Abstract
Significant evidence suggests that exposure to traumatic and/or acute stress in both mice and humans results in compromised immune function that in turn may affect associated brain processes. Additionally, recent studies in mouse models of immune deficiency have suggested that adaptive immunity may play a role during traumatic stress exposure and that impairments in lymphocyte function may contribute to increased susceptibility to various psychogenic stressors. However, rodent studies on the relationship between maladaptive stress responses and lymphocyte deficiency have been complicated by the fact that genetic manipulations in these models may also result in changes in CNS function due to the expression of targeted genes in tissues other than lymphocytes, including the brain. To address these issues we utilized mice with a deletion of recombination-activating gene 2 (Rag2), which has no confirmed expression in the CNS; thus, its loss should result in the absence of mature lymphocytes without altering CNS function directly. Stress responsiveness of immune deficient Rag2−/− mice on a BALB/c background was evaluated in three different paradigms: predator odor exposure (POE), fear conditioning (FC) and learned helplessness (LH). These models are often used to study different aspects of stress responsiveness after the exposure to an acute stressor. In addition, immunoblot analysis was used to assess hippocampal BDNF expression under both stressed and non-stressed conditions. Subsequent to POE, Rag2−/− mice exhibited a reduced acoustic startle response compared to BALB/c mice; no significant differences in behavior were observed in either FC or LH. Furthermore, analysis of hippocampal BDNF indicated that Rag2−/− mice have elevated levels of the mature form of BDNF compared to BALB/c mice. Results from our studies suggest that the absence of mature lymphocytes is associated with increased resilience to stress exposure in the POE and does not affect behavioral responses in the FC and LH paradigms. These findings indicate that lymphocytes play a specific role in stress responsiveness dependent upon the type, nature and intensity of the stressor.
Keywords: Stress, Anxiety, Fear, BDNF, Western Blots, lymphocytes, Open Field Test, Acoustic Startle
1. Introduction
Pathological responses to stress, as a result of a traumatic event, are known to be related to a combination of genetic and environmental factors that determine susceptibility or resilience to develop exacerbated fear responses (Gillespie et al., 2009; Skelton et al., 2012). Recent research suggests that impairments in immune function may be a central mechanism determining susceptibility or resilience to the development of these responses (Baker et al., 2012; Neylan et al., 2011) with a higher incidence of maladaptive responses among those with pre-existing inflammatory conditions (LeardMann et al., 2009; O’Toole and Catts, 2008). Additionally, a number of studies have shown a myriad of immune abnormalities, along with specific epigenetic modifications in genes associated with immune responses, in people suffering from conditions such as posttraumatic stress disorder (Glatt et al., 2013; Rusiecki et al., 2013; Smith et al., 2011; Uddin et al., 2010; Zovkic et al., 2013). This link between stress exposure and the immune system appears to be bi-directional, in which traumatic stress exposure is associated with a higher risk for developing a significant number of chronic inflammatory conditions (Lemieux et al., 2008; O’Toole and Catts, 2008; Plantinga et al., 2013). Despite mounting evidence implicating the immune system in pathological stress responses, specific mechanisms linking traumatic stress and immune function remain poorly understood.
Research using various animal models of immune deficiency suggests a role for the adaptive arm of the immune system in determining resilience to stress via mechanisms mediated through the actions of T cells (Cohen et al., 2006; Lewitus et al., 2008; Lewitus and Schwartz, 2009). These studies have found that the absence of adaptive immunity is associated with increased fear and anxiety responses after stress exposure (Cohen et al., 2006) and have culminated in the proposal that mature T cells help maintain homeostasis and confer protection against stress exposure by a mechanism involving down-regulation of pro-inflammatory cytokines and the production of brain-derived neurotrophic factor (BDNF) in the hippocampus (Lewitus et al., 2008; Lewitus and Schwartz, 2009; Schwartz and Ziv, 2008). Nevertheless, certain behavioral traits seen in the immune deficiency models employed in these studies may be due to the expression of targeted genes in the CNS rather than in peripheral immune cells alone (Fang et al., 2013; Rattazzi et al., 2013). Of particular concern is recombination-activating gene 1 (RAG1), which is highly expressed in the hippocampus and cerebellum, as well as lymphocytes (Chun et al., 1991; Sun et al., 2007) and whose deletion induces behavioral deficits independent of lymphocyte function (Fang et al., 2013; McGowan et al., 2011).
The purpose of the present study was to further clarify the role of the adaptive immune system in stress responsiveness by employing the Rag2−/− mouse model of immune deficiency. Similar to Rag1−/− mice, the loss of RAG2 in these mice inactivates the variable (diverse) recombination (V[D]J) process of the immunoglobulin and T cell and B cell receptors. However, in contrast to RAG1, RAG2 has no confirmed expression in the CNS (Chun et al., 1991; Shinkai et al., 1992) (Supplemental figures 1 and 2) and thus, the impact of its loss should be restricted to peripheral lymphocytes. As a result, Rag2−/− mice lack mature T and B lymphocytes while maintaining normal hematopoiesis (Shinkai et al., 1992). To examine the role of lymphocytes in several models of stress responsiveness after acute stress exposure, mice were tested in the following fear and stressor paradigms: 1) predator odor exposure (POE), 2) Pavlovian fear conditioning (FC), and 3) learned helplessness (LH). Additionally, the expression of BDNF was examined in order to explore the potential relationship between lymphocytes, stress exposure and the regulation of this neurotrophic factor. Our results indicate that immune deficient and immune competent mice display similar behavior in the FC and LH paradigms while the absence of lymphocyte function contributes to resilience in the POE paradigm. Moreover, hippocampal BDNF levels for the mature form of the protein were higher in Rag2−/− mice under basal conditions and following LH, but not POE. The present studies suggest that the impact of lymphocyte function on stress responsiveness is dependent on the nature of the stressor and type of response involved.
