Abstract
Human copper transporter 1 (hCTR1) is the high-affinity copper influx transporter in mammalian cells that also mediates the influx of cisplatin. Loss of hCTR1 expression has been implicated in the development of resistance to this cancer chemotherapeutic agent. It has turned out to be very difficult to develop antibodies to hCTR1 and polyclonal antibodies produced by different laboratories have yielded conflicting results. We have characterized a newly-available rabbit monoclonal antibody that reacts with an epitope on the N-terminal end of hCTR1 that now permits rigorous identification and quantification of hCTR1 using Western blot analysis. Postnuclear membrane (PNM) preparations made from cells engineered to express high levels of myc-tagged hCTR1, and cells in which the expression of hCTR1 was knocked down, were used to characterize the antibody. The identity of the bands detected was confirmed by immunoprecipitation, surface biotinylation and deglycosylation of myc-tagged hCTR1. Despite the specificity expected of a monoclonal antibody, the anti-hCTR1 detected a variety of bands in whole cell lysates (WCL), which made it difficult to quantify hCTR1. This problem was overcome by isolating post-nuclear membranes and using these for further analysis. Three bands were identified using this antibody in PNM preparations that migrated at 28, 33–35 and 62–64 kDa. Multiple lines of evidence presented here suggest that the 33–35 and 62–64 kDa bands are hCTR1 whereas the 28 kDa band is a cross-reacting protein of unknown identify. The 33–35 kDa band is consistent with the expected MW of the glycosylated hCTR1 monomer. This analysis now permits rigorous identification and quantification of hCTR1.
Keywords: copper transporter 1, copper, monoclonal antibody, transporter, Western blot
Introduction
Copper is an essential micronutrient important for many cellular processes including cell signaling, metabolism and embryologic development (1, 2) . Maintaining copper homeostasis is a critical cellular function that is mediated by evolutionarily-conserved copper transporters and chaperones. One of the most important of these is human copper transporter 1 (hCTR1) which is the high-affinity transporter responsible for most of the copper uptake into the cell (3). Along with copper, hCTR1 also appears to transport the platinum-containing chemotherapeutic agents, and loss of hCTR1 expression has been implicated in the development of resistance to the platinum-containing drugs (4–6). The expression of hCTR1 may serve as a biomarker of platinum drug sensitivity (7, 8). Moreover, attempts have been made to manipulate hCTR1 cell surface expression to overcome resistance to platinum-based chemotherapy (9–12). For these reasons, being able to accurately identify and quantify changes in hCTR1 expression has become important to further our understanding of both copper biology and chemotherapy resistance.
It has proven very difficult to develop high-quality polyclonal antibodies capable of rigorously identifying and quantifying hCTR1 expression in mammalian cells. This has resulted in problems in reproducing results from one laboratory to another leading to confusion in the literature. The calculated molecular weight of the hCTR1 monomer is 21 kDa; however, it has generally been detected with different polyclonal antibodies as a smear around 35 kDa (13–15). This is consistent with the observation that hCTR1 is modified with both N- and O-linked sugars in its N-terminal region (14, 16). hCTR1 appears to exist as a trimer when fully assembled in membranes (17). hCTR1 is found on both the plasma membrane and a variety of internal membranes, and the relative distribution of cell surface to intracellular hCTR1 is highly variable among cell lines (6). hCTR1’s membrane expression, glycosylation, tendency to exist in multimeric forms and low level expression in many cell types has further complicated its identification and quantification by Western blot analysis. While a number of studies using polyclonal antibodies have been published (18–22), it is not clear that the antibodies were really detecting hCTR1 and this has been a major problem in the field. We present here the characterization of a new and commercially-available rabbit monoclonal antibody that detects an epitope in the N-terminal part of hCTR1 that now permits rigorous identification of hCTR1 in human cells.
Materials and Methods
Antibodies and Reagents
Primary antibodies used included a rabbit monoclonal anti-hCTR1 antibody (Epitomics catalog # 5773–1/Abcam catalog # AB129067), mouse monoclonal anti-myc antibody from Cell Signaling (Danvers, MA) and a mouse monoclonal anti-human transferrin receptor from Invitrogen (Carlsbad, CA). The rabbit monoclonal anti-hCTR1 antibody from Epitomics recognizes amino acids 1–30 of the hCTR1 N-terminal. All primary antibodies were used at a dilution of 1:1000; gels were blocked by incubation with 5 % powdered milk, 0.1 % Tween-20. Secondary antibodies consisted of goat anti-rabbit (LI-COR Biosciences; Lincoln, NE) and goat anti-mouse (LI-COR Biosciences; Lincoln, NE). All secondary antibodies were incubated with 5% powdered milk, 0.1% Tween-20 and 0.02% SDS. Copper sulfate was obtained from Sigma-Aldrich (St. Louis, MO) and cisplatin (cDDP) was obtained from Teva Parenteral Medicines, Inc (Irvine, CA). Blastocidin and puromycin were both obtained from Thermo Fisher Scientific (Waltham, MA).
Cell Culture
A2780 and A2780/myc-hCTR1 ovarian cancer cells were cultured in RPMI 1640 medium (HyClone; Logan, Utah) supplemented with 10% fetal bovine serum (HyClone; Logan, Utah) and 100 U/mL penicillin/100 μg/mL streptomycin (HyClone; Logan, Utah). HEK293T/myc-CTR1 cells were cultured in Dulbecco’s minimal essential media - High Glucose (DMEM, HyClone; Logan, Utah) supplemented with 10 % fetal bovine serum, 2 mM L-glutamine (HyClone; Logan, Utah), 1 mM sodium pyruvate (Corning; Manassas, VA), and 100 U/mL penicillin/100 μg/mL streptomycin. The A2780 ovarian cancer cell line was obtained from Dr. Thomas Hamilton at the Fox Chase Cancer Center and the HEK293T cells were obtained from the American Type Culture Collection.
