Abstract
Despite progress in recent years, pancreatic cancer still remains a major clinical challenge. Its incidence and mortality rates have been on consistent rise, thus underscoring the critical need for novel diagnostic, prognostic and therapeutic tools for its effective management. Recent studies have demonstrated that microRNAs (miRNAs/miRs) are deregulated in a variety of malignancies, including pancreatic cancer, and play a significant role in the initiation, progression and metastasis. Furthermore, their vital involvement in the therapeutic resistance of cancer has also been established. Hence, there has been enormous interest worldwide in investigating the roles of miRNAs in pancreatic cancer pathogenesis and exploiting their utility for clinical benefit. In this review, we summarize current knowledge on the role of miRNAs in pancreatic cancer and discuss their potential use as diagnostic and prognostic biomarkers, and as novel targets for development of effective therapeutic strategies.
Keywords: MicroRNAs, miRNAs, pancreatic cancer, metastasis, drug resistance, diagnosis, prognosis and therapy
Introduction
Pancreatic cancer is a highly lethal malignancy and fourth leading cause of cancer-related death in the United States [1]. The median survival after diagnosis is 2–8 months, and approximately 3–6% of all patients with pancreatic cancer survive 5 years after diagnosis [2]. This is mostly due to the fact that it is diagnosed at a stage when it is either locally advanced or has already metastasized to the distant organs [3]. The treatment of this malignancy also remains a clinical challenge due to inherent or acquired resistance mechanisms [4]. Hence, there is a paramount need to understand the molecular mechanisms underlying its initiation, aggressive progression and therapy resistance. Such information can aid in developing better diagnostic, prognostic and therapeutic approaches for efficient clinical management. The recent discovery of microRNAs (miRNAs or miRs) has revealed a novel mechanism of gene regulation and provided new avenues for cancer research. miRNAs are small, non-coding RNA molecules, which regulate the gene expression at post-transcription level [5, 6]. miRNAs bind to the 3’-untranslated regions (3’-UTRs) of target messenger RNAs (mRNAs) and subsequently results in either target degradation or translational inhibition. To date, more than 700 miRNAs have been identified in humans [7]. Moreover, miRNAs regulate more than one-third of all human genes, which suggest their remarkable influence on human biology [8]. miRNAs are involved in the regulation of various biological processes including proliferation, apoptosis, differentiation and development [7]. Several studies have shown that miRNAs exhibit differential expression pattern in various human diseases, including cancer, and are functionally involved in the disease processes [9]. Indeed, substantial evidence has been provided by several groups to suggest a clear link between miRNAs and cancer. It is known that more than 50% of miRNA genes are localized within genomic regions that are either frequently amplified or deleted, resulting in deregulation of miRNAs [9, 10]. Furthermore, miRNAs exhibit tissue-specific and disease-specific expression that could provide the basis for developing miRNAs as novel diagnostic, prognostic and therapeutic targets [11, 12]. In this review, we focus on the recent advances in miRNA research as it pertain to pancreatic cancer. Our specific emphasis will be on the biological and clinical implications of miRNAs in cancer in order to envision their potential translational significance.
Aberrant expression of miRNAs in pancreatic cancer
A significant number of studies have shown that miRNAs are highly deregulated in pancreatic cancer (Table 1). An earlier study performed on pancreatic ductal adenocarcinoma (PDAC) samples, normal pancreas and chronic pancreatitis reported significant differences in the miRNA expression profiles among the normal and diseased pancreas. The investigation revealed differential expression of several miRNAs in PDAC and pancreatitis samples, and cancer cell lines [13]. Based on this study, authors further concluded that miR-196a and miR-217 could be used as potential markers in differentiating PDAC from normal pancreas and chronic pancreatitis. An investigation by Bloomston and coworkers revealed that twenty one miRNAs were overexpressed and four miRNAs were downregulated in pancreatic cancer, and this expression profile perfectly discriminated pancreatic cancer from benign pancreatic tissue. Furthermore, it was suggested that fifteen miRNAs with increased expression and eight with significantly decreased expression could differentiate pancreatic cancer from chronic pancreatitis [14]. In 2009, Zhang and coworkers performed miRNA profiling, and their findings demonstrated that eight miRNAs (miR-95, miR-186, miR-196a, miR-190, miR-200b, miR-221, miR-222, and miR-15b) were significantly overexpressed in majority of the pancreatic cancer clinical samples and cell lines [15]. Olson and coworkers demonstrated that expression of miR-142-3p, miR-142-5p, miR-155, and miR-146a were upregulated in human pancreatic neuroendocrine tumors (PNETs) when compared to normal human islets [16]. miR-155 and miR-21, were significantly upregulated in the intraductal papillary neoplasms (IPMN) as compared to normal ducts [17]. Since IPMN are non-invasive, precursor lesions of pancreatic cancer, it was concluded that aberrant activation of these miRNAs is an early event in pancreatic cancer progression. In a similar study, Ryu and coworkers showed that miR-155 was significantly overexpressed in pancreatic intraepithelial neoplasia-2 and -3 (PanIN-2/PanIN-3) lesion, and that miR-155 level increased progressively from PanIN-2 to PanIN-3 lesions. These authors also suggested that miR-155 activation was an ‘early’ event in the multistep progression model [18]. Recently, Yu and colleagues investigated that several miRNAs including miR-378, miR-130b, miR-133a, miR-151-5p, miR-148a/b, miR-185, miR-331-3p/5p, miR-200c, miR-330-3p, miR-34c-5p, miR-129-3p, and miR-423-5p were significantly overexpressed in low-grade PanINs (PanIN-1 or PanIN-2). Furthermore, authors also identified miR-196b to be most significantly overexpressed miRNA in advanced PanIN-3 lesions, while no expression was seen in low grade PanIN lesions. Consequently, this study suggested that miR-196b could be a useful marker for the identification of high-grade PanIN and invasive PDACs [19]. Recently, He et al [20] demonstrated that the expression of miR-218 decreased during the progression of pancreatic cancer. Together, all these findings suggest that miRNAs are aberrantly expressed in pancreatic cancer, and their deregulation could play an important role in the development of pancreatic cancer, and be useful in clinical management of the disease.