2. Methods
2.1 Animals
Rag2−/− mice were originally developed by the Alt laboratory by targeting the RAG2 gene in CCE embryonic stem cells and transferring targeted cells into blastocysts (Shinkai et al., 1992). For this study, Rag2−/− mice were acquired from Taconic Farms, Inc. (Hudson, NY) where they have been backcrossed onto the BALB/c background for twelve generations and maintained by homozygous pairings. Male Rag2−/− and wild type (WT) BALB/c mice were obtained at 5–6 weeks of age and housed under normal conditions (12 hr light/dark cycle, 4–5 mice per cage) with ad libitum access to food and water. All experiments were conducted when mice were between 8–12 weeks old. Prior to beginning any experiments all animals were handled daily for several days to habituate the animals to the experimenter and to monitor overall health. Any cages exhibiting severe signs of fighting between cage mates either before or after stress exposure were excluded. Verification of immune status was conducted by flow cytometry for all mice at the completion of each experiment (Supplemental Figure 3). All procedures were carried out under approved IACUC protocols and institutional guidelines at the University of Maryland, School of Medicine and Baltimore VA Health Care System.
2.2 Basal Behavioral Assessments
To determine if basal behavioral responses were comparable between WT and Rag2−/− mice, an independent group of animals was first tested in the open field test (day 1) followed by the elevated plus maze test (day 2). In addition, to ensure that the olfactory system was not compromised by the absence of lymphocytes a group of mice was evaluated in a buried food test. All tests were conducted between 10 am and 3 pm under 5 lux illumination and constant background white noise (~65–70dB).
Open field test (OFT)
Individual mice (WT: n = 12; Rag2−/−: n = 14, 8 weeks old) were placed in square arenas (50 × 50 cm) and allowed to explore for 30 min while being recorded overhead. Total distance traveled and time in center (interior 50% of the arena) were analyzed with the use of TopScan (Cleversys; Reston, VA).
Elevated plus maze test (EPM)
The EPM is an apparatus raised 50cm above the ground with two enclosed arms (35 × 5 × 15 cm) perpendicular to two open arms (39.5 × 5 cm) intersected by an open central area (5 × 5 cm). Individual mice were placed in the center facing one of the two open arms and recorded with an overhead camera for 10 min as they freely explored the maze. An observer was present for the entire session and any mouse that fell off of the maze was returned to the same arm, in the same position. TopScan was used to determine total distance traveled, the number of entries into each arm and the proportion of time spent in the open arms.
Olfaction Test
To evaluate whether there are differences in olfaction between WT and Rag2−/− mice that may influence the effect of predator odor exposure, a buried food test (Yang and Crawley, 2009) was conducted to compare the latency of WT (n = 8) and Rag2−/− mice (n = 9) to sniff out and begin consuming a treat that they had been familiarized with in their home cage. For the test a Honey Teddy Graham (Nabisco) was buried in the bedding at one end of a clean cage and a single mouse was placed at the opposite end of the cage. The animal was then allowed to freely explore the cage for 15 min; the time to find and retrieve the buried food was recorded by an observer. To avoid any scent traces, mice were placed in a new cage separate from any remaining cage mates upon completion of the test and clean cages with fresh bedding were used for each round of testing.
2.3 Experiment 1: Predator odor exposure (POE) and behavioral assessments
This paradigm, based on an innate fear response to feline urine odor present in rodent species, has been validated in rats and mice (Blanchard et al., 2003a; Blanchard et al., 1990) and used in immune deficient mice by Cohen et al (2006). These studies have shown that a short exposure to cat odor is sufficient to elicit long term behavioral changes, including increased anxiety as measured by the EPM and heightened startle responsiveness in the acoustic startle test.
Predator Odor Exposure (POE)
Age-matched WT and Rag2−/− mice (8–12 weeks) were divided into control non-exposed (WT: n = 13; Rag2−/−: n = 14) and exposed (WT: n = 18, Rag2−/−: n = 20) groups. All exposure sessions were conducted in a secluded room separate from the colony and behavioral testing rooms. Following transport to the exposure room animals were allowed to acclimate for approximately 30 minutes. To avoid any contact with aversive odors, all control mice were ran through the paradigm and removed from the testing room prior to exposing experimental mice to predator odor. In brief, mice were individually placed in a clear acrylic, covered chamber (40 × 40 × 40 cm) with ~1 inch of cat litter for 10 minutes; control mice were exposed to fresh cat litter (Tidy Cat, Non-clumping “Breathe Easy” cat litter with anti-microbial additives), while POE mice were exposed to sifted cat litter that had been used for two weeks by both a male and female cat. Upon removal from the chamber, mice were placed in a clean cage separate from remaining cage mates to avoid transference of stress to non-exposed mice; once all cage mates had been processed they were returned to their home cage. Following completion of exposure all mice were returned to the colony room where they remained undisturbed for one week at which point the mice were tested in the EPM as previously described. Twenty-four hours after the EPM test they were evaluated for acoustic startle responses.
Acoustic Startle Test
All mice were individually tested in startle chambers equipped with an animal enclosure mounted on a piezoelectric accelerometer (SR-LAB, San Diego Instruments, San Diego, CA). Following previously published protocols testing acoustic startle response following POE (Cohen et al, 2006; Lewitus et al, 2008) mice were allowed to acclimate to the startle chambers for 5 min (68 dB background noise) and then underwent 30 acoustic startle trials with an average inter-trial interval of 30s. Each trial consisted of 60 ms of background noise followed by a 40 ms 110dB tone. The response window, during which maximum and average startle response was determined, lasted for 65ms after stimulus onset. Data are presented as the initial startle response (average of first 2 trials) and the average startle response across trial bins (5 trials/bin).
Re-exposure to POE context
Three weeks after the acoustic startle test, a subset of mice (control non-exposed WT: n = 7; Rag2−/−: n = 8 and exposed WT: n = 8, Rag2−/−: n = 8) were tested for maintenance of fear memory by returning them to the exposure chambers and assessing exploratory behavior. For this test, all mice were individually placed in control chambers with clean cat litter and video recorded for 10 minutes. Exploratory behavior was manually scored as the number of rearings, with both front paws raised, in the open area of the chamber. Topscan was utilized to determine total distance traveled. Two hours after re-exposure to the context mice were euthanized; brains extracted, snap frozen and stored at −80° until used for BDNF analysis.