The wild-type myc-tagged hCTR1 was constructed in the vector pENTR/D-TOPO and then transferred to the pLenti6/V5-DEST by LR reaction as previously described (23). The myc-CTR1WT lentiviral particles were produced in HEK293T cells using the ViraPower™ packaging kit (Invitrogen; Carlsbad, CA) and then transduced into the HEK293T and A2780 cells under 8 μg/mL and 10 μg/mL blastocidin selection, respectively. All cells were grown at 37 ºC in 5 % CO2.
hCTR1 was knocked-down using lentiviral-based short-hairpin RNA transduction. The lentiviral particles were obtained from Sigma-Aldrich (CTR1 shRNA Clone ID# TRCN0000043348). Cells were transduced at a ratio of 10 transducing lentiviral particles per cell after reaching 50–80% confluency. After overnight incubation, the viral particle-containing media was removed and the cells were cultured in selection media (10 μg/mL puromycin) for two weeks and were subsequently maintained in 2 μg/mL puromycin. The efficiency of knock down was then confirmed via Western blot and qRT-PCR using GAPDH as an internal control as previously reported (24).
Postnuclear Membrane Isolation
Cells were grown to 80–90% confluency, scraped into suspension and pelleted by centrifugation at 1,000 x g for 10 minutes at 4 ºC. The pellet was resuspended in 750 μL of ice-cold homogenizing buffer (250 mM sucrose, 10 mM Tris HCl, pH 7.4 and 2 mM EDTA) containing a protease inhibitor cocktail tablet (Roche complete; Indianapolis, IN), and dounced 40 times using a homogenizer plunger. The suspension was then sucked through a 28 gauge needle and gently expelled thrice. The cellular debris was pelleted by centrifugation at 1,000 x g for 10 minutes at 4 ºC and the supernatant was then transferred to a ½ x 2 inch polyallomer centrifuge tube (Beckman Coulter; Brea, CA) and centrifuged at 135,000 x g for 30 minutes in a Beckman Optima™ TL ultracentrifuge using a Beckman TLA 100.3 fixed-angle rotor. The post-nuclear membrane (PNM) pellet was then re-suspended in 80 μL of ice-cold homogenizing buffer, the protein concentration was determined by the DC™ Protein assay (Bio-Rad; Hercules, CA) the sample was stored at −80 ºC.
Thirty μg of protein was added per well when using PNM preparations. Laemmli’s 2x sample buffer (125 mM Tris HCl, pH 6.8, 2 mM EDTA, 6 % SDS, 20 % glycerol and 0.25 % bromophenol blue) with fresh 5 % β-mercaptoethanol was added to the sample and allowed to sit at room temperature for 30 minutes. The sample was then separated on a 4–15% Criterion™ TGX™ precast gel (Bio-Rad; Hercules, CA). The gel was then transferred to an Immobilon-FL transfer membrane (Millipore; Billerica, MA) and then blocked using 5% powdered milk and tris-buffered saline (TBS) for one h. Membranes were then incubated with primary antibody at least overnight and washed in TBS, 0.1% Tween-20, thrice. Following washing, membranes were incubated with secondary antibody for one h followed by washing thrice with TBS, 0.1 % Tween-20. The membrane was then analyzed using the Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences; Lincoln, NE).
Whole Cell Lysis
Cells were grown until 80–90% confluent, washed with once with PBS, trypsinized and pelleted by centrifugation at 1,500 x g. RIPA buffer containing 50 mM Tris, 150 mM NaCl, 0.1 % SDS, 0.5 % sodium deoxycholate, 1 % Triton X-100 and supplemented with the protease and phosphatase inhibitor cocktail (Thermo Scientific; Logan, UT) was then added to the cell pellet which was re-suspended and centrifuged at 10,000 x g for 10 min to remove insoluble cellular debris. The protein concentration of the supernatant was then determined by DC™ Protein Assay (Bio-Rad; Hercules, CA).
Biotinylation of Plasma Membrane Proteins
Assay of cell surface levels of hCTR1 in HEK293T/myc-CTR1 and A2780/myc-CTR1 was performed with and without exposure to 100 μM copper sulfate for 30 minutes using the Cell Surface Protein Isolation kit (Thermo Scientific; Rockford, IL). Cells grown to 80–90% confluence in a 145 mm plate were biotinylated, lysed and cell surface proteins isolated and eluted according to the manufacturer’s instructions. The eluted proteins were subjected to Western blot analysis with anti-CTR1 and anti-myc antibodies with anti-transferrin receptor used as a loading control.
Immunoprecipitation (IP) of Myc-tagged hCTR1
PNM preparations were resuspended in 0.1 M phosphate, pH 7.2, 150 mM NaCl, 5 mM dithiothreitol and 1 % n-dodecyl-β-D-maltoside for 1 hour at room temperature. The supernates were diluted into IP buffer (50 mM phosphate, pH 7.2, 200 mM NaCl, 2.5 mM dithiothreitol, and 0.5 % n-dodecyl-β-D-maltoside) after which they were precleared with protein A/G plus agarose beads (Thermo Scientific; Rockford, IL) and then centrifuged at 1,200 x g for 5 minutes. Anti-CTR1 antibody was added to precleared supernatant at 1/100 and rotated at 4 ºC for 60 minutes, after which 10 μL of protein A/G plus agarose beads were added, and the mixture was rotated overnight at 4 ºC. The beads were then washed 5 times in IP buffer and bound proteins were eluted at 37 ºC with 2X sample buffer (125 mM Tris, pH 6.8, 2 mM EDTA, 6 % SDS, 20 % glycerol, 0.25 % bromophenol blue and 5 % β-mercaptoethanol). Myc-CTR1 was immunoprecipitated with the ProFound™ c-Myc Tag IP/Co-IP Kit (Thermo Scientific, Rockford, IL). The beads on which the proteins were captured were washed with 0.5 mL TBS with 0.05 % Tween-20 thrice and the bound proteins were eluted at 37 ºC with 2X sample buffer.
Protein Deglycosylation
PNGase F and total protein deglycosylation kits (New England Biolabs; Ipswich, MA) were used to removed sugars from hCTR1 in PNM preparations. To remove N-linked sugars with PNGase F, glycoprotein denaturing buffer was added to 30 μg of protein sample and allowed to incubate at room temperature for 20 min. G7 reaction buffer and 10% NP40 buffer were then added and incubated at room temperature for 5 min. PNGase F was added and incubated at 37 ºC for 1 h followed by addition of 2X sample buffer and Western blot analysis. The Total Protein Deglycosylation kit was used remove both N-linked and O-linked sugars using the same procedure with substitution of the deglycosylation enzyme mix for the PNGase.
