Abstract
Background
Voltage-gated calcium channel α2δ1 subunit is the binding site for gabapentin, an effective drug in controlling neuropathic pain states including thermal hyperalgesia. Hyperalgesia to noxious thermal stimuli in both spinal-nerve-ligated (SNL) and voltage-gated calcium channel α2δ1 over-expressing transgenic (Tg) mice correlates with higher α2δ1 levels in dorsal root ganglia and dorsal spinal cord. In this study, we investigated whether abnormal synaptic transmission is responsible for thermal hyperalgesia induced by elevated α2δ1 expression in these models.
Methods
Behavioral sensitivities to thermal stimuli were test in L4 SNL and sham mice, as well as in α2δ1 Tg and wild-type mice. Miniature excitatory (mEPSC) and inhibitory (mIPSC) postsynaptic currents were recorded in superficial dorsal spinal cord neurons from these models using whole-cell patch clamp slice recording techniques.
Results
The frequency, but not amplitude, of mEPSC in superficial dorsal horn neurons was increased in SNL and α2δ1 Tg mice, which could be attenuated by gabapentin dose dependently. Intrathecal α2δ1 antisense oligodeoxynucleotide treatment diminished increased mEPSC frequency and gabapentin's inhibitory effects in elevated mEPSC frequency in the SNL mice. In contrast, neither the frequency, nor the amplitude, of mIPSC was altered in superficial dorsal horn neurons from the SNL and α2δ1 Tg mice.
Conclusions
Our findings support a role of peripheral nerve injury-induced α2δ1 in enhancing presynaptic excitatory input onto superficial dorsal spinal cord neurons that contributes to nociception development.
1. Introduction
Neuropathic pain syndromes, including hypersensitivity to noxious stimuli (hyperalgesia), dramatically reduce the life quality of patients (Woolf & Mannion, 1999; Zimmermann, 2001; Costigan et al., 2009; Baron et al., 2010). Some ligands for the α2δ1 subunit of voltage-gated calcium channels (VGCC), such as gabapentin (GBP) and pregabalin (Gee et al., 1996; Lynch et al., 2006), are among limited drugs that are effective in pain relief in a subpopulation of neuropathic pain patients (Field et al., 2007), suggesting that α2δ1 proteins are critical in neuropathic pain processing.
α2δ subunits are auxiliary subunits of VGCC (Tanabe et al., 1987; Yaksh, 2006; Park & Luo, 2010), which include at least four distinctive genes, α2δ1-4 (Klugbauer et al., 1999). α2δ1 is the most abundant subtype in the spinal cord and dorsal root ganglia (DRG) (Marais et al., 2001; Cole et al., 2005). Co-expression of α2δ1 with α1 and β VGCC subunits in vitro results in increased calcium current densities, accelerated activation and inactivation kinetics, and hyperpolarized activation threshold (Klugbauer et al., 2003). Similar effects were also found in DRG neurons from transgenic mice (Tg) over-expressing α2δ1 in neuronal cells (Li et al., 2006).
Accumulating evidence shows that α2δ1 proteins contribute to both induction and maintenance of nerve injury-induced neuropathic pain states through a presynaptic mechanism. For instance, α2δ1 proteins are highly upregulated in DRG after spinal nerve ligation (SNL) injury, then translocated to primary afferent presynaptic terminals in dorsal spinal cord (Li et al., 2004; Bauer et al., 2009). This correlates with neuropathic pain states sensitive to intrathecal treatment with α2δ1 antisense oligodeoxynucleotides, or GBP (Luo et al., 2002; Li et al., 2004; Bauer et al., 2009). Since α2δ1 Tg mice also show hyperalgesia with a similar pharmacology profile as that in the SNL model (Chaplan et al., 1997; Luo et al., 2002; Li et al., 2006; Nguyen et al., 2009), it is highly likely that increased α2δ1 expression may mediate behavioral hypersensitivities through a similar presynaptic mechanism in both models. However, this hypothesis has not been tested thoroughly.
Peripheral nerve injuries have been shown in some studies to change excitatory (Millan, 1999; Chen et al., 2009; Sandkuhler, 2009) or inhibitory (Moore et al., 2002b; Scholz et al., 2005) synaptic transmission in superficial dorsal horn (SDH) where projection neurons receive periphery nociceptive inputs mainly from myelinated Aδ and non-myelinated C fibers as well as modulation from interneurons (Usunoff et al., 2006; D'Mello & Dickenson, 2008). However, it is not known whether these changes are mediated by dysregulated α2δ1 since nerve injuries also cause dysregulation of other factors (Wang et al., 2002; Valder et al., 2003; Kim et al., 2009). In this study, we examined if increased α2δ1 expression alone in the Tg mice (Li et al., 2006) was sufficient to induce similar changes in miniature excitatory (mEPSC) or inhibitory (mIPSC) postsynaptic currents in superficial dorsal horn neurons as that in the SNL model (Kim & Chung, 1992).
2. Methods and Materials
2.1 Animals
Male adult 129sv mice (6-8 weeks) were purchased from Charles River laboratories, Inc. (Wilmington, MA). Male, age-matched (8-12 weeks) α2δ1 Tg and their wild-type (WT) littermates had been backcrossed over 10 generations to the 129sv background before the experiments. All animal care and experiments were performed according to protocols approved by the Institutional Animal Care Committees of the University of California, Irvine.
2.2 Spinal nerve ligation surgery
Unilateral SNL was performed as described by Kim and Chung (1992). Briefly, under deep isoflurane anesthesia (5% for induction, and 2% for maintenance), the mouse left L4 spinal nerve, which is equivalent anatomically to L5 spinal nerve in rat (Rigaud et al., 2008), was exposed and ligated tightly with a 6.0 silk suture. We performed sham ligation by exposing the left L4 spinal nerve and loosely winding a suture around the nerve without ligation.
