Abstract
LAY ABSTRACT
Mutations in neuroligin genes have been identified in association with autism. This manuscript provides characterization of a specific mutation in the neuroligin-3 gene that has been associated with autism in two brothers. The resulting autism model has alterations in social and cognitive function reminiscent of autism in patients. This model will be useful to understand the role of neuroligin dysfunction as a rare cause of autism.
SCIENTIFIC ABSTRACT
Multiple candidate genes have been identified for autism spectrum disorders. While some of these genes reach genome-wide significance, others, such as the R451C point mutation in the synaptic cell adhesion molecule neuroligin-3, appear to be rare. Interestingly, two brothers with the same R451C point mutation in neuroligin-3 present clinically on seemingly disparate sides of the autism spectrum. These clinical findings suggest genetic background may play a role in modifying the penetrance of a particular autism-associated mutation. Animal models may contribute additional support for such mutations as functionally relevant and can provide mechanistic insights. Previously, in collaboration with the Südhof laboratory, we reported that mice with an R451C substitution in neuroligin-3 displayed social deficits and enhanced spatial learning. While some of these behavioral abnormalities have since been replicated independently in the Südhof laboratory, observations from the Crawley laboratory failed to replicate these findings in a similar neuroligin-3 mutant mouse model and suggested that genetic background may contribute to variation in observations across laboratories. Therefore we sought to replicate our findings in the neuroligin-3 R451C point mutant knockin mouse model (NL3R451C) in a different genetic background. We backcrossed our NL3R451C mouse line onto a 129S2/SvPasCrl genetic background and repeated a subset of our previous behavioral testing. NL3R451C mice on a 129S2/SvPasCrl displayed social deficits, enhanced spatial learning, and increased locomotor activity. These data extend our previous findings that NL3R451C mice exhibit autism-relevant behavioral abnormalities and further suggest that different genetic backgrounds can modify this behavioral phenotype through epistatic genetic interactions.
INTRODUCTION
Mutations in the neuroligin and neurexin genes encoding for synaptic cell- adhesion molecules have been implicated as causes of autism (Jamain et al., 2003; Chih et al., 2004; Comoletti et al., 2004; Yan et al., 2005; Kim et al., 2008) In collaboration with the Südhof laboratory, we created and characterized a novel neuroligin-3 (NL3) point mutant model on a hybrid genetic background (Tabuchi et al., 2007) based on an inherited, X-linked mutation identified in two brothers, one with autism and intellectual disability and one with Asperger Syndrome (Jamain et al., 2003). In that initial study, our laboratory at The University of Texas Southwestern Medical Center in Dallas identified enhanced spatial learning on the Morris water maze and deficits in social interaction (Tabuchi et al., 2007). Social interaction abnormalities in the same NL3R451C mutant model on a similar hybrid genetic background have since been independently replicated in the laboratory of Dr. Thomas Südhof at Stanford University in Palo Alto (Etherton et al., 2011a). A separately generated NL3R451C mutant model generated and tested on a pure C57BL6 genetic background was characterized in the laboratory of Dr. Jacqueline Crawley at NIMH (Chadman et al., 2008). In that study, they identified neurodevelopmental abnormalities in the righting response and in ultrasonic vocalizations in pups but no changes in either spatial learning or social interaction (Chadman et al., 2008). These differences could be accounted for by the different genetic background, differences in behavioral protocol sensitivity among laboratories, a lack of robustness in the behavioral deficits (Bozdagi et al., 2010), or other factors. In an effort to determine the effect of genetic background on our observed phenotype, we backcrossed our NL3R451C mutant mice onto a pure 129S2/SvPasCrl (Charles River; 129-E) genetic background for 10 backcrosses and verified the genetic background using Jackson Laboratory Congenic Services testing (Jax® Mice and Services, Bar Harbor, Maine 04609 USA). We have now examined both social and spatial learning behaviors in this new genetic background and find deficits in both the social and spatial learning domains similar to our original report (Tabuchi et al., 2007), as well as an additional increase in locomotor activity on this background. These behaviors were performed by a completely independent behavioral core technician who was not aware of the goals of the experiment or of the genotype of the mice being tested. Our findings suggest that both social deficits and enhanced spatial learning are reproducible in the NL3R451C mutant mice under similar testing conditions, implicating either genetic background or differences in behavioral protocols among the labs as potential causes of the discrepant results.