TABLE 1.
List of aberrantly expressed miRNAs in pancreatic cancer.
| Upregulated microRNAs | References |
|---|---|
| miR-190, miR-186, miR-221, miR-222, miR-200b, miR-15b, and miR-95 |
[15] |
| miR-142-3p, miR-142-5p, miR-155, and miR-146a | [16] |
| miR-155 and miR-21 | [17] |
| Let-7f-l, Let-7d, miR-15b, miR-16-1, miR-21, miR-24, miR-100, miR-107, miR-125, miR-155, miR-181a, miR-181c, miR-376a, miR-424 |
[90] |
| miR-10, miR-23, miR-99, miR-103, miR-107, miR-125, miR-143, miR-148a&b, miR-181a,b,c&d, miR-199a, miR-205, miR-210, miR-213, miR-220, miR-221, miR-222, miR-223 |
[14] |
| miR-21, miR-194, miR-200b, miR-200c, miR-221, miR-429 | [38] |
| miR-196b | [15, 19] |
| miR-31, miR-143, miR-145, miR-146, miR-196a&b,miR-205, miR-222, miR-375 |
[13] |
| Downregulated microRNAs | |
| miR-223 | [91] |
| miR-29c, miR-30a-3p, miR-96, miR-141, miR-216, miR-217, miR-494 |
[13] |
| miR-130b, miR-148a, miR-148b, miR-375 | [13, 14] |
| miR-139, miR-142-P, miR-345 | [90] |
| miR-150 | [21] |
| miR-96 | [13, 14, 26] |
| Let7 | [30] |
| miR-20a | [92] |
| miR-34 | [23] |
| miR-126, miR-16 | [93] |
| miR-345, miR-142-P, miR-139 | [90] |
| miR-23b | [94] |
Biological significance of miRNAs in pancreatic cancer
With emerging data, it is now becoming evident that miRNAs regulate a variety of processes involved in the development, progression and chemoresistance of pancreatic cancer (Figure 1). In the following sections, we have provided detailed description about the biological significance of miRNAs in pancreatic cancer.
Figure 1. Multifaceted roles of miRNAs in pancreatic cancer pathobiology.
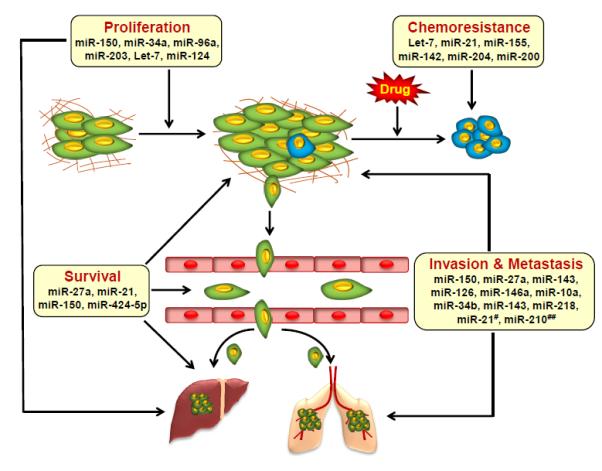
Illustrative overview of the roles miRNAs play in carcinogenic processes. Selective representation of a set of aberrantly expressed miRNAs associated with pancreatic cancer cell proliferation, survival, chemo-resistance invasion and metastasis. #Overexpressed in fibroblast cells as a result of their interaction with tumor cells; ##Overexpressed in pancreatic tumor cells through their cross-talk with pancreatic stellate cells.
Role of miRNAs in pancreatic cancer proliferation and survival
Emerging experimental studies suggest that miRNAs play pivotal role in the cancer cell proliferation and survival by altering the expression of oncogenes and tumor suppressor genes [21]. miR-34a is downregulated in various malignancies including pancreatic cancer [22]. It is known that miR-34a exerts its anti-cancer effects by altering the expression of several genes involved in cell cycle progression, DNA repair, angiogenesis and apoptosis [22]. It was revealed in an earlier study that restoration of miR-34a resulted in the inhibition of pancreatic cancer growth and clonogenicity by causing G1 and G2/M cell cycle arrests and apoptosis induction [23]. Moreover, this study demonstrated that restoration of miR-34a caused dramatic reduction (87%) of the tumor-initiating cell population. Recently, Liu et al [24] identified that miR-34b is also downregulated in pancreatic cancer and its restoration significantly inhibited pancreatic cancer progression in vitro and in vivo. Another study has shown that miR-27a, an oncogenic miRNA, is significantly overexpressed in pancreatic cancer and its inhibition diminishes growth and malignant behavior of pancreatic cancer cells by inducing tumor suppressor Spry2 [25]. miR-96 is also shown to be significantly downregulated in pancreatic cancer and its restoration inhibits pancreatic cancer cell proliferation, migration, and invasion and causes apoptosis. Mechanistically, it was demonstrated that miR-96 imparted its effect by inhibiting K-RAS and Akt signaling [26]. In a recent study, Xu et al [27] demonstrated the potential of miR-203 in the inhibition of Survivin, which is overexpressed in majority of cancers, including pancreatic cancer and plays a role in cytoprotection, apoptosis resistance and cell-cycle regulation. Their study showed that overexpression of miR-203 effectively induced apoptosis, inhibited cell proliferation and caused cell cycle arrest. Furthermore, in vivo study also demonstrated a potent effect of miR-203 on tumor growth inhibition [27]. Farhana et al [28] provided evidence that modulation of miR-150* and miR-630 expression resulted in diminished cellular growth and induced apoptosis. Numerous studies have shown that the expression of Let-7 is either significantly downregulated or completely lost in pancreatic cancer [29, 30]. A study on its function demonstrated that its ectopic expression led to the inhibition of pancreatic cancer cell proliferation by negative regulation of K-Ras oncogene and MAPK signaling [30]. Recently, we provided evidence that miR-150 is downregulated in pancreatic cancer and negatively regulates the expression of MUC4 oncoprotein. Moreover, we demonstrated that miR-150 mediated MUC4 downregulation was, in part, responsible for the suppression of growth and malignant potential of pancreatic cancer cells [21]. Sicard et al 2013 [31] demonstrated that depletion of miR-21 diminished the progression of aggressive model of pancreatic cancer and induced apoptosis in vivo. miR-424-5p is significantly overexpressed in pancreatic cancer and its upregulation is associated with enhanced proliferation and apoptosis resistance [32]. Using pyrosequencing, Wang and coworkers identified that miR-124 gene is highly methylated in pancreatic cancer tissues and its downregulation is significantly correlated with poor survival. Furthermore, they demonstrated that it inhibited pancreatic cancer cell proliferation by inhibiting Rac1 oncogene and MKK4-JNK-c-Jun pathway [33]. Altogether, these findings indicate that miRNAs play important roles in proliferation and survival of pancreatic cancer cells.
