Abstract
Objective
To test the hypothesis that low bispectral index (BIS) scores and low sedative requirements during therapeutic hypothermia predict poor neurologic outcome.
Design, Setting, and Patients
Observational study of a prospectively collected cohort of 160 consecutive cardiac arrest patients treated with therapeutic hypothermia.
Interventions
None
Measurements and Results
Eighty-four (60%) of the 141 subjects that survived hypothermia induction were discharged from the ICU with poor neurologic outcome, defined as a cerebral performance category (CPC) score of 3, 4, or 5. These subjects had lower BIS (p<0.001) and sedative requirements (p<0.001) during hypothermia compared to the 57 subjects discharged with good outcome. Early prediction of neurologic recovery was best seven hours after ICU admission, and median BIS scores at that time were 31 points lower in subjects discharged with poor outcome [11 (IQR 4 to 29) vs. 42 (37 to 49), p<0.001]. Median sedation requirements decreased by 17% (IQR −50% to 0%) seven hours after ICU admission in subjects with poor outcome but increased by 50% (95% CI 0% to 142%) in subjects with good outcome (p<0.001). Each 10-point decrease in BIS was independently associated with a 59% increase in the odds of poor outcome (95% CI: 32% to 76%, p<0.001). The model including BIS and sedative requirement correctly reclassified 15% of subjects from good to poor outcome and 1% of subjects from poor to good outcome. The model incorrectly reclassified 1% of subjects from poor to good outcome but did not incorrectly reclassify any from good to poor outcome.
Conclusions
BIS scores and sedative requirements early in the course of therapeutic hypothermia improve the identification of patients who will not recover from brain anoxia. The ability to accurately predict non-recovery after cardiac arrest could facilitate discussions with families, reduce patient suffering, and limit use of ICU resources in futile cases.
Keywords: Cardiac arrest, therapeutic hypothermia, neurologic function, sedation, BIS, ischemia reperfusion, ICU
Introduction
Emergency medical services respond to 382,000 cardiac arrests each year in the United States (1), but 90% of these patients die in the field or during hospitalization (2). Therapeutic hypothermia improves neurologic function and survival following cardiac arrest and is now standard of care for patients that remain unconscious following return of spontaneous circulation (3, 4). Unfortunately, nearly 60% of patients treated with hypothermia following cardiac arrest die during post-resuscitation care (5). Advanced age, an initial cardiac rhythm of asystole or pulseless electrical activity (PEA), and a long time to return of spontaneous circulation are associated with poor neurologic recovery, but these predictors lack the specificity required to withhold treatments (6, 7). Moreover, due to the neurophysiologic effects of hypothermia, sedatives, and muscle relaxants, neurologic recovery cannot be accurately assessed by physical examination until at least 72 hours after return to normothermia and discontinuation of sedatives (8, 9). Clinicians have noted the paucity of data to help identify patients early in the course of care that will not recover from cerebral anoxia (10).
The bispectral index (BIS) is a processed EEG signal that was initially developed to measure anesthetic depth but is used in some intensive care units (ICU) to monitor patient brain activity and titrate sedatives, including those exposed to therapeutic hypothermia. Sedative use is required to limit awareness for patients exposed to therapeutic hypothermia, but deep sedation has been associated with death in ICU patients (11). A more severely injured brain might require less sedation than a less injured brain. BIS scores and sedation requirements during therapeutic hypothermia may reflect neurologic function and could be used to clinically predict neurologic recovery. We performed this study to test the hypothesis that low BIS measurements and low sedative requirements during therapeutic hypothermia following cardiac arrest predict poor neurologic function at ICU discharge.
Materials and Methods
Study Population
All comatose adult patients successfully resuscitated from sudden cardiac arrest and treated with therapeutic hypothermia in the Vanderbilt University Medical Center's cardiovascular ICU between May 15, 2007 and September 18, 2011 were eligible for inclusion in this study, regardless of their location of arrest, cause of arrest, or initial cardiac rhythm. Patients were not eligible for therapeutic hypothermia if they demonstrated spontaneous awakening with purposeful movement, had a known terminal illness, had active do-not-resuscitate orders, arrested following traumatic head injury, had an initial post-arrest body temperature less than 34°C, had an estimated time from arrest to return of spontaneous circulation greater than 60 minutes, or were pregnant. Patients treated with sedatives outside of protocol or who died before the target temperature was reached were also excluded from the study.
