Abstract
The precise expression and timely delivery of connexin 43 (Cx43) proteins to form gap junctions are essential for electrical coupling of cardiomyocytes. Growing evidence supports a cytoskeletal-based trafficking paradigm for Cx43 delivery directly to adherens junctions at the intercalated disc. A limitation of Cx43 localization assays in cultured cells, in which cell-cell contacts are essential, is the inability to control for cell geometry or reproducibly generate contact points. Here we present a micropatterned cell pairing system well suited for live microscopy to examine how the microtubule and actin cytoskeleton confer specificity to Cx43 trafficking to precisely defined cell-cell junctions. This system can also be adapted for other cell types and used to study dynamic intracellular movements of other proteins important for cell-cell communication‥
Keywords: connexin 43, micropatterning, trafficking, cytoskeleton
Introduction
Gap junctions (GJs) contain connexin (Cx) channels formed by the pairing of two hexameric hemichannels, known as connexons, from adjacent cells [1]. Cx43 is the predominant connexin in ventricular myocytes and is localized to the intercalated disc (ID) at longitudinal ends of the cell, along with adhesion proteins and other ion channels [2–8]. This polarized Cx43 localization is important to facilitate rapid and directional action potential propagation along the long axis of cardiac myofibers, which is essential for effective ventricular contraction with each heartbeat [9–20]. Heart failure is a growing epidemic in the United States [21], and is characterized in part by altered Cx43 localization and arrhythmogenesis [22–25].
Cx43 is constantly made, transported, and degraded leading to a short half-life of only 1 to 5 hours [26–28]. Critical to ventricular myocyte coupling is Cx43 forward transport from the Golgi apparatus to form GJs at the cell-cell junction. We and others have found that Cx43 hemichannels can be directly targeted to the GJ plaque with specificity of delivery directed by the Cx43 hemichannel, the microtubule plus-end tracking proteins EB1 and p150(Glued), and the adherens junction structure including β-catenin [24, 28, 29]. In this paradigm, dynamic microtubules serve as highways that anchor at adherens junction complexes to allow Cx43 cargo delivery to the GJ plaque. More recently, we found that the actin cytoskeleton also regulates Cx43 delivery to the cell-cell junction [30]. What remains is the challenge to integrate the implicated cytoskeletal proteins in an overall model to address how Cx43 gets its “postal address”, or where in the subcellular trafficking pathway from Golgi exit to channel delivery does Cx43 become destined to arrive at the GJ plaque.
Studies of membrane Cx43 localization typically utilize cultured cardiomyocytes that can undergo internal rearrangement once in vitro [31–33], or cultured non-cardiac cells with various geometries and number cellular contacts. This variability in the shape and contacts of cultured cells limits the isolation of a particular behavior or event that may be important to Cx43 transport from the Golgi apparatus to a point of cell-cell contact. Here we present a micropatterning technique that reproducibly defines a rectangular cell geometry and restricts cell-cell contacts to a single edge. The work is based on the pioneering studies of André Kléber, who developed multiple cell patterning techniques that have been used to study Cx43 function and intercellular coupling [34–41].
Using cell lines and isolated cardiomyocytes, our approach is designed to generate longitudinally oriented cell pairs with a defined border similar to those found in ventricular working myocardium. In our system, dynamic Cx43 movement toward the cellular junction, and the underlying cytoskeletal delivery apparatus, can be scrutinized in real-time by confocal microscopy and with single-particle tracking [42] without the complication of varied cell shapes and contacts. Specifically, we find that Cx43 cargo and components of its trafficking machinery, including EB1, microtubules and actin [24, 28, 30], are reproducibly recruited to micro-patterned cell-cell junctions containing N-cadherin. Further exploration of actin and microtubule interactions in micro-patterned cell pairs should uncover how the “postal address” is applied to Cx43 hemichannels for delivery to the GJ plaque.