2.4 Experiment 2: Pavlovian Fear Conditioning (FC)
The FC paradigm has been instrumental in exploring both the neurochemical underpinnings, as well as potential treatments, for the inability to extinguish a learned fear. In this protocol, which provides a moderate to severe stressor (Gafford and Ressler, 2011), mice are exposed to a non-aversive conditioned stimulus (CS) paired with an aversive unconditioned stimulus (US); repeated exposures (2–5) result in the development of a cued response (CR), such as freezing. Mice are then presented with the CS only in a new context and tested for extinction of the CR, a measure of the animal’s ability to learn to dissociate the CS and the US in a neutral environment. Spontaneous recovery, or renewal, of the CR can then be assessed in a third context after several days to weeks to determine if extinction is context specific.
Day 1 - Conditioning
Acquisition of fear conditioning (WT: n = 12; Rag2−/−: n = 9, age 11–12 weeks) took place in Context 1: one side of a shuttle box with a grid shock floor within a sound attenuating chamber (Coulbourn Instruments; Whitehall, PA). The front and back walls of the chamber were clear acrylic, while the inner and side walls were metallic. A speaker was mounted at the top of the side wall and lighting was provided by a house light (~12 lux) mounted in the ceiling of the chamber. Mice were individually placed in the cage and acclimated for three minutes. The conditioning session consisted of five CS-US paired trials separated by a 5 min inter-trial interval. Each 30 s trial consisted of an auditory CS (80 dB, 1000 Hz) that coterminated with a 1 s US foot shock (500 μA). To remove any scent traces between sessions each chamber was cleaned with Vimoba (Quip Laboratories, Inc.; Wilmington, DE).
Days 2 and 3 - Extinction
Twenty-four hours after conditioning, mice were individually placed in a test cage within an isolation chamber (Coulbourn Instruments). This new context consisted of four clear acrylic walls, a light mounted on the side of the chamber (~9 lux), a wire mesh floor, and the addition of a drop of diluted almond extract (1:10 in water) in the drop pan under the floor. Cages were cleaned with 70% ethanol between sessions to avoid olfaction cues imparted by the cleanser during the acquisition stage. To evaluate extinction, mice were placed in the test cage and underwent a 3 minute habituation period followed by 15 trials of CS presentation only (80dB tone, at 1000Hz, for 30s) with a 90s inter-trial interval. All sessions were recorded with a camera mounted in the ceiling of each test cage and automatically scored with FreezeFrame (Actimetrics; Wilmette, IL).
Day 26, Spontaneous recovery
Contextual cues were changed in the test cage to create a third context. Side walls were covered with black and white vertical striped paper; the floor was replaced by a solid wood, black panel and the dimensions of the cage changed by the insertion of a 1in. thick white wooden panel at the back of the cage. No olfaction cue was added and the cages were cleaned with MB-10 (Quip Laboratories) between sessions. The test session was then conducted as previously described.
2.4 Experiment 3: Learned helplessness (LH)
The LH paradigm utilizes an exposure to inescapable stress to induce the development of behavioral interference when provided an option to escape (Maier and Watkins, 2005). The protocol employed was based on comparative studies on different strains of mice, including BALB/c mice (Shanks and Anisman, 1988). For this experiment WT and Rag2−/− mice, age 11–13 weeks (n = 10–12/group), were subjected to one session of inescapable stress and tested 24 hours and 7 days later to assess behavioral interference. An additional group of non-stressed controls (5/genoptype) was included to confirm the inducement of behavioral interference. The inescapable stress session (day 1) took place in a shuttle cage with grid shock floor (Coulbourn Instruments) and consisted of 360, 2s foot shocks at 150μA (average inter-trial interval of 8s); the door separating the two sides of the shuttle box remained closed to prevent any escape. The test sessions (days 2 and 8) consisted of returning the mice to the shuttle box, subjecting them to a series of foot shocks (150 μA) while providing an option to escape upon opening of the shuttle door; the foot shock was terminated upon escape through the door or after 24s. The test session consisted of a total of 30 trials with an average 15s inter-trial interval. During the first five test trials (p1–5), the foot shock and the opening of the shuttle door commenced simultaneously, allowing the animal to immediately move to the other side of the box and terminate the shock. Trials 6–30 included a 2s delay between the initiation of the foot shock and the opening of the door. The animal’s coping response was analyzed as a measure of escape latency (the average time it took to terminate a shock) and number of escape failures over the course of the last 25 trials. Each shuttle box was cleaned with 70% ethanol between individual runs. Twenty-four hours after the retest mice were euthanized and brains extracted for BDNF analysis.
2.5 Hippocampal BDNF expression
The expression of BDNF was studied in dissected hippocampi from non-stressed mice as well as a subset of mice that underwent the POE and LH paradigms. Relative amounts of hippocampal pro-BDNF and mature BDNF were determined by immunoblot analysis. Protein extraction was performed on a single hippocampus (right or left hemisphere at random) with RIPA buffer (Life Technologies Corp.) supplemented with protease and phosphatase inhibitors (1:100, Sigma-Aldrich). Tissue was manually dissociated, sonicated and centrifuged at 4°C for 30 min at 14000rpm. The supernatant was removed and stored at −80°C until ready for use. Protein concentration was determined using the microplate procedure for the Pierce BCA Protein Assay Kit (Thermo Scientific). Immunoblot analysis was conducted using the NuPAGE SDS-PAGE gel system (Life Technologies Corp.) with 10% Bis-Tris gels. Briefly, 10μg of protein was loaded per lane and electrophoresed for 45 min at 170V. Proteins were then transferred onto PVDF membranes for 1 hour at 40V. Membranes were washed in Tris-buffered saline with Tween-20 (TBST) and blocked with 5% non-fat dry milk (NFM) in TBST for 1 hr. The membranes were incubated overnight at 4°C in a 5% NFM/TBST containing anti-BDNF (1:5000; Santa Cruz Biotechnology, #sc-546) or anti-GAPDH (1:50000; Cell Signaling, #5174), then washed and incubated in HRP-conjugated secondary antibody for 1 hour at room temp. Bound antibody was detected via chemiluminescence (SuperSignal West Pico, Thermo Scientific) and exposure to x-ray film. Quantification of signals was accomplished using ImageJ software (NIH; Bethesda, MD). All values were normalized to a loading control and standardized to a blot control across gels as necessary and are presented as the optical density relative to the average WT control values for comparison. Specificity and accuracy of the anti-BDNF antibody was evaluated by pre-absorption with a blocking peptide (Santa Cruz, sc-546P) and comparison with both a human SH-SY5Y cell lysate (50μg) and mouse hippocampal extract (10μg) as positive controls. The antibody recognizes both pro-BDNF (28–32 kDa) and mature BDNF (14 kDa) in the mouse hippocampus.