Standardized Patient Treatment
Therapeutic hypothermia was performed according to an institutional protocol that dictates standardized cooling, rewarming, ventilation, and sedation. Upon arrival to the ICU, external cooling pads are placed on the torso and thighs of the patient, who is then rapidly cooled to 32–34°C as measured by an esophageal temperature probe. Twenty-four hours after the time of arrest, patients are actively rewarmed using the external pads to a temperature of 37°C at a rate of 0.25°C per hour. Rewarming requires 12–20 hours. At induction of hypothermia, midazolam and fentanyl infusions are initiated at 2 mg/h and 100 μg/h, respectively. Bedside nurses titrate midazolam and fentanyl infusions at their discretion to achieve a BIS score between 40 and 60, purported to represent moderate to deep sedation and amnesia. The institutional protocol suggests that midazolam and fentanyl doses be adjusted in 1 mg/h and 50 μg/h increments and dictates that infusions not decrease below 1 mg/h midazolam and 50 μg/h fentanyl at any time during hypothermia. Following return to normothermia, sedation infusions are withheld. A cisatricurium infusion, titrated to maintain 2 out of 4 twitches using train-of-four technique monitoring on the ulnar nerve, is administered to reduce shivering. Patients are mechanically ventilated with a tidal volume of 8 ml/kg predicted body weight, a respiratory rate titrated to an arterial pCO2 of 40 mmHg, and a fraction of inspired oxygen and positive end expiratory pressure titrated to an arterial oxygen saturation greater than 92%.
After approval from the Institutional Review Board, patient data were collected prospectively on all therapeutic hypothermia patients using an Utstein style template, which provides a standardized uniform method for collecting and reporting cardiac arrest and resuscitation data (12). For patients with out of hospital cardiac arrest, the rhythm of arrest and time to return of spontaneous circulation were recorded as reported by emergency medical personnel. Hourly BIS scores and infusion rates of midazolam and fentanyl were recorded in the electronic medical record by the bedside ICU nurse. Any decision to withdraw care was made by the patient's physicians and family based on the patient's specified wishes and clinical condition. BIS scores and sedation requirements were not incorporated into any decision to withdraw care, particularly since physicians and investigators were unaware of any associations among BIS scores, sedation requirements, and neurologic outcome during clinical care of these patients.
The primary outcome for this prospective observational study was neurologic function at the time of ICU discharge, as defined by the Cerebral Performance Category (CPC) score. The CPC score was developed as a measure of central nervous system function after cardiac arrest (13), and is the most commonly used post-resuscitation outcome measurement (4, 12). The CPC score reflects three domains of function: neurologic impairment, level of activities performed, and level of participation. A CPC score of 1 indicates good cerebral performance (i.e. conscious, alert, independent). A CPC score of 2 indicates moderate cerebral disability (i.e. conscious, sufficient cerebral function for independent activities of daily life, able to work in a sheltered environment). A CPC score of 3 indicates severe cerebral disability (i.e. conscious, dependent on others for daily support, function ranges from ambulatory state to severe dementia or paralysis). A CPC score of 4 indicates coma or vegetative state. A CPC score of 5 indicates death. Poor neurologic outcome was defined as a CPC score of 3, 4, or 5, and good neurologic outcome as a CPC score of 1 or 2. Follow-up CPC assessments were also performed six months after cardiac arrest or when subjects returned to clinic if not at six months. The primary outcome for each subject was independently and retrospectively determined using chart review by two physicians responsible for all data adjudication. These physicians were blinded to subject BIS and sedative requirements.
Statistical Analysis
Descriptive statistics are presented as mean +/− standard deviation for continuous variables and number (percentage) for categorical variables. The Wilcoxon rank sum test and the Pearson Chi-square test were used to compare baseline characteristics between patients with good and poor neurologic outcomes. Spearman's correlation test was used to compare continuous variables with each other, for example to compare BIS scores to sedation requirements. A logistic regression base model was developed to assess the associations between neurologic outcome and a priori determined risk factors age, time to return of spontaneous circulation, and rhythm of arrest. For each of the first 30 hours of ICU admission for therapeutic hypothermia, the BIS scores and sedation requirements at each hour were then added to the model, and the added predictive value for each of these 30 models was determined using the likelihood ratio test against the base model. Each patient's hourly BIS and sedation requirement were calculated as the average at that hour +/− 1 hour, except hour 30, which was calculated as the average of hours 30 and 29. The final model (presented in Table 2) was validated and calibrated using R functions validate() and calibrate() through Bootstrapping, with 300 replicates (14). Following model calibration, the bias-corrected R2 was 0.698, the slope shrinkage factor was 0.841, the mean absolute error was 0.03, and the 0.9 quantile of the absolute error was 0.066.
Table 2.