METHODS
Behavioral Overview
All mice tested were male, littermate progeny of matings between 14 wild-type males and heterozygous females (neuroligin-3 is an X-linked gene). Due to litter effects on behaviors such as aggression (Barnard et al., 1991) and development of stereotypies (Powell et al., 1999), using littermate pairs from multiple litters minimizes such effects. Our behavior cohort of 20 littermate pairs was derived from 12 total litters. 6 mouse pairs (1 WT, 1 NL3R451C) came from 6 separate litters or one pair per litter. 8 mouse pairs came from 4 litters (2 pairs per litter) and 6 mouse pairs from 2 litters (3 mouse pairs per litter). All mice were group-housed with 2 littermates in each cage and were provided with ad lib food and water. Mice were kept on a 12 hr light: 12 hr dark cycle, and behavioral testing was conducted during the light cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
All behavioral testing was conducted by experimenters blind to genotype, and every effort was made to test less stressful behaviors before more stressful behaviors. Mice were transported from their housing room and allowed to habituate at the site of behavioral testing for approximately 1 hr prior to testing.
NL3R451C mutant mice were generated as previously described (Tabuchi et al., 2007) and then backcrossed for 10 generations onto a uniform 129S2/SvPasCrl genetic background. Uniformity of the genetic background to 99.5% was confirmed using Jackson Laboratory Congenic Services testing (Jax® Mice and Services, Bar Harbor, Maine 04609 USA). Genotyping to distinguish WT from KO was performed using the following primers (written 5′-3′) NL3R451C forward – TGT ACC AGG AAT GGG AAG CAG, NL3R451C reverse – GGT CAG AGC TGT CAT TGT CAC. WT mice displayed a 250bp band while KO displayed a 290bp band on a 2% agarose gel. For the NL3R451C cohort, mice ranged from 111 days to 162 days of age (3.7 to 5.4 months of age) at the start of behavioral testing, and the number of NL3R451C littermate pairs was 20 (total of 40 mice), except for 3-box social interaction test (n=17 littermate pairs, total of 34 mice) due to errors in mouse placement/handling during these experiments (data removed prior to analysis by behavioral technician blind to genotype). The order of tests for NL3R451C mice was as follows: Day 1 elevated plus maze, Day 2 open field (Days 3–7 off), Day 8 3-chamber sociability and preference test, Days 9 and 12 social interaction with a juvenile (Days 10–11 off), and Days 13–27 Morris water maze. Results are not presented in the order of testing to ease in the presentation and interpretation of the findings. All behavioral testing of NL3R451C mice was performed by a behavioral core technician (AP) managed by Dr. Shari Birnbaum outside of the auspices of the Powell Laboratory. The technician had no prior knowledge of any differences in behavioral outcomes in NL3R451C mice (Tabuchi et al., 2007; Chadman et al., 2008; Etherton et al., 2011a) and was completely blind to both the genotypes and to the purpose of the study.