2.6 Statistical Analysis
All statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). Students t-tests were utilized for group comparisons to analyze basal behavior in the EPM, as well as paired comparisons for BDNF expression. Two-way ANOVAs comparing immune status and treatment were used to analyze the effect of POE and average escape latencies in the LH paradigm. Repeated measure 2-way ANOVAs were used to analyze trial bin data from the OFT, POE, FC and LH paradigms. As appropriate, these tests were followed up with Fisher’s LSD post hoc analysis; p ≤ 0.05 was considered significant. Any outliers (2 SD from group mean) were excluded from analysis.
3. Results
3.1 Basal behavioral profile
Analysis of behavior in the OFT revealed significant main effects of immune status (F (1, 24) = 4.425, p = 0.046) and time (F (2, 48) = 46.25, p < 0.0001) with no interaction, indicating that Rag2−/− mice display greater locomotion with comparable habituation to the arena as compared to WT mice (Figure 1A). While no significant effect of either immune status or time was detected for time spent in the center of the arena, there was a significant interaction (F (2, 48) = 5.018, p = 0.011). Fisher’s LSD post-hoc analysis indicated that significant differences were restricted to the first 10 minutes of the test, with Rag2−/− mice spending more time in the center of the arena than WT mice (Figure 1B). In contrast, analysis of behavior in the EPM showed no significant differences for either the time spent in the open arms or total distance traveled in the maze (Figure 1C). Furthermore, distance traveled in the open arms and the ratio of open arm entries to total arm entries were also comparable (data not shown). These findings suggest that behavioral differences between WT and Rag2−/− mice may be test specific, with Rag2−/− mice exhibiting reduced basal levels of anxiety and increased locomotor activity in the OFT that is not evident in the EPM test. Finally, there is no difference in olfaction between WT and Rag2−/− mice as indicated by the lack of significant differences in the latency to retrieve buried food during the olfaction test (Figure 1D).
Figure 1. Basal behavioral assessments.
A) Rag2−/− mice exhibit significantly greater locomotor activity than WT mice with comparable habituation to the arena in the OFT (2-way repeated measures ANOVA: immune status: p = 0.046; time: p < 0.0001; interaction: ns). B) Rag2−/− mice spend significantly more time in the center of the arena during the first ten minutes of the OFT session (immune status × time: p = 0.011; Fisher’s LSD post hoc). C) No significant differences were found in either the time spent in the open arms or total distance traveled in the EPM. D) There are no evident differences in olfaction between WT and Rag2−/− mice as determined by the latency to retrieve buried food. * p < 0.05, ns: non-significant
3.2 Experiment 1: Predator odor exposure (POE)
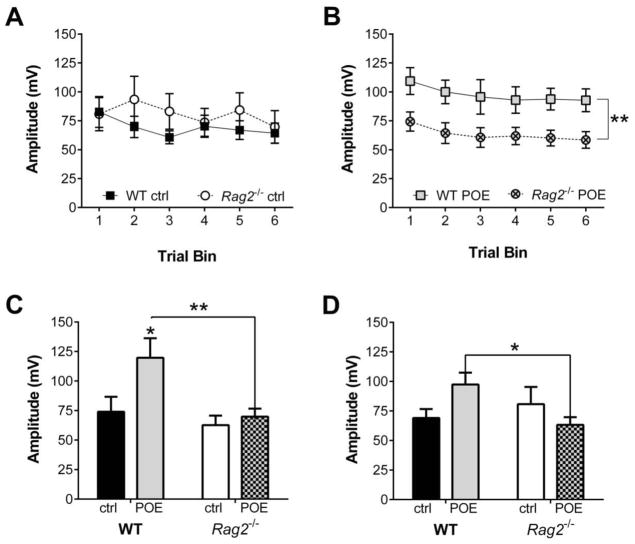
Assessment of the effect of POE on anxiety-like behavior one week after exposure revealed no significant effects in any measure of the EPM (data not shown). However, when evaluated for stress reactivity in the acoustic startle test (8 days post POE) WT mice exhibited an enhanced startle response compared to Rag2−/− mice. Non-exposed WT and Rag2−/− mice display similar startle behavior, with no significant differences evident for average startle responses or habituation to the tone (Figure 2A, 2C and 2D). In contrast, POE mice exhibited a significant effect of immune status (F (1, 33) = 8.26, p = 0.007) and time (F (5, 165) = 2.60, p = 0.027) with no significant interaction over the course of the session (Figure 2B). Additionally, analysis of the initial startle response (Figure 2C) indicated significant main effects for both immune status (F (1, 56) = 6.402, p = 0.014) and POE (F (1, 56) = 4.804, p = 0.033), with no significant interaction. Finally, there was a significant interaction between immune status and POE (F (1, 56) = 1.253, p = 0.025) when analyzing the mean startle amplitude for the entire session (Figure 2D). Fisher’s LSD post hoc analysis revealed that the effect of POE is dependent upon immune status, with WT POE mice displaying a significant increase in startle amplitude compared to Rag2−/− POE mice (p = 0.011). These results indicate that while WT mice display greater reactivity than Rag2−/− mice following POE, habituation to the acoustic startle test is comparable between the two groups. Finally, a subgroup of mice was returned to the exposure context three weeks after the initial exposure to assess fear memory. A significant decrease in exploratory behavior, reflected by reduced rearing in the open area of the chamber, was seen in the POE mice compared to non-exposed controls (F (1, 27) = 10.56, p = 0.0031; Figure 3A). Concomitant with these findings, POE mice also exhibited greater locomotion than controls (F (1, 27) = 18.10, p = 0.0002; Figure 3B); there were no significant effect of immune status or an interaction between immune status and treatment.