2.3 Cold test
Each mouse was acclimatized in a test compartment with a mash floor for about 30 min. Acetone (50 μL) was gently applied onto the plantar surface of the hindpaw using a pipette. The foot withdrawal responses were graded to a four–point scale: 0, no response; 1, brisk withdrawal or flick of the paw; 2, repeated flicking of the paw; 3, repeated flicking and licking of the paw (Choi et al., 1994). The test was repeated 3 times separated approximately by a 5-min interval on each mouse. Data were averaged for analysis.
2.4 Hargreaves test
Thermal paw withdrawal latencies were measured using a Hargreaves apparatus (Hargreaves et al., 1988). Briefly, each mouse was acclimatized in a test compartment on a glass surface maintained at 30 °C for at least 30 min. A radiant heat light source was positioned over the plantar surface of the hindpaw. Paw withdraw latency was recorded automatically as the duration between the time when the light was turned on and when the animals removed the paw from the thermal stimulation. The cut-off time was set to 20 sec to limit the risk of tissue damage. Three test results were recorded to calculate the average paw withdrawal latency for each mouse.
All behavioral tests were performed in a blind manner until the examiners needed to decode the data for analysis. For the SNL model, data from the injury side were compared between the SNL and sham mice. For the transgenic mouse model, data from both hindpaws were averaged, and compared between the WT and α2δ1 Tg mice.
2.5 Intrathecal antisense oligodeoxynucleotides treatment
Mouse α2δ1 (Accession number: NM_001110846) antisense (AGCCATCTTCGCGATCGAAG) and mismatch (CGATACCTCGCTGGCTAAAG) oligodeoxynucleotides with phosphothioate modification on three nucleotides at each end were synthesized by GineLink (Hawthorne, NY), precipitated, washed in 75% ethanol solutions and dissolved in sterile saline before use. Seven days after SNL when mice showed behavioral hypersensitivities, each solution (5 μL/mouse) was intrathecally injected between the L4/L5 regions (Li et al., 2006; Nguyen et al., 2009) once a day for 4 days. Similar treatments in the SNL model with the same antisense oligodeoxynucleotides resulted in specific knockdown of injury-induced increase of α2δ1 in spinal cord and behavioral hypersensitivity without causing any detectable toxicity (Li et al., 2004; Boroujerdi et al., 2008; Nguyen et al., 2009; Boroujerdi et al., 2011).
2.6 Patch clamp recording
Briefly, a mouse was decapitated under deep isoflurane anesthesia, and the L4 lumber spinal cord was removed after laminectomy, sliced (300 μm) with a vibratome (VT-1200, Leica Inc.) in ice-cold sucrose-based artificial cerebral spinal fluid (SACSF) saturated with 95% O2/5% CO2 (carbogen). The SACSF contained (mM): 250 sucrose, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3 and 11 glucose. Slices were first recovered at 31 °C for at least one hr in SACSF, then kept at room temperature (22-24 °C) in carbogenated ACSF containing (mM) 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3 and 11 glucose for at least 30 min. Then a slice was placed in a recording chamber and superfused (1.5-2.0 mL/min) with carbogen-saturated ACSF continually. 1 μM tetrodotoxin (TTX) was applied continually with perfusate when mEPSC/mIPSC was recorded. Drugs were applied with the perfusate as needed. Neurons located within or dorsal to lamina II (Substantia gelatinosa) were visualized with an upright microscope (Eclipse FN1, Nikon) with near-infrared illumination, and the lamina location of recorded sites was confirmed under low magnification after recording. Whole-cell patch clamp recording was performed at 32 ± 0.5°C with MultiClamp 700B amplifiers (Axon Instruments, Molecular Devices, Union City, CA), Digidata 1440 analog-to-digital converters (Axon Instruments) and pClamp 10.2 software (Axon Instruments). Data were sampled at 10 kHz and filtered at 2 kHz. The patch electrode had a resistance of 5-7 MΩ when filled with pipette solution 1 containing (mM): 135 potassium gluconate, 5 KCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.1 GTP; or as indicated, with solution 2 containing (mM): 70 potassium gluconate, 65 KCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, 0.1 GTP. The pH of these solutions was adjusted to 7.2 with Tris-base, and the osmolarity was adjusted to 300 mOsm with sucrose. The junction potential between the patch pipette and bath solution was nulled before gigaseal formation. Series resistance was monitored without compensation throughout the experiment (Multiclamp 700B). The data were discarded if the series resistance (15-30 MΩ) changed by more than 20% during whole-cell recording. mEPSC/mIPSC were recorded for 5 min after stabilization, counted and analyzed using clampfit 10.3 (Molecular Devices) after the traces were low-pass filtered at 2 kHz. Waveform templates were defined according to the rise and decay times and only events that match the waveform and above amplitude threshold (4 pA) were analyzed. For drug treatment, the baseline mean values of mEPSC frequency were obtained for 5 min, while the mean values during drug application were obtained for 2 min over the peak drug response. The drug effects on mEPSC frequency were compared to the baseline mean values, and expressed as percentage changes over the baseline values.
2.8 Western blots
Western blots were performed as described (Boroujerdi et al., 2011). Briefly, lumbar dorsal spinal cord samples from sham or SNL mice one-week post injury were extracted a ice-cold buffer (pH 7.5) containing 50 mM Tris, 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, and protease inhibitor cocktail (Roche Applied Science). Protein concentrations were measured using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc., Rockford, IL). Equal amounts of proteins were separated on polyacrylamide gels, transferred to PVDF membranes. Membranes were blocked with 5% milk solution, cut in half based on the molecular weight of the α2δ1 and β-actin, then probed with primary antibodies against α2δ1 (1:1000; Sigma-Aldrich, St. Louis, MO) or β-actin (1:10,000; Novus, Littleton, CO), respectively, overnight at 4°C. After washing, membranes were incubated with HRP-conjugated secondary antibodies for one hr at room temperature. Protein bands were detected using chemiluminescence reagents (Pierce Biotechnology, Inc., Rockford, IL) followed by X-ray film exposure. The band densities were quantified within the linear range of the film using Image J 1.45 (NIH). Co-detection of β-actin on the same membrane served as a loading control. The ratio of α2δ1 to β-actin band densities was taken within each sample before comparisons were made between the control and experimental samples.