Social interaction and Social learning Behaviors
3-chamber sociability and social preference tests were conducted as previously described (Moy et al., 2004; Nadler et al., 2004; Blundell et al., 2009) except the 3-chamber apparatus dimensions differed (15 × 90 × 18.5 cm divided into three compartments of 15 × 29 cm separated by dividers with a central 3.8 × 3.8 cm door). Video tracking software from Noldus Ethovision 3.1 was used to record mouse behavior. The interaction zone (3.75 cm on each side of the cage and 9 cm in front on the cage) is defined as a rectangular zone around a rectangular cage (7.5 cm × 5 cm). In the test, mice were initially allowed to explore the apparatus for 10 min. Then, mice were allowed to interact with an empty cage in one compartment vs. a caged social target (i.e. novel mouse) in the far compartment for another 10 min. The test for social novelty involved a subsequent 10 min test in which the mouse from the previous 10 min test (Familiar mouse) is in one compartment and a novel mouse is placed in a cage in the opposite compartment. Location of empty cages and target mouse as well as novel vs. familiar mouse was counterbalanced.
Social interaction with a juvenile mouse was conducted as previously described (Blundell et al., 2009) with some modifications. Briefly mice were habituated in their home cages for 15 min under red light. Following habituation mice were placed in an empty home cage for 2 min and allowed to interact with a juvenile novel mouse. Social learning was assessed 3 days later by allowing the mice to interact with the same juvenile mouse for an additional 2 min. All mice were scored live during testing and videotaped for backup. Target mice used for the 3-chamber sociability and preference test were 4–6 month old C57BL/J6 male mice purchased from Jackson Laboratory (Bar Harbor, ME). Target mice used for the social interaction with juvenile test were 3–4 weeks old C57Bl/6J male mice also purchased from Jackson Laboratory (Bar Harbor, ME).
Anxiety-like Behaviors
The open field and elevated plus maze (EPM) tests were conducted precisely as described previously (Etherton et al., 2009), as was the dark/light test (Powell et al., 2004; Blundell et al., 2009) For the open field test, mice were placed along the edge of an open field arena (44 × 44 × 44 cm) and allowed to freely explore for 10 minutes. For EPM, mice were placed in the center of the maze (each arm was 30 cm long and 5 cm wide with 25 cm high walls on the closed arms) and allowed to freely explore for 5 min. The elevated plus maze was conducted under dim white light measured at ~7 lux. For the dark/light test, mice were placed in the dark chamber (each chamber was 25 × 26 cm with 1,700 lux on the light side and ~0.1 lux on the dark side) and allowed to habituate for 2 min. After habituation, mice were allowed to freely explore both chambers for 10 minutes. Noldus Ethovision version 3.1 was used to track and record mouse behavior.
Locomotor Activity
Locomotor activity was measured as described previously (Powell et al., 2004; Tabuchi et al., 2007; Etherton et al., 2009) Briefly, mice were placed in novel cages (a clean cage with the same dimensions as their home cage; L x W x H = 27.3 cm × 16.5 cm × 12.7 cm) with minimal bedding and allowed to freely explore for 2 hours. Horizontal locomotor activity (i.e. the number of photobeam breaks) was measured by computer software (San Diego Instruments; San Diego, CA), and data were analyzed in 5 min bins.
Morris water maze
The Morris water maze and visible platform tests were performed as described (Powell et al., 2004) except that the probe trial was performed on day 9. Mice were trained to find a submerged platform in a pool (144 cm in diameter) of opaque water, with a thigmotaxis zone 9 cm from the wall of the pool. The water temperature was 22°C and was made opaque using gothic white, non-toxic tempra paint. Mice received 4 training trials per day. In all trials, mice were allowed to swim until they reached the platform or 60 seconds had elapsed. On day 9, 24 hours following the conclusion of training, a probe test was conducted with the platform removed from the pool. In this initial probe trial, one of the mutant mice was deemed a “floater” and both it and its littermate were excluded from the probe trial analysis in a blinded fashion by the behavioral technician (N=19 for initial probe). Following the probe test, the platform was submerged in the opposite quadrant for reversal training. Following four days of reversal training a probe trial was conducted. In this reversal probe trial, 4 WT mice were deemed “floaters” and one mutant was deemed a “floater” and the 5 corresponding littermate pairs were excluded from the reversal probe trial analysis in a blinded fashion by the behavioral technician (N=15 for reversal probe). Reversal training was conducted similar to the initial training. Following the reversal probe trial a visible platform test was conducted. If during any of the swimming test a mouse did not swim but floated when placed in the pool that mouse was labeled a “floater” and he and his littermate pair were excluded from analysis for that test blindly by the technician.