CCK8 assay of cell survival
The sensitivity of HEK293T/myc-EV and HEK293T/myc-CTR1 cells to a 96 h exposure to increasing concentrations of cDDP was determined using the CCK8 assay (Cell Counting Kit-8, CCK-8; Dojindo Molecular Technologies, Rockville, MD) as previously reported (25)
Statistical Analysis
Tests for statistical significance were performed using the t test function in Microsoft Excel. Group means were compared using Student’s t-test and presented as mean ± SEM. A p value of < 0.05 was considered as statistically significant.
Results
Characterization of hCTR1 by Western Blot
Human embryonal kidney HEK293T and human ovarian carcinoma A2780 cells were selected for use in these studies as the expression of hCTR1 with polyclonal antibodies has previously been reported (13–15, 26–31) . Using the monoclonal anti-hCTR1, multiple bands were observed in whole cell lysates (WCL) from the HEK293T and A2780 cells. Most of these bands were faint, but a sharp band at 28 kDa was consistently present and was one of the most intense bands on the blots. At the expected molecular weight of the glycosylated hCTR1 monomer (33–35 kDa), only a faint band was identified using WCLs. Using postnuclear membranes (PNM), three bands were consistently identified that migrated at 28, 33–35 and 62–64 kDa (Figure 1). The 33–35 and 62–64 kDa bands were relatively more intense and sharply-defined in the PNMs than in the WCLs and the 28 kDa band was substantially less prominent suggesting that much of this protein was cytosolic or nuclear.
Figure 1.
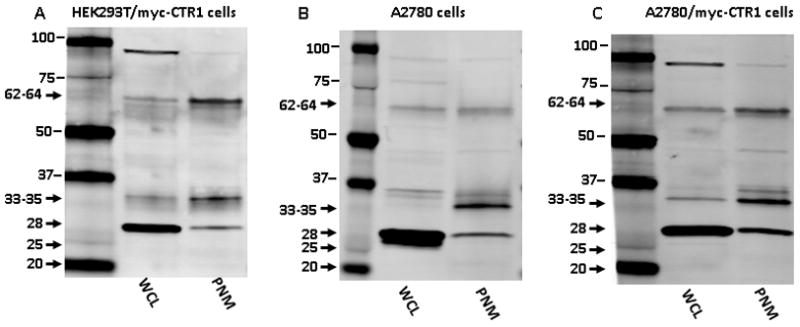
Comparison of hCTR1 expression in whole cell lysate (WCL) versus postnuclear membrane (PNM) preparations as detected with the rabbit monoclonal anti-hCTR1 antibody. (A) HEK293T/myc-CTR1; (B) A2780; and, (C) A2780/myc-CTR1 cell lines. The expected size of the glycosylated hCTR1 monomer ranges from 33–35 kDa.
To assist in positive identification of hCTR1, the HEK293T and A2780 cells were molecularly engineered to express hCTR1 containing a myc tag at its N-terminal end. Over-expression of hCTR1 in these two cell lines consistently intensified the 33–35 and 62–64 kDa bands found in PNM but did not have a similar effect on the 28 kDa band when using an anti-transferrin receptor (TR) antibody as a lane loading control (Figure 2). A 6.5-fold and 2.4-fold increase in hCTR1 was obtained in the HEK293T/myc-CTR1 and A2780/myc-CTR1 cell lines, respectively, whereas there was only a 1.3-fold and 0.7-fold change in the 28 kDa band in the PMN of the HEK293T/myc-CTR1 and A2780/myc-CTR1 cell lines, respectively. In HEK293T cells expressing only the empty vector (HEK293T/myc-EV), the average CTR1/TR intensity ratio was 0.04 ± 0.01 while the average CTR1/TR intensity ratio in the HEK293T/myc-CTR1 cells was 0.26 ± 0.07 (p = 0.03; Figures 2A and B). In the HEK293T/myc-EV cells, the average 28 kDa band/TR intensity ratio was 0.03 ± 0.01 while the average 28 kDa band/TR intensity ratio in the HEK293T/myc-CTR1 cells was 0.04 ± 0.003 (p = 0.15, Figure 2C). Moreover, in the A2780 cell line, the average CTR1/TR intensity ratio was 0.12 ± 0.01 while the ratio in the A2780/myc-CTR1 cells was 0.28 ± 0.02 (p = 0.03; Figure 2D and E). In the A2780 cells, the average 28 kDa band/TR intensity ratio was 3.00 ± 1.03 while the ratio in the A2780/myc-CTR1 cells was 2.20 ± 0.86 (p = 0.58, Figure 2F) indicating no significant change. These results provide one line of evidence that the 32–34 and 62–64 kDa bands are truly hCTR1 whereas the 28 kDa band is not.
Figure 2.
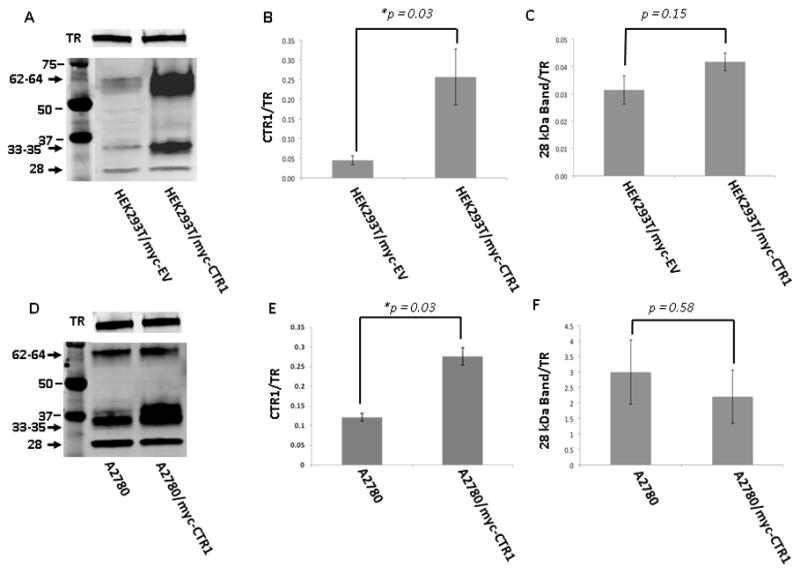
Detection of hCTR1 in cells molecularly engineered to over-express myc-CTR1. Representative Western blots (panels A and D) and histograms summarizing the results of 4 independent experiments on HEK293T/myc-CTR1 (panels B and C) and A2780/myc-CTR1 cells (panels E and F). Analyzes were performed on PNM preparations using the Epitomics anti-hCTR1 antibody. Blots were concurrently probed with anti-transferrin receptor (TR) antibody to provide a lane-loading control.