miRNAs as regulators of pancreatic cancer invasion and metastasis
Metastasis is the major cause of death in cancer patients. Biologically, it is a multi-step process involving dissociation of cancer cells from the primary site, invasion through the extracellular matrix into the blood or lymphatic system, and eventually extravasation and colonization at distant sites. To date, several miRNAs have been characterized for their potential role in regulating the metastatic processes, and such miRNAs are sometimes referred as “metastamiRs” [34, 35]. Weiss and coworkers identified that miR-10a is a key regulator of metastasis. Their study demonstrated that miR-10a overexpression promoted pancreatic cancer metastasis by downregulation of HOXB1 and HOXB3 genes [12]. Based on microarray and microdissection analysis, Nakata et al [36] observed significantly higher level of miR-10b in pancreatic cancer cells as compared to normal pancreatic ductal cells. Furthermore, their study revealed that high miR10b expression was associated with poor survival. Using microarray analysis, Huang and coworkers (2013) examined the levels of miRNAs in metastatic and non-metastatic pancreatic cancer cell lines and identified miR-100 to be significantly overexpressed in all the metastatic pancreatic cancer cell lines suggesting its association with the metastatic potential [37]. In clinical samples of pancreatic cancer, Liu C and coworkers identified that miR-34b was down-regulated in cancer tissues and that correlated with the specific stage of tumor-node-metastasis (TNM) and lymph-node metastasis. Mechanistically, it was revealed that miR-34b acted as a suppressor of tumor metastasis through negative regulation of Smad3 [24]. In another study, it was found that miR-194, miR-200b, miR-200c and miR-429, were significantly overexpressed in highly metastatic pancreatic cancer cells as compared to low or non-metastatic cell lines. Moreover, these miRNAs are known to target EP300, a metastatic suppressor gene. Their study further suggested that miR-194, miR-200b, miR-200c and miR-429, might regulate the metastatic behavior of pancreatic cancer cells by targeting metastasis-specific suppressor genes [38]. miR-143 is a tumor suppressor miRNA whose expression is lost in various malignancies, including pancreatic cancer [39-41]. Hu Y and coworkers (2012) showed that restoration of miR-143 significantly downregulated the expression of MMP-2, MMP-9, K-RAS, and GEF1 and GEF2. Further, their findings demonstrated that miR-143 drastically diminished the invasion and metastasis of pancreatic cancer cells [41]. Frampton and coworkers demonstrated that loss of miR-126 is a crucial event for invasive ductal adenocarcinoma as its restoration resulted in inhibition of invasive capacity and reversed epithelial to mesenchymal transition (EMT). It was also established that ADAM9 is a direct target of miR-126 [42]. In another recent study, it was shown that miR-224 and miR-486 are overexpressed in highly invasive and metastatic pancreatic cancer suggesting their possible involvement in promoting metastasis [7]. Expression of miR-146a has been reported to be significantly low in metastatic pancreatic cancer cell lines as compared to a normal pancreatic duct epithelial cell line [43]. It was shown that its ectopic expression led to efficient inhibition of invasiveness. The overexpression of miR-27a is also reported in gastric, breast, colon and pancreatic cancer [44], and its inhibition decreases the growth and malignant potential of pancreatic cancer cells [25, 39]. He and coworkers (2013) performed miRNA microarray analysis and identified that expression of miR-218 decreases progressively from normal acinar/ductal epithelium, IPMN, pancreatic cancer to metastatic lymph nodes. Authors further suggested that downregulation of miR-218 might promote pancreatic cancer metastasis [20]. Roldo et al [45] have shown that high expression of oncogenic miR-21 in pancreatic cancer is associated with a high Ki67 proliferation index and liver metastasis. More importantly, recent discoveries have suggested an important role of tumor-stromal interactions in pancreatic cancer pathobiology [46-48]. In that regard, it was recently demonstrated that pancreatic tumor-associated fibroblasts (TAFs) have an upregulated expression of miR-21, which is likely induced by their interaction with pancreatic tumor cells [49]. Furthermore, it was shown that miR-21 expression in TAFs promoted invasion and metastasis of pancreatic tumor cells. In another study, investigators have shown that pancreatic stellate cells (PSCs) induces the expression of miR-210 in pancreatic cancer cells, which facilitates EMT and promotes their migration [50]. Altogether, these findings identify a number of miRNAs to be of potential significance in the metastatic progression of pancreatic cancer.
