Abstract
Objectives
To assess the associations among age, health status, and resting metabolic rate (RMR) in a large population of older adults.
Design
Cross-Sectional Analysis
Setting
Community-dwelling volunteers from the Baltimore Longitudinal Study of Aging (BLSA)
Participants
Four hundred twenty persons aged 40 – 96 (mean 68.2 ± 11.0) who underwent a comprehensive physical examination, cognitive assessment, resting metabolic rate testing, body composition assessment, and physical function testing during a three-day clinic visit.
Measurements
Participants were assigned to “IDEAL” (Insight into the Determination of Exceptional Aging and Longevity) or “non-IDEAL” categories based on health status. IDEAL participants were defined by the absence of: physical and cognitive impairments, chronic conditions/comorbidities and blood profile alterations. A three-stage linear regression model was used to assess the relationship between RMR and age, using IDEAL classification as a predictor, adjusting for sex and body composition.
Results
RMR averaged 1512.4 (± 442.9) kcal/day and was lower with advancing age (β = −8.55, p < 0.001). After adjusting for age, sex, and body composition RMR was 109.6 kcals/day lower in IDEAL than non-IDEAL participants (p < 0.005).
Conclusions
Individuals who are fully functional and free of major medical conditions have lower RMR than those affected by disease and functional impairments. These findings suggest that health status plays a role in energy utilization and regulation independent of age and body composition and that elevated RMR may be a global biomarker of poor health in older persons.
Keywords: Aging, Resting Metabolic Rate, Comorbidities
INTRODUCTION
Resting metabolic rate (RMR) is the minimal energy required for life (1). In 1908, Rubner proposed his “rate of living theory” based on the observation that a slower rate of metabolism was associated with greater longevity in animals (1). Rubner’s theory has been at the center of discussions for more than a century and conflicting views continue to emerge (2). In 2000, Brand proposed the “uncoupling to survive” hypothesis, which postulates that greater mitochondrial uncoupling (and greater metabolic rate) leads to lower reactive oxygen species (ROS) and longer lifespan (3). In conflict with this view, recent studies have shown that higher RMR is a risk factor for mortality in humans (4, 5) although the true mechanism of this association is not well understood.
Previous studies have found that RMR is highest at one year of age and declines rapidly after word, with the rate of decline slowing after puberty (6). This decline is explained partially by a decline in lean body mass (7). Conversely, there are also many reasons to believe the energetic requirement for maintaining life may increase with age. Aging is characterized by agradual loss of physiologic reserve, impaired response to stress and unstable homeostasis (8), which contribute to disease susceptibility and impaired recovery from disease and injury (9). Comorbid conditions common with aging may challenge biological homeostasis and trigger counteracting responses that require extra energy (10). Eventually this chronic elevation of metabolic rate may contribute to organ damage at the cellular level and, especially in older persons with comorbidities and frailty, attenuate the well-documented age-related decline in resting metabolic rate as the body works harder to maintain the biological processes that sustain life (11–13). Over time, this chronic elevation may contribute to the increased risk of mortality observed in recent studies (4, 5).
Assessing the separate contributions of aging and morbidity to changes in RMR is complex as the concept of “healthy aging”, as originally defined by Rowe and Kahn in 1987 (14), and expanded upon by multiple researchers (15, 16), is difficult to operationalize. In recent years, the Baltimore Longitudinal Study of Aging (BLSA) has developed an operational definition of “IDEAL aging” using an extensive assessment of physical and cognitive function, health status and medical history to investigate the separate effects of aging and disease. These criteria comprise the inclusion criteria to enroll new participants into the BLSA, are highly standardized, and encompass both objective and subjective measurements. In simple terms, participants are considered IDEAL if they have no physical or cognitive impairments, no chronic diseases except controlled hypertension, and no abnormal blood tests.
The aim of this paper is to shed light on the association between RMR and aging by health status, and to understand whether common age-related comorbidities and conditions may be associated with elevated RMR in mid-to-late life. We hypothesize that disease burden (non-IDEAL status) will be associated with higher metabolic rate as the body attempts to repair itself and return to homeostasis.