Methods
Mice
C57BL/6 mice were maintained under sterile barrier conditions. Animal care and procedures are in accordance with national and institutional requirements
Molecular Biology
Plasmids encoding fluorescently tagged human Cx43-EGFP and LifeAct-mCherry were generated using the Gateway cloning system (Life Technologies) as previously described [30]. The Cx43-EGFP destination clone was made using the previously described pDEST-eGFP-N1 vector [43]. All plasmids published by the Shaw Lab are available at the non-profit Addgene repository.
Cell Culture and Cardiomyocyte Isolation
HeLa cells were obtained from ATCC and maintained in fully supplemented DMEM media which contains 10% FBS, nonessential amino acids, sodium pyruvate (Life Technologies), and 1x Mycozap-CL (Lonza). One day prior to seeding, HeLa cells were transfected with Lipofectamine 2000 (Life Technologies) in 10cm dishes with 6µg of LifeAct-mCherry and Cx43-EGFP plasmids for live cell imaging, or Cx43-EGFP for fixed cell studies. On the following day, micro-patterned 35mm glass-bottom dishes (In Vitro Scientific) were incubated with 3 mLs of 1% (w/v) Pluronic F108 (BASF) surfactant for 1 hour at room temperature to prevent cell adhesion to unstamped regions. Treated dishes were gently rinsed with 3 mLs of PBS and incubated in culture media for 1 hour at 37 degrees. Transfected cells were detached with 1mL of 0.02% EDTA in PBS for 5–10 minutes at 37 degrees, and seeded at a density of 1×105 cells/mL in 3mLs of culture media. After allowing cells to adhere for 1 hour, non-attached cells were removed by three 5-minute washes in warm media. Cells were washed every half hour as needed to remove remaining non-attached cells. Cells were collected at 3, 9, or 12 hours post seeding prior to imaging or fixation.
Neonatal mouse ventricular cardiomyocytes were isolated and maintained in DMEM:F12 media supplemented with 5% fetal bovine serum (FBS) and Mycozap-PR (Lonza) as described [30, 44]. Briefly, ventricles were dissected from postnatal day 1 to 4 hearts and digested with 0.2 mg/mL trypsin (Invitrogen) and 50 U/mL type II collagenase (Worthington) in Hank's Balanced Salt Solution (HBSS, Life Technologies) at 37°C with gentle agitation using micro magnetic stir bars (Fisher Science). Dispersed cells were collected and stored in 5% FBS on ice, while fresh pre-warmed isolation buffer was added every 10 minutes until tissues were completely digested. Cells were pre-plated on 10cm BD Primaria dishes for 30 minutes in DMEM:F12 (Life Technologies) with 5% FBS and 1x Micozap-PR (Lonza) to remove fibroblasts, before they are seeded in micro-patterned 35mm glass-bottom dishes (In Vitro Scientific) at a density of 1×105 cells/mL in 3 mLs of media supplemented with Arabinofuranosyl Cytidine (AraC) and 5-bromo-2'-deoxyuridine (BrdU) to inhibit proliferation of non-myocytes. Prior to seeding, micro-patterned dishes were treated with Pluronic as described for HeLa cells above. Dishes were washed 3 times for 5 minutes each with warm media to remove non-attached cells at 5 hours post seeding. At 8, 12 and 24 hours post seeding, cell pairs can be collected for fixation and antibody labeling.
Soft lithography and micro-patterning
Soft lithographic masks were designed in AutoCAD (Autodesk Inc.). Micro-contact with surface areas of 40, 60, 80 and 100×12 µm2 were tested and optimized for HeLa and neonatal ventricular myocytes. A SU8 mold was made by SU8 2002 (Microchem) spin-coating, baked, exposed with a photomask, and developed. Polymer stamps were replicated from the SU8 mold with polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning). The replicated PDMS stamps were then incubated with 50 µg/mL fibronectin (Life Technologies) and 1% gelatin (BD Biosciences) for 3 hours. Glass-bottom dishes (MatTek) were O2-plasma cleaned prior to stamping. Micro-patterned dishes are sealed with Parafilm and stored at 4 degrees for up to three weeks in the dark prior to seeding cell pairs.