Multivariable Logistic Regression Model for the Prediction of Poor Neurologic Outcome at ICU Discharge, using BIS and sedation requirement data seven hours following ICU admission for cardiac arrest
| Variable | Adjusted Odds Ratios (95% CI) for Poor Outcome | P-value |
|---|---|---|
| BIS score, 10 point increase | 0.41 (0.24 – 0.68) | <0.001 |
| Sedation requirement, 50% increase | 0.52 (0.26 – 1.06) | 0.07 |
| Age, 10 year increase | 0.86 (0.54 – 1.38) | 0.54 |
| Time to return of spontaneous circulation, 5 min increase | 1.79 (1.16 – 2.77) | 0.009 |
| Initial post-arrest PEA or asystolic rhythm, compared to VT or VF | 4.33 (0.94 – 20.04) | 0.06 |
CI, confidence interval; PEA, pulseless electrical activity; VT, ventricular tachycardia; VF, ventricular fibrillation; BIS, bispectral index.
After determining the optimal time for predicting neurologic outcome [earliest hour with the largest area under the receiver operator characteristic (ROC) curve (C-index)] we reclassified subjects from the base model's outcome prediction using the model with BIS and sedation requirement at that hour added to the base model. We report the reclassification rate of the two models using the threshold of 0.773 for the predicted probability of having a poor outcome. The threshold 0.773 was chosen to maximize the sum of sensitivity and specificity of the final model. All tests were two-tailed, with a significance level of 0.05. Statistical software R (version 3.0.1, Vienna, Austria) was used for the analyses (15).
Results
One hundred sixty patients received therapeutic hypothermia during the study period. Nineteen patients were excluded from analysis because six died before the target temperature was achieved, and thirteen received sedative medications outside of protocol. One hundred forty-one patients comprised the study cohort.
Eighty-four of the 141 study subjects (59.6%) died or were discharged from the ICU with poor neurologic function; 77 died in the ICU (CPC 5), two were discharged in a persistent vegetative state (CPC 4), and five were discharged with severe disability (CPC 3). Fifty-seven of the 141 subjects (40.4%) were discharged from the ICU with good neurologic function; 39 were discharged without any apparent neurologic deficits (CPC 1), and 18 were discharged with mild to moderate disability (CPC 2). Subjects discharged with good neurologic function (CPC 1 or 2) were younger, less likely to have preexisting chronic kidney disease, more likely to have had an initial cardiac rhythm amenable to defibrillation, had a shorter time to medical personnel CPR, had a shorter time to return of spontaneous circulation, and took longer to reach target temperature, compared to subjects discharged with poor neurologic function (Table 1).
Table 1.
Patient Characteristics According to Good and Poor Neurologic Function at ICU Discharge
| Characteristic | Good Neurologic Outcome (n=57) | Poor Neurologic Outcome (n=84) | P-value |
|---|---|---|---|
| Age, years | 53 ± 14 | 60 ± 15 | 0.005 |
| Men, n (%) | 41 (72%) | 51 (61%) | 0.17 |
| Medical history, n (%) | |||
| Coronary artery disease | 16 (28%) | 32 (38%) | 0.22 |
| Hypertension | 27 (47%) | 43 (51%) | 0.66 |
| Chronic kidney disease | 1 (1.8%) | 10 (11.9%) | 0.027 |
| Chronic obstructive pulmonary disease | 5 (8.8%) | 15 (17.9%) | 0.13 |
| CPC prior to arrest, n (%) | 0.36 | ||
| CPC 1 | 55 (96.5%) | 81 (98.8%) | |
| CPC 2 | 2 (3.5) | 1 (1.2) | |
| CPC 3, 4, or 5 | 0 (0) | 0 (0) | |
| Arrest site, n (%) | 0.89 | ||