2.9 Statistic analysis
Two-tailed Student's t-test was used to evaluate the statistical significance of differences in the frequency and amplitude of mEPSC/mIPSC between α2δ1 Tg and SNL neurons and their respective controls with or without drug treatments. One-way or Two-way ANOVA analysis with Bonferroni post hoc test was used for multi-group comparisons. p < 0.05 was considered statistically significant.
3. Results
3.1 Elevated α2δ1 induces hyperalgesia
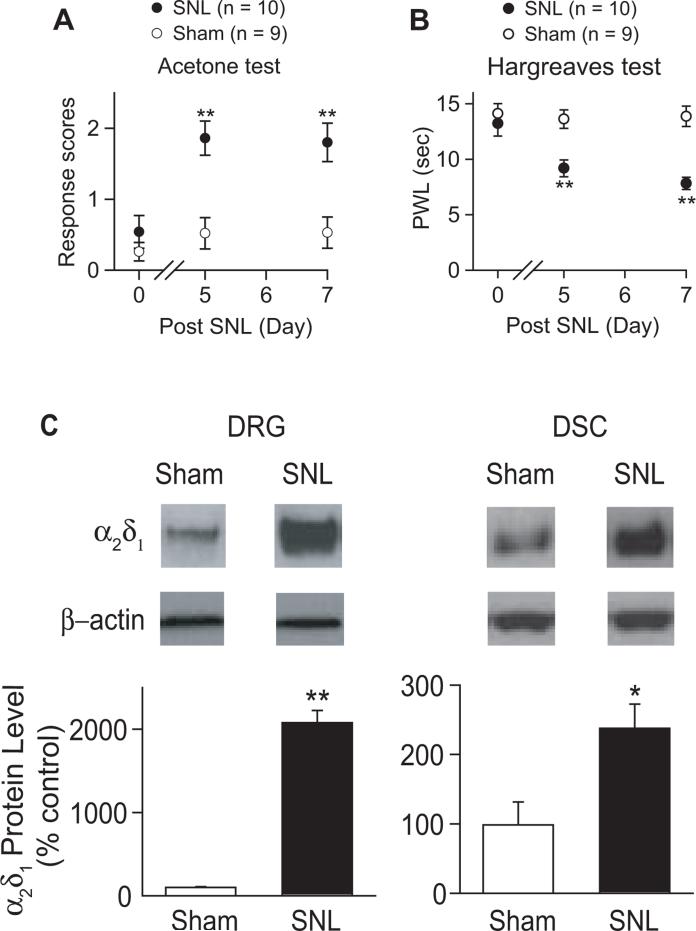
Behavioral sensitivities to noxious cold and heat were tested in left L4 SNL and sham mice 5-7 days after surgery. SNL, but not sham surgery, led to cold hyperalgesia (Fig. 1A) and heat hyperalgesia (Fig. 1B) with an onset time similar to that previously reported (Luo et al., 2001; Luo et al., 2002; Li et al., 2004). Data from Western blots indicated that SNL, but not sham surgery, increased α2δ1 protein levels in DRG and dorsal spinal cord at the injury side (Fig. 1C), similar to that reported in SNL rats (Luo et al., 2001; Luo et al., 2002; Li et al., 2004).
Figure 1. Unilateral L4 SNL injury caused nociceptions that correlated with α2δ1 upregulation in dorsal spinal cord and DRG.
Responses to noxious cold (A), and paw withdrawal latency (PWL) to heat (B) stimuli in the hindpaws of injury-side from sham and SNL mice were tested blindly at designated time points post surgery. Data presented are the means ± SEM from the number of animals indicated. **p < 0.01 compared with control values by one-way ANOVA test. The α2δ1 levels in injury-side of dorsal spinal cord and DRG were examined one-week post sham or SNL surgery with Western blots (C). β-actin was used as loading control as described in the Method. Representative Western blots were shown on top of each corresponding bar graph presented as the means ± SEM from 8 each of sham and SNL mice in 3 independent Western blots. *p < 0.05, **p < 0.01, compared with control values by Student's t test.
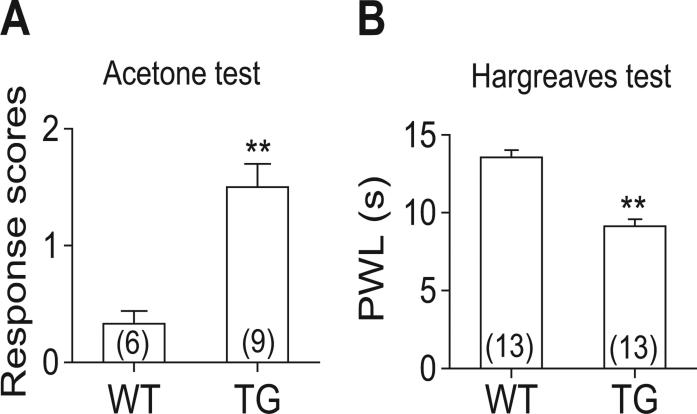
Based on these data, we hypothesized that α2δ1 upregulation could contribute to SNL-induced hypersensitivity. To determine whether the increase of α2δ1 alone, without other injury factors, is sufficient to induce behavioral hypersensitivities, we tested similar behavioral sensitivities in a Tg mouse line with α2δ1 protein overexpression in neuronal tissues (Li et al., 2006). The α2δ1 Tg, but not WT littermate, mice showed similar cold (Fig. 2A), and heat (Fig. 2B) hyperalgesia as the SNL mice (Fig. 1A-B). These findings suggest that behavioral hypersensitivities in both models are likely mediated through elevated spinal α2δ1 levels, forming the basis for electrophysiology studies
Figure 2. Over-expression of α2δ1 in the Tg mice caused similar behavioral hypersensitivities as in the SNL mice.