Immunofluorescence
Cell pairs were fixed with 4% paraformaldehyde (Electron Microscopy Services) in phosphate-buffered saline (PBS) for 30 minutes, or with ice-cold 100% methanol (Sigma) for 5min. After PBS washes, cells were permeabilized for 5 minutes using 0.1% Triton X-100 in PBS. Cells were then blocked for 1 hour at room temperature in 5% normal goat serum (Life Technologies) before addition of primary antibodies. Mouse monoclonal anti-N-cadherin (BD Biosciences), chick anti-GFP (Abcam), rabbit anti-Cx43 (Sigma), mouse monoclonal anti-EB1 (BD Biosciences), mouse monoclonal anti-αTubulin (Sigma), and Alexa Fluor 555 Phalloidin (Life Technologies) were incubated for 1 hour with 5% normal goat serum in PBS at a dilution of 1:500 each. Following three washes with PBS, cell pairs were incubated for 1 hour with goat Alexa Fluor secondary antibodies (Life Technologies) and TO-PRO-3 nuclear counterstain (Life Technologies). ProLong gold (with DAPI) mounting reagent (Life Technologies) was added prior to confocal imaging.
Live cell imaging
Culture media was replaced with warm HBSS (Life Technologies) containing 10% FBS prior to imaging HeLa cell pairs at 12 hours post seeding. Live-cell spinning-disk confocal microscopy was performed at 37°C using a Yokogowa CSU-X1 spinning disk confocal unit with 486 and 561 nm laser sources, a x100/1.49 Apo TIRF objective, and a Coolsnap HQ2 camera controlled by NIS Elements software as previously described [24, 30]. An exposure of 200ms was used to capture LifeActmCherry and Cx43-EGFP fluorescence for a total of 2 minutes.
Image analysis
Data were converted from Elements .nd2 files to .tif image sequences using ImageJ (NIH). For analysis of Cx43-EGFP cargo, the u-Track 2.0 (Danuser lab, Harvard) Matlab software was used to autonomously determine cargo trajectories [42].
Results
Soft lithography was used to generate micro-contacts for cell pair formation on a glass surface (Figure 1A). A photomask was created using selective exposure of a thin film of a light-sensitive organic polymer (photoresist) to light [45], and was used to generate an inverse master mold from which silicone rubber stamps were made with poly(dimethylsiloxane) (PDMS). Using the stamps, fibronectin and gelatin were picked up and transferred onto glass-bottom dishes, which were appropriate for high-resolution confocal imaging. We tested several dimensions for optimal cell-cell contact formation and found that 80×12 µm2 to 100×12 µm2 micro-contacts separated by a 2µm gap allowed for efficient and reproducible HeLa and neonatal ventricular myocyte cell pairs. We have noted that cell pairs prefer micro-contacts >80µm in length. Shorter length contacts attract single cells to adhere and expand over both rectangles. Figure 1B shows micro-patterned cell contacts containing a mixture of FITC-labeled fibronectin and gelatin. The Pluronic F108 surfactant was applied prior to cell seeding to cell adhesion to non-patterned areas. At 1 hour post seeding and after several washes, HeLa cell pairs begin to flatten and fill in the micro-contacts but not the surrounding glass surface treated with Pluronic (Figure 1B).
Figure 1. Cell pair formation using a micro-patterning approach.
A). Micro-contacts 40 to 100 µm in length and 12 µm in width, separated by a 2 µm gap, were used to optimize photomask design. B) Micro-contacts with the surface area of 100 ×12 µm2 are visualized by stamping FITC-fibronectin and gelatin onto glass-bottomed dishes. HeLa cell pairs adhering to stamped contacts can be visualized 1 hour post seeding. Scale bar: 100µm.