Figure 2. Startle responsiveness one week post POE.
A) Startle behavior in control WT and Rag2−/− mice is comparable. B) WT POE mice exhibit a significant increase in startle amplitude compared to Rag2−/− POE with similar habituation over the course of the entire session (2-way repeated measures ANOVA: immune status: p = 0.007; time: p = 0.027; interaction: ns). C) Analysis of the initial startle response revealed significant main effects for immune status (p = 0.014) and POE (p = 0.033); WT POE mice displayed a significantly enhanced response compared to both WT control mice and Rag2−/− POE mice (Fisher’s LSD post hoc). D) Evaluating the mean startle amplitude for all trials reveals a significant interaction (immune status × POE: p = 0.025; Fisher’s LSD post hoc) indicating that the effect of POE is dependent upon immune status. * p < 0.05, ** p < 0.01, ns: non-significant
Figure 3. Re-exposure to POE context.
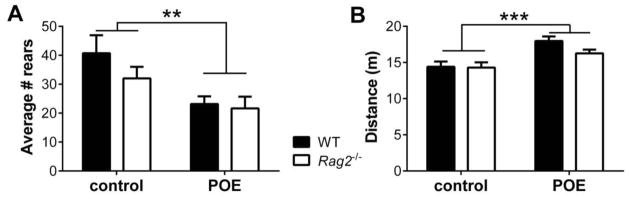
A) POE induces long-term changes in behavior in both WT POE and Rag2−/− POE mice as shown by a significant reduction in rearing in the open area of the exposure chamber three weeks after exposure (2-way ANOVA; POE: p = 0.0031, immune status: ns, interaction: ns). B) POE mice also exhibited greater locomotor activity compared to control mice (p = 0.0002) with no effect of immune status. ** p < 0.01, *** p < 0.001, ns: non-significant
3.3 Experiment 2: Pavlovian Fear Conditioning (FC)
Following FC in Context 1, all mice were tested for the presence and extinction of freezing behavior in response to the CS over the course of three sessions (Figure 4). For the first two sessions mice were placed in a new context where they exhibited low levels of freezing during the habituation period (H) indicative of a lack of contextual fear (Figure 4A and 4B). Quantification of freezing during the tone presentation indicated that WT and Rag2−/− mice display similar stress responses to FC, which decreased from the first session to the second suggesting extinction of the CR (Figure 4A and 4B). Manifestation of a fear response when presented with a similar stimulus, particularly within a novel, non-aversive context, is a maladaptive fear response; thus, we retested mice for spontaneous recovery of the CR three weeks after the initial extinction test to determine if either group were more susceptible to developing this trait. Overall, WT and Rag2−/− mice failed to display renewal of the CR in a new context as revealed by the lack of significant difference in freezing (Figure 4C).
Figure 4. Fear Conditioning.

A) WT and Rag2−/− mice exhibit a similar degree of freezing in a new context 1 day after fear conditioning. A lack of freezing during the habituation period “H” indicates that the CR is driven by the cue and not the context. B) A test for extinction in the same context 1 day later showed decreased, yet comparable, freezing in WT and Rag2−/− mice. C) To test for spontaneous recovery of the CR, all mice were retested in a third context 3 weeks after conditioning. Neither WT nor Rag2−/− mice displayed a significant renewal of the CR.
3.4 Experiment 3: Learned helplessness (LH)
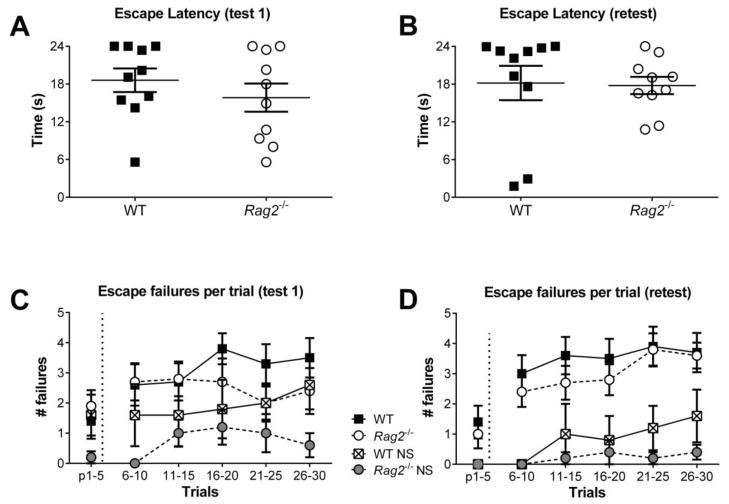
After exposure to inescapable stress mice were assessed for the development and maintenance of behavioral interference in the LH paradigm as determined by the latency to escape and the number of failed escapes when given the opportunity to terminate a foot shock. Mice were initially tested twenty-four hours after the inescapable stress session. As seen in Figure 5A, escape latencies for WT and Rag2−/− mice are not significantly different, with both groups showing a similar distribution of responses. Evaluation of escape failures by trial bin (Figure 5C) shows that WT and Rag2−/− mice exposed to inescapable stress had a similar number of escape failures and failed to escape more often than non-stressed controls (stress versus non-stressed: F (1,28) = 5.24, p = 0.0298), a verification of the development of behavioral interference. In order to determine if lymphocytes participate in long-term effects of traumatic stress exposure, mice were retested one week after the first test. As seen in Figure 5B and 5D, behavioral interference persisted in both WT and Rag2−/− mice, with no significant differences in escape latency or escape failures between groups. In contrast, the non-stressed controls display improved coping responses and exhibited significantly fewer escape failures during the retest than stressed animals (F (1,28) = 22.87, p < 0.0001; Figure 5D).
Figure 5. Behavioral interference following exposure to inescapable stress.
WT and Rag2−/− mice exhibit similar escape latencies and distribution of responses in the LH paradigm when tested one day (A) and one week (B) after inescapable stress exposure. The number of escape failures, shown by binned trials, was also similar for WT and Rag2−/− mice during test 1 (C) and the retest (D). The development and maintenance of behavioral interference is evident from the comparison to non-stressed controls that display significantly fewer escape failures during test 1 (2-way ANOVA, p = 0.0298) and the retest (p < 0.0001).