| Out of hospital | 46 (81%) | 67 (80%) | |
| In hospital | 11 (19%) | 17 (20%) | |
| Arrest witnessed, n (%) | 52 (91.2%) | 68 (81.0%) | 0.093 |
| Time to medical personnel CPR, min | 8.2 ± 5.9 | 12.0 ± 9.6 | 0.059 |
| Initial rhythm of arrest, n (%) | <0.001 | ||
| VF/VT | 47 (85.5%) | 40 (49.4%) | |
| PEA | 6 (10.9%) | 23 (28.4%) | |
| Asystole | 2 (3.6%) | 18 (22.2%) | |
| Time from arrest to return of spontaneous circulation, min | 14.3 ± 9.4 | 30.1 ± 19.9 | <0.001 |
| Time from arrest to hypothermia initiation, min | 240 ± 141 | 250 ± 169 | 0.89 |
| Time from arrest to target temperature, min | 509 ± 202 | 416 ± 223 | 0.02 |
| Initial temperature, °C | 36.5 ± 1.1 | 36.1 ± 1.3 | 0.18 |
| ICU length of stay, days | 8.5 ± 7.0 | 4.5 ± 2.9 | <0.001 |
| CPC at ICU discharge | <0.001 | ||
| CPC 1 | 39 (68.4) | 0 (0) | |
| CPC 2 | 18 (31.6) | 0 (0) | |
| CPC 3 | 0 (0) | 5 (6.0) | |
| CPC 4 | 0 (0) | 2 (2.4) | |
| CPC 5 (died in ICU) | 0 (0) | 77 (91.7) | |
| Cause of death/withdrawal, n (%) | |||
| Neurologic futility | 0 (0) | 58 (69.0) | |
| Circulatory collapse | 0 (0) | 8 (9.5) | |
| Multi-organ failure | 0 (0) | 6 (7.1) | |
| Undetermined | 0 (0) | 5 (6.0) | |
| Time to follow-up, months* | 3.7 ± 3.4 | 3.4 ± 4.0 | 0.86 |
| CPC at follow-up† | <0.001 | ||
| CPC 1 | 50 (92.6) | 1 (1.2) | |
| CPC 2 | 1 (1.9) | 3 (3.6) | |
| CPC 3 | 0 (0) | 0 (0) | |
| CPC 4 | 0 (0) | 0 (0) | |
| CPC 5 (died in ICU or by follow-up) | 4 (7.4) | 80 (95.2) |
among subjects that survived to hospital discharge.
two subjects were lost to follow-up, both of whom were discharged with a CPC score of 1. Data are presented as mean +/− standard deviation or number (percent). ICU, intensive care unit; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation; VT, ventricular tachycardia; PEA, pulseless electrical activity.
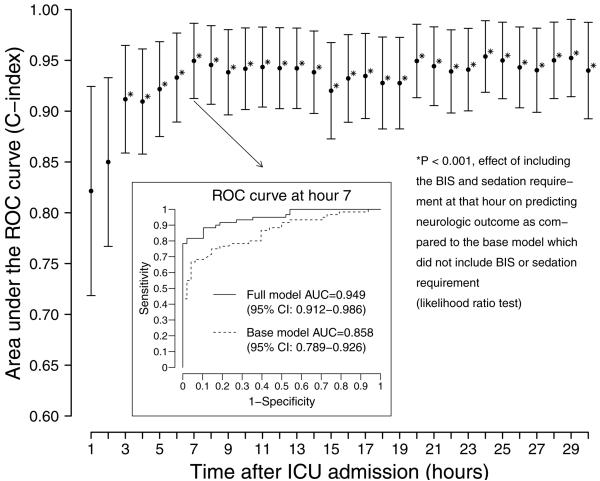
In subjects discharged with poor neurologic outcome, BIS scores and sedation requirements were lower beginning three hours after ICU admission compared to subjects discharged with good neurologic outcome (Figure 1). To determine the best time to predict neurologic outcome early in the course of care, we built prediction models using BIS scores and sedative requirements at each of the first 30 hours, in addition to age, time to return of spontaneous circulation, and initial rhythm of arrest. The earliest time with the greatest ability to predict neurologic function at ICU discharge was seven hours after ICU admission. The seven-hour model had a C-index equal to 0.95 (95% CI: 0.91 to 0.99), significantly better (likelihood ratio test, p<0.001) than the base model that contained established predictors only [C-index = 0.83 (95% CI: 0.76 to 0.91)]. The seven-hour model demonstrated 0.80 sensitivity (95% CI 0.68 – 0.88) and 0.98 specificity (0.89 – 1.00) to predict poor neurologic function. Some models from earlier time periods (hours 3–6) also predicted neurologic function better than the base model (p<0.001) but not as well as hour seven in our cohort. Some models from subsequent time periods (hours 8–30) predicted neurologic function as well as the seven-hour model and significantly better than the base model (p<0.001) but were longer into ICU stay and therefore less valuable for the early prediction of neurologic outcome (Figure 2). Thus the seven-hour model provided the earliest predictive ability of neurologic outcomes, while ensuring a high degree of sensitivity and specificity.
Figure 1.