Statistical Analyses
Statistical analyses were conducted using StatPlus (AnalystSoft Inc.) or Excel data analysis tool. All data were initially analyzed using unpaired Student’s t-test, 2-way or 1-way repeated measures ANOVA, as appropriate. Genotype was always used as the between-subjects factors, and for the repeated measures ANOVAs, Interaction Target (for social interaction with a caged conspecific and 3-box social interaction test), Trial (for social interaction with a juvenile), Day (for Morris water maze), or Bin (for locomotor activity) were used as a within-subjects factor. Further planned comparisons were conducted using paired or unpaired t-tests, as appropriate. A thorough description of statistical analyses can be found in Supplementary Table 1.
RESULTS
NL3R451C Mutants Exhibit Altered Social Interaction
Similar, to our previous findings on a hybrid genetic background (Tabuchi et al., 2007), NL3R451C mutants on a 129S2/SvPasCrl genetic background show decreased sociability in a 3-chamber task of sociability (Moy et al., 2004). When presented with a social target and an inanimate target simultaneously in a 3-chamber sociability task, WT littermate controls demonstrated a clear preference for the social target compared to the inanimate target (Fig. 1A). WT preference for social target versus inanimate target was statistically significant using both post-hoc Bonferonni test as well as planned comparison with paired and unpaired t-tests (Table 1) indicating significant “sociability”. In contrast, NL3R451C mutants showed no preference for social versus inanimate targets using post-hoc Bonferonni test, paired t-test, and unpaired t-test task (Table 1) indicating lack of “sociability”. It should be noted that when using ANOVA with genotype and zone as independent variables, no main effect of and no interaction between zone and genotype was observed (Table 1).
Figure 1. NL3R451C Mutants Exhibit Differences in Social Interaction.

(A) NL3R451C mutants lack preference for social vs. inanimate objects in the 3-chamber sociability task. Social approach in WT and NL3R451C mice was assessed using a 3-chamber apparatus that contained an inanimate interaction target (empty cage) in one of the end-chambers and a social interaction target (caged, sex-matched adult mouse) in the opposite end-chamber. Average time spent interacting with the target ± S.E.M. is shown. N=17 littermate pairs. (B) WT mice exhibit a trend toward preference for social novelty while NL3R451C mice show an opposite trend toward preference for a familiar social target. In this task, the inanimate target was replaced with a novel social target adult mouse while the previous social interaction target (now a familiar mouse) remained the same. Location of novel and familiar targets were counterbalanced within each genotype group in pseudorandom fashion. N=17 littermate pairs. (C) NL3R451C mice display increased locomotor activity during all 3-box testing trials. Distance travelled during each trial ± S.E.M. is plotted for WT and NL3R451C groups. N=17 littermate pairs. (D) NL3R451C and WT mice displayed similar social interaction with a juvenile mouse during the reciprocal juvenile social interaction task. When placed in the presence of the same juvenile three days later, NL3R451C and WT mice displayed similar times interacting. N=20 littermate pairs. *P<0.05, **P<0.01, ***P<0.001
Curiously, a subsequent test of preference for social novelty did not result in a significant preference for the novel social target versus the familiar social target in either group, although the WT littermate trend was in the direction of a preference for social novelty whereas the NL3R451C trend was opposite that of the WT littermates (Fig. 1B). All locations of inanimate and social targets were counterbalanced within both NL3R451C and littermate WT groups and no initial preference for either side of the 3-box chamber was noted during the habituation phase (data not shown). Interestingly, we identified an increase in locomotor activity in this task as the total distance travelled during baseline, sociability, and social preference portions of the task was significantly increased in the NL3R451C group, though both groups demonstrated habituation of locomotor activity during the task (Fig. 1C). Given the simultaneous presentation of both social and inanimate targets in this task and the relatively high degree of interaction with both social and inanimate targets in the NL3R451C mutants, the increased locomotor activity is not a likely cause of the lack of sociability in this task.