Role of miRNAs in chemoresistance in pancreatic cancer
Chemotherapy remains the primary line of treatment for most of the cancer cases diagnosed at an advanced stage. However, in significant number of cases, we witness therapeutic failure due to chemoresistance resulting in a poor clinical outcome of the patients [51]. With accumulating data, it is now becoming evident that miRNAs play an important role in the therapeutic resistance of tumor cells. It has been demonstrated recently that let-7, a tumor suppressor miRNA negatively regulates ribonucleotide reductase subunit M2 (RRM2), a key determinant of chemoresistance to nucleoside drugs such as gemcitabine and capacitabine [52]. It was shown that let-7 expression inversely correlated with RRM2 expression in gemcitabine-resistant pancreatic cancer cells. Additionally, silencing RRM2 or overexpression of let-7 was shown to sensitize pancreatic cancer cells to gemcitabine [52]. Emerging research indicates that miR-21, besides its oncogenic role in tumor progression and metastasis, plays an important role in the chemoresistance of cancer cells. Dongand coworkers (2011) provided experimental evidence for a role of miR-21 in imparting chemoresistance to pancreatic cancer cells by directly regulating BCL-2 expression [53]. In a separate study, Hwang and coworkers (2010) demonstrated that inhibition of miR-21 in pancreatic cancer led to increased sensitivity to anticancer drug [54]. The role of miR-155 as an oncogenic miRNA is very well known [55]. Sheng et al [56] demonstrated that gemcitabine treatment induced the expression of miR-155 in pancreatic cancer cells suggesting its role in acquired chemoresistance. Moreover, miR-142-5p and miR-204 were also reported to be significantly downregulated in gemcitabine-resistant pancreatic ductal adenocarcinoma and established cell lines [57]. miR-200 is a tumor suppressor miRNA that plays a vital role in the development of metastases [58]. Li et al [59] identified that the entire miR-200 family of miRNAs are downregulated in gemcitabine-resistant pancreatic cancer cells. They demonstrated that restoration of miR-200 resulted in the reversal of drug resistance and sensitizes pancreatic cancer cells to gemcitabine cytotoxicity. Moreover, it was also revealed that induction of miR-200 by some nontoxic natural agents, such as DIM and curcumin, sensitizes the pancreatic cancer cells to gemcitabine cytotoxicity [59, 60]. Thus, these data provide clear evidence for the role of miRNAs in pancreatic cancer drug resistance.
Clinical potential of miRNAs in pancreatic cancer
The advent of miRNAs and current knowledge on their role in the development and progression of pancreatic cancer has created ample opportunities for translating the miRNA research into clinical settings. Recently, several studies have highlighted the relevance of these miRNAs in the diagnosis, prognosis and therapy of pancreatic cancer as described in the following sections.
miRNAs as novel diagnostic biomarkers
The poor outcome in treating pancreatic cancer can be attributed to its late diagnosis due to the asymptomatic nature of disease at the early stage. Therefore, there is an urgent need for the development of biomarkers for early detection to effectively manage this malignancy. Considering the vigorous involvement of miRNAs in cancer initiation and progression; miRNA profiling of serum, pancreatic juice and cyst fluid engenders a potential avenue for miRNA-based biomarker development. So far, several miRNAs have been identified that could be potentially used as diagnostic biomarkers for pancreatic cancer. At present, CA19-9 is the most common conventional diagnostic maker for pancreatic cancer patients; however, the sensitivity and the specificity of utilizing a combination of miRNAs (plasma miR-16+ miR- 196a +CA19-9) has been determined to be better than CA19-9 alone for screening of early pancreatic cancer [61]. Several studies also demonstrate the usefulness of specific miRNA signatures in differentiating tumors from normal/benign tissue. Szafranska et al [13] concluded that downregulation of miR- 217 accompanied by a high level of miR-196a can distinguish normal pancreas and chronic pancreatitis from PDAC. Combined analyses of four miRNAs, namely miR-21, miR-210, miR-155, and miR-196a, have also been reported to differentiate PDAC from healthy control group [62]. Earlier in our study, we identified that miR-150 was significantly downregulated in pancreatic cancer tissues as compared to normal pancreas suggesting the potential of miR-150 in differentiating between normal and pancreatic cancer samples [21]. Detection of miR-210 in the serum revealed four-fold higher expression in pancreatic cancer patients as compared to the normal controls [63]. Likewise, plasma concentrations of miR-18a were significantly higher in pancreatic cancer patients than in the normal controls [64]. miR-155 has also been identified as one of the potential biomarker for detecting early pancreatic neoplasia [65]. Furthermore, Yu et al [19] evaluated 700 miRNAs in PanIN lesions and identified aberrant expression of 35 miRNAs in PanIN-3, including overexpression of let-7f/g, -18a, -15b, -21, -29a/b/c, -31, -93, -95, miR-101, -103, -106b, -146a, -155, -182, -190, -193b, -194, -196b, -200a/b, -203, -222, -338-3p, -429, and 486-3p, and no or weak expression of miR-107, -139-3p/5p, -216a/b, -217, -218 and -483-5p in PanIN-3. Based on this study, miR-196b emerges as the most useful biomarker in discriminating PanIN-3 lesions. Although, the above studies demonstrate the potential implication of miRNA profiling as a diagnostic tool for distinguishing PDAC from normal pancreas or chronic pancreatitis, it is important to classify/categorize miRNAs according to stage, grade and cellular origin to establish them as biomarker in clinical settings.
miRNAs as prognostic biomarkers
Besides the implication of miRNAs for diagnosis, data from emerging studies also provide compelling evidence for their potential utility as prognostic biomarkers in pancreatic cancer. Several studies have identified specific miRNA expression profiles that can predict outcome of the disease. miR-142-5p and miR-204 are downregulated in gemcitabine-resistant PDAC samples and higher expression of these miRNAs is correlated with prolonged survival of pancreatic cancer patients [57]. This study concluded that miR-142-5p is a predictive marker for gemcitabine responsivness in patients with resected pancreatic cancer. miR-320c is also found to be significantly overexpressed in gemcitabine-resistant pancreatic cancer cells suggesting its potential use as a marker for predicting clinical response [66]. Yu J et al [67] showed that patient group exhibiting high miR-200c expression had median survival of 33.5%, while it was only 11.2% in the patient group with low miR-200c expression. Greither and coworkers investigated the prognostic value of miR-155, miR-203, miR-210 and miR-222 in a cohort of 56 PDAC samples [68]. Their study identified a significant correlation between elevated levels of miRNAs and overall patient survival. Tumors from patients exhibiting high expression of all the 4 miRNAs exhibited a 6.2-fold increased risk of tumor-related death as compared to those with low expression [68]. Using in situ hybridization, Dillhoff et al [69] demonstrated that miR-21 is overexpressed in PDAC cells and is associated with the shorter survival in node-negative patients. In a separate study, Giovannetti et al [70] determined that high expression of miR-21 correlated with poor outcome in pancreatic cancer patients treated with gemcitabine. Recently, Kadera et al examined the expression of miR-21 in 153 PDAC specimens and reported that miR-21 was significantly overexpressed in tumor associated fibroblast in around 78% of tumor cases [49]. Moreover, their study also revealed that high miR-21 expression was correlated with lymph node metastasis and decreased overall survival [49]. In another study, it was demonstrated that six miRNAs (miR-452, miR-105, miR-127, miR-518-a-2, miR-187 and miR-309-3p) could effectively distinguish long-term survivors in patients with node-positive disease dying within 24 months [45]. Further, their study also revealed that high expression of miR-196a-2 could predict poor survival [14]. In a separate study, miR-203 was identified as a prognostic marker in PDAC patients, who underwent resection [71]. Thus, these reports underscore the potential of miRNAs for prognostic assessments in pancreatic cancer.