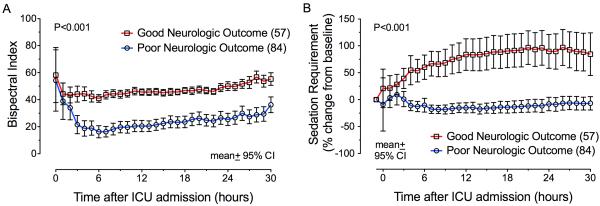
BIS scores (Panel A) and sedation requirements (Panel B) were higher during hypothermia in subjects discharged from the intensive care unit with good vs. poor neurologic recovery. Baseline sedation infusions were 2 mg/h midazolam and 100 μg/h fentanyl.
Figure 2.
Area under the ROC curve (C-index) for the prediction of poor neurologic recovery at each of the first 30 hours following intensive care unit (ICU) admission. These models included BIS scores and sedative requirements at each hour, in addition to the base model covariates age, time to return of spontaneous circulation, and initial rhythm of arrest. The optimal early prediction time occurred seven hours after ICU admission (C-index = 0.95, 95% CI: 0.91 – 0.99). The ROC curve for hour seven is shown.
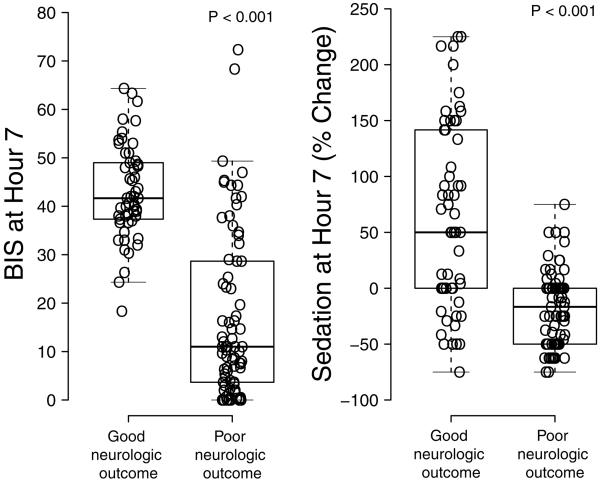
Median BIS scores seven hours after ICU admission were 31 points lower in subjects discharged with poor outcomes as compared to those with good outcomes (p<0.001), and median sedation requirements decreased by 17% in subjects with poor outcome seven hours after ICU admission but increased by 50% in subjects with good outcome (p<0.001, Figure 3). The fentanyl and midazolam doses at this time were 78 ± 41 and 1.7 ± 0.7 in subject with poor outcome versus 155 ± 85 μg/h and 3.6 ± 2.0 mg/h in subjects with good outcome (p<0.001 for both fentanyl and midazolam at this time). Ninety-eight percent of subjects that had a BIS score less than 20 seven hours after ICU admission were discharged from the ICU with poor neurologic outcome, while 96% of subjects that required greater than a 50% increase in sedation seven hours after ICU admission were discharged from the ICU with good neurologic function.
Figure 3.
BIS scores (Panel A) and sedation requirements (Panel B) seven hours after ICU admission were increased in subjects discharged from the intensive care unit with good vs. poor neurologic recovery.
After adjusting for established risk factors for poor neurologic function following cardiac arrest in our logistic regression model, each 10-point increase in BIS score remained independently associated with 59% lower odds of poor neurologic outcome (Odds Ratio: 0.41, 95% CI 0. 32 to 0.76, p<0.001, Table 2). While not statistically significant, each 50% increase in sedation requirement was independently associated with a trend towards 48% lower odds of poor neurologic outcome (Odds Ratio: 0.52, 95% CI 0.26 to 1.06, p=0.07). Despite the influence of BIS score on sedation titration, BIS scores did not perfectly correlate with sedation requirement (r = 0.57, R2=0.33, P<0.001). Thirty-three percent of the variation in sedation requirement was due to changes in BIS, and BIS scores predicted neurologic outcome independent of sedation requirement. Increased time to return of spontaneous circulation also independently predicted poor neurologic outcome, initial post-arrest asystolic or PEA cardiac rhythm had an insignificant association but trend towards poor neurologic function, and age had no independent association with neurologic function at ICU discharge (Table 2).