To further confirm band identity, the expression of hCTR1 was constitutively knocked down in the A2780 cells (Figure 3A) and the CTR1 mRNA was quantified by qRT-PCR. As shown in Figure 3B, the A2780/CTR1KD348 cells were found to express only 44 ± 6.2% (p = 0.021) as much as CTR1 mRNA as the control A2780/SCRKD cells, indicating 56% reduction in hCTR1 mRNA level. As shown in Figure 3C, a 60% reduction in hCTR1 protein expression was attained for both the 33–35 and 62–64 kDa bands. In 3 independent experiments, the average CTR1/TR intensity ratio in the A2780 cells was 0.25 ± 0.05 while the average ratio in the A2780/CTR1KD348 cells was 0.10 ± 0.03 (p = 0.04). The average 28 kDa band/TR intensity ratio in the A2780 cells was 0.16 ± 0.07 while the average ratio in the A2780/CTR1KD348 cells was 0.10 ± 0.05 (p = 0.47, Figure 3D) indicating no significant change. These results provide a second line of evidence that the 32–34 and 62–64 kDa bands are CTR1.
Figure 3.
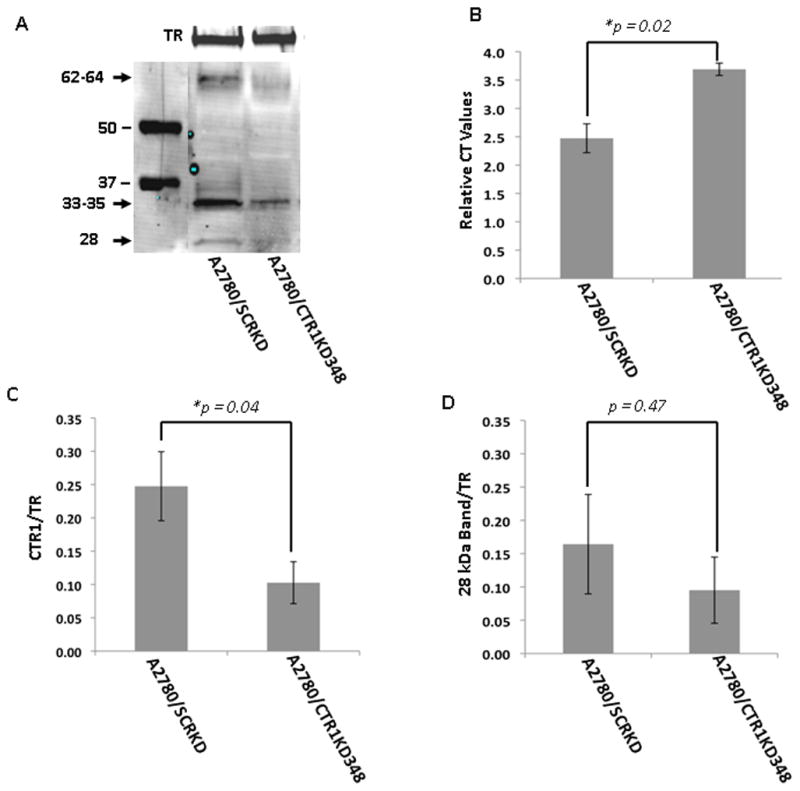
Detection of hCTR1 in A2780 cells molecularly engineered to knockdown hCTR1 expression. A) representative Western blot; B) a histogram showing the mean relative mRNA expression level determined from independent qRT-PCR measurements; C) histogram summarizing the results of 4 independent Western blots. Analyzes were performed on PNM preparations using the Epitomics anti-hCTR1 antibody. Blots were concurrently probed with anti-transferrin receptor (TR) antibody to provide a lane-loading control.
hCTR1 contains both N-linked and O-linked sugars in its N-terminal domain. Removal of the N-linked sugars is expected to decrease the molecular weight of hCTR1 by approximately 7–8 kDa whereas removal of the O-linked sugar reduces the MW by only 1–2 kDa. Figure 4 shows that when the PNM isolated from the HEK293T/myc-CTR1 cells were deglycosylated with enzymes that removed just the N-linked, or both the N-linked and O-linked sugars, both the 62–64 kDa and 33–35 kDa bands shifted to lower MW consistent with the conclusion that they represent hCTR1 as has been previously reported (14). This provides a third line of evidence indicating that these two bands are actually hCTR1. The 28 kDa band did not shift in MW following treatment with deglycosylating enzymes.
Figure 4.