Materials and Methods
Participants
The BLSA is a study of human aging, established in 1958 and conducted by the National Institute on Aging Intramural Research Program. A general description of the sample and enrollment procedures and criteria has been reported (17). Briefly, the BLSA continuously enrolls healthy individuals aged 20 years and older to maintain an adequate balance of active participants across age groups, sex and ethnicities. All participants are community volunteers who must pass a comprehensive health and functional screening evaluation and be free of major chronic conditions and cognitive and functional impairment at enrollment. Participants are followed for life regardless of the development of comorbidities and conditions and undergo extensive testing every one to four years depending on age. In 2007, highly standardized criteria for excellent or “IDEAL” (Insight into the Determinants of Exceptional Aging and Longevity) health were established for the BLSA study enrollment. IDEAL criteria, are based on physical and cognitive function, absence of major diseases (except controlled hypertension and cancer that has been clinically silent for more than 10 years), and laboratory blood values. At the end of each week, the BLSA research team reviews pertinent data collected on each BLSA participant to adjudicate the “IDEAL” status.
The sample for the current study consists of 420 men and women who visited the BLSA between July 2007 and December 2011 and underwent a comprehensive health history and physical examination, physical performance testing, cognitive testing, and RMR assessment. The Internal Review Board of the Med star Research Institute approved the study protocol and all participants provided written informed consent.
Measurements
Participants were examined at the National Institute on Aging Intramural Research Program Clinical Research Unit in Baltimore, Maryland over three days of testing. Certified nurse practitioners and specialized technicians administered all assessments following standardized protocols. Consistent with previous concepts of successful aging (14, 16), IDEAL aging status was defined using a combination of subjective and objective measures designed to assess presence of specific health conditions and functional limitations. Participants were classified as IDEAL if they met all of the following criteria: no reported difficulty completing activities of daily living (ADLs) or independent activities of daily living (IADLs) and a short physical performance batter score (SPPB) of 12 if younger than age 80, 11 if aged 80–89, or 10 if aged 90 or over; no reported mobility limitation defined as difficulty walking 400 meters, climbing 10 steps, or shortness of breath after climbing one flight of stairs; no reported medical history of cardiovascular disease, congestive heart failure, stroke, bypass surgery, kidney disease, diabetes, or cancer within the past ten years; measured systolic blood pressure of <145 mmHg and diastolic blood pressure of <90 mmHg; a Blessed Mental score of <4 and a Mini-Mental State Examination score of>26; measured hemoglobin of ≥ 11.0 g/dL for men or ≥ 10.5 g/dL for women; measured albumin of ≥ 3.2 g/dL, and no history of medical condition(s) requiring an absolute need for long-term treatment with antibiotic, antiviral, corticosteroids, immunosuppressants, or pain medications.
Certified examiners administered the SPPB (18) in a dedicated area of the clinic. The gait speed component was conducted over 6 meters and two trials were performed with the faster used for scoring. Time to complete 5 repeated chair stands used the faster of the first or second split of 5 out of 10 stands for scoring. Standing balance score was derived from performance on three progressively more difficult positions - side-by-side and semi- and full-tandem stands and the ability to hold each for up to 10 seconds.
A detailed health examination and interview was administered by a nurse practitioner during the physical examination. Information from the interview was used to derive functional status and history of chronic conditions. Blood pressure was assessed in the supine position and measured three times on each arm, alternating right and left with one minute in between each measurement. Mean overall blood pressure value was used to define hypertensive status.
Screening for cognitive impairment was performed by the nurse practitioners using the Blessed Dementia Rating Scale (19) and by certified cognitive testers using the Mini-Mental State Examination (20). Laboratory values were assessed using blood samples collected after an overnight fast. Albumin and hemoglobin were determined using Vitros-Dye Binding BCG, Vitros-enzymatic, and SYSMEX XE-2100 methods, respectively.