Responses to noxious cold (A), and paw withdrawal latency (PWL) to heat (B) stimuli in the hindpaws of adult injury-free WT and α2δ1 Tg mice were tested blindly. Data presented are the means ± SEM from the number of animals indicated in the parentheses. **p < 0.01 compared with WT mice by Student's t-test.
3.2 Upregulated α2δ1 enhances mEPSC frequency in superficial dorsal spinal cord
Since most nociceptive transmission is processed first in SDH, and SNL causes α2δ1 upregulation in DRG neurons, followed by subsequent translocation to axonal terminals in dorsal spinal cord (Li et al., 2004; Bauer et al., 2009), we hypothesized that SNL induced α2δ1 could affect presynaptic neurotransmission in SDH.
First, we examined α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/Kainate receptor mediated mEPSC from voltage-clamped (Vm = −60 mV) SDH neurons of L4 spinal cord slices from 5-7 day SNL mice with behavioral hypersensitivities (Fig. 1). N-methyl-D-aspartate (NMDA) receptors were blocked with antagonist AP-5 (50 μM) while IPSC were blocked with 10 μM biccuculine, a GABAA receptor antagonist, and 1 μM strychnine, a glycine receptor antagonist. Recorded mEPSC could be blocked by 20 μM DNQX, an AMPA/Kainate receptor antagonist (data not shown), confirming that mEPSC from dorsal spinal cord neurons are mediated through AMPA/Kainate receptor activation by action potential-independent presynaptic release of glutamate.
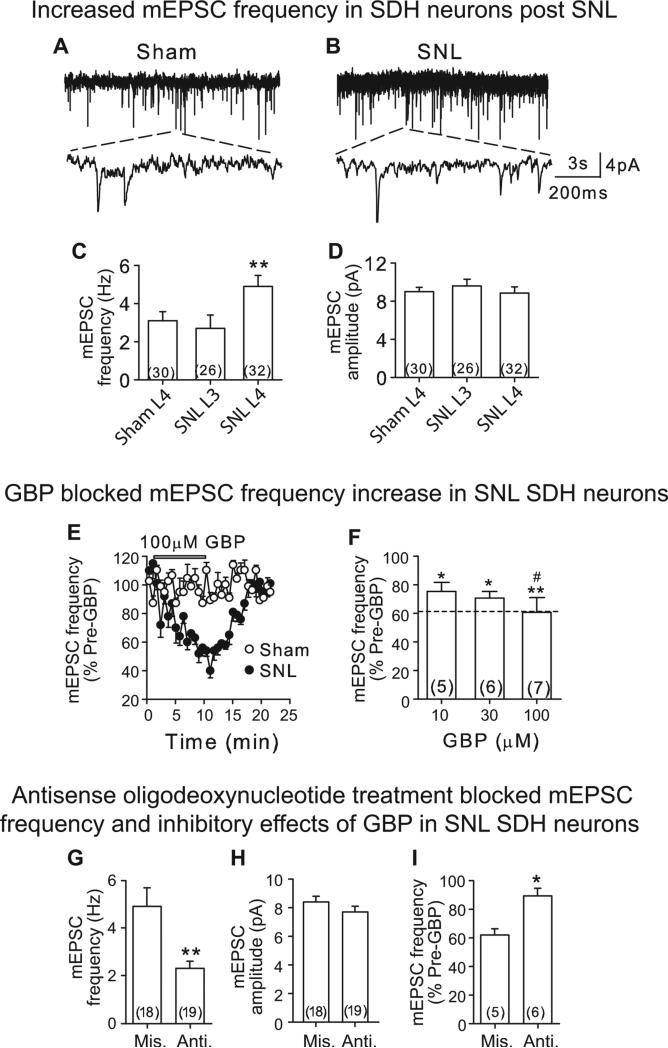
SNL injury did not change the membrane properties, including resting membrane potential and input resistance, of SDH neurons compared with that from sham neurons (Table S1). Compared with sham control, SNL increased significantly mEPSC frequency, but not amplitude, in injured SDH neurons (Figs 3A and B, S1A and B), but not in non-injured adjacent SDH neurons (Fig. 3C and D). Thus, SNL enhances presynaptic excitatory synaptic transmission in SDH of the injury segment, which may derive from increasing glutamate release.
Figure 3. SNL injury led to elevated mEPSC frequency in SDH neurons that was sensitive to α2δ1 blockade.
A and B. Representative mEPSC traces from injury-side of L4 spinal cord slices of sham (A) and SNL (B) mice 5-7 days after surgery. The upper panels were 10 s traces and the lower panels were 600 ms traces derived from each top trace as indicated by the dot lines. C and D. Summary of averaged mEPSC frequency (C) and amplitude (D) in L3 and L4 SDH neurons from injury side of sham and SNL mice. **p < 0.01 compared with L4 sham neurons by Student's t-test. E. Time courses for the effects of 100 μM GBP on mEPSC frequency from sham and SNL neurons. F. Dose-dependent normalization of mEPSC frequency on SNL neurons by GBP. *p < 0.05, **p < 0.01 compared with pre-GBP treatment, #p < 0.05 compared with 10 μM GBP by one-way ANOVA test. Dot-line represents baseline mEPSC frequency level in L4 sham neurons shown in C. G and H. Summary of averaged mEPSC frequency (G) and amplitude (H) data in SDH neurons from SNL mice that were treated with intrathecal α2δ1 antisense (Anti.) or mismatch (Mis) oligodeoxynucleotides (5 μg/day) for 4 days, starting 1-week post injury. I. Effects of GBP (100 μM) on elevated mEPSC frequency in L4 SDH neurons from injury-side of SNL mice post α2δ1 antisense or mismatch oligodeoxynucleotide treatments. Summarized data are shown as the means ± SEM from the number of neurons indicated in the parentheses. *p < 0.05, **p < 0.01 compared with α2δ1 mismatch treated groups by Student's t-test.
