Abstract
Advanced age is associated with chronic low-grade inflammation (i.e. inflamm-aging) and poor macrophage function that includes a weak pro-inflammatory cytokine response to bacteria and diminished phagocytosis (i.e. age-dependent macrophage dysfunction [ADMD]). One reason for this is that ADMD is associated with poor NFκB and MAPK activation following Toll-like receptor stimulation (Boyd et al., 2012)(Boyd et al., 2012)(Boyd et al., 2012)[1][1][1](Boyd et al., 2012). Herein, we tested the hypothesis that inflamm-aging induces production of A20, a cytosolic and homeostatic suppressor of the NFκB and MAPK signaling cascades that deubiquitinates (i.e. inactivates) the common upstream signaling molecule TRAF6, and this is responsible for ADMD. Western blots and immunohistochemistry comparing tissues from young, mature, and aged C57BL/6 mice indicated that A20 was strongly elevated in the lungs of aged mice but not in other tissues. Elevated A20 was also detected in alveolar macrophages (AM) from aged mice. In contrast CYLD, a second deubiquitinase that also negatively regulates the NFκB pathway was decreased with aging. Following co-incubation of AM with the bacteria Streptococcus pneumoniae, TRAF6 polyubiquitination was diminished in AM isolated from aged versus young mice. A20 production was inducible in the J774A.1 macrophage cell line and C57BL/6 AM by overnight incubation with TNFα but not IL-6. Retrovirus-induced expression of A20 in J774A.1 cells resulted in their diminished production of IL-6 following exposure to S. pneumoniae but had no effect on levels of phagocytosis. Overnight incubation of AM from young mice with TNFα also resulted in a dampened IL-6 response to S. pneumoniae. Finally, dietary supplementation of aged mice with anti-inflammatory n-3 polyunsaturated fatty acids in the form of fish oil lowered lung A20 levels and enhanced resistance, including a 100-fold reduction in bacterial titers in the lungs, to experimental challenge with S. pneumoniae. We conclude that elevated A20 due to TNFα partially explains the ADMD phenotype and that ADMD is potentially reversible.
Keywords: aging, macrophage, pneumonia, inflammation, infection, innate immunity
1. INTRODUCTION
Advanced age is associated with the inability of macrophages to kill bacteria and produce the pro-inflammatory cytokines necessary to recruit and activate other immune cells (i.e. age-dependent macrophage dysfunction [ADMD] (Boyd and Orihuela 2011). This is a major problem that is best exemplified by the fact that the case-fatality rate for seniors (>65 years) hospitalized with Streptococcus pneumoniae, the leading cause of community-acquired pneumonia (van der Poll and Opal 2009), infection is 37-fold greater than for young adults (18–34 years) in the United States (Prevention 2013).
Published studies suggest that ADMD is the result of weak nuclear factor kappa B (NFκB) and mitogen-activated protein kinase (MAPK) activation following macrophage exposure to bacterial products (Boehmer et al., 2004; Boyd et al., 2012; Ding et al., 1994; Renshaw et al., 2002; van Duin et al., 2007; Yoon et al., 2004). NFκB is the central transcription factor necessary for the expression of pro-inflammatory genes encoding cytokines, chemokines and defensins (Gilmore 2006). Activated MAPK p38 and JNK (i.e. phosphorylated) are involved in directing the cellular responses to diverse stimuli including the modulation of gene expression and the stability of mRNA transcribed from pro-inflammatory genes (Doyle et al., 2004; Johnson and Lapadat 2002; Winzen et al., 1999). Importantly, Mahbub et al. have recently shown that macrophages derived from the bone-marrow of aged mice do not have age-related defects in their cytokine response to bacterial products (Mahbub et al., 2012). This suggests that ADMD is not intrinsic to the aging process, but instead a result of immune cells residing within the aged tissue environment. One potential explanation for ADMD is thereby inflamm-aging; the persistent low-grade tissue inflammation that accompanies advanced age (Franceschi 2007; Franceschi et al., 2000).
A causal link between low-grade inflammation and susceptibility to infectious diseases is supported by a positive correlation between elevated Tumor necrosis factor (TNF)α and Interleukin (IL)-6 and a greater incidence of community-acquired pneumonia in otherwise healthy seniors over a 6.5-year period (Yende et al., 2005). It is further corroborated by the fact that patients with co-morbidities that are established risk factors for pneumonia have increased circulating levels of these pro-inflammatory cytokines (Lexau et al., 2005; Naucler et al., 2013). Directly, implicating TNFα as a key cytokine that enhances susceptibility to infection, we have demonstrated that young mice (4–5 month) infused with age-relevant physiological levels of TNFα for 5 days had 100-fold more S. pneumoniae in their lungs one day after experimental infection than mice receiving saline (Hinojosa et al., 2009).