RMR was assessed first thing in the morning for 16 minutes in a quiet, thermo-neutral environment, in a fasted, rested state via indirect calorimetry (21, 22), using a Cosmed k4b2 portable metabolic analyzer (Cosmed, Rome, Italy). Prior to testing, the Cosmed was calibrated using a 3.0 liter flow syringe and gases of known concentrations. The Cosmed collects gas-exchange data on a breath-by-breath basis averaged over 30 second intervals to reduce variability. The first five minutes of data were discarded and the remaining minutes were averaged to arrive at a single measure of resting metabolic rate.
Body composition was determined using dual-energy x-ray absorptiometry (DEXA) and computed tomography (CT). Total body DEXA was performed using a Prodigy Scanner (GE, Madison, WI) and analyzed with version 10.51.006 software. Total nonbone, nonadipose tissue and adipose tissue at midfemur were obtained from a cross-sectional 10mm CT image using a Somatom Sensation 10 computed tomography scanner (Siemens, Malvern, PA) and quantified using Geanie software version 2.1 (BonAlyse Oy, Jyvaskyla, Finland). Although DEXA provides a global assessment of lean and fat mass distribution, it is not sensitive to variations in muscle density caused by fat infiltration. In contrast, CT provides an indicator of muscle density but is limited to a few regional assessments to minimize radiation exposure in a research setting. To take advantage of the benefits of these two methods, we calculated “adjusted lean mass” by multiplying the lean mass of the right leg (grams, DEXA) by the muscle density of the right thigh (CT scan) to more accurately account for the age-related fat infiltration of lean mass (23, 24). A similar calculation was performed to adjust fat mass (fat mass of the right leg (DEXA) x fat density of the right thigh (CT scan)) to maintain fat mass and lean mass on the same scale in the analysis.
Statistical Analysis
All analyses were performed using Stata MP, version 10 (Statacorp, College Station, TX) and p-values <.05 were considered significant. Participants were classified as IDEAL or non-IDEAL and T-tests and chi-square tests were used to evaluate differences in continuous and categorical variables, respectively between groups. Unadjusted relationships between variables were explored using scatter plots and locally weighted regression smoothers, and a histogram was used to assess the distribution of RMR. Based on these results, the cross-sectional relationship between RMR and age was modeled using linear models with the IDEAL classification as a predictor and sex, CT-adjusted lean body mass and CT-adjusted fat mass as covariates. Interactions between variables were not significant and not included in the final models.
RESULTS
Participant characteristics are detailed in Table 1. Of the 420 participants in the analyses, 218 qualified as IDEAL and 202 as non-IDEAL. IDEAL participants tended to be younger and female, and had lower BMI, lower Blessed Mental score, higher MMSE score, higher hemoglobin, and higher albumin (p <0.05). There were seven IDEAL participants self-reporting ADL disability and one self-reporting IADL disability however because the IDEAL criteria use a combination of subjective and objective measures of physical function, they were classified as IDEAL based on high SPPB performance and absence of any chronic conditions.
Table 1.