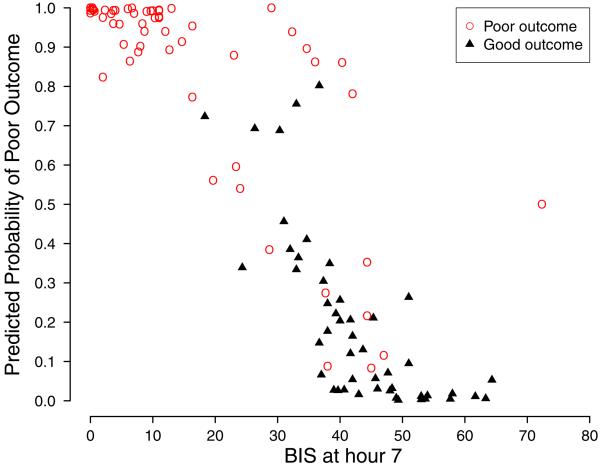
Using the seven-hour post ICU admission BIS and sedative requirement prediction model, 97.9% of patients predicted to have an 80% or greater probability of poor neurologic function at ICU discharge had poor neurologic function at ICU discharge, and 93.8% of patients predicted to have an 80% or greater probability of good neurologic function at ICU discharge had good neurologic function at ICU discharge (Figure 4). In comparison to the base model that only contained established neurologic function risk factors, 16 subjects (14.8%) were correctly reclassified from good to poor outcome, one subject (0.9%) was correctly reclassified from poor to good outcome, and one subject (0.9%) was incorrectly reclassified from poor to good outcome. The BIS and sedation requirement model did not incorrectly reclassify any patients from good to poor outcome.
Figure 4.
Predicted probability of poor neurologic recovery and BIS score seven hours after ICU admission in subjects discharged from the ICU with good and poor neurologic function.
Average time to follow-up was 3.7 ± 3.4 months among patients that did not die in the ICU. CPC scores at follow-up were similar to scores at ICU discharge (Table 1). The BIS and sedation requirement model predicted neurologic function at follow-up with similar precision as it did at ICU discharge (sensitivity 0.75, 95% CI: 0.62 to 0.84; specificity 0.98, 95% CI: 0.89 to 1.00; C-index 0.93, 95% CI: 0.88 to 0.97). BIS scores seven hours following ICU admission remained a strong predictor of poor neurologic outcome at follow-up [54% increase in the odds of poor outcome for every 10 point decrease in BIS score (95% CI: 28% to 71%, p=0.001)] but sedation requirement less so [40% increase in the odds of poor outcome for every 50% decrease in sedation requirement (95% CI: −10% to 67%, p=0.10)]. The seven-hour interval following ICU admission was the best time (highest C-index) within the first day of hypothermia to predict neurologic function at follow-up.
Discussion
We report that high BIS scores and increased sedative requirements during the initial period of therapeutic hypothermia are almost universally associated with good neurologic function at ICU discharge and long-term follow-up, while low BIS scores and sedative requirement are almost universally associated with poor neurologic function at ICU discharge and long-term follow-up.
The addition of BIS scores and sedative requirements to previously validated resuscitation-related predictors of neurologic outcome significantly improves the ability to identify patients that will die or be discharged from the ICU with severe neurologic deficits, with the optimal time of prediction occurring seven hours after ICU admission. Inclusion of these data lead to the accurate reclassification of 15% of patients from good to poor prognosis without sacrificing specificity for predicting poor outcome. In addition, for the first time we have demonstrated that sedative requirements during therapeutic hypothermia might be associated with neurologic recovery. This study demonstrates a simple method for bedside practitioners to guide care and counsel families. Importantly, use of early BIS scores and sedative requirements to predict neurologic recovery did not incorrectly reclassify any patients from a good- to a poor-predicted neurologic outcome.
Some prior studies have examined the relationship between electroencephalographic data during hypothermia and neurologic recovery. Seder et al reported that the initial BIS score following neuromuscular blockade was associated with a 14% increase in odds of good neurologic outcome at discharge per unit increase in BIS score, adjusted for witnessed arrest, initial cardiac rhythm, and time to return of spontaneous circulation (16). They did not measure the ability of BIS scores at sequential periods to predict outcome as brain function evolves, however, and specifically excluded a subject whose BIS score was used to affect patient care. By examining the ability of BIS scores at sequential hours to predict outcome, we were able to identify an optimal time for prognostication. Furthermore, our study intentionally incorporated BIS scores into sedation management and found that both BIS scores and sedation requirements are associated with neurologic recovery. Another study found in unadjusted analyses that BIS values were higher one day after the initiation of hypothermia in subjects with good outcome and that a BIS score of zero at any point was associated with death (17). Cloostermans et al. reported that low-voltage or isoelectric EEG patterns 24 hours following initiation of hypothermia predicted poor neurologic outcomes with 100% specificity, while continuous, diffuse slow patterns at 12 hours predicted good neurologic outcomes with 100% specificity (18). Unfortunately, the sensitivities of these EEG patterns were 40% and 43%, respectively, limiting their applicability to the majority of patients undergoing therapeutic hypothermia. Our model demonstrated 80% sensitivity for predicting poor neurologic function while preserving 98% specificity. Moreover, use of the BIS monitor provides continuous usable data to clinicians at the bedside and requires little training, unlike EEG interpretation. EEG interpretation requires training that is uncommon in bedside nurses and non-neurology physicians. No prior studies have prospectively examined the relationship between sedative use during hypothermia and neuronal activity or neurologic recovery.