Therapeutic potential of miRNAs
It is now understood that miRNAs are involved in the development and progression of pancreatic cancer. Consequently, there are plenty of opportunities to exploit these miRNAs for the development of novel therapeutic strategies. Although, the data from clinical trial studies involving miRNAs in cancer therapy are limited [72], preclinical data is available from several studies to advocate miRNA replacement as a therapy for pancreatic cancer. It was demonstrated recently that viral-mediated delivery of miR-143 or miR-145 could block the tumor formation by pancreatic cancer cells [73]. Similarly, in another study adenovirus-mediated transduction of miR-143 significantly inhibited pancreatic cancer metastasis [74]. Also, miR-96 restoration remarkably diminished the tumorigenesis in subcutaneous implanted MiaPaCa cells [26]. In our recently published study, we have also demonstrated that restoration of tumor suppressor miR-150 in pancreatic cancer cells efficiently diminishes pancreatic cancer cell growth and malignant potential [21]. Delivery of let-7 mimics has also been shown to inhibit the pancreatic cancer growth [30]. Similarly, restoration of miR-34 using specific mimic blocked pancreatic cancer cell growth, metastatic potential and sensitizes them to chemo- and radiation- therapy [75]. Targeting oncogenic miRNAs, using antisense technology or other specific inhibitors is also shown to be a promising therapeutic strategy. For example, inhibition of oncogenic miR-21 and miR-221 by this approach causes significant growth inhibition through upregulation of tumor suppressor genes such as PTEN, RECK and p27 [76]. Repression of miR-10a function has also been shown to effectively suppress pancreatic cancer metastasis [77]. Silencing of miR-132 and miR-212, two significantly upregulated miRNAs in pancreatic cancer, using anti-sense miRNA oligonucleotides also led to diminished pancreatic tumor growth [78]. Altogether, the above studies provide strong rationale to exploit miRNAs as a novel therapeutic approach against pancreatic cancer.
Translational challenges and emerging strategies
Above mentioned studies strongly advocate potential usefulness of miRNAs in cancer diagnosis, prognosis and therapy. Despite these promises, there are several challenges hindering the path of their translational applicability. For example, small size of mature miRNAs, their low occurrence in clinical samples and sequence similarity are the major constraints for their diagnostic or prognostic utility. To overcome these limitations, some novel methods have recently been developed that could aid in the development of clinically feasible approaches for miRNA detection with high sensitivity and specificity [5]. Here, we are discussing a few that carry great promise to overcome the associated challenges. Zhang et al [79] developed an electrical detection based method using silicon nanowires (SiNW). This method uses peptide nucleic acids (PNA) immobilized on the surface of the SiNW device for miRNA detection. This method is rapid and highly sensitive for miRNA detection. Yang et al [80] later modified and developed more sensitive detection method (10 amol detection limit), using a Fe-Ru redox pair as a reporter. Following similar principle, another strategy was developed in which the sensor was capable of detecting as low as 1 fM target miRNA, and could identify completely matched versus mismatched miRNA sequences [81]. Ma et al [82] compared the efficiency of digital PCR and conventional qPCR for measuring the low abundant miRNAs in the circulating body fluid. Their study demonstrated that digital PCR exhibited higher sensitivity and reproducibility than qPCR [82]. Thus, these emerging advances leading to refined methodologies for detecting miRNAs with excellent efficiency and reproducibility for diagnosis and prognosis hold great promise for the future. On parallel roads, efforts are also being made to develop effective miRNA-based therapies for the management of cancer. To achieve therapeutic efficacy of miRNAs systemically, the desired system should be able to pass several major obstacles viz. rapid clearance from blood, poor cellular uptake and off target effects. To overcome these challenges, viral and non-viral delivery systems have been developed. Kota and colleagues have shown that adenoviral mediated delivery of miR-26a resulted in inhibition of hepatocellular carcinoma progression in mouse model [83]. In two different studies, delivery of let-7 by viral-based vector system inhibited Kras-induced lung cancer progression in xenograft and autochthonous mouse models [84, 85]. Although, viral vector-based systems show high gene transfer efficiency but undesired immunogenic responses is a critical issue. Therefore, nonviral mediated strategies using liposomes and polymeric nanoparticles have been developed and tested. Liposome-mediated delivery has an advantage that it protects the oligonucleotides from degradation by nucleases, improves the half-life and enhances their cellular uptake. miRNA therapeutics developed liposomal delivery system for miR-34 and its pre-clinical study on prostate and lung cancer yielded therapeutic effects in vivo. In a separate study, liposomal formulations have been used for systemic delivery of miR-34a and miR-143/145 in pancreatic tumors [86]. The miR-34a- and miR-143/145-encapsulated liposomes inhibited the growth of MiaPaCa-2 subcutaneous xenografts as well orthotopically grown tumors [86]. However, despite being a very potent delivery system, cellular toxicity of liposomes remains a major concern. Thus, for the safe and effective delivery of miRNAs, polymeric nanoparticle-based strategies are also being explored. Since off target effect is also one of the major challenges of miRNA based therapy; researchers are trying to develop targeted nanoparticles by conjugating nanoparticle-miRNA complexes with antibodies that bind specifically to the desired target cells. For instance, Gaca and coworkers [87] developed a miRNA-loaded nanoparticle system conjugated with Hsp70 antibody on the surface to target Hsp70 positive glioblastoma cells. Ephrin-A1 conjugated nanoparticles of let-7a were shown to significantly inhibit the tumor growth in lung cancer as compared to either ephrin-A1 or let-7a nanoparticle alone [88]. Hu et al developed miR-34a-delivering nanocomplexes with a tumor-targeting and -penetrating bifunctional CC9 peptide for pancreatic cancer therapy. Their study demonstrated that this nanocomplex significantly induced apoptosis and inhibited pancreatic tumor growth [89]. Thus, the delivery of miRNA mimics, using liposomes or polymer-based nanoparticle formulations, has emerged as a big hope in the field of medicine and pharmaceutical industry.