3.5 Hippocampal BDNF expression
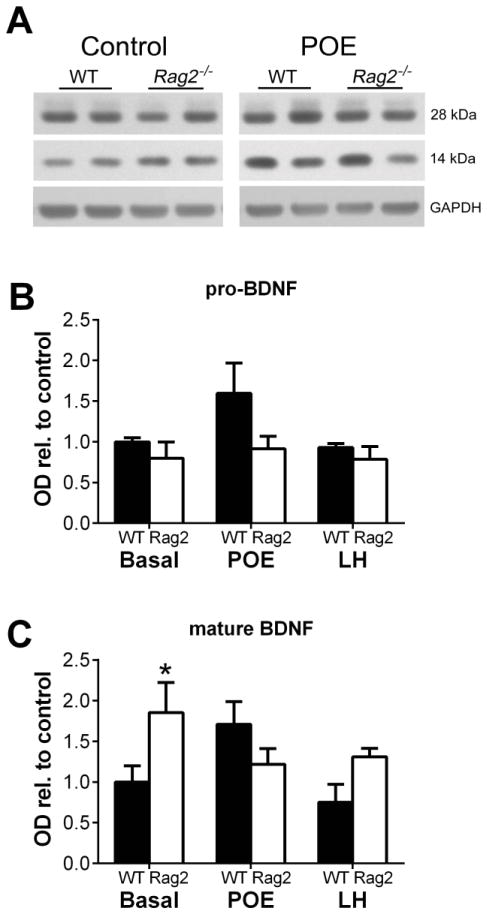
Immunoblot analysis for BDNF expression in the hippocampus was utilized to assess levels of both the precursor form of BDNF (pro-BDNF) and the cleaved, mature form of BDNF (Figure 6A). As seen in Figures 6B and 6C, under basal conditions, Rag2−/− mice have significantly higher levels of mature BDNF compared to WT mice (t (2) = 6.631, p = 0.022) with no difference in pro-BDNF expression. Exposure to predator odor in WT mice induced a notable, although not significant, increase over Rag2−/− mice for both mature and pro-BDNF (t (6) = 2.363, p = 0.056). In contrast, BDNF levels following LH remained relatively unchanged, though slightly lower than under basal conditions, with Rag2−/− mice maintaining a slight, albeit not significant increase in mature BDNF compared to WT mice.
Figure 6. Immunoblot analysis of hippocampal BDNF.
A) Representative bands of pro-BDNF and mature BDNF after POE. B) Semiquantitative analysis of optical density relative to WT control values indicates no significant difference in expression of pro-BDNF under basal conditions or after either POE or LH. C) However, under basal condition there is a significant increase in mature BDNF (Student’s t test: p = 0.022), with no significant difference seen following POE or LH. *p < 0.05
4. Discussion
This study demonstrates that, compared to WT BALB/c mice, Rag2−/− mice on a BALB/c background display reduced startle reactivity following exposure to predator odor. This can be interpreted as resilience to develop specific aspects of maladaptive responses to stress, elicited by an inability to engage adaptive immune responses. While differences were seen in the POE paradigm, no significant findings were present in the LH or FC tests. Furthermore, assessment of basal behavior revealed that Rag2−/− mice display increased locomotion and decreased anxiety in the OFT, but not the EPM. Finally, compared to their WT counterparts, Rag2−/− mice had elevated levels of mature BDNF in the hippocampus under basal conditions. Taken together, these results suggest that mature lymphocyte function may participate in certain facets of stress responsiveness by promoting and maintaining heightened reactivity to stressors, perhaps via modulation of the production or processing of BDNF.
Our primary behavioral difference between Rag2−/− and WT mice was observed in the POE paradigm. This finding may be related to specific aspects of stress responsiveness modeled by POE. While the POE, FC and LH paradigms all rely upon exposure to an acute and uncontrollable stressor, each models different long-term effects of acute stress exposure. The POE paradigm was developed as an ethologically valid procedure which results in the reproduction of key aspects of posttraumatic stress including heightened anxiety and startle responses in a context distinct from the original traumatic experience (Adamec, 1997; Adamec et al., 2006; Blanchard et al., 2003b; Blanchard et al., 1990; Blanchard et al., 1998; Blanchard et al., 2001; Cohen et al., 2008; Cohen et al., 2013). It is based on the innate fear responses of rodents to feline scents, which elicits long-lasting behavioral and hormonal alterations (Matar et al., 2013).
The FC paradigm also reproduces important endophenotypes of posttraumatic stress, but is based on associative learning of an aversive event. As the paradigm depends on the timing and schedule of cue presentations it models different aspects of stress responsiveness than the POE paradigm, namely development and extinction of a cued fear response (Balogh and Wehner, 2003; Choi et al., 2010; Gafford and Ressler, 2011; Myers and Davis, 2007; Myers et al., 2006). The FC procedure utilized for the present experiments measured several facets of extinction and recall over different sessions, including consideration of the context in which the original stressor occurred. Thus the present results suggest that the resilience of Rag2−/− mice evident in the POE paradigm may be related to resilience to the development or maintenance of innate fear, rather than differential associative learning. In support of this view, both WT and Rag2−/− mice manifested comparable levels of increased locomotion and rearing when re-exposed to the POE context, indicating similar recall to the context of the traumatic event in both genotypes.
In regards to the LH paradigm, a lack of effect on associative fear learning may also explain the absence of differential responses between WT and Rag2−/− mice. While the LH is a more accepted model of behavioral inhibition or behavioral depression, the intensity of the stressor has led to the use of this model to study specific neurobehavioral, hormonal and immunological alterations caused by an acute or traumatic stress exposure (Anisman and Merali, 2001; King et al., 2001; Maier and Watkins, 2005; Petty et al., 1997). In particular, the fact that re-exposure to the context alone is sufficient to maintain behavioral and hormonal alterations caused by inescapable shock suggests a significant associative learning component (Maier, 2001). Nevertheless, the genetic background should be considered when interpreting the lack of effects in the LH paradigm. BALB/c mice display high levels of behavioral interference in the LH when compared with other strains of mice (Shanks and Anisman, 1988). Thus, it is possible that the LH procedure is not a good model to detect behavioral differences in genetically modified BALB/c mice. Further studies employing different strains of mice may provide better understanding on the role of lymphocytes in the behavioral inhibition elicited with this paradigm.