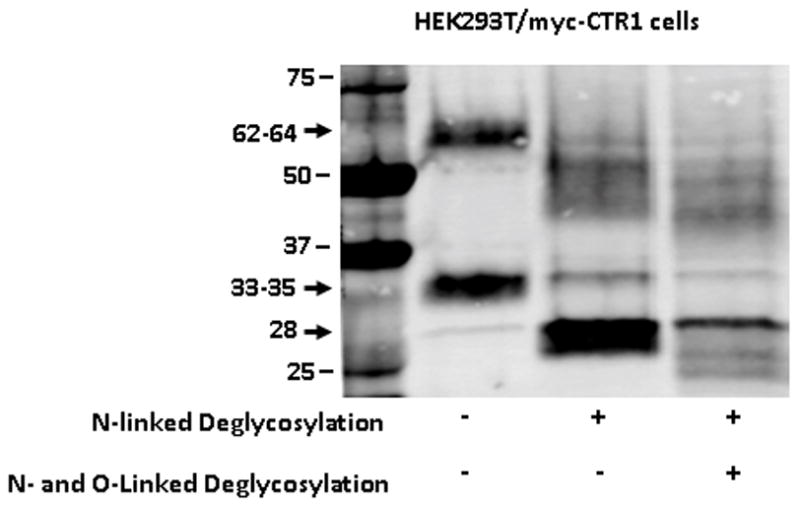
Effect of deglycosylation on detection of hCTR1 by Western blot analysis. (A), HEK293T/myc-CTR1 cells. Treatment of the HEK293T/myc-CTR1 cells with PNGase to remove the N-linked sugars reduced the MW of the monomer by 7–8 kDa; subsequent removal of the O-linked sugars resulted in a smaller 1–2 kDa shift. The blot was probed with the Epitomics anti-hCTR1 antibody.
hCTR1 Immunoprecipitation
hCTR1 was immunoprecipitated from PNMs prepared from the HEK293T/myc-CTR1 cell line using either an anti-myc antibody or the anti-hCTR1 monoclonal antibody coupled to agarose beads. As shown in Figure 5, both antibodies were able to enrich the 33–35 and 62–64 kDa bands. The 28 kDa band was not enriched in the immunoprecipitates produced with either antibody. The 28 kDa band was observed in the flow-through of both the immobilized anti-myc and anti-CTR1 antibodies when the flow through was probed with the anti-CTR1 antibody indicating that it failed to be captured by either antibody. Consistent with this conclusion, the 28 kDa band was not detected in the flow-through when it was probed with the anti-myc antibody indicating that this protein does not contain a myc tag. Thus, although the anti-hCTR1 monoclonal antibody detects the 28 kDa band in PNM preparations on Western blot analysis, it failed to immunoprecipitate this protein. These results provide a fourth line of evidence that the 33–35 and 62–64 kDa bands are hCTR1.
Figure 5.

Detection of hCTR1 in immunoprecipitates prepared from HEK293T/myc-CTR1 cells using anti-hCTR1 and anti-myc antibodies. Myc-CTR1 was precipitated from PNM preparations with anti-myc or anti-hCTR1 coupled to beads. Immunoprecipitates were probed with anti-hCTR1 (upper panel) or anti-myc (lower panel). Lanes 1 and 3: CTR1 in PNM samples prior to immunoprecipitation. Lanes 3 and 4: myc-CTR1 captured by and eluted from beads coated with anti-myc antibody. Lane 5 and 6: captured by and eluted from beads coated with anti-CTR1 antibody myc-CTR1. Lane 7 and 8: proteins not completely captured by the beads coated with anti-myc antibody and present in the flow through (FT). Lane 9 and 10: proteins not completely captured by the beads coated with anti-CT1 antibody and present in the flow through (FT). *Endogenous Myc; ** IgG light chain.
Two other bands of 19 and 23 kDa that were not observed in PNM preparations of either the HEK293T/myc-EV or the HEK293T/myc-CTR1 cells were found to be enriched in the immunoprecipitates from the HEK293T/myc-CTR1 cells. Enrichment was produced by immunoprecipitation with either the anti-myc or the anti-hCTR1 antibody, and these bands were detected in these immunoprecipitates when they were probed with either antibody indicating that they contained both the hCTR1 epitope and the myc tag. These bands appear to be truncated forms of myc-CTR1.
hCTR1 Biotinylation
To determine what forms of hCTR1 on the plasma membrane were detected by the anti- hCTR1 antibody, the surface proteins of the HEK293T/myc-CTR1 and A2780/myc-CTR1 cells were biotinylated with a reagent that was not cell permeable and the biotinylated proteins were captured on streptavidin beads. As shown in Figure 6, when the biotinylated proteins were subjected to Western blot analysis only the 33–35 and 62–64 kDa bands were identified using the anti-CTR1 antibody. The 28 kDa band was not observed in either cell line. When the cells were treated with 100 μM copper sulfate for 30 minutes, both the 33–35 and 62–64 kDa bands decreased in intensity. This effect was consistent in both cell lines studied.
Figure 6.
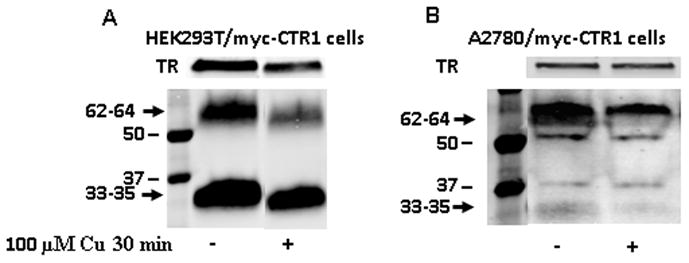
Detection of hCTR1 in biotinylated plasma membrane preparations and the effect of copper exposure. (A) Western blot of biotinylated plasma membrane protein derived from HEK293T/myc-CTR1 cells; (B) preparation made from A2780/myc-CTR1 cells. The Western blots of biotinylated proteins were concurrently probed with anti-hCTR1 and anti-transferrin receptor antibody to provide a lane loading control.
Technical Considerations
The 62–64 kDa band was further characterized by varying the amount of reducing agent added to the sample buffer prior to gel loading. When fresh β-mercaptoethanol or dithiothreitol was not added to the 2X sample buffer immediately prior to mixing with the protein sample, the band observed at 62–64 kDa was more predominant than the hCTR1 monomer at 33–35 kDa. As the amount of reducing agent added to the sample buffer was increased, the 62–64 kDa band decreased in intensity whereas the 33–35 kDa band became more predominant (Supplemental Figure 1A and B); there was no effect on the 28 kDa band. Furthermore, the ability to identify hCTR1 was improved by not boiling the protein sample after the sample buffer was added and prior to gel loading. As shown in Supplemental Figure 1C, boiling the PNM sample prior to gel loading reduced the intensity of the 33–35 kDa band with no effect on the 28 kDa band.
hCTR1 effect on the cDDP sensitivity of HEK cells
The level of expression of hCTR1 regulates sensitivity to the cytotoxic effect of cDDP in several other cell types (reviewed in (6)). However, HEK cells are derived from the renal epithelium whose transporter systems, including the expression of organic cationic transporters known to transport cDDP (32, 33) are quite different from most tumor cells. To determine whether forced expression of CTR1 in the HEK cells modulates cDDP sensitivity, survival as a function of cDDP concentration was determined for both the HEK293T/myc-EV and HEK293T/myc-CTR1 cells. Figure 7A shows that forced expression of hCTR1 increased cDDP sensitivity by 2.5-fold when the cells were exposed for 96 h; the IC50 for the HEK293T/myc-EV cells was 3.8 ± 0.2 um whereas it was 1.5 ± 0.2 for the HEK293T/myc-CTR1 cells (N = 3, p = 0.00067). Thus, the effect of hCTR1 was predominant over other influx transport mechanisms potentially present in these cells.