We generated rectangular HeLa and neonatal ventricular cardiomyocyte cell pairs with defined cellular borders using the micro-patterning approach. HeLa cell pairs begin take shape, organize the cytoskeleton, and express actively trafficked Cx43-EGFP cargo in just a few hours after cells are seeded onto micro-contacts. Figure 2A shows formation of a defined cell-cell junction between HeLa cells transfected with Cx43-EGFP at 3 and 9 hours post seeding At the early time point, cells have grown over the 2µm space between the micro-contacts to form a cell-cell junction expressing Cx43-EGFP and N-cadherin. Over time, long strands of F-actin align along the longitudinal axis of the cell pair as Cx43-EGFP plaques accumulate at the cell-cell border.
Figure 2. Micro-patterned HeLa and neonatal ventricular myocyte cell pairs contain a defined cellular border containing N-cadherin and GJ plaques.
A) Cx43-EGFP transfected HeLa cell pairs at 3 and 9 hours post seeding were collected and fixed for antibody detection. F-actin is labeled by Phalloidin staining (red). N-cadherin (blue) is detected at the cell-cell border (arrowheads), which co-localizes with Cx43-EGFP (green) plaques at 9 hours post seeding. B) A fixed neonatal ventricular myocyte pair at 12 hours post seeding is shown. Phalloidin is used to resolve F-actin (red). Endogenous Cx43 (green) and N-cadherin (blue) co-localize at the cell-cell border (arrowheads). C) At 24 hours post seeding, a fixed cardiomyocyte pair contains Cx43 plaques (green) at the cellular border (arrowheads). Sarcomere organization is detected by α-Actinin labeling (red), while Phalloidin is used to resolve F-actin fibers (blue). Scale bar: 5µm.
Micro-patterned neonatal mouse ventricular myocyte pairs can also be generated using this approach. Cell pairs taken at 12 hours post seeding begin to adhere to the micro-contacts and express F-actin fibers aligning along the long axis (Figure 2B). Endogenous Cx43 plaques are co-localized with N-cadherin at the nascent cell-cell border (arrowheads). At 24 hours post seeding, cell pairs fill out the rectangular micro-contacts and express organized sarcomeric α–actinin along cables of longitudinally arranged F-actin labeled with Phalloidin, as well as larger Cx43 plaques aligned at the cellular border (Figure 2C, arrowheads). We have observed that α–actinin becomes increasingly organized as the cells are cultured over longer periods of time.
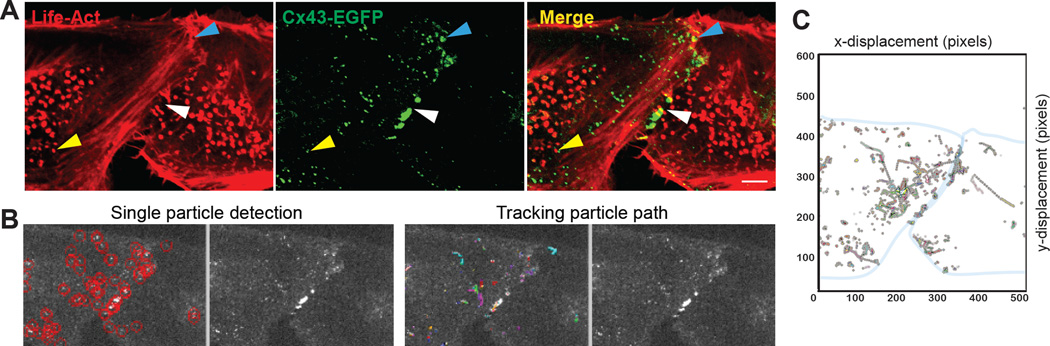
Using this micro-patterning technique, real-time movements of Cx43-EGFP cargo, along with F-actin labeled with LifeAct-mCherry which binds F-actin without affecting its dynamics [46], can be tracked in live HeLa cell pairs using confocal microscopy (Figure 3, Supplemental Video). We find a near stationary population of Cx43-EGFP co-localizing with actin structures in the cell interior (yellow arrowhead), and another comprising large plaques at the cellular border (white arrowhead). A more dynamic cargo population can be seen traveling along F-actin cables aligned toward an area of the cell-cell border that is, over a two minute period, accumulating Cx43-EGFP (blue arrowhead). This rapid directional movement is usually associated with microtubule-based vesicular transport, and suggests potential crosstalk between the microtubule and actin networks in Cx43 trafficking.
