrophy syndrome: a meta-analysis of randomized trials. BMC infectious diseases. 2010;10:183. doi: 10.1186/1471-2334-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]