An increased sedation requirement to achieve a target BIS may be a marker of preserved neuronal activity, or increased sedative exposure may protect the brain as it recovers from hypoxia. Gamma-aminobutyric acid receptor agonists, which include benzodiazepines and propofol, inhibit neuron depolarization and reduce oxygen consumption (19, 20). Post anoxic status epilepticus, on the other hand, is neuronal hyperactivity and strongly predicts poor neurologic function following cardiac arrest (21). Increased benzodiazepine administration might have reduced seizures or cerebral oxygen consumption in patients, leading to improved neurologic outcomes. The time-dependent nature of our findings, with the greatest early predictive strength occurring seven and not zero, one, two, or three hours following initiation of hypothermia, implies that processes occur in the brain during the first few hours following arrest that impact neurologic recovery. In experimental models of brain ischemia and reperfusion, mitochondrial dysfunction, reactive oxygen species overproduction, neuroexcitotoxicity, cellular apoptosis and tissue necrosis occur in the first 6 hours (22, 23). BIS scores and sedative requirements during this period may help differentiate between brains that will or will not recover function.
The current study has several limitations, most related to the protocol used for administering sedatives, administering neuromuscular antagonists, and monitoring sedation during therapeutic hypothermia. BIS monitoring may not consistently measure patient sedation or decrease awareness during anesthesia (24). But unlike in the operating room, where measurement of end-tidal anesthetic gas concentration is used to gauge sedation, no such monitors exist in the ICU. Patients subjected to iatrogenic hypothermia and mechanical ventilation require sedation (25), excess sedation is harmful (11), and the BIS monitor provides a means to measure sedation. The imperfect correlation between sedation requirement and BIS score may be due to the relatively broad target range for BIS score (40–60) or sedative titration by bedside nurses outside of protocol. The current study demonstrates an additional use of the BIS monitor during therapeutic hypothermia – neurologic recovery prognostication – but accurate BIS monitoring may require the concomitant use of neuromuscular receptor antagonists. Neuromuscular receptor antagonists are not required but are frequently used during hypothermia to prevent shivering which otherwise increases oxygen consumption and antagonizes hypothermia (25, 26). Neuromuscular receptor antagonism also improves BIS monitor fidelity (27), but prolonged exposure to steroidal based neuromuscular receptor antagonist compounds such as vecuronium or pancuronium has been reported to increase critical illness myopathy (28). Cisatricurium, a benzylisoquinolinium neuromuscular receptor antagonist compound, has not been associated with myopathy and possesses consistent pharmacokinetics in a variety of patients since it does not require hepatic or renal metabolism (29). Midazolam, the benzodiazepine sedative used in the current study, has been associated with an increased risk of delirium in other ICU patient populations (30). A study assessing the association between propofol sedation during hypothermia and neurologic recovery is warranted. The methods employed in the current study could aid studies of alternative sedatives since percent change in sedation requirement rather than sedative-specific dosing was used to predict neurologic outcome. These limitations may restrict the generalizability of our results to patients that receive midazolam, fentanyl, neuromuscular receptor antagonists, and BIS monitoring during hypothermia. And finally, the present study would be strengthened by validating the predictive power of BIS scores and sedation requirements early in the course of hypothermia in additional cohorts. Nonetheless we have demonstrated a novel means to prognosticate neurologic recovery during therapeutic hypothermia.
Conclusions
BIS scores and sedative requirements early in the course of therapeutic hypothermia following cardiac arrest improve the identification of patients who may not recover from brain anoxia. The ability to accurately predict neurologic non-recovery after cardiac arrest could facilitate discussions with families about outcomes and expectations early during the course of care, reduce unnecessary patient suffering, and limit use of ICU resources in futile cases.
Acknowledgments
Copyright Form Disclosures: Dr. Billings IV's institution received grant support from the National Institutes of Health (K23 career development grant). Dr. Billings IV received support for article research from NIH (K23GM102676). Dr. Burjek received support for travel from the Society of Critical Care Anesthesiologists; is employed by Northwestern Memorial Hospital, and was previously employed by Vanderbilt University Medical Center. Dr. Wagner consulted and lectured for Imacor. Dr. Hollenbeck's institution received grant support from NIH (K23GM102676 (FTB) and UL1RR024975). Dr. Hollenbeck received support for article research from NIH. Dr. Wang's institution received grant support (Clinical and translational science award to Vanderbilt). Dr. Wang received support for article research from NIH. Dr. Yu's institution received grant support (Clinical and translational science award to Vanderbilt). Dr. Yu received support for article research from NIH. Dr. McPherson consulted for Velomedix Corp.