Figure 3. Real-time Cx43-EGFP cargo movements can be tracked in HeLa cell pairs.
A) A HeLa cell pair at 12 hours post seeding contains Cx43-EGFP plaques (white arrowhead), near stationary interior cargo collections (yellow arrowhead), and fast-moving cargo along F-actin fibers that accumulate at the cellular border (blue arrowhead). Still images represent a single frame from a 2 minute video (Supplemental Video) of a live cell pair transfected with Cx43-GFP and Life-Act-mCherry. B-C) A single particle-tracking algorithm [42] is used to detect and track cargo trajectories along the×and y axis over the 2 minute interval. Scale bar: 2µm
Using a single particle tracking algorithm [42], we can detect and track Cx43 cargo trajectories in time-lapse sequences. This autonomous algorithm detects cargo movement across each frame by first linking cargos between consecutive frames, before combining the resulting track segments to generate complete Cx43 cargo trajectories (Figure 3B). The trajectories of Cx43-EGFP cargo captured at 2 second intervals for 2 minutes (with 200 ms exposure per frame) is shown in Figure 3C. This approach defines a single cell-cell contact region for GJ formation so that cargo moving toward GJ plaques can be isolated and studied in comparison to those traveling along non-junctional regions.
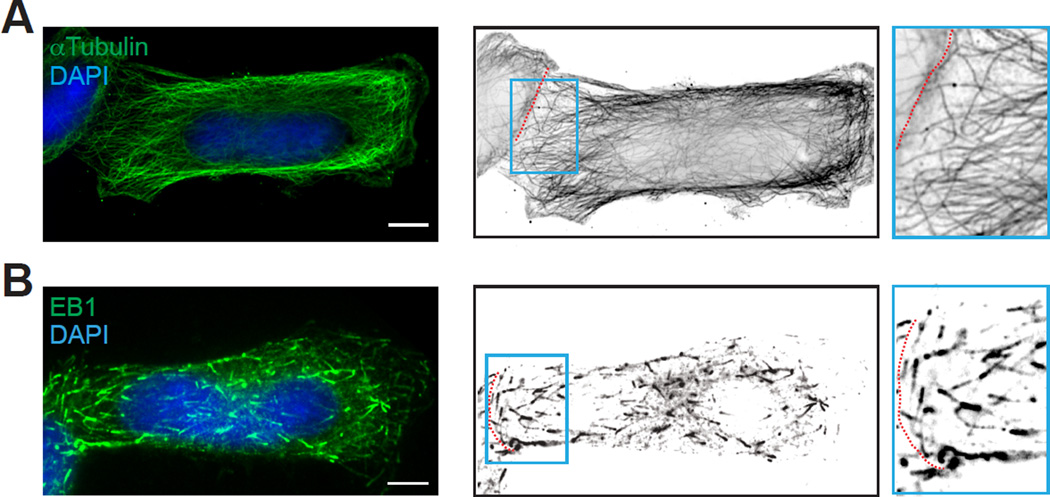
EB1 and microtubules are specific components of the cytoskeleton that have been previously implicated in Cx43 forward trafficking [24, 28]. In our system, this set of trafficking machinery preferentially extends toward the micro-patterned cell-cell junction (Figure 4). Specifically, microtubules labeled with anti-αtubulin, along which Cx43 cargo is transported [28], are highly oriented toward the defined cellular border (Figure 4A). Moreover, comet-shaped trajectories of the plus-end-tracking protein EB1 are also aligned toward the micro-patterned cell contact in antibody labeled fixed cell pairs (Figure 4B). Taken together, this approach can be used to reproducibly generate polarized cell pairs with a defined cellular border, a well-organized cytoskeleton, and preserved trafficking machinery, and is thus well suited for live-cell studies of cytoskeletal-based vesicular trafficking.
Figure 4. EB1 and microtubule components of the Cx43 forward trafficking machinery are oriented toward the cell-cell border.