CONCLUSION
Discovery of microRNAs has brought revolution in the field of life science and has impacted multiple areas of significance to us. Novel information on the miRNAs and their biological implications is emerging at a logarithmic rate and the field holds enormous potential to influence biological science and cross-disciplinary areas of research. Accumulating data have greatly highlighted the role of miRNAs in the pathobiology of cancer and provided evidence that miRNAs could be exploited for clinical benefit. Extensive genome-wide miRNA profiling studies have demonstrated that specific miRNAs/miRNA signatures are frequently dysregulated in pancreatic cancer, and could serve as basis for early diagnosis and prognosis of pancreatic cancer. Furthermore, ability of miRNAs to regulate multiple genes involved in pancreatic cancer etiology, progression, metastasis and therapy-resistance makes them attractive targets for the efficient management and treatment of pancreatic cancer. It is noteworthy that within a short time span since miRNAs were first associated with cancer, we have made significant progress in our understanding of their involvement in the disease processes. However, it may still take a while to exploit their advantage in clinical settings, and may require many more studies to develop a refined understanding and application approaches. Cancer being a complex disease necessitates validation in prospective studies if miRNAs were to become biomarkers of choice. Furthermore, therapeutic approaches based on miRNA replacement or inhibition of their functions needs to be tested. In particular, pancreatic tumors poses additional barrier based on their unique physical characteristics, and thus will need additional manipulation in therapeutic approaches. Nonetheless, the progress has been promising, and will in due course of time result in development of miRNA-based clinical management approaches for this devastating malignancy.
Highlights.
MicroRNAs are novel post-transcriptional regulators of gene expression
MicroRNAs are aberrantly expressed in pancreatic cancer
MicroRNAs play key roles in pancreatic cancer pathogenesis
MicroRNAs can be exploited as novel diagnostic and prognostic biomarkers
MicroRNAs can serve as novel targets for pancreatic cancer therapy
Figure 2. Potential implications of miRNAs in pancreatic cancer diagnosis, prognosis and therapy.
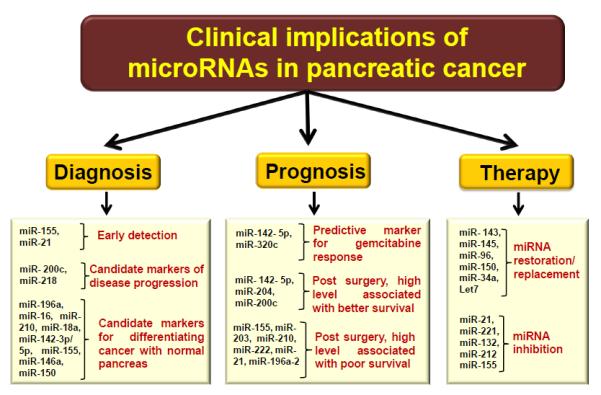
Schematic representation showing that differentially expressed miRNAs (signatures miRNAs) could serve as basis for early diagnosis and prognosis of pancreatic cancer. Moreover, restoration of tumor suppressor miRNAs and or inhibition of oncogenic miRNAs could be used as a novel therapeutic approach against pancreatic cancer.
Acknowledgments
#Grant support: NIH/NCI (CA137513, CA167137, CA169829, CA175772) and USAMCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No potential conflict of interest to disclose
Conflict of Interest Statement
None
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Bhardwaj A, Singh S, Srivastava SK, McClellan S, Nirodi CS, Piazza GA, Grizzle WE, Owen LB, Singh AP. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J. Biol. Chem. 2013;19(288):21197–21207. doi: 10.1074/jbc.M113.484576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J. Am. Coll. Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuramitsu Y, Taba K, Ryozawa S, Yoshida K, Zhang X, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Identification of up- and down-regulated proteins in gemcitabine-resistant pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2010;30:3367–3372. [PubMed] [Google Scholar]
- 5.Srivastava SK, Bhardwaj A, Leavesley SJ, Grizzle WE, Singh S, Singh AP. MicroRNAs as potential clinical biomarkers: emerging approaches for their detection. Biotech. Histochem. 2013;88:373–387. doi: 10.3109/10520295.2012.730153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhardwaj A, Arora S, Prajapati VK, Singh S, Singh AP. Cancer "stemness"-regulating microRNAs: role, mechanisms and therapeutic potential. Curr. Drug Targets. 2013;14:1175–1184. doi: 10.2174/13894501113149990190. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Jamaluddin MS, Weakley SM, Yao Q, Chen C. Roles and mechanisms of microRNAs in pancreatic cancer. World J. Surg. 2011;35:1725–1731. doi: 10.1007/s00268-010-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj A, Singh S, Singh AP. MicroRNA-based Cancer Therapeutics: Big Hope from Small RNAs. Mol. Cell Pharmacol. 2010;2:213–219. doi: 10.4255/mcpharmacol.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Bhatti I, Lee A, Lund J, Larvin M. Small RNA: a large contributor to carcinogenesis? J. Gastrointest. Surg. 2009;13:1379–1388. doi: 10.1007/s11605-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Marin-Muller C, Bharadwaj U, Chow KH, Yao Q, Chen C. MicroRNAs: control and loss of control in human physiology and disease. World J. Surg. 2009;33:667–684. doi: 10.1007/s00268-008-9836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, Heidecke CD, Lerch MM, Bagowski CP. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137:2136–2145. doi: 10.1053/j.gastro.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 13.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 14.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, Chen C. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J. Surg. 2009;33:698–709. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol. Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu JK, Hong SM, Karikari CA, Hruban RH, Goggins MG, Maitra A. Aberrant MicroRNA-155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology. 2010;10:66–73. doi: 10.1159/000231984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 2012;18:981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, Di Y, Liang M, Yang F, Yao L, Hao S, Li J, Jiang Y, Jin C, Fu D. The microRNA-218 and ROBO-1 signaling axis correlates with the lymphatic metastasis of pancreatic cancer. Oncol. Rep. 2013;30:651–658. doi: 10.3892/or.2013.2516. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, Grizzle WE, Singh AP. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS. One. 4(2009):e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Cheng H, Shi S, Cui X, Yang J, Chen L, Cen P, Cai X, Lu Y, Wu C, Yao W, Qin Y, Liu L, Long J, Xu J, Li M, Yu X. MicroRNA-34b inhibits pancreatic cancer metastasis through repressing Smad3. Curr. Mol. Med. 2013;13:467–478. doi: 10.2174/1566524011313040001. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Yu S, Zhao W, Lu Z, Chen J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298:150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W, Liu J, Yu J, Chen J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Wang Q, An Y, Xu L. MiR203 regulates the proliferation, apoptosis and cell cycle progression of pancreatic cancer cells by targeting Survivin. Mol. Med. Rep. 2013;8:379–384. doi: 10.3892/mmr.2013.1504. [DOI] [PubMed] [Google Scholar]