Participant characteristics, combined and stratified by IDEAL status
| ALL N=420 |
IDEAL N=218 |
NON-IDEAL N=202 |
P value | |
|---|---|---|---|---|
| Group characteristics | ||||
| Age (years), mean (SD) | 68.2 (11.0) | 64.9 (10.8) | 71.7 (10.2) | <0.001 |
| Male sex, no. (%) | 232 (55.2) | 110 (50.5) | 122 (60.4) | 0.04 |
| Body Mass Index (kg/m2), mean (SD) | 27.6 (4.7) | 26.9 (4.4) | 28.3 (4.9) | 0.003 |
| Body fat (%) | 34.6 (0.1) | 33.9 (0.1) | 35.3 (0.1) | 0.10 |
| RMR (kcal/day), mean (SD) | 1512.4 (442.9) | 1476.1 (415.4) | 1551.5 (468.6) | 0.08 |
| IDEAL characteristics | ||||
| SPPB score, mean (SD) | 11.4 (1.2) | 12.0 (0.2) | 10.8 (1.5) | <0.001 |
| ADL disability, no. (%) | 36 (8.6) | 7 (3.2) | 29 (14.4) | <0.001 |
| IADL disability, no. (%) | 11 (2.6) | 1 (0.1) | 10 (5.0) | 0.04 |
| Mobility difficulty, no. (%) | 20 (4.8) | 0 (0.0) | 20 (9.9) | <0.001 |
| Cardiovascular disease, no. (%) | 51 (12.1) | 0 (0.0) | 51 (25.2) | <0.001 |
| Congestive heart failure, no. (%) | 5 (1.2) | 0 (0.0) | 5 (2.5) | 0.02 |
| Stroke, no. (%) | 23 (5.5) | 0 (0.0) | 23 (11.4) | <0.001 |
| Bypass, no. (%) | 33 (7.9) | 0 (0.0) | 33 (16.3) | <0.001 |
| Kidney disease, no. (%) | 17 (4.0) | 0 (0.0) | 17 (8.4) | <0.001 |
| Diabetes, no. (%) | 52 (12.4) | 0 (0.0) | 52 (25.7) | <0.001 |
| Cancer, no. (%) | 50 (11.9) | 0 (0.0) | 50 (24.8) | <0.001 |
| Hypertension, no. (%) | 11 (2.6) | 0 (0.0) | 11 (5.4) | <0.001 |
| Blessed mental score, mean (SD) | 1.5 (1.7) | 1.2 (1.3) | 1.8 (2.0) | <0.001 |
| MMSE score, mean (SD) | 28.6 (1.6) | 28.9 (1.5) | 28.4 (1.6) | 0.02 |
| Hemoglobin (g/dL), mean (SD) | 13.8 (1.4) | 14.0 (1.2) | 13.6 (1.5) | 0.002 |
| Albumin (g/dL), mean (SD) | 4.21 (0.3) | 4.24 (0.3) | 4.17 (0.3) | 0.04 |
SD, Standard deviation; RMR, Resting Metabolic Rate; SPPB, Short Physical Performance Battery; ADL, Activities of Daily Living; IADL, Independent Activities of Daily Living; MMSE, Mini-Mental State Examination
The first column shows the combined characteristics of all participants in the analysis. The second and third columns show the participant characteristics as stratified by IDEAL and non-IDEAL criteria, respectively.
Consistent with prior research, unadjusted RMR averaged 1512.4 (± 442.9) kcal/day, was lower with advancing age (β =−8.55, p<0.001), and higher in men than in women (β =348.86, p <0.001) (11, 12). Adjusted lean mass was negatively associated with age (r= −0.31, p <0.001) and adjusted fat mass was positively associated with age (r= 0.23, p <0.001).