While these results differ from previous studies that found increased susceptibility to stress in immune deficient mice (Cohen et al., 2006; Lewitus et al., 2008; Lewitus and Schwartz, 2009) it is important to note that there are numerous dissimilarities, the most important of which was the use of a different mouse model of lymphocyte deficiency. Studies using the SCID Rag1/Rag2−/− mouse, as well as those examining Rag1−/− mice (McGowan et al., 2011; Rattazzi et al., 2013) report deficits in these models when exposed to stressors. Nevertheless, several of these neurobehavioral alterations have been attributed to a potential role for the RAG1 gene in hippocampal function rather than lymphocyte function (Cushman et al., 2003; Fang et al., 2013; McGowan et al., 2011). Specifically, the RAG1 gene is expressed in the hippocampus and has been proposed to be involved in hippocampal function including learning and memory (Chun et al., 1991; Fang et al., 2013; McGowan et al., 2011). Thus, the utilization of the Rag2−/− model of lymphocyte deficiency described here may represent a more precise model to address specific peripheral lymphocyte modulation of stress responsiveness and brain function.
An important consideration with respect to previously published studies is that the present studies did not involve lymphocyte stimulation by means of antigen challenge. Thus, the concept of a protective role for lymphocytes under stress remains valid under mechanisms engaging specific immune responses as shown in cases of vaccination with CNS specific peptides (Lewitus et al., 2008; Lewitus and Schwartz, 2009; Lewitus et al., 2009). Moreover, it is possible that preventing the function of specific T cell subsets results in impaired stress responsiveness (Na et al., 2012; Rattazzi et al., 2013). As is the case for a myriad of immunological responses involving different lymphocytes subsets, the relationship (protective or detrimental) between stress and lymphocyte function is likely very selective and specific (Beurel et al., 2013; Cohen et al., 2006; Na et al., 2012; Rattazzi et al., 2013). Nevertheless, the present results indicate that immune deficient Rag2−/− mice behave remarkably similar to their immune competent counterparts in a considerable number of behavioral tests. They are comparable in several measures of basal anxiety, including their performance in the EPM test, and also perform similarly in the FC paradigm showing normal association learning of paired stimuli. While the extinction effect was not evident during single sessions there was a progressive attenuation of fear responses associated with time, indicating that memory consolidation processes are normal in both groups of mice. Moreover, coping responses were also comparable in the LH paradigm, a stressor of significant demand, applied in different sessions, and lasting more than a week after initial stress exposure. These results clearly indicate that deletion of the RAG2 gene with the consequent impairment in adaptive immune function does not necessarily result in impaired stress responsiveness. Furthermore, taken in consideration with previous studies using RAG1 knockout mice, our findings indicate that the role of RAG1 in the CNS may be quite substantial and warrants further investigation.
Previous studies have indicated that peripheral lymphocytes may contribute to the production of BDNF, particularly in the hippocampus, as measurements of total BDNF via immunofluorescent staining (Lewitus et al., 2008) or ELISA (Wolf et al., 2009) indicated that CD4+ T cell depletion or immune deficiency led to a significant reduction in this neurotrophic factor. For this study we chose to examine BDNF via immunoblot analysis allowing for the determination of both pro-BDNF and mature BDNF levels. Pro-BDNF preferentially binds to the pan-neurotrophin receptor p75NTR while mature BDNF binds with high affinity to TrkB receptors resulting in differential and often opposite effects on neurons (Pang et al., 2004; Woo et al., 2005). Using this approach it was found that levels of pro-BDNF did not appear to depend upon immune status while mature BDNF hippocampal content was higher in Rag2−/− mice relative to WT mice. Moreover, consistent with previous findings that stress decreases hippocampal BDNF, a tendency towards reduced BDNF was found in both WT and Rag2−/− mice after the LH paradigm suggesting similar regulatory mechanisms after stress. These results indicate that basal constitutive levels of hippocampal mature BDNF are dependent upon functional peripheral recombination processes while its modulation by stress is probably independent of peripheral lymphocyte function. Thus, increased basal levels of mature BDNF may be related to some of the behavioral differences observed between WT and Rag2−/− mice in the POE paradigm as well as the OFT. It must be noted however that the significance of these findings is still unclear as the precise role of hippocampal BDNF in behavior following different stressors remains elusive. For instance, increased or decreased BDNF expression in rodents has been shown dependent on the type of stressor employed, the time of BDNF measurement and sex and age of the animals (Bath et al., 2013). While most of the studies report decreased hippocampal BDNF expression following stress, other studies report the opposite. For instance elevated levels of pro-BDNF, were observed immediately after acute restraint stress in rats (Marmigere et al., 2003; Rage et al., 2002) and after footshocks in female rats (Lin et al., 2009). Whether the behavioral traits observed in our study are related to differential processing or utilization of BDNF remains to be determined, along with the precise mechanisms that control this pathway.
Among the potential mechanisms by which peripheral lymphocytes may influence brain function, a particular mention must be given to the production of acetylcholine (ACh) by mature CD4+ T cells and B cells (Reardon et al., 2013) along with their interaction with components of the innate immune system, including toll-like receptors (TLRs) (Zimmerman et al., 2012). It has been shown that vagal stimulation results in the production of ACh by splenic CD4+ T cells as a part of an anti-inflammatory loop governed by the CNS to control inflammation in response to antigen stimulation (Rosas-Ballina et al., 2011). Similarly, B cells can transiently express choline acetyltransferase (ChAT) and limit inflammation in local microenvironments (Reardon et al., 2013). Thus, it is possible that reduced anxiety in response to innate fear exposure may be related to the lack of ACh input from peripheral mature CD4+ T cells and B cells. This is supported by the recently demonstrated ability of systemically administered acetylcholinesterase (AChE) inhibitors to promote anxiety in mice (Mineur et al., 2013). Moreover, involvement of TLRs in stress responsiveness through the engagement of lymphocyte responses (Zimmerman et al., 2012) will not be functional in Rag2−/− mice. Thus, downstream mechanisms that promote anxiety by this pathway should be limited, further supporting a TLR involvement in stress-induced anxiety. Consequently, these potential mechanisms deserve future investigation.