Figure 7.
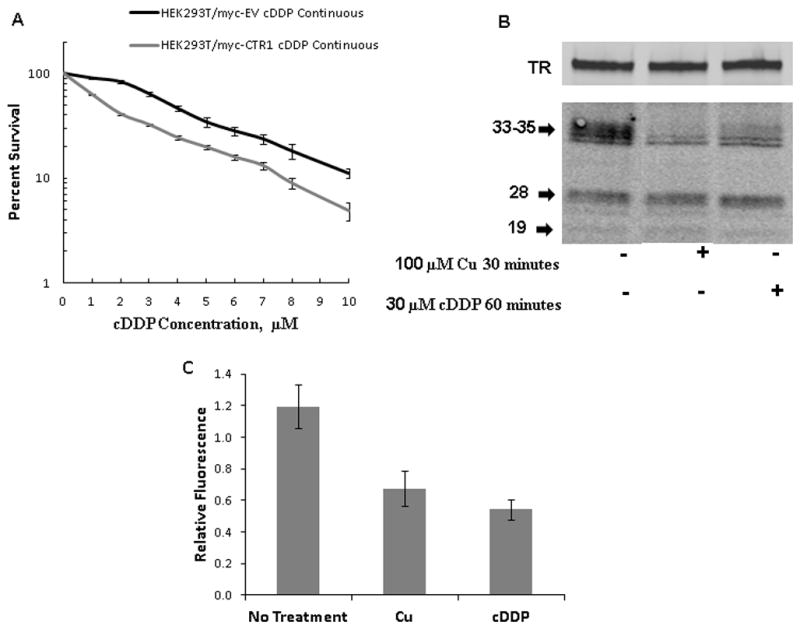
Effect of hCTR1 over-expression on cDDP sensitivity and of cDDP on hCTR1 expression. (A) Survival of HEK293T/myc-EV and HEK293T/myc-CTR1 cells following a 96 h exposure to increasing concentrations of cDDP. (B) Representative Western blot showing the effect of a 30 min exposure to 100 μM Cu and a 1 h exposure to 30 μM cDDP on hCTR1. (C) Histogram showing the quantification of CTR1 in PNM as determined from 5 independent Western blot analyses.
Down-regulation of hCTR1 by cDDP
Documentation of the ability of the Epitomics monoclonal antibody to detect hCTR1 in PNM preparations allowed us to address the question of whether cDDP triggers the down-regulation of hCTR1; we sought to determine the effect of cDDP on hCTR1 expression in the HEK293T/myc-CTR1 cells and used exposure to Cu as a control. cDDP has previously been reported to trigger the down-regulation of CTR1 in some types of cells (27) but not in others (30, 34). These prior studies were done with 3 different polyclonal antibodies; one of these has been exhausted and the other two of which are not widely available. As shown in Figure 7B, exposure of the HEK293T/myc-CTR1 cells to 100 μM Cu for 30 min reduced the ratio of CTR1 to transferrin receptor in PNM preparations by 56% from 1.20 ± 0.14 to 0.67 ± 0.11 (p = 0.025, N = 5). Exposure of the cells to 30 μM cDDP for 1 h, at which time all cells were still viable, reduced the ratio of CTR1 to transferrin receptor by 65% to 0.54 ± 0.06 (p = 0.008, N = 5). Thus, as we previously reported using a polyclonal antibody to hCTR1 and ovarian carcinoma cells (27), cDDP triggers the loss of hCTR1 from the postnuclear membrane fraction in HEK293Tcells as well.
Discussion
hCTR1 is important for both the copper homeostasis and cancer chemotherapy but its characterization and expression has been impeded by the lack of specific antibodies. For unknown reasons it has been difficult to produce highly specific antibodies to this transporter. Those antibodies that have been produced detect multiple bands on Western blots making interpretation and quantification difficult. Reproducibility has been poor and the problem is further compounded by the relative lack of abundance of hCTR1 in whole cell lysates, glycosylation of the protein, multimer stability and the presence of degradation fragments. These difficulties prompted us to undertake a thorough characterization of the new rabbit monoclonal anti-hCTR1 antibody commercially available from Epitomics.
When whole cell lysates were studied, multiple bands were observed and only a faint, ill-defined band was detected at the expected molecular weight of the glycosylated hCTR1 monomer at 33–35 kDa. Much cleaner Western blots were obtained when starting with partially-purified postnuclear membranes, and major bands were observed at 28, 33–35, and 62–64 kDa. Five lines of evidence indicate that the 33–35 and 62–64 kDa bands are genuine hCTR1 but that the 28 kDa band is not. First, forced expression of myc-CTR1 in both the HEK293T and A2780 cells increased the intensity of both the 33–35 and 62–64 kDa bands, and these same bands were detected using both the anti-hCTR1 and anti-myc antibodies consistent with the expected presence of the myc tag in the exogenously-expressed protein. The intensity of the 28 kDa band did not change, and the anti-myc antibody failed to detect this band. Second, constitutive knockdown of hCTR1 in the A2780 cells reduced the intensity of both the 33–35 and 62–64 kDa bands, but did not affect the intensity of the 28 kDa band. Third, treatment of the PNM preparations with deglycosylating enzymes produced a shift in the MW of both the 33–35 and 62–64 kDa bands but failed to shift the 28 kDa band. It has previously been demonstrated that hCTR1 is N-linked glycosylated at Asn-15 and O-linked glycosylated at Thr-27, with the N-linked sugars contributing 7–8 kDa in MW and the O-linked sugars 1–2 kDa (14, 16). Deglycosylation resulted in the expected shift in the MW of both the 33–35 and 62–64 kDa bands. Fourth, the 33–35 and 62–64 kDa bands were detected in immunoprecipitates prepared with both the anti-CTR1 and anti-myc antibodies whereas the 28 kDa band was not. Finally, fifth, whereas the 33–35 and 62–64 kDa bands were detected in preparations of biotinylated plasma membrane proteins, the 28 kDa band was not. Additionally, whereas the 33–35 and 62–64 kDa bands had the fuzzy quality characteristic of glycosylated membrane proteins, the 28 kDa band appeared much more discrete in all of the types of preparations analyzed.