- 28.Farhana L, Dawson MI, Murshed F, Das JK, Rishi AK, Fontana JA. Upregulation of miR-150* and miR-630 induces apoptosis in pancreatic cancer cells by targeting IGF-1R. PLoS. One. 8(2013):e61015. doi: 10.1371/journal.pone.0061015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev. Res. (Phila) 2012;5:355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrisani J, Bournet B, du Rieu MC, Bouisson M, Souque A, Escourrou J, Buscail L, Cordelier P. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum. Gene Ther. 2009;20:831–844. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- 31.Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu K, Hu G, He X, Zhou P, Li J, He B, Sun W. MicroRNA-424-5p suppresses the expression of SOCS6 in pancreatic cancer. Pathol. Oncol. Res. 2013;19:739–748. doi: 10.1007/s12253-013-9637-x. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Chen L, Zhang J, Chen H, Fan J, Wang K, Luo J, Chen Z, Meng Z, Liu L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene. 2013;23(33):514–24. doi: 10.1038/onc.2012.598. [DOI] [PubMed] [Google Scholar]
- 34.Edmonds MD, Hurst DR, Welch DR. Linking metastasis suppression with metastamiR regulation. Cell Cycle. 2009;8:2673–2675. doi: 10.4161/cc.8.17.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69:7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakata K, Ohuchida K, Mizumoto K, Kayashima T, Ikenaga N, Sakai H, Lin C, Fujita H, Otsuka T, Aishima S, Nagai E, Oda Y, Tanaka M. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery. 2011;150:916–922. doi: 10.1016/j.surg.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Huang JS, Egger ME, Grizzle WE, McNally LR. MicroRNA-100 regulates IGF1-receptor expression in metastatic pancreatic cancer cells. Biotech. Histochem. 2013;88:397–402. doi: 10.3109/10520295.2012.762460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mees ST, Mardin WA, Wendel C, Baeumer N, Willscher E, Senninger N, Schleicher C, Colombo-Benkmann M, Haier J. EP300--a miRNA-regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas. Int. J. Cancer. 2010;126:114–124. doi: 10.1002/ijc.24695. [DOI] [PubMed] [Google Scholar]
- 39.Ma Q, Jiang Q, Pu Q, Zhang X, Yang W, Wang Y, Ye S, Wu S, Zhong G, Ren J, Zhang Y, Liu L, Zhu W. MicroRNA-143 inhibits migration and invasion of human non-small-cell lung cancer and its relative mechanism. Int. J. Biol. Sci. 2013;9:680–692. doi: 10.7150/ijbs.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H, Chen Y, Hu X, Qian G, Ge S, Zhang J. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Mol. Cancer. 2013;12:77–12. doi: 10.1186/1476-4598-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Ou Y, Wu K, Chen Y, Sun W. miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour. Biol. 2012;33:1863–1870. doi: 10.1007/s13277-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 42.Frampton AE, Krell J, Jacob J, Stebbing J, Castellano L, Jiao LR. Loss of miR-126 is crucial to pancreatic cancer progression. Expert. Rev. Anticancer Ther. 2012;12:881–884. doi: 10.1586/era.12.67. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Vandenboom TG, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng SY, Dong CG, Wu WK, Wang XJ, Qiao J, Shao JF. Lentiviral expression of anti-microRNAs targeting miR-27a inhibits proliferation and invasiveness of U87 glioma cells. Mol. Med. Rep. 2012;6:275–281. doi: 10.3892/mmr.2012.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 46.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin. Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, Grizzle WE, Owen LB, Singh S. CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor kappaB: implications for bidirectional tumor-stromal interactions. J. Biol. Chem. 2012;287:39115–39124. doi: 10.1074/jbc.M112.409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadera BE, Li L, Toste PA, Wu N, Adams C, Dawson DW, Donahue TR. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS. One. 8(2013):e71978. doi: 10.1371/journal.pone.0071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takikawa T, Masamune A, Hamada S, Nakano E, Yoshida N, Shimosegawa T. miR-210 regulates the interaction between pancreatic cancer cells and stellate cells. Biochem. Biophys. Res. Commun. 2013;437:433–439. doi: 10.1016/j.bbrc.2013.06.097. [DOI] [PubMed] [Google Scholar]
- 51.Warsame R, Grothey A. Treatment options for advanced pancreatic cancer: a review. Expert. Rev. Anticancer Ther. 2012;12:1327–1336. doi: 10.1586/era.12.115. [DOI] [PubMed] [Google Scholar]
- 52.Bhutia YD, Hung SW, Krentz M, Patel D, Lovin D, Manoharan R, Thomson JM, Govindarajan R. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: role of LIN-28 and SET oncoprotein. PLoS. One. 2013;8:e53436. doi: 10.1371/journal.pone.0053436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong J, Zhao YP, Zhou L, Zhang TP, Chen G. Bcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cells. Arch. Med. Res. 2011;42:8–14. doi: 10.1016/j.arcmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH, Kim SW, Del CM, Peters GJ, Giaccone G. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS. One. 2010;5:e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgs G, Slack F. The multiple roles of microRNA-155 in Oncogenesis. J. Clin. Bioinforma. 3(2013):17. doi: 10.1186/2043-9113-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia QS, Ishigaki Y, Zhao X, Shimasaki T, Nakajima H, Nakagawa H, Takegami T, Chen ZH, Motoo Y. Human SMG-1 is involved in gemcitabine-induced primary microRNA-155/BIC up-regulation in human pancreatic cancer PANC-1 cells. Pancreas. 2011;40:55–60. doi: 10.1097/MPA.0b013e3181e89f74. [DOI] [PubMed] [Google Scholar]