In summary, lymphocyte deficiency in Rag2−/− mice on a BALB/c background does not result in enhanced susceptibility to stress exposure or reductions in BDNF when compared to immune competent BALB/c mice under the tested conditions. In contrast, Rag2−/− mice display resilience to deleterious effects of exposure to predator odor when compared with immune competent BALB/c mice and express elevated levels of hippocampal mature BDNF. Our findings suggest that the influence of peripheral lymphocytes is dependent upon the nature and intensity of the stressor. Further studies are necessary to address specific mechanisms of interaction between peripheral lymphocytes and the CNS that may mediate these effects.
Supplementary Material
Acknowledgments
Supported by the VA Research Merit Award BX000935 and by the National Institute of Mental Health Research Grant R01MH097676.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R. Transmitter systems involved in neural plasticity underlying increased anxiety and defense--implications for understanding anxiety following traumatic stress. Neuroscience and biobehavioral reviews. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiology & behavior. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Rodent models of depression: learned helplessness induced in mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 8. Chapter 8. 2001. p. 10C. [DOI] [PubMed] [Google Scholar]
- Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behavioural brain research. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biological psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Progress in neuro-psychopharmacology & biological psychiatry. 2003a;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. European journal of pharmacology. 2003b;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Rodgers J, Weiss SM. The characterization and modelling of antipredator defensive behavior. Neuroscience and biobehavioral reviews. 1990;14:463–472. doi: 10.1016/s0149-7634(05)80069-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiology & behavior. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yang M, Li CI, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neuroscience and biobehavioral reviews. 2001;25:587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z. Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008;11:331–349. doi: 10.1017/S1461145707007912. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Joseph Z. Animal models of post-traumatic stress disorder. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 9. Chapter 9. 2013. p. 45. [DOI] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. Journal of neurobiology. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral Changes Resulting from Recombinase Activation Gene 1 Deletion. Clinical and Vaccine Immunology. 2003;10:13–18. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Yin Y, Chen H, Hu Z, Davies H, Ling S. Contribution of Rag1 to spatial memory ability in rats. Behavioural brain research. 2013;236:200–209. doi: 10.1016/j.bbr.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Ressler KJ. Fear Conditioning and Extinction as a Model of PTSD in Mice. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Springer Science+Business Media, LLC; 2011. pp. 171–184. [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH, Baker DG, Lohr JB, Kremen WS, Litz BT, Tsuang MT. Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2013;162B:313–326. doi: 10.1002/ajmg.b.32167. [DOI] [PubMed] [Google Scholar]
- King JA, Abend S, Edwards E. Genetic predisposition and the development of posttraumatic stress disorder in an animal model. Biological psychiatry. 2001;50:231–237. doi: 10.1016/s0006-3223(01)01071-x. [DOI] [PubMed] [Google Scholar]
- LeardMann CA, Smith TC, Smith B, Wells TS, Ryan MA. Baseline self reported functional health and vulnerability to post-traumatic stress disorder after combat deployment: prospective US military cohort study. BMJ (Clinical research ed ) 2009;338:b1273. doi: 10.1136/bmj.b1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain, behavior, and immunity. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain, behavior, and immunity. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Molecular psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biological psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebral cortex (New York, NY : 1991) 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biological psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and biobehavioral reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Matar MA, Zohar J, Cohen H. Translationally relevant modeling of PTSD in rodents. Cell and tissue research. 2013 doi: 10.1007/s00441-013-1687-6. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain research. 2011;1383:187–195. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning & memory (Cold Spring Harbor, NY) 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Sun B, Rempel H, Ross J, Lenoci M, O’Donovan A, Pulliam L. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain, behavior, and immunity. 2011;25:524–531. doi: 10.1016/j.bbi.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole BI, Catts SV. Trauma, PTSD, and physical health: an epidemiological study of Australian Vietnam veterans. Journal of psychosomatic research. 2008;64:33–40. doi: 10.1016/j.jpsychores.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Wu J, Davis LL. Posttraumatic stress and depression. A neurochemical anatomy of the learned helplessness animal model. Annals of the New York Academy of Sciences. 1997;821:529–532. doi: 10.1111/j.1749-6632.1997.tb48322.x. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rage F, Givalois L, Marmigere F, Tapia-Arancibia L, Arancibia S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience. 2002;112:309–318. doi: 10.1016/s0306-4522(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson PS, Rosas-Ballina M, Tracey KJ, Mak TW. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M, Yan L, Baccarelli A. PTSD and DNA Methylation in Select Immune Function Gene Promoter Regions: A Repeated Measures Case-Control Study of U.S. Military Service Members. Frontiers in psychiatry/Frontiers Research Foundation. 2013;4:56. doi: 10.3389/fpsyt.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Ziv Y. Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends in immunology. 2008;29:211–219. doi: 10.1016/j.it.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Shanks N, Anisman H. Stressor-provoked behavioral changes in six strains of mice. Behavioral neuroscience. 1988;102:894–905. doi: 10.1037//0735-7044.102.6.894. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature neuroscience. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 8. Chapter 8. 2009. p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G, Shaltiel G, Barbash S, Cohen J, Gasho CJ, Shenhar-Tsarfaty S, Shalev H, Berliner SA, Shelef I, Shoham S, Friedman A, Cohen H, Soreq H. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFkappaB pathway. Translational psychiatry. 2012;2:e78. doi: 10.1038/tp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Meadows JP, Kaas GA, Sweatt JD. Interindividual Variability in Stress Susceptibility: A Role for Epigenetic Mechanisms in PTSD. Frontiers in psychiatry/Frontiers Research Foundation. 2013;4:60. doi: 10.3389/fpsyt.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.