One key and shared signal transducer in the TNF receptor family, Toll-like/IL-1 receptor family, and T-cell receptor pro-inflammatory cell signaling pathways is TRAF6. Following appropriate cell stimulation, pro-inflammatory cell signaling cascades are initiated that converge and result in the polyubiquitination of TRAF6 (i.e. activation). Activated TRAF6 functions as a positive signal transducer in the NFκB pathway and is also necessary for activation of the MAPK (Hinz et al., 2010; Landstrom 2010; Mu et al., 2011; Yamashita et al., 2008). Indeed, de-ubiquitination of TRAF6 is essential to terminate/down-regulate the activation of these pro-inflammatory pathways (Boone et al., 2004). A20 is the key homeostatic suppressor of TRAF6 and is expressed as a result of TNFα induced GSK3 kinase activity and NFκB activation (Park et al., 2011). A20 is an ubiquitin-editing enzyme that interrupts TRAF6 signaling through disruption of the ubiquitin complexes attached to TRAF6 (Shembade et al., 2010). Importantly, recent studies have shown that macrophages exposed to TNFα have elevated levels of A20 and that these cells no longer function normally. In particular, they no longer produce IL-6 following lipopolysaccharide stimulation. This anergic phenotype is thought to be part of a homeostatic response meant to prevent tissue damage during prolonged/excessive inflammatory states (Baeuerle and Henkel 1994; Fan and Cook 2004; Janssen-Heininger et al., 2000; Kelly et al., 2011; Martin and Dixit 2011; Park et al., 2011).
Herein, we tested the hypothesis that ADMD is the result of basally elevated A20 levels in aged macrophages due to inflamm-aging. Because of the tremendous morbidity and mortality associated with pneumonia in the elderly, our research was focused on A20 levels in alveolar macrophages (AM), the resident innate immune cell within the lungs, and in response to S. pneumoniae. Our experimental results shed new light on the molecular mechanisms underlying ADMD and suggest that ADMD is potentially reversible.
2. MATERIALS AND METHODS
2.1. Mice, tissues, and bacteria
For experiments requiring live animals, young (4 months), mature (12 months), and aged (21 months) C57BL/6 mice of both sexes were obtained from the National Institute for Aging (NIA) Aged Rodent Colony (Charles River) and The Jackson Laboratory (a gift from Drs. David E. Harrison and Kevin Flurkey). All animal experiments were performed in compliance with approved Institutional Animal Care and Use Committee protocols at the University of Texas Health Science Center at San Antonio. Infection experiments were performed using S. pneumoniae serotype 4 strain TIGR4 (Tettelin et al., 2001).
2.2 Western blot and immunohistochmical analysis of tissues
Flash frozen tissues from male C57BL/6 mice of the designated age were obtained from the NIA Aged Rodent Tissue Bank. Tissues were homogenized in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M NaH2PO4) containing 1% (vol/vol) protease inhibitor cocktail and protein concentrations were determined using a bicinchoninic acid assay (Sigma). For each sample, 35 μg of protein was separated by SDS-PAGE under reducing conditions and transferred onto nitrocellulose using a semi-dry electrophoretic system (Bio-Rad). Immunoblot analysis was performed using monoclonal antibodies against A20 (Santa Cruz) and CYLD (Cell Signaling). Detection was performed using goat anti-mouse horseradish peroxidase conjugated secondary antibodies (Jackson ImmunoResearch) and HyGLO Chemiluminescent Substrate (Denville Scientific). To confirm equal protein load, membranes were stripped and probed with antibody against actin (Bethyl Laboratories). Band intensities on autoradiographs were determined using Quantity One software (Bio-Rad).
For visualization of A20 within the lungs, immunohistochemistry on paraffin embedded lung sections from uninfected young and aged mice was performed. Sections were de-paraffinized with xylene, rehydrated with ethyl alcohol, and then water. Samples were blocked with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS) with 0.05% Tween (T-PBS) for 1 hour. Sections were incubated with A20 rabbit antiserum (ProSci) for 1 hour. After washing three times with PBS, sections were covered with 1% BSA in T-PBS containing biotinylated goat anti-rabbit antibody (Jackson ImmunoResearch) at 1:1000. Samples were washed and incubated with streptavidin conjugate horseradish peroxidase. Sections were stained with DAB (Sigma) and counterstained with hematoxylin (Sigma). Specimens were washed and mounted with FluorSave (Millipore). Images were acquired at 40X using a Leica DFC 300FX microscope
2.3 Immunoblot analysis of isolated alveolar macrophages
Mice were asphyxiated with isoflurane in a candle jar and bronchoalveolar lavage was performed using 3 mL of ice cold PBS. Bronchoalveolar lavage fluid (BALF) was passed through a 40 μM cell strainer to remove debris, centrifuged, and the pelleted cells were then suspended in Dulbecco’s Modified Eagle Medium with 10% BSA and 1% penicillin/streptomycin (DMEM+). Those with macrophage morphology were counted using a hemocytometer and seeded in tissue culture plate at the required number. Following 1 h of adherence at 37 °C in 5% CO2, non-adherent cells were removed by gently washing and the remaining AM were allowed to rest overnight. For measurement of basal A20, CYLD, and GPR120 levels, 2.5 x 105 AM in 96-well plates were lysed with 50 μl RIPA buffer and samples were then processed for Western blot as described above. For measurement of TRAF6, 2.5 x 105 AM in 96-well plates were stimulated with media containing 105 CFU/ml of live S. pneumoniae for 20 minutes. Cells were washed then immediately lysed with RIPA buffer containing protease inhibitor. For experiments examining TRAF6 activation, an ubiquitin enrichment kit was used following the manufacture’s instruction (Thermo Scientific). For each mouse, 25 μg of infected AM protein lysate was incubated with anti-ubiquitin capture beads in spin columns overnight at 4 °C with end-over-end rotation. The following day the spin columns were centrifuged to separate unbound lysate, washed 3X with saline, and the ubiquitinated proteins were eluted from beads by boiling in 2X SDS sample buffer for 15 minutes. Samples were then analyzed by Western blot. Commercially available antibodies against A20, CYLD, GPR120 (Santa Cruz), and TRAF6 (Santa Cruz) were used.