A) Microtubule tracks, detected by anti-αtubulin antibody labeling, are preferentially aligned toward the defined cell-cell junction in a fixed HeLa cell pairs at 12 hours post seeding. B) The EB1 plus-end-tracking protein is also aligned toward cellular junction in antibody labeled fixed cell pairs. DAPI was used to visualize the cell nucleus. Cell-cell borders are marked by dashed lines. Scale bar: 5µm.
Discussion
Ventricular cardiomyocytes have exquisitely organized cellular architecture including the highly organized sarcomere, the noncontractile cytoskeleton, as well as polarized GJ localization at the intercalated disc. However, current in vitro approaches for examining Cx43 localization to the cell-cell junction are limited in that cells lose polarity, alter their cytoskeletal organization and have variable number of contacts with neighboring cells. Here we present a micro-patterning approach allowing for the reliable assessment and quantification of dynamic Cx43 trafficking that controls for cytoskeletal and membrane organization.
Our system is based on a micro-patterning techniques pioneered by the Kléber lab [34–41]. This approach has been used to explore how cell geometry and organization influence impulse conduction, gap junction distribution, and intercalated disc protein expression to reveal that micro-patterning can be used to recapitulate cardiomyocyte characteristics in vitro. In particular, it was used to quantify how Cx43 deficiency affected cell-cell coupling [35, 36, 41]. A more recent study, stemming from a tissue repair perspective, characterized Cx43 GJ coupling and colocalization with N-cadherin at cell-cell contacts between heterologous cell pairs [47]. Building on these studies, we have developed a precise method to study Cx43 trafficking and how specific components of the cytoskeleton regulate trafficking dynamics.
In addition to its role in maintaining cellular architecture, F-actin is highly dynamic and regulates vesicular transport through motor protein-based trafficking in plants and mouse oocytes [48–52]. In cardiomyocytes, α actin isoforms comprise the thin filaments of the sarcomere [53], while β and γ actin form F-actin not associated with generating contractile force [54]. In our previous study, we determined that F-actin is required for Cx43 forward trafficking to cell-cell junctions, and that this trafficking mechanism is disrupted in the setting of actin-depolymerization and ischemia [30]. However, how specificity of Cx43 delivery to the GJ plaque is conferred by actin is unknown.
In this study, we observed fast-moving Cx43 cargo along F-actin tracks oriented toward the cell-cell junction (Figure 3A, Supplemental Video, blue arrowhead), as well as near stationary cargo associated with actin collections in the cell interior (yellow arrowhead) and GJ plaques (white arrowhead). Cx43-EGFP cargo deposited along actin reservoirs in the cell interior could be recruited for delivery in response to cellular needs. It is also possible that the long F-actin tracks oriented toward the cellular border could help shape the delivery paths of microtubules. Interestingly, it has been identified that F-actin directly affects microtubule transport and dynamics during axonal growth [55]. Future studies using this micropatterning approach are needed to elucidate the precise mechanism of how actin and potentially other cytoskeletal players govern directionality of Cx43 cargo trafficking.
In summary, our approach permits precise control of the cellular microenvironment and preserves internal cellular organization in reproducibly generated cell pairs. Cell-cell contacts and components of the Cx43 trafficking machinery are well defined in this micropatterning system, which serves as an important tool in unlocking specific Cx43 trafficking routes, and in determining how and when newly formed Cx43 hemichannel are targeted to the GJ plaque. This technique can be adapted for other cell types and used to study intracellular movements of additional proteins or channels important for cardiac function.
Supplementary Material
Live confocal imaging of a HeLa cell pair expressing LifeAct-mCherry and Cx43-EGFP at 12 hours post seeding is shown. Images are captured at 2 second intervals for 2 minutes (with 200 ms exposure per frame). Cx43-GFP is associated with near stationary actins structures in the cell interior (yellow arrowhead) and the cellular border region with GJ plaques (white arrowhead). Cargos exhibit fast directional movement along actin tracks oriented toward the cellular border region actively accumulating Cx43-EGFP signal (blue arrowhead).