As blocking SNL-induced α2δ1 with intrathecal GBP and α2δ1 antisense oligodeoxynucleotides can reverse behavioral hypersensitivities (Luo et al., 2001; Luo et al., 2002; Li et al., 2004), we next tested whether these treatments could attenuate elevated mEPSC frequency in SDH neurons from SNL mice, which would support the involvement of elevated α2δ1 in mediating this neuroplasticity and behavioral hypersensitivities. GBP, within the effective dose range (10-100 μM) reported in brain and spinal cord slice preparations (Fink et al., 2000; Patel et al., 2000), dose-dependently and reversibly normalized mEPSC frequency in SDH neurons at the injury side to a level similar to that in the sham control without altering the mEPSC frequency in sham neurons (Figs. 3C, E and F).
Intrathecal treatments with α2δ1 antisense, but not mismatch, oligodeoxynucleotides (5 μg/mouse, once per day) for 4 days in 1-week SNL mice also caused a significant reduction in averaged frequency (Figs. 3G, S1C), but no significant change in amplitude (Figs. 3H, S1D) of mEPSC in L4 injury side SDH neurons. The antisense oligodeoxynucleotide effect was specific since it also blocked the inhibitory effects of GBP (100 μM) on SNL-induced mEPSC frequency (Fig. 3I). Similar antisense oligodeoxynucleotide treatments in SNL rats have been shown to block SNL-induced behavioral hypersensitivity and α2δ1 upregulation in dorsal spinal cord (Li et al., 2004; Boroujerdi et al., 2008; Boroujerdi et al., 2011). Together, these data further support that upregulated α2δ1 likely contributes to SNL-induced increase of mEPSC frequency, which contributes to behavioral hypersensitivity.
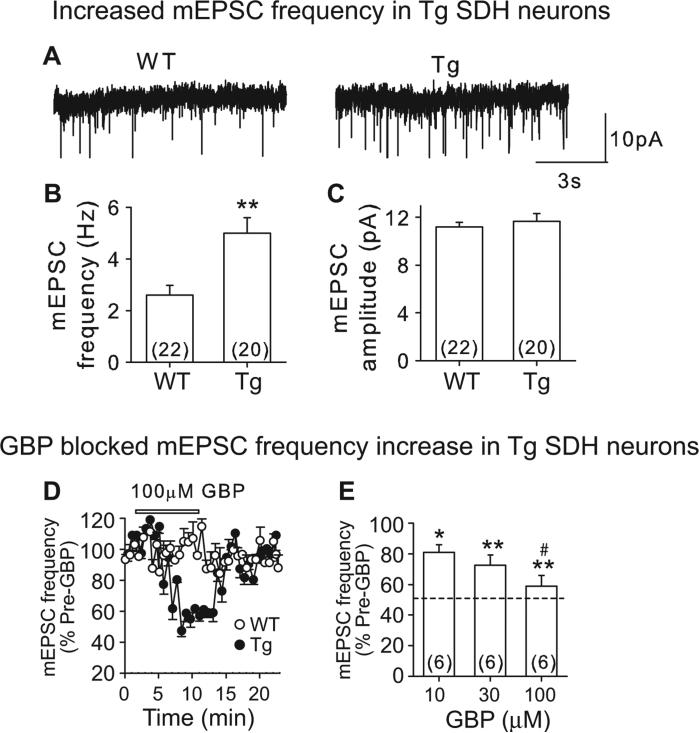
To test whether α2δ1 overexpression alone was sufficient to enhance mEPSC frequency in SDH neurons, we performed similar recordings in L4 spinal cord slices from the α2δ1 Tg and WT mice. Similar to that seen for the SNL mice, Tg SDH neurons have similar resting membrane potential and input resistance as WT neurons (Table S1). The averaged frequency (Figs. 4A and B, S2A), but not amplitude (Figs. 4A and C, S2B), of mEPSC in Tg neurons appeared significantly higher than that from WT neurons (Fig. 4B). Thus, α2δ1 upregulation alone is sufficient to increase mEPSC frequency in SDH neurons. GBP dose-dependently and reversibly normalized mEPSC frequency in Tg SDH neurons to a level similar to that in WT control without changing the mEPSC frequency in WT SDH neurons (Fig. 4B, D and E). These results suggest that GBP modulates mEPSC only when α2δ1 is upregulated, consistent with findings from a previous study (Moore et al., 2002a).
Figure 4. Enhanced mEPSC in SDH neurons from α2δ1 Tg mice.
A. Representative mEPSC traces from WT (left) and α2δ1 Tg (right) SDH neurons. B and C. Summary of averaged mEPSC frequency (B) and amplitude (C) data. **p < 0.01 compared with WT group by Student's t-test. D. Time courses for the effects of 100 μM GBP on mEPSC frequency from WT and Tg L4 SDH neurons. E. Dose-dependent normalization of mEPSC frequency in Tg SDH neurons by GBP. Dot-line represents baseline mEPSC frequency level in WT SDH neurons shown in B. Summarized data are shown as the means ± SEM from the number of neurons indicated in the parentheses. *p < 0.05, **p < 0.01 compared with pre-GBP treatment, #p < 0.05 compared with 10 μM GBP, by one-way ANOVA test.