2.4 Overexpression of A20 in J774A.1 cells and testing for ADMD
Retroviral particles that induced the expression of either myc-tagged A20 or Glutathione S-transferase (GST) were obtained from 293T cells previously transfected with plasmids encoding the retrovirus particles and myc-A20 or GST (a gracious gift from Dr. James Bliska, Stony Brook NY) (Zhang et al., 2005). J774A.1 cells were incubated in retrovirus containing 293T-conditioned media at 32° C for 24 h to allow for retroviral transduction. Transduced J774A.1 cells were then grown at 37° C for 24 h and then subjected to selection with puromycin (2 μg/ml) for 10 days with daily medium changes. The puromycin-resistant cells were maintained in DMEM-10% BSA with 2 μg/ml puromycin and used for experiments within five passages. J774A.1 cells were washed and changed to fresh medium without puromycin 3 h before any experimentation. Changes in levels of A20 in these cells were examined by Western blot as described above. For measurement of de novo IL-6 production, 2.0 x 105 J774A.1 cells were exposed to ethanol-killed pneumococci at multiplicity of infection (MOI) of .1, 1, and 10 for 4 hours, then supernatants were collected and examined by ELISA (BD Biosciences). For experiments examining macrophage phagocytosis, 106 J774A.1 cells were incubated with phRODO labeled pneumococci at an MOI of 10 for 30 minutes (Molecular Probes). Cells were placed on ice, detached, and immediately examined by flow cytometry for internalized bacteria. phRODO is only fluorescent in acidic environments such as within the phagolysosome of macrophages. This dye therefore allows for the exclusion of attached but non-internalized bacteria (Fabbrini et al., 2012).
2.5 Treatment of primary macrophages with cytokines and testing for the ADMD phenotype
Alveolar macrophages from young mice were obtained via lavage as previously described, allowed to rest overnight, and then treated with 10 ng/ml of recombinant mouse TNFα or IL-6 (Sigma) for 18 hours. The same was done for J774A.1 cells. Levels of A20 were assessed by Western blot as described above. Alternatively and following overnight TNFα treatment, young AM were exposed to 105 CFU equivalents of ethanol-killed pneumococci for 4 hours. Cells were washed, lysed, and levels of intracellular IL-6 determined by ELISA.
2.6 Treatment of aged mice with fish oil and bacterial challenge
Female C57BL/6J mice, 18-month old, were fed 5g per day of AIN-93M rodent chow supplemented with 5% corn oil (control diet; MP Biomedicals) or 1% corn oil and 4% fish oil containing 18% eicosapentaenoic acid and 12% docosahexaenoic acid (Ocean Nutrition) for two months. Mice were anesthetized with 2.5% vaporized isoflurane, and then infected intranasally with 105 cfu TIGR4 in 25 μl PBS. After 24 hours mice were euthanized and bacterial burden in the lungs was then assessed by their excision, homogenization, serial dilution of homogenates and plating, and extrapolation of colony counts following overnight incubation (Shivshankar et al., 2011). Levels of IL-6, TNF-α and Chemokine (C-X-C Motif) Ligand 1 (KC) in whole lung homogenates of the right inferior lobe were determined by ELISA (BD Biosciences). Pathological examination of Hematoxylin and Eosin (H&E) stained lung sections was completed as previously stated (Shivshankar et al., 2011).
The presence of macrophages and neutrophils in lung sections from infected mice that had received fish or control oil were examined by immunofluorescence. Sections were de-paraffinized with xylene, rehydrated with ethyl alcohol and then cold tap water. Samples were blocked in 1% BSA in T-PBS for 1 hour. Sections were incubated with antibody against F4/80 (Serotec) or Ly6G (BD Biosciences) for 1 hour. After washing three times with PBS, sections were covered with 1% BSA in T-PBS containing goat anti-rabbit Cy3 conjugated antibody (