The anti-hCTR1 monoclonal antibody was not useful for detecting hCTR1 in WCL preparations, but was able to detect hCTR1 in PNM preparations and in preparations of biotinylated plasma membrane proteins. It was also able to immunoprecipitate hCTR1. We have not conducted any studies of its ability to detect hCTR1 by immunocytochemical analysis. The exact epitope to which the anti-CTR1 antibody binds has not been determined. However, the observation that the antibody was able to detect hCTR1 following deglycosylation suggests that it is not a sugar-related epitope. The identity of the cross-reacting protein that migrates at 28 kDa is not known, but it appears to be largely cytoplasmic or nuclear as it was easily detected in WCL but much less apparent in PNM preparations. Despite the fact that it presumably contains the same epitope, the 28 kDa protein was not immunoprecipitated by the anti-hCTR1 antibody.
The size of the 33–35 kDa band is consistent with the fully glycosylated hCTR1 monomer, but the identity of the 62–64 kDa band is less certain. Higher MW bands similar to the 62–64 kDa have been observed in previous studies using anti-hCTR1 antibodies and reported to be a dimer (35, 36). However, if it is a dimer, it is not apparent why the trimeric form of hCTR1 was also not detected. The stability of this higher MW hCTR1 multimer under denaturing conditions has also been observed by other laboratories (36–38). The observation that the intensity of the 62–64 kDa band decreased and that of the 33–35 kDa band increased as the concentration of reducing agent was increased suggests that the 62–64 kDa band is formed from the hCTR1 monomer by oxidation.
To optimally visualize the 33–35 kDa hCTR1 monomer it was found to be important to add fresh reducing agent to the sample buffer immediately prior to adding the sample buffer to the protein sample and also to avoid boiling the sample prior to loading on the gel. When fresh reducing agent was not added to the sample buffer, a faint band was observed at 33–35 kDa with a more intense band at 62–64 kDa indicating that this protein is highly sensitive to its redox environment. Moreover, boiling the sample also decreased the intensity of the band at 33–35 kDa. Membrane proteins may aggregate with boiling so it is not unexpected that boiling might hinder binding of the anti-CTR1 antibody to its epitope on the N-terminus of the hCTR1 molecule although no high molecular weight complexes were detected in boiled samples.
One of the reasons for extensively qualifying the rabbit monoclonal anti-CTR1 antibody was to permit a more precise analysis of the effect of Cu and cDDP exposure on the expression of CTR1. That the over-expressed myc-CTR1 in the HEK293T cells was functional was established by showing that the HEK293T/myc-CTR1 cells were 2.5-fold more sensitive to the cytotoxic effect of cDDP. The Western blot analysis indicated that both agents produced a clear and substantial reduction of hCTR1 in the postnuclear HEK293T/myc-CTR1 membranes relative to the level of the transferrin receptor. This result is consistent with prior studies showing that the level of hCTR1 ubiquitination is increased upon Cu and cDDP exposure (39), and that pretreatment with the proteosome inhibitors can block degradation and enhance cDDP accumulation (27, 40). However, this analysis did not include assessment of the loss of hCTR1 from the plasma membrane specifically and it remains possible that CTR1, like the Cu efflux transporter ATP7A, is extracted from membranes by proteins such as sec61β (41) and thus lost from the postnuclear membrane preparations.
This analysis has documented the ability of the commercially available Epitomics rabbit monoclonal anti-hCTR1 to detect and quantify the expression of hCTR1 in PNM, immunoprecipitates and biotinylated membrane protein preparations of mammalian cells by Western blot analysis and thus addresses what has been a longstanding problem in the field. This antibody can now be used as a reference standard against which to compare the ability of polyclonal and mouse monoclonal antibodies to detect this important transporter.
Supplementary Material
Effect of reducing agent on relative expression of the 33–35 and 62- 64 kDa bands in PNM preparations made from the HEK293T/myc-CTR1 cells. A, effect of β-mercaptoethanol; B, effect of DTT. (C) Effect of boiling the protein sample on detection of hCTR1 in the HEK293T/myc-CTR1 cells. Boiled PNM were heated to 100º C for 10 minutes. Western blots were probed with the anti-hCTR1 antibody and anti-transferrin receptor antibody to provide a lane loading control.
Comparison of the CTR1 band pattern in whole cell lysates made from A2780/myc-CTR1 and HEK293/myc-CTR1 cells.