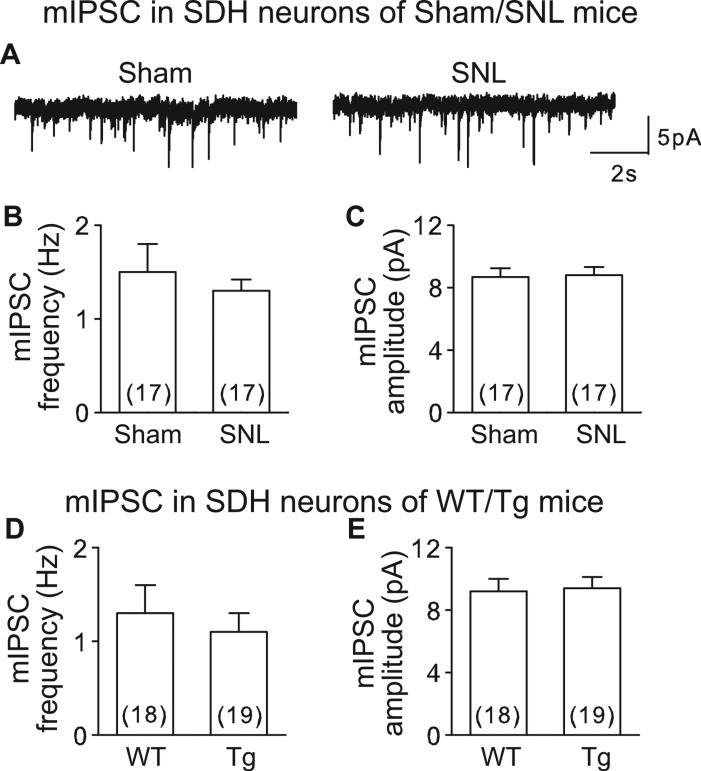
3.3 Inhibitory synaptic transmission in superficial dorsal horn is not altered in SNL and α2δ1 Tg mice
Inhibitory interneurons in spinal dorsal horn release glycine and GABA, which activate glycine and GABAA receptors, and cause hyperpolarization of postsynaptic neurons. To test whether α2δ1 upregulation affected inhibitory synaptic transmission in SDH neurons from SNL (5-7 days post injury) and α2δ1 Tg mice, we examined mIPSC from L4 spinal slices of these models using high Cl− (65 mM) intrapipette solution (solution 2 in Methods) to set Cl− reversal potential at −18 mV so that at −60 mV, both mEPSC and mIPSC are inward currents. We blocked mEPSC with 20 μM DNQX and 50 μM APV, and the remaining mIPSC could be eliminated by 10 μM bicuculline and 1 μM strychnine (data not shown), confirming that the recording condition was suitable for recording mIPSC (Fig. 5A).
Figure 5. mIPSC was not altered in SDH neurons from SNL and α2δ1 Tg mice.
A. Representative mIPSC traces from a sham (left) or SNL (right) SDH neuron. B-E. Summary of averaged mIPSC frequency (B, D) and amplitude (C, E) from sham/SNL (B, C) or WT/Tg (D, E) mice. Summarized data are shown as the means ± SEM from the number of neurons indicated in the parentheses. p > 0.05 by Student's t-test for all the comparisons between sham and SNL as well as WT and Tg mice.
Interestingly, both SNL (Figs. 5A, B and C, S3A and B) and Tg (Figs. 5D and E, S3C and D) mice showed similar mIPSC frequencies and amplitudes in SDH neurons as corresponding controls (SNL vs Sham; Tg vs WT). These data suggest that increased α2δ1 does not alter SDH inhibitory synaptic transmission in these models.
4. Discussion
SNL increases α2δ1 proteins in DRG neurons, and subsequent axonal transport of α2δ1 proteins to presynaptic central terminals in dorsal spinal cord that contributes to neuropathic pain states (Li et al., 2004; Bauer et al., 2009). SNL also causes dysregulation of other genes in DRG and spinal cord (Wang et al., 2002; Valder et al., 2003; Kim et al., 2009). To study the mechanism of α2δ1 mediated nociception without the influence from other injury factors, we included the non-injury α2δ1 Tg mice as a complementary model in which α2δ1 expression is elevated in spinal cord and DRG neurons in addition to other neuronal cells (Li et al., 2006). Our data show that both the SNL and α2δ1 Tg models have similar cold and heat hyperalgesia, validating the use of these models for our studies.
Taking the advantage of spinal cord slice patch-clamp recording from both models, we could focus on changes in spinal cord synaptic neurotransmission without worrying about influence from the peripheral and descending contributions. Since elevated α2δ1 proteins and behavioral hypersensitivities are common correlates in the SNL and α2δ1 Tg mouse models, we tested if elevated α2δ1 proteins contribute to SDH neuron sensitization through a similar synaptic mechanism in these models. Our data showed that SDH neurons in both models received higher frequency of glutamate input than their respective controls (Figs. 3, 4, S1, 2). The mEPSC frequency increase occurred only in injured L4, but not adjacent non-injured L3 SDH neurons from the SNL model (Fig. 3C), supporting that this change is injury segment specific. Intrathecal GBP, which blocks behavioral hypersensitivities in both models secondary to its binding to α2δ1 proteins (Luo et al., 2001; Luo et al., 2002; Field et al., 2006; Li et al., 2006; Lynch et al., 2006), also diminishes elevated mEPSC frequency dose-dependently in SDH neurons from both models (Figs. 3, 4). The specificity of elevated α2δ1 in this injury-induced neuroplasticity is further confirmed by the following findings. Intrathecal α2δ1 antisense oligodeoxynucleotide treatment, which can block injury-induced behavioral hypersensitivities and α2δ1 upregulation in dorsal spinal cord (Li et al., 2004; Boroujerdi et al., 2008; Boroujerdi et al., 2011), also diminishes (1) increased mEPSC frequency (Fig. 3G), and (2) the GBP's inhibitory effects on elevated mEPSC (Fig. 3I) in SDH neurons from SNL mice.