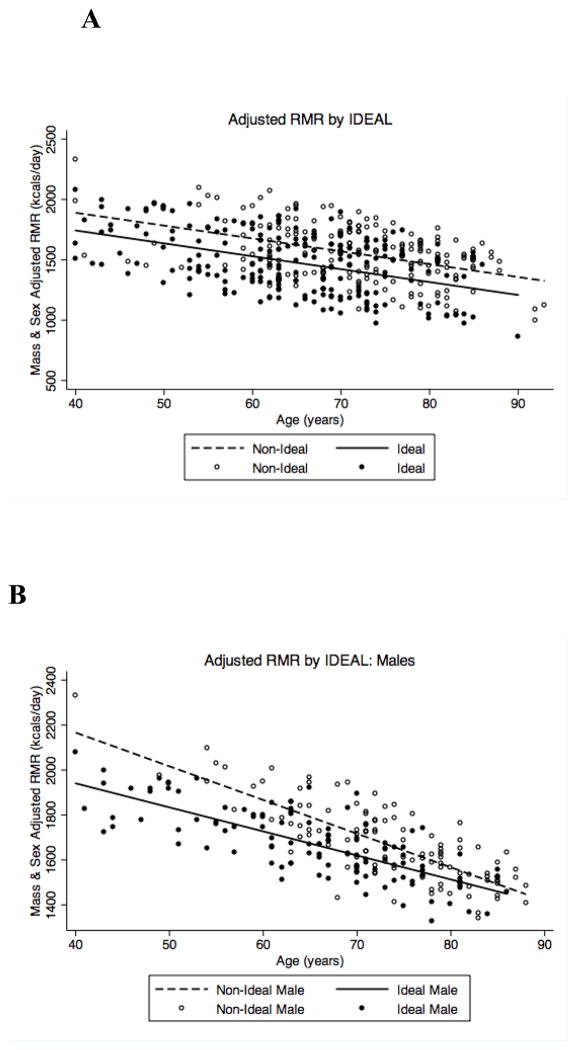
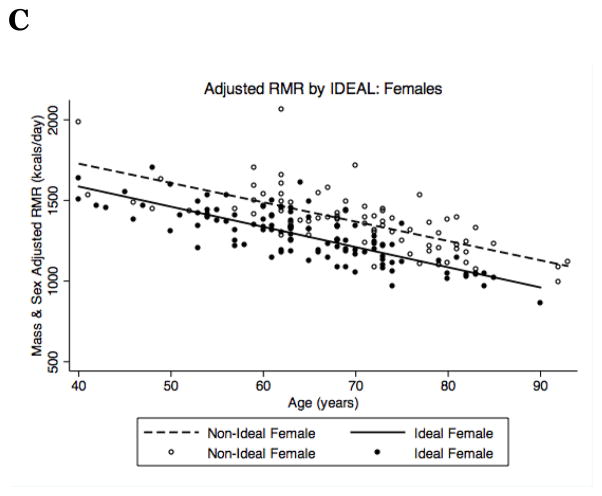
To model the association between IDEAL status and RMR, we performed a three-stage linear regression model (Table 2). Stage one modeled the unadjusted relationship between IDEAL status and RMR. This revealed a non-significant trend towards lower RMR in IDEAL participants (p = 0.08) of 75.4 kcals/day. Stage two expanded the model to account for age (β = −12.56, p <0.001) and sex (β = 374.11, p <0.001), which increased the difference between IDEAL and non-IDEAL participants to 123.59 kcals/day (p = 0.002). Finally, stage three added covariates for lean mass (β = 0.001, p <0.001) and fat mass (β = −0.0004, p <0.001) to account for differences in body composition. These additions decreased the difference between IDEAL and non-IDEAL participants to 109.6 kcals/day, but the association between RMR and IDEAL status remained highly significant (p = 0.005). When stratified by sex, the difference in RMR between IDEAL and non-IDEAL participants was 125 kcals/day in men (n=232, p = 0.03) and 99 kcals/day in women (n=188, p = 0.05). Although the interaction between IDEAL status and sex was not significant, there appeared to be a trend towards a survival bias in the oldest non-IDEAL males (Figure 1B).
Table 2.
Association between resting metabolic rate and IDEAL status (N=420) modeled using a three-stage linear regression model.
| Dependent Variable: Resting Metabolic Rate (kcal/day) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent Variables: | β | P – value | β | P-value | β | P-value |
| IDEAL status | −75.39 | 0.08 | −123.59 | 0.002 | −109.58 | 0.005 |
| Intercept | 1551.48 | <0.001 | 2226.64 | <0.001 | 1068.60 | <0.001 |
| Age | −12.56 | <0.001 | −5.73 | 0.008 | ||
| Male sex | 374.11 | <0.001 | 257.89 | <0.001 | ||
| Adjusted lean mass | 0.001 | <0.001 | ||||
| Adjusted fat mass | −.0004 | <0.001 | ||||
IDEAL, Insight into the Determination of Exceptional Aging and Longevity
Model 1 shows the beta-coefficient and p-value from a simple linear regression model assessing the relationship between IDEAL status and resting metabolic rate. Model 2 expands upon model 1 by adding age and sex to the model. Model 3 expands upon model 2 by adding variables to account for body composition.
Figure 1.
RMR (kcal/day) stratified by IDEAL status and adjusted for age, sex, and body composition in (A) all participants, (B) males and (C) females.