Highlights.
A micro patterning technique to constrain cell pair geometry and contact points.
HeLa and cardiomyocyte pairs express N-cadherin and Cx43 at cell-cell junctions.
The cytoskeletal delivery machinery for Cx43 orients to the cell-cell border.
Our technique allows analysis of real-time Golgi to cell border Cx43 trafficking.
Acknowledgements
We thank Roger Chang for setting up the single-particle tracking software in Matlab, and Drs. James W. Smyth and Matthew L. Wheeler for critical review of this manuscript. SS Zhang and RM Shaw are supported by the American Heart Association (Postdoctoral and Established Investigator Awards), as well as by the NIH/NHLBI (RO1).
Abbreviations
- Cx43
Connexin 43
- GJ
Gap junction
- ID
Intercalated disc
- PDMS
poly(dimethylsiloxane)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
We have no conflict of interest to disclose.
References
- 1.Unwin PN, Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283:545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- 2.Delmar M. The intercalated disk as a single functional unit. Heart Rhythm. 2004;1:12–13. doi: 10.1016/j.hrthm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Severs NJ. Gap junction shape and orientation at the cardiac intercalated disk. Circ Res. 1989;65:1458–1462. doi: 10.1161/01.res.65.5.1458. [DOI] [PubMed] [Google Scholar]
- 4.Palatinus JA, Gourdie RG. Xin and the art of intercalated disk maintenance. American journal of physiology Heart and circulatory physiology. 2007;293:H2626–H2628. doi: 10.1152/ajpheart.00954.2007. [DOI] [PubMed] [Google Scholar]
- 5.Forbes MS, Sperelakis N. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell. 1985;17:605–648. doi: 10.1016/0040-8166(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 6.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2012;1818:1831–1843. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth JW, Shaw RM. The gap junction life cycle. Heart Rhythm. 2012;9:151–153. doi: 10.1016/j.hrthm.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SS, Shaw RM. Multilayered regulation of cardiac ion channels. Biochim Biophys Acta. 2013;1833:876–885. doi: 10.1016/j.bbamcr.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 10.Huang GY, Wessels A, Smith BR, Linask KK, Ewart JL, Lo CW. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev Biol. 1998;198:32–44. doi: 10.1006/dbio.1998.8891. [DOI] [PubMed] [Google Scholar]
- 11.Ya J, Erdtsieck-Ernste EB, de Boer PA, van Kempen MJ, Jongsma H, Gros D, Moorman AF, Lamers WH. Heart defects in connexin43- deficient mice. Circ Res. 1998;82:360–366. doi: 10.1161/01.res.82.3.360. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas SA, Schuessler RB, Berul CI, Beardslee MA, Beyer EC, Mendelsohn ME, Saffitz JE. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation. 1998;97:686–691. doi: 10.1161/01.cir.97.7.686. [DOI] [PubMed] [Google Scholar]
- 14.Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res. 2001;51:681–690. doi: 10.1016/s0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 15.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 16.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–110. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 23.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Norstrand DW, Asimaki A, Rubinos C, Dolmatova E, Srinivas M, Tester DJ, Saffitz JE, Duffy HS, Ackerman MJ. Connexin43 mutation causes heterogeneous gap junction loss and sudden infant death. Circulation. 2012;125:474–481. doi: 10.1161/CIRCULATIONAHA.111.057224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 27.Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110:978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisler SB, Green KJ, Isom LL, Meshinchi S, Martens JR, Delmar M, Russell MW. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes. J Biomed Biotechnol. 2010;2010:624719. doi: 10.1155/2010/624719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostin S, Hein S, Bauer EP, Schaper J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ Res. 1999;85:154–167. doi: 10.1161/01.res.85.2.154. [DOI] [PubMed] [Google Scholar]
- 33.Kostin S, Schaper J. Tissue-specific patterns of Gap junctions in adult rat atrial and ventricular cardiomyocytes in vivo and in vitro. Circ Res. 2001;88:933–939. doi: 10.1161/hh0901.089986. [DOI] [PubMed] [Google Scholar]