Funding Sources: United States National Institutes of Health [K23GM102676 (FTB) and UL1RR024975] and the Vanderbilt University Department of Anesthesiology.
Footnotes
Author statements of competing financial interests: No disclosures
References
- 1.Writing Group Members. Roger VL, Go AS, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. MMWR Surveill Summ. 2011;60:1–19. [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Fugate JE, Brinjikji W, Mandrekar JN, et al. Post-Cardiac Arrest Mortality Is Declining: A Study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–934. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa K, Tasaki O, Hamasaki T, et al. Prognostic indicators and outcome prediction model for patients with return of spontaneous circulation from cardiopulmonary arrest: The Utstein Osaka Project. Resuscitation. 2011;82:874–880. doi: 10.1016/j.resuscitation.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Blondin NA, Greer DM. Neurologic Prognosis in Cardiac Arrest Patients Treated With Therapeutic Hypothermia. The Neurologist. 2011;17:241–248. doi: 10.1097/NRL.0b013e318224ee0e. [DOI] [PubMed] [Google Scholar]
- 9.Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation Confounds Outcome Prediction in Cardiac Arrest Survivors Treated with Hypothermia. Neurocrit Care. 2010;15:113–119. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scirica BM. Therapeutic Hypothermia After Cardiac Arrest. Circulation. 2013;127:244–250. doi: 10.1161/CIRCULATIONAHA.111.076851. [DOI] [PubMed] [Google Scholar]
- 11.Shehabi Y, Bellomo R, Reade MC, et al. Early Intensive Care Sedation Predicts Long-Term Mortality in Ventilated Critically Ill Patients. American Journal of Respiratory and Critical Care Medicine. 2012 doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. [Google Scholar]
- 15.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- 16.Seder DB, Fraser GL, Robbins T, et al. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med. 2009;36:281–288. doi: 10.1007/s00134-009-1691-1. [DOI] [PubMed] [Google Scholar]
- 17.Leary M, Fried DA, Gaieski DF, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation. 2010;81:1133–1137. doi: 10.1016/j.resuscitation.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Cloostermans MC, van Meulen FB, Eertman CJ, et al. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Critical Care Medicine. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 19.Cotev S, Shalit MN. Effects on diazepam on cerebral blood flow and oxygen uptake after head injury. Anesthesiology. 1975;43:117–122. doi: 10.1097/00000542-197507000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Vandesteene A, Trempont V, Engelman E, et al. Effect of propofol on cerebral blood flow and metabolism in man. Anaesthesia. 1988;43(Suppl):42–43. doi: 10.1111/j.1365-2044.1988.tb09067.x. [DOI] [PubMed] [Google Scholar]
- 21.Legriel S, Hilly-Ginoux J, Resche-Rigon M, et al. Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation. 2012 doi: 10.1016/j.resuscitation.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Pundik S, Xu K, Sundararajan S. Reperfusion brain injury: focus on cellular bioenergetics. Neurology. 2012;79:S44–51. doi: 10.1212/WNL.0b013e3182695a14. [DOI] [PubMed] [Google Scholar]
- 23.Sims NR, Muyderman H. Biochimica et Biophysica Acta. BBA - Molecular Basis of Disease. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Avidan MS, Jacobsohn E, Glick D, et al. Prevention of intraoperative awareness in a high-risk surgical population. N. Engl. J. Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 25.Neumar RW, Nolan JP, Adrie C, et al. Post-Cardiac Arrest Syndrome: Epidemiology, Pathophysiology, Treatment, and Prognostication A Consensus Statement From the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 26.Badjatia N, Kowalski RG, Schmidt JM, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit Care. 2007;6:186–191. doi: 10.1007/s12028-007-0011-2. [DOI] [PubMed] [Google Scholar]
- 27.Vivien B, Di Maria S, Ouattara A, et al. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 29.de Jonghe B, Lacherade J-C, Sharshar T, et al. Intensive care unit-acquired weakness: Risk factors and prevention. Critical Care Medicine. 2009;37:S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 30.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and Risk Factors for Development of Delirium in Surgical and Trauma Intensive Care Unit Patients. The Journal of Trauma: Injury, Infection, and Critical Care. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]