DISCUSSION
These findings demonstrate that individuals aged 40 to 96 who are fully functional and free of major medical conditions have significantly lower RMR than those affected by disease and/or functional impairments. This supports previous research examining the metabolic and energetic cost of disease and expands on it to include age-related comorbidities and conditions (4, 25). Further, these findings emphasize the important role health status plays in energy utilization and regulation throughout mid-to-late life independent of age and body composition.
Although transient increases in metabolic rate due to physical activity are beneficial (25, 26), persistently elevated metabolic rate due to chronic diseases and conditions may be harmful and lead to accelerated disease progression and early mortality (4, 5, 9). The mechanisms for these effects are not completely understood and may include increased oxidative stress, a shifting of energetic needs for other homeostatic mechanisms, a rising catabolic state associated with weight loss, and chronic fatigue. Alternatively, high RMR may simply be a biomarker; a bystander of impending deterioration of health that eventually results in adverse outcomes.
Previous research on the health effects of aging and metabolic rate has been limited to smaller samples with younger participants or confined to a single health related condition (4, 27). Our study examined the relationship between metabolic rate and both mobility and common disease-driven health-related factors over a wide age spectrum. Accordingly, this approach provides a more comprehensive analysis of the multitude of factors associated with the energetic cost of life. Finally, we employed a widely accepted method of measuring RMR (21, 22) rather than group equation estimates to increase accuracy and minimize bias (13, 28).
This study has several potential limitations. First, due to the demands of attending a clinic visit and enduring performance testing the study population is healthier than the general population and may underestimate the difference in metabolic rate between IDEAL and non-IDEAL persons. The true relationship may indeed exceed 109 kcals/day, which suggests that disease and co-morbidities may confer even greater energy costs. Further, the trend of a survival bias among males may indicate that men who are non-IDEAL in midlife may not survive into older age. Because of sample size limitations, we could not establish the contribution of specific diseases, functional or cognitive limitations, or medications but believe this an important next step for future research. Second, although this study employed two highly accurate measures of body composition to minimize the confounding effects of body mass on RMR, residual confounding and/or measurement error may still exist, as body composition was only assessed using the right leg. Finally, this study uses cross-sectional data to estimate age-related change and our findings should be confirmed in a longitudinal design. Previously published data from the BLSA indicates the relationship between age and RMR predicts longevity (5) and warrants further exploration by IDEAL aging status to further clarify the relationship among RMR, comorbidities, and longevity.
A fundamental component of living is the ability to access and utilize energy. Theoretically, individuals who are most efficient at utilizing energy should survive the longest, which may explain the previously established association between RMR and longevity (4, 5, 29). Although the 109 kcal/day difference between IDEAL and non-IDEAL participants may seem modest, it is important to note that 100 kcals is roughly equivalent to the energy it takes an average sized individual (150 lbs) to walk one mile. In older individuals, this may translate to shortages in the energy available for physical tasks, as energy is directed elsewhere to regulate comorbid conditions. An important next step in this area of research is to understand the individual contributions of diseases and impairments to energetic efficiency, as they may provide novel targets for intervention. Further, the findings from this research indicate RMR may be a possible candidate biomarker for global health status, a tool that could be useful in clinical practice, particularly for early diagnosis or monitoring the effects of treatments in older persons with complex comorbidities. These questions should be addressed in future studies performed in larger samples and with longitudinal design.
Elevated RMR signals metabolic inefficiency as more energy is required to maintain life. This sustained disease-driven metabolic inefficiency may contribute to accelerated aging, increased disability, and death as energy reserves cease to meet daily needs. The current study supports these hypotheses and emphasizes the importance of healthy aging in the compression of morbidity and promotion of longevity.
Acknowledgments
The authors would like to acknowledge Magda Tolea, PhD for her assistance in developing the IDEAL criteria.
Funding:
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
Footnotes
Conflicts of Interest:
There are no conflicts of interest to report.
Original Data:
Dr. Schrack had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the manuscript.
Dr. Schrack performed and is responsible for the statistical analysis for this study.