Consistent with our previous findings (Tabuchi et al., 2007), in a task of direct, reciprocal social interaction with a juvenile target mouse, NL3R451C mice exhibited no differences compared to WT littermates (Fig. 1D). Initial time spent interacting with the juvenile target mouse was equivalent in both NL3R451C and WT littermate groups (Fig. 1D). Both WT and NL3R451C mice demonstrated significant reductions in time spent interacting with the same, familiar juvenile target 3 days later, demonstrating normal social recognition in both groups (Fig. 1D).
NL3R451C Mutants Exhibit Increased Spatial Learning in the Morris Water Maze Task
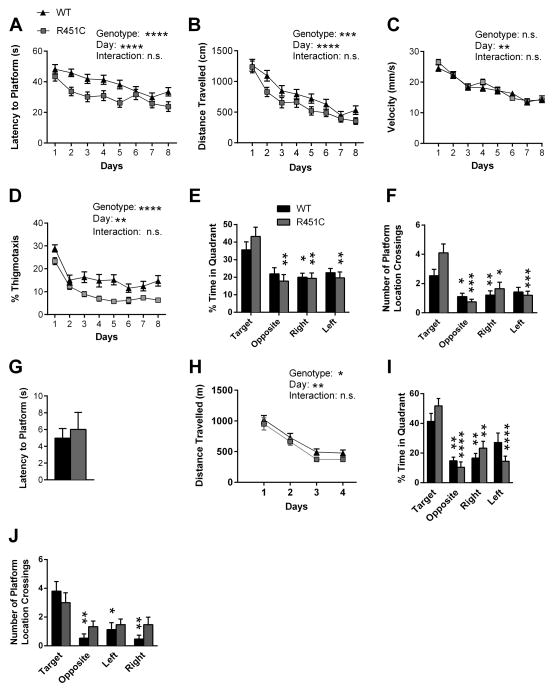
We also observed a significant increase in NL3R451C mutant spatial learning in the Morris water maze task similar to that observed previously (Tabuchi et al., 2007). NL3R451C mutants demonstrated a significantly increased rate of learning during training to find the hidden platform using both latency to reach the hidden platform (Fig. 2A) and distance travelled prior to reaching the hidden platform (Fig. 2B) which eliminates swim speed as a confounding factor. Swim speed throughout the task was not significantly different between the NL3R451C and WT littermates (Fig. 2C). Interestingly, thigmotaxis across training trials was persistently increased in WT compared to NL3R451C (Fig. 2D) which parallels the slower learning curve in WT compared to NL3R451C mutants. On the probe trial, only NL3R451C mutants demonstrated a significant preference for the target quadrant versus all three other quadrants (Fig. 2E). Using a more narrowly defined endpoint, the number of platform location crossings, both groups preferred the target location to all other phantom platform locations (Fig. 2F). Total distance travelled during the probe trial was equivalent in both groups eliminating swim speed and increased activity as confounds (not shown). Both groups performed equivalently on the visible platform version of the Morris water maze (Fig. 2G). These data suggest that the NL3R451C mutants on a pure 129 background acquire the water maze task more rapidly than their WT littermates.
Figure 2. NL3R451C Mutants Exhibit Increased Spatial Learning in the Morris Water Maze Task.
(A–C) NL3R451C mice exhibit increased spatial learning in the Morris water maze during training compared to WT littermates using both latency (N=20 littermate pairs) (A) and distance travelled (N=20 littermate pairs) (B) prior to reaching the hidden platform. (C) Average swim speed did not differ between WT and NL3R451C during training trials (N=20 littermate pairs). (D) NL3R451C mice spent less time swimming near the wall of the water maze (thigmotaxis) compared to WT littermates during training trials (N=20 littermate pairs). (E) WT and NL3R451C mice both show a statistically significant preference for the Target quadrant over other quadrants during a probe trial (N=19 littermate pairs; 1 littermate pair was removed because 1 mouse was considered a “floater”). (F) Using the measure of platform location crossing, NL3R451C mice displayed an increase in crossings of the target platform location during the probe trial when compared to their WT littermates (N=19 littermate pairs). (G) All mice performed equivalently on the visible platform version of the Morris water maze (N=20 littermate pairs). (H) When platform location was reversed and mice were re-trained, WT and NL3R451C mice acquire the reversal learning task equally well (N=15 littermate pairs). (I-J) No difference in spatial preference was observed during the probe trial on either time spent in quadrant measure (I) or number of platform crossings measure (J). *P<0.05, **P<0.01 ***P<0.001, ****P<0.0001
We next performed a reversal platform water maze task in which our previous results of increased spatial reversal learning in the NL3R451C mice (Tabuchi et al., 2007) were not replicated. Using distance travelled to reach the hidden platform, no difference in rate of reversal learning was observed in NL3R451C mice compared to WT littermates (Fig. 2H). Using time spent in quadrant (Fig. 2I) during the probe trail following reverse training, both groups demonstrated a significant preference for the target quadrant. However, when using frequency of platform crossings (Fig. 2J) during the same probe trail only WT demonstrated a significant preference for the target location (Fig. 2J).
NL3R451C Mutants Exhibit Increased Locomotor Activity and Decreased Anxiety-like Behavior
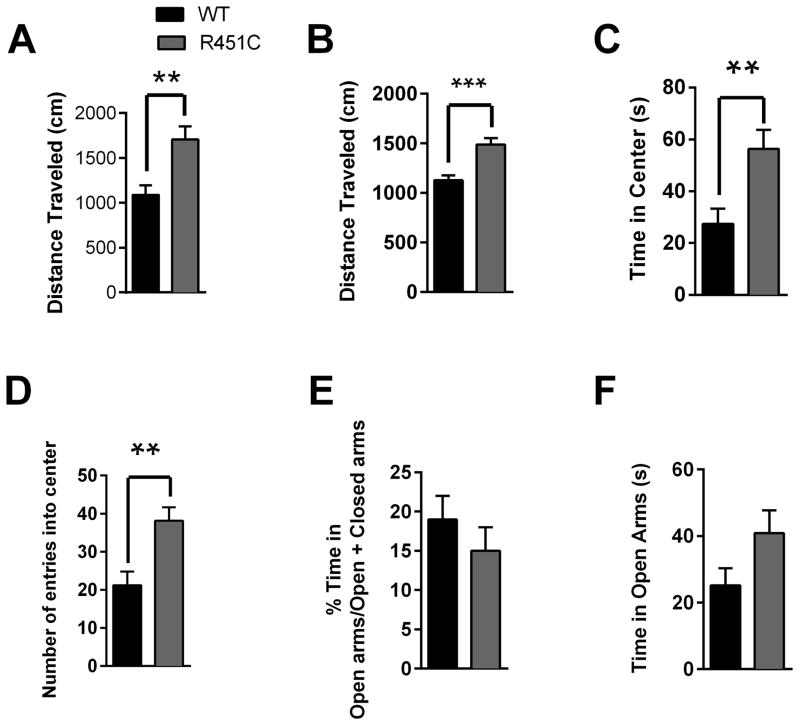
Because NL3R451C mutants exhibited increased locomotor activity during the social interaction task, we examined this phenotype in open field and elevated plus maze tasks as well. NL3R451C mutants on a 129 background exhibited consistently increased distance travelled in the open field (Fig. 3A) and in the elevated plus maze (Fig. 3B). We also observed increased time spent in the center of the open field (Fig. 3C), and a corresponding increased frequency of entries into the center of the open field (Fig. 3D) consistent with a decrease in anxiety-like behavior. In the elevated plus maze, there was no change in anxiety-like behavior as measured in any number of ways. This includes the ratio of time spent in the open arms/time spent in both arms (Fig. 3E, Table 1), absolute time spent in open arms (Fig. 3F, Table 1), ratio of (open arm time)/(closed arm time) (WT 21% + 5%, NL3R451C 19% ± 3%, p=0.39, and ratio of (open arm time – closed arm time)/(open arm time + closed arm time) (WT 70% ± 6%, NL3R451C 61% ± 6%, p=0.29 Additionally, we observed no significant change in time spent in center area of the elevated plus maze (data not shown).
Figure 3. NL3R451C Mutants Exhibit Increased Locomotor Activity.
(A) NL3R451C mice exhibited increased locomotor activity compared to WT mice in the open field test. (B) An increase in locomotor activity in the NL3R451C mice was also observed in the elevated plus maze (EPM). (C) Time spent in the center of an open field is increased in NL3R451C mice compared to WT mice. (D) Number of entries into the center of an open field area is also increased in NL3R451C mice. (E) There was no change in time spent in open arms of the EPM. (F) Time spent in closed arms of the EPM was unchanged between WT and NL3R451C mice. N=20 littermate pairs. **P<0.01 and ****P<0.0001
DISCUSSION
In the present study we replicated a previous finding that mice with an R451C substitution in NL3 exhibit impaired social behaviors and enhanced initial spatial learning. While previous studies (Tabuchi et al., 2007; Etherton et al., 2011b) have reported abnormalities in NL3 R451C mice on a hybrid background, this study is the first to examine NL3 R451C mice on a pure 129S2/SvPasCrl. The 129 background was chosen because different results had already been published in NL3R451C mice on a C57 genetic background. Thus, we felt that if genetic background were important, the 129 background component in our 129/C57 hybrid mice might account for the differences. More importantly the studies described here represent the third time mice with a NL3 R451C mutation display behavioral abnormalities congruent with an autism-like, social phenotype. We recognize that it will also be important to study the NL3R451C mutant mice on the C57 background using our own behavioral protocols to further resolve any remaining discrepancies.
At variance with these three replicated findings is a separately generated NL3R451C mutant model generated and tested on a pure C57BL6 genetic background and characterized in the laboratory of Dr. Jacqueline Crawley at NIMH (Chadman et al., 2008). While the Crawley group found mild behavior differences suggestive of developmental anomalies and reduced ultrasonic vocalizations, they did not observe social deficits or enhanced spatial learning (Chadman K et al., 2008). It is not uncommon for mutant mouse models to have variable phenotypes across strains (Spencer et al., 2011), therefore it is likely that some of these differences are attributable to differences in genetic background among laboratories (i.e. use of a hybrid line generated by the Südhof laboratory, the Crawley laboratory’s use of pure C57BL6 line, and our present use of a pure 129S2/SvPasCrl line). In addition it is also important to consider differences in methodology among the laboratories. The initial behavioral findings of social deficits have now been replicated in three separate experiments using three independently generated cohorts, one at Stanford University in Palo Alto and two at The University of Texas Southwestern Medical Center in Dallas. Each of these replications identified slightly different abnormalities in the social domain on different tasks, though all found at least one significant social abnormality. In both our original description (Tabuchi et al., 2007) and in the present study, we identify differences using the 3-chamber sociability or social preference task but no differences in direct, reciprocal interactions with a juvenile target. The nature of these two tasks is very different. In the 3-box sociability task, the experimental mouse is able to choose interaction/approach or no interaction/approach and can choose between interacting with a social target versus an inanimate target. In the juvenile social interaction task, the experimental mouse may have no choice in interacting with the juvenile target mouse as the target mouse is free to approach the experimental mouse at any time. These inherent differences in the two tasks may account for why the NL3R451C mice show abnormalities in the 3-chamber sociability task, but not the juvenile reciprocal interaction task. Furthermore, the slight variations in social abnormalities across different cohorts may reflect the difficulty in examining spontaneous, baseline, innate behavior that is passively observed and measured rather than intentionally elicited.