- 34.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 35.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res. 2004;95:170–178. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 36.Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, Kleber AG. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–1224. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 37.McCain ML, Desplantez T, Geisse NA, Rothen-Rutishauser B, Oberer H, Parker KK, Kleber AG. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: relation between Cx43 immunofluorescence and intercellular electrical conductance. American journal of physiology Heart and circulatory physiology. 2012;302:H443–H450. doi: 10.1152/ajpheart.01218.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCain ML, Lee H, Aratyn-Schaus Y, Kleber AG, Parker KK. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proc Natl Acad Sci U S A. 2012;109:9881–9886. doi: 10.1073/pnas.1203007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohr S, Scholly DM, Kleber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991;68:114–130. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- 40.Thomas SP, Bircher-Lehmann L, Thomas SA, Zhuang J, Saffitz JE, Kleber AG. Synthetic strands of neonatal mouse cardiac myocytes: structural and electrophysiological properties. Circ Res. 2000;87:467–473. doi: 10.1161/01.res.87.6.467. [DOI] [PubMed] [Google Scholar]
- 41.Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kleber AG. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ Res. 2003;92:1209–1216. doi: 10.1161/01.RES.0000074916.41221.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, Danuser G. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang SS, Kim KH, Rosen A, Smyth JW, Sakuma R, Delgado-Olguin P, Davis M, Chi NC, Puviindran V, Gaborit N, Sukonnik T, Wylie JN, Brand-Arzamendi K, Farman GP, Kim J, Rose RA, Marsden PA, Zhu Y, Zhou YQ, Miquerol L, Henkelman RM, Stainier DY, Shaw RM, Hui CC, Bruneau BG, Backx PH. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His-Purkinje network. Proc Natl Acad Sci U S A. 2011;108:13576–13581. doi: 10.1073/pnas.1106911108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi Y, Hong S, Lee LP. Shadow overlap ion-beam lithography for nanoarchitectures. Nano Lett. 2009;9:3726–3731. doi: 10.1021/nl901911p. [DOI] [PubMed] [Google Scholar]
- 46.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. American journal of physiology Heart and circulatory physiology. 2008;295:H390–H400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers SL, Gelfand VI. Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol. 2000;12:57–62. doi: 10.1016/s0955-0674(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 50.Schuh M. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol. 2011;13:1431–1436. doi: 10.1038/ncb2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akkerman M, Overdijk EJ, Schel JH, Emons AM, Ketelaar T. Golgi body motility in the plant cell cortex correlates with actin cytoskeleton organization. Plant Cell Physiol. 2011;52:1844–1855. doi: 10.1093/pcp/pcr122. [DOI] [PubMed] [Google Scholar]
- 52.Egea G, Serra-Peinado C, Salcedo-Sicilia L, Gutierrez-Martinez E. Actin acting at the Golgi. Histochem Cell Biol. 2013;140:347–360. doi: 10.1007/s00418-013-1115-8. [DOI] [PubMed] [Google Scholar]
- 53.Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res. 2008;77:667–675. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- 54.Hayakawa K, Ono S, Nagaoka R, Saitoh O, Obinata T. Differential assembly of cytoskeletal and sarcomeric actins in developing skeletal muscle cells in vitro. Zoolog Sci. 1996;13:509–517. doi: 10.2108/zsj.13.509. [DOI] [PubMed] [Google Scholar]
- 55.Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24:11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Live confocal imaging of a HeLa cell pair expressing LifeAct-mCherry and Cx43-EGFP at 12 hours post seeding is shown. Images are captured at 2 second intervals for 2 minutes (with 200 ms exposure per frame). Cx43-GFP is associated with near stationary actins structures in the cell interior (yellow arrowhead) and the cellular border region with GJ plaques (white arrowhead). Cargos exhibit fast directional movement along actin tracks oriented toward the cellular border region actively accumulating Cx43-EGFP signal (blue arrowhead).