SDH projection neurons receive inputs mainly from small myelinated Aδ and nonmyelinated C-fibers carrying nociceptive information such as cold and heat hyperalgesia (Light & Perl, 1979; Brown, 1982; Millan, 1999; Graham et al., 2007), and form synaptic connections with heterogeneous neurons (Millan, 1999; Prescott & De Koninck, 2002; Lu & Perl, 2005). In combination with pharmacological findings that intrathecal injection of glutamate receptor antagonists (Chaplan et al., 1997; Nguyen et al., 2009), or similar GBP and α2δ1 antisense oligodeoxynucleotides (Luo et al., 2001; Luo et al., 2002; Li et al., 2004) relieves behavioral hypersensitivity in these models, our data support that increased presynaptic α2δ1 at the injury segment is likely contributing to the central sensitization and nociception development in the SNL model.
It has been reported recently that transient overexpression of α2δ1 with P/Q-type VGCC subunits in vitro renders the accumulation of P/Q-type VGCC at presynaptic boutons and subsequent higher probability of vesicle release (Hoppa et al., 2012). We provide complementary in vivo evidences here indicating that this α2δ1-associated phenomenon also occurs in mouse spinal cord after peripheral nerve injury that mediates nociceptions. We have shown that GBP attenuates increased mEPSC frequency in SNL and α2δ1 mice, suggesting GBP's presynaptic modulatory role. However, GBP fails to affect mEPSC in sham and WT mice, which is similar to that observed previously by Moore, et al. (Moore et al., 2002a), and in line with animal behavioral results showing that intrathecal GBP has no effect on behavioral sensitivities under control conditions (Stanfa et al., 1997; Luo et al., 2001; Li et al., 2006). While in vitro data support that α2δ1 is critical in regulating VGCC trafficking to the cell membrane and nerve terminals and GBP disrupts this process (Hendrich et al., 2008; Bauer et al., 2009), the onset time of gabapentin's inhibitory action in our experiments (mins) is faster than the expected time for regulating VGCC trafficking, which takes at least hrs. Thus, our data support a fast action of GBP in blocking synaptic hyperexcitability and behavioral hypersensitivity in neuropathic pain models (Hunter et al., 1997; Hwang & Yaksh, 1997; Abdi et al., 1998; Chapman et al., 1998; Luo et al., 2002; Li et al., 2004; Mixcoatl-Zecuatl et al., 2004; Suzuki et al., 2005; Li et al., 2006; Suzuki & Dickenson, 2006) through a yet clearly defined mechanism secondary to its binding to α2δ1 proteins (Lynch et al., 2006). This could be different from its effects in inhibiting VGCC trafficking. Recent findings supporting this notion include that SNL injury-induced and α2δ1-mediated behavioral hypersensitivities are modulated by descending serotonergic facilitation at the spinal level (Suzuki et al., 2005; Chang et al., 2013), which is indeed sensitive to blockade by gabapentinoids (Suzuki et al., 2005; Bee & Dickenson, 2008). Interestingly, the descending serotonergic facilitatory pathway is thought to play a critical role in recruitment and activation of silent glutamatergic synapses in the spinal cord (Li & Zhuo, 1998), which could also facilitate the priming of dorsal spinal cord neurons for sensitization (Nguyen et al., 2009). It is possible that priming of dorsal horn neurons by increased presynaptic α2δ1 proteins (Nguyen et al., 2009) triggers recruitment of silent synapses through a yet identified process that promotes the formation of descending serotonergic facilitatory circuit (Li & Zhuo, 1998) in mediating behavioral hypersensitivity. Further investigations are warranted for testing this hypothesis.
GABAA and glycine receptors mediate IPSC in the spinal cord (Todd & Sullivan, 1990; Yoshimura & Nishi, 1993). However, experimental data on whether inhibitory synaptic transmission is altered in the spinal cord post peripheral nerve injuries are not consistent. Some studies show neither GABAergic nor glycinergic tone changes in neuropathic rodent models (Somers & Clemente, 2002; Polgar et al., 2003; Polgar et al., 2005; Wang et al., 2007), while some show loss of GABAergic inhibition in peripheral nerve injury models (Ibuki et al., 1997; Moore et al., 2002b; Yowtak et al., 2011). We found no change in mIPSC in SDH neurons from SNL and α2δ1 Tg mice. In addition to the differences in recording conditions and animal strains used, this discrepancy may be due to the fact that we did not separate GABAA and glycine receptor mediated IPSC. Thus, any change in GABAA receptor mediated IPSC could have been diluted to a level below the sensitivity of detection in our studies. However, our findings is consistent with that from studies indicating, for instance, neither GABAergic nor glycinergic IPSC changes in diabetic neuropathic or SNL rats (Polgar et al., 2003; Polgar et al., 2005; Wang et al., 2007). Our data are also supported by a previous electron microscopy study showing that SNL causes elevation of α2δ1 in excitatory, but not inhibitory, synapses (Bauer et al., 2009).
In this study, we systematically compared AMPA receptor mediated excitatory and Glycine/GABAA receptor mediated inhibitory synaptic transmission in SDH of the SNL model. Our conclusion is consistent with findings from several other studies indicating that neuropathic pain syndromes in various peripheral nerve injury animal models may result from enhanced excitatory synaptic transmission in the spinal cord, even though the reported endpoint measurements in different peripheral nerve injury models may differ (Kohno et al., 2003; Wang et al., 2007; Zhang et al., 2009; Zhou et al., 2011). However, our data would not allow us to exclude the contribution of other postsynaptic mechanisms, such as NMDA receptor-mediated dorsal spinal cord neuron hyperexcitability, to behavioral hypersensitivity in the nerve injury model (Tao et al., 2003a; Tao et al., 2003b) since NMDA-receptor was blocked in our recording conditions.