Increased initial water maze acquisition rate has now been replicated in two separate genetic backgrounds using similar versions of the Morris water maze. While the initial hybrid genetic background demonstrated both initial and reversal training water maze differences, the present study demonstrated only increased acquisition on initial water maze training. It was also noted that neither group of mice reached as low a latency to reach the hidden platform over 8 days of training as might be typical for mice on a hybrid or pure C57BL6J genetic background, though they clearly demonstrated a steady learning curve. These differences are likely to reflect genetic background differences.
In the present studies and in Tabuchi et al, great care was taken to breed mice to generate a large cohort (N=17-20) of littermate pairs born to WT dams within weeks of one another so that all mice could be tested in a single cohort of littermate pairs with equivalent numbers of WT and mutant animals run in each behavior in an interleaved fashion with each animal experiencing the same set of behavioral tasks in the same sequence. Careful examination of the sequence of behavioral testing reported in (Chadman et al., 2008), indicates that data were combined among different cohorts that had undergone 3 variations of behavioral testing sequences. For example, data used to infer conclusions on spatial learning and memory involved mice from two cohorts, one that had undergone acoustic startle response testing (prepulse inhibition) and response to painful stimuli in the hot plate and tail flick assay prior to the water maze and another cohort whose initial testing only involved social interaction testing prior to water maze. For social interaction tests, one cohort was relatively naïve while another cohort had experienced manipulations/handling as pups and experienced prepulse inhibition, hot plate, and tail flick tests prior to social approach testing. In addition, each cohort of mice included different numbers of WT and mutants rather than strict littermate pairs, further complicating interpretation. While such variances may not have statistically affected WT behavior across cohorts on these tasks, it remains difficult to interpret pooled data across such disparately treated cohorts.
In summary, we have replicated mild social and spatial learning phenotypes in NL3R451C mutant mice for the third and second time respectively using a different, relatively pure, background strain (Tabuchi et al., 2007; Etherton et al., 2011b). Genetic background did subtly modify these phenotypes, consistent with variation among siblings with the same NL3R451C mutation in patients. Differing results demonstrating no phenotype in social or spatial learning domains (Chadman et al., 2008) may be the result of genetic background differences, variances in behavioral experiences across cohorts, variances in number of mice across cohorts, and difference in use of strict littermate pairs among other differences in behavioral tasks across laboratories.
Supplementary Material
Acknowledgments
This work was supported by the NIMH MH081164 (CMP), Autism Speaks (CMP), and The Hartwell Foundation (CMP). TCJ and CMP conceived and designed the experiments; TCJ, SL, & AP carried them out with input from CMP & SB; TCJ performed statistical analysis; TCJ & CMP wrote the paper with input from all authors. We thank Dr. Thomas C. Südhof for the gift of neuroligin 3 mutant mice.
Footnotes
The authors declare no competing interests.
References
- Barnard CJ, Hurst JL, Aldhous P. Of mice and kin: the functional significance of kin bias in social behaviour. Biological reviews of the Cambridge Philosophical Society. 1991;66:379–430. doi: 10.1111/j.1469-185x.1991.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes, brain, and behavior. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M, Foldy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Sudhof TC. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A. 2011a;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011b;30:2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors. Physiology & behavior. 1999;66:355–363. doi: 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism research : official journal of the International Society for Autism Research. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH, Jr, Vicente A, Sommer SS. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.