- 57.Ohuchida K, Mizumoto K, Kayashima T, Fujita H, Moriyama T, Ohtsuka T, Ueda J, Nagai E, Hashizume M, Tanaka M. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann. Surg. Oncol. 2011;18:2381–2387. doi: 10.1245/s10434-011-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 2010;70:6401–6406. doi: 10.1158/0008-5472.CAN-10-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Vandenboom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, Wang X, Gong Y, Wang W, Kong X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int. J. Cancer. 2012;131:683–691. doi: 10.1002/ijc.26422. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev. Res. (Phila) 2009;2:807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, Koong AC. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl. Oncol. 2010;3:109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br. J. Cancer. 2011;105:1733–1740. doi: 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol. Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwagami Y, Eguchi H, Nagano H, Akita H, Hama N, Wada H, Kawamoto K, Kobayashi S, Tomokuni A, Tomimaru Y, Mori M, Doki Y. miR-320c regulates gemcitabine-resistance in pancreatic cancer via SMARCC1. Br. J. Cancer. 2013;109:502–511. doi: 10.1038/bjc.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol. Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greither T, Grochola LF, Udelnow A, Lautenschlager C, Wurl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int. J. Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 69.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giovannetti E, Funel N, Peters GJ, Del CM, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A, Danesi R, Campani D, Verheul HM, Boggi U. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70:4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 71.Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Kayashima T, Sakai H, Fujita H, Nakata K, Tanaka M. MicroRNA-203 expression as a new prognostic marker of pancreatic adenocarcinoma. Ann. Surg. Oncol. 2010;17:3120–3128. doi: 10.1245/s10434-010-1188-8. [DOI] [PubMed] [Google Scholar]
- 72.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Y, Ou Y, Wu K, Chen Y, Sun W. miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour. Biol. 2012;33:1863–1870. doi: 10.1007/s13277-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 75.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS. One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 77.Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, Heidecke CD, Lerch MM, Bagowski CP. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137:2136–2145. doi: 10.1053/j.gastro.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 78.Park JK, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, Postier RG, Brackett DJ, Schmittgen TD. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem. Biophys. Res. Commun. 2011;406:518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang GJ, Chua JH, Chee RE, Agarwal A, Wong SM. Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009;24:2504–2508. doi: 10.1016/j.bios.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 80.Yang H, Hui A, Pampalakis G, Soleymani L, Liu FF, Sargent EH, Kelley SO. Direct, electronic microRNA detection for the rapid determination of differential expression profiles. Angew. Chem. Int. Ed Engl. 2009;48:8461–8464. doi: 10.1002/anie.200902577. [DOI] [PubMed] [Google Scholar]
- 81.Zhang GJ. Silicon nanowire biosensor for ultrasensitive and label-free direct detection of miRNAs. Methods Mol. Biol. 2011;676:111–121. doi: 10.1007/978-1-60761-863-8_9. [DOI] [PubMed] [Google Scholar]
- 82.Ma J, Li N, Guarnera M, Jiang F. Quantification of Plasma miRNAs by Digital PCR for Cancer Diagnosis. Biomark. Insights. 2013;8:127–136. doi: 10.4137/BMI.S13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG, Slack FJ. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 85.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaca S, Reichert S, Multhoff G, Wacker M, Hehlgans S, Botzler C, Gehrmann M, Rodel C, Kreuter J, Rodel F. Targeting by cmHsp70.1-antibody coated and survivin miRNA plasmid loaded nanoparticles to radiosensitize glioblastoma cells. J. Control Release. 2013;172:201–206. doi: 10.1016/j.jconrel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 88.Lee HY, Mohammed KA, Kaye F, Sharma P, Moudgil BM, Clapp WL, Nasreen N. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. Int. J. Nanomedicine. 2013;8:4481–4494. doi: 10.2147/IJN.S41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu QL, Jiang QY, Jin X, Shen J, Wang K, Li YB, Xu FJ, Tang GP, Li ZH. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model. Biomaterials. 2013;34:2265–2276. doi: 10.1016/j.biomaterials.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 90.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, Miele L, Sarkar FH, Xia J, Wang Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr. Drug Targets. 2013;14:1150–1156. doi: 10.2174/13894501113149990187. [DOI] [PubMed] [Google Scholar]
- 92.Yan H, Wu J, Liu W, Zuo Y, Chen S, Zhang S, Zeng M, Huang W. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum. Gene Ther. 2010;21:1723–1734. doi: 10.1089/hum.2010.061. [DOI] [PubMed] [Google Scholar]
- 93.Jiao LR, Frampton AE, Jacob J, Pellegrino L, Krell J, Giamas G, Tsim N, Vlavianos P, Cohen P, Ahmad R, Keller A, Habib NA, Stebbing J, Castellano L. MicroRNAs targeting oncogenes are down-regulated in pancreatic malignant transformation from benign tumors. PLoS. One. 2012;7:e32068. doi: 10.1371/journal.pone.0032068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P, Zhang J, Zhang L, Zhu Z, Fan J, Chen L, Zhuang L, Luo J, Chen H, Liu L, Chen Z, Meng Z. MicroRNA 23b Regulates Autophagy Associated With Radioresistance of Pancreatic Cancer Cells. Gastroenterology. 2013;145:1133–1143. doi: 10.1053/j.gastro.2013.07.048. [DOI] [PubMed] [Google Scholar]