Acknowledgments
The authors would like express their appreciation to Dr. Jack Kaplan for thoughtful discussions and advice. This work was supported by grants CA152185 and CA095298 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6:171–80. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 2.Tsai CY, Finley JC, Ali SS, Patel HH, Howell SB. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem Pharmacol. 2012;84:1007. doi: 10.1016/j.bcp.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wee NK, Weinstein DC, Fraser ST, Assinder SJ. The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int J Biochem Cell Biol. 2013;45:960–3. doi: 10.1016/j.biocel.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–52. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]
- 5.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–94. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abada P, Howell SB. Regulation of cisplatin cytotoxicity by Cu influx transporters. Met Based Drugs. 2010;2010:317581. doi: 10.1155/2010/317581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YY, Choi CH, Do IG, Song SY, Lee W, Park HS, et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. 2011;122:361–5. doi: 10.1016/j.ygyno.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–34. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo MT, Fu S, Savarak N, Chen HHW. Role of the High-Affinity Copper Transporter (hCTR1) in Cisplatin Sensitivity in Cancer Chemotherapy. Cancer Res. 2012;72:4616–21. doi: 10.1158/0008-5472.CAN-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang ZD, Long Y, Tsai WB, Fu S, Kurzrock R, Gagea-Iurascu M, et al. Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol Cancer Ther. 2012;11:2483–94. doi: 10.1158/1535-7163.MCT-12-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Naing A, Fu C, Kuo MT, Kurzrock R. Overcoming Platinum Resistance Through the Use of a Copper-Lowering Agent. Overcoming Platinum Resistance Using a Copper-Lowering Agent. Mol Cancer Ther. 2011;11:1221–5. doi: 10.1158/1535-7163.MCT-11-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing Tumor-Specific Uptake of the Anticancer Drug Cisplatin with a Copper Chelator. Cancer Cell. 2010;17:574–83. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maryon EB, Zhang J, Jellison JW, Kaplan JH. Human copper transporter 1 lacking o-linked glycosylation is proteolytically cleaved in a rab-9 positive endosomal compartment. J Biol Chem. 2009;284:28104–14. doi: 10.1074/jbc.M109.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem. 2007;282:20376–87. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 15.Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285:32385–92. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364:497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci. 2009;106:4237–42. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Teramae M, Yamauchi M, Fukuda T, Yasui T, Sumi T, et al. Association of copper transporter expression with platinum resistance in epithelial ovarian cancer. Anticancer Res. 2013;33:1409–14. [PubMed] [Google Scholar]
- 19.Ogane N, Yasuda M, Kameda Y, Yokose T, Kato H, Itoh A, et al. Prognostic value of organic anion transporting polypeptide 1B3 and copper transporter 1 expression in endometrial cancer patients treated with paclitaxel and carboplatin. Biomed Res. 2013;34:143–51. doi: 10.2220/biomedres.34.143. [DOI] [PubMed] [Google Scholar]
- 20.Narayanan G, RBS, Vuyyuru H, Muthuvel B, Konerirajapuram Natrajan S. CTR1 Silencing Inhibits Angiogenesis by Limiting Copper Entry into Endothelial Cells. PLoS One. 2013;8:e71982. doi: 10.1371/journal.pone.0071982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Benedetto ML, Capo CR, Ferri A, Valle C, Polimanti R, Carri MT, et al. Glutaredoxin 1 is a major player in copper metabolism in neuroblastoma cells. Biochim Biophys Acta. 2013;1840:255–61. doi: 10.1016/j.bbagen.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Davies KM, Hare DJ, Cottam V, Chen N, Hilgers L, Halliday G, et al. Localization of copper and copper transporters in the human brain. Metallomics. 2013;5:43–51. doi: 10.1039/c2mt20151h. [DOI] [PubMed] [Google Scholar]
- 23.Larson CA, Adams PL, Jandial DD, Blair BG, Safaei R, Howell SB. The role of the N-terminus of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Biochem Pharmacol. 2010;80:448–54. doi: 10.1016/j.bcp.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang X, Lin X, Manorek G, Howell SB. Claudin-3 and Claudin-4 Regulate Sensitivity to Cisplatin by Controlling Expression of the Copper and Cisplatin Influx Rransporter CTR1. Mol Pharmacol. 2013;83:84–94. doi: 10.1124/mol.112.079798. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X, Lin X, Manorek G, Howell SB. Challenges associated with the targeted delivery of gelonin to claudin-expressing cancer cells with the use of activatable cell penetrating peptides to enhance potency. BMC Cancer. 2011;11:61. doi: 10.1186/1471-2407-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalayda GV, Wagner CH, Jaehde U. Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem. 2012;116C:1–10. doi: 10.1016/j.jinorgbio.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Holzer AK, Katano K, Klomp LW, Howell SB. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res. 2004;10:6744–9. doi: 10.1158/1078-0432.CCR-04-0748. [DOI] [PubMed] [Google Scholar]
- 28.Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2004;66:817–23. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- 29.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–11. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivy KD, Kaplan JH. A re-evaluation of the role of hCTR1, the human high-affinity copper transporter, in platinum-drug entry into human cells. Mol Pharmacol. 2013;83:1237–46. doi: 10.1124/mol.113.085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem. 2009;284:29704–13. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–80. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–86. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 34.Song I, Savaraj N, Siddik Z, Liu P, Wei Y, Wu C, et al. Role of copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- 35.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutrition. 2006;136:21–6. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277:29162–71. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 37.Haas KL, Putterman AB, White DR, Thiele DJ, Franz KJ. Model Peptides Provide New Insights into the Role of Histidine Residues as Potential Ligands in Human Cellular Copper Acquisition via Ctr1. JACS. 2011;133:4427–37. doi: 10.1021/ja108890c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277:4380–7. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 39.Safaei R, Maktabi MH, Blair BG, Larson CA, Howell SB. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem. 2009;103:333–41. doi: 10.1016/j.jinorgbio.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jandial DD, Farshchi-Heydari S, Larson CA, Elliot GI, Wrasidlo WJ, Howell SB. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res. 2009;15:553–60. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abada PB, Larson CA, Manorek G, Adams P, Howell SB. Sec61beta controls sensitivity to platinum-containing chemotherapeutic agents through modulation of the copper-transporting ATPase ATP7A. Mol Pharmacol. 2012;82:510–20. doi: 10.1124/mol.112.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of reducing agent on relative expression of the 33–35 and 62- 64 kDa bands in PNM preparations made from the HEK293T/myc-CTR1 cells. A, effect of β-mercaptoethanol; B, effect of DTT. (C) Effect of boiling the protein sample on detection of hCTR1 in the HEK293T/myc-CTR1 cells. Boiled PNM were heated to 100º C for 10 minutes. Western blots were probed with the anti-hCTR1 antibody and anti-transferrin receptor antibody to provide a lane loading control.
Comparison of the CTR1 band pattern in whole cell lysates made from A2780/myc-CTR1 and HEK293/myc-CTR1 cells.