In summary, combinational analysis with data from the SNL and α2δ1 Tg models supports our hypothesis that α2δ1 upregulation at the presynaptic central terminals of primary afferents is sufficient to enhance excitatory glutamatergic neurotransmission in superficial dorsal spinal cord that contributes to nociception. Therefore, blocking injury-induced α2δ1 upregulation and glutamatergic pathways in a sensory neuron specific manner may improve therapeutic efficacy and reduce drug side effects in neuropathic pain management.
Supplementary Material
It is known that elevated α2δ1 proteins in spinal cord contribute to nerve injury induced neuropathic pain states.
Findings from this study added electrophysiological evidences to support that elevated α2δ1 proteins contribute to central sensitization by increasing excitatory presynaptic inputs onto superficial dorsal spinal cord neurons, leading to the development of behavioral hypersensitivities.
Acknowledgements
We thank Dr. C. Xiao for help in electrophysiological experiments, and comments on the manuscript, Drs. K-W Li, X-G Chen and J. Zeng for assistance in some initial experiments.
Funding Sources: This study was supported in part by grants NS064341 and DE021847 from the National Institutes of Health (Z.D. Luo).
Footnotes
All authors have no potential conflicts of interest to this study.
References
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesthesia and Analgesia. 1998;87:1360–1366. [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140:209–223. doi: 10.1016/j.pain.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Boroujerdi A, Kim HK, Lyu YS, Kim DS, Figueroa KW, Chung JM, Luo ZD. Injury discharges regulate calcium channel alpha-2-delta-1 subunit upregulation in the dorsal horn that contributes to initiation of neuropathic pain. Pain. 2008;139:358–366. doi: 10.1016/j.pain.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroujerdi A, Zeng J, Sharp K, Kim D, Steward O, Luo ZD. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain. 2011;152:649–655. doi: 10.1016/j.pain.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982;67:193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Chang EY, Chen X, Sandhu A, Li CY, Luo ZD. Spinal 5-HT3 receptors facilitate behavioural hypersensitivity induced by elevated calcium channel alpha-2-delta-1 protein. Eur J Pain. 2013;17:505–513. doi: 10.1002/j.1532-2149.2012.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chapman V, Suzuki R, Chamarette HL, Rygh LJ, Dickenson AH. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998;75:261–272. doi: 10.1016/s0304-3959(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Balasubramanyan S, Lai AY, Todd KG, Smith PA. Effects of sciatic nerve axotomy on excitatory synaptic transmission in rat substantia gelatinosa. J Neurophysiol. 2009;102:3203–3215. doi: 10.1152/jn.00296.2009. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Li Z, Schwarz JB. Ca2+ channel alpha2-delta ligands for the treatment of neuropathic pain. J Med Chem. 2007;50:2569–2575. doi: 10.1021/jm060650z. [DOI] [PubMed] [Google Scholar]
- Fink K, Meder W, Dooley DJ, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol. 2000;130:900–906. doi: 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. Moving from an averaged to specific view of spinal cord pain processing circuits. J Neurophysiol. 2007;98:1057–1063. doi: 10.1152/jn.00581.2007. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, Fontana DJ. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. European Journal of Pharmacology. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected]. Pain. 2009;143:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Honore P, Anderson DJ, Bunnelle WH, Mortell KH, Zhong C, Wade CL, Zhu CZ, Xu H, Marsh KC, Lee CH, Jarvis MF, Gopalakrishnan M. (L)-Phenylglycine, but not necessarily other alpha2delta subunit voltage-gated calcium channel ligands, attenuates neuropathic pain in rats. Pain. 2006;125:136–142. doi: 10.1016/j.pain.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mixcoatl-Zecuatl T, Medina-Santillan R, Reyes-Garcia G, Vidal-Cantu GC, Granados-Soto V. Effect of K+ channel modulators on the antiallodynic effect of gabapentin. Eur J Pharmacol. 2004;484:201–208. doi: 10.1016/j.ejphar.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Moore KA, Baba H, Woolf CJ. Gabapentin-- actions on adult superficial dorsal horn neurons. Neuropharmacology. 2002a;43:1077–1081. doi: 10.1016/s0028-3908(02)00226-5. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002b;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Deng P, Matthews EA, Kim DS, Feng G, Dickenson AH, Xu ZC, Luo ZD. Enhanced pre-synaptic glutamate release in deep-dorsal horn contributes to calcium channel alpha-2-delta-1 protein-mediated spinal sensitization and behavioral hypersensitivity. Mol Pain. 2009;5:6. doi: 10.1186/1744-8069-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010;4:510–517. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MK, Gonzalez MI, Bramwell S, Pinnock RD, Lee K. Gabapentin inhibits excitatory synaptic transmission in the hyperalgesic spinal cord. Brit. J. Pharmacol. 2000;130:1731–1734. doi: 10.1038/sj.bjp.0703530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol. 2002;539:817–836. doi: 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002;323:171–174. doi: 10.1016/s0304-3940(02)00157-x. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–590. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006;198:72–80. doi: 10.1016/j.expneurol.2005.10.032. Epub 2005 Dec 2005. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Mao P, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience. 2003a;117:731–739. doi: 10.1016/s0306-4522(02)00801-1. [DOI] [PubMed] [Google Scholar]
- Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci. 2003b;23:6703–6712. doi: 10.1523/JNEUROSCI.23-17-06703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Popratiloff A, Schmitt O, Wree A. Functional neuroanatomy of pain. Adv Anat Embryol Cell Biol. 2006;184:1–115. [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87:560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience. 2009;158:875–884. doi: 10.1016/j.neuroscience.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J Pharmacol Exp Ther. 2011;336:254–264. doi: 10.1124/jpet.110.173112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.