References
- 1.Rubner M. Machinery of metabolism. JAMA. 1916;66:1879. [Google Scholar]
- 2.Miller RA. Genes against aging. J Gerontol A Biol Sci Med Sci. 2012;67A:495–502. doi: 10.1093/gerona/gls082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 4.Jumpertz R, Hanson RL, Sievers ML, et al. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab. 2011;96:E972–E979. doi: 10.1210/jc.2010-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: The baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batisse-Lignier M, Rousset S, Labbé A, et al. Growth velocity in infancy influences resting energy expenditure in 12–14 year-old obese adolescents. Clin Nutr. 2012;31:625–629. doi: 10.1016/j.clnu.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Molnar DSY. The effect of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur J Pediatr. 1997;156:376–381. doi: 10.1007/s004310050618. [DOI] [PubMed] [Google Scholar]
- 8.Shock N. Homeostatic disturbances and adaptations in aging. Bulletin der Schweizerischen Akademie der Medizinischen Wissenschaften. 1969;24:284–98. [PubMed] [Google Scholar]
- 9.Frisard M, Ravussin E. Energy metabolism and oxidative stress. Endocrine. 2006;29:27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- 10.Floyd RA, Towner RA, He T, et al. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51:931–941. doi: 10.1016/j.freeradbiomed.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughn L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–5. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- 12.Fukagawa NK, Bandini LG, Young JB. Effect of age on body composition and resting metabolic rate. Am J of Physiol. 1990;259:E233–E238. doi: 10.1152/ajpendo.1990.259.2.E233. [DOI] [PubMed] [Google Scholar]
- 13.Weiss CO, Cappola AR, Varadhan R, et al. Resting metabolic rate among old-old women with and without frailty: Variability and estimation of energy requirements. J Am Geriatr Soc. 2012;60:1695–1700. doi: 10.1111/j.1532-5415.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 15.Depp CA, Jeste DV. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin SJ, Jette AM, Connell CM. An examination of healthy aging across a conceptual continuum: Prevalence estimates, demographic patterns, and validity. J of Gerontol A Biol Sci Med Sci. 2012;67:783–789. doi: 10.1093/gerona/glr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone JL, Norris AH. Activities and attitudes of participants in the baltimore longitudinal study. J Gerontol. 1966;21:575–580. doi: 10.1093/geronj/21.4.575. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Rumpler WV, Seale JL, Conway JM, et al. Repeatability of 24-h energy expenditure measurements in humans by indirect calorimetry. Am J Clin Nutr. 1990;51:147–152. doi: 10.1093/ajcn/51.2.147. [DOI] [PubMed] [Google Scholar]
- 22.Mcanena OJ, Harvey LP, Katzeff HL, et al. Indirect calorimetry: Comparison of hood and mask systems for measuring resting energy expenditure in healthy volunteers. JPEN J Parenter Enteral Nutr. 1986;10:555–557. doi: 10.1177/0148607186010006555. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Ruts E, Kim J, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly african american women. Am Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 24.Cree MG, Newcomer BR, Katsanos CS, et al. Intra muscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 25.Duncan GE, Perri MG, Theriaque DW, et al. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557–562. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- 26.Pan X, Li G, Hu Y, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The da qing IGT and diabetes study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 27.Weyer C, Bogardus C, Pratley RE. Metabolic factors contributing to increased resting metabolic rate and decreased insulin-induced thermogenesis during the development of type 2 diabetes. Diabetes. 1999;48:1607–1614. doi: 10.2337/diabetes.48.8.1607. [DOI] [PubMed] [Google Scholar]
- 28.Luhrmann PM, Neuhauser-Berthold M. Are the equations published in the literature for predicting resting metabolic rate accurate for use in the elderly? J Nutr Health Aging. 2004;8:144–149. [PubMed] [Google Scholar]
- 29.Lotka AJ. Contribution to the energetics of evolution. Proc N A S. 1922;8:147–151. doi: 10.1073/pnas.8.6.147. [DOI] [PMC free article] [PubMed] [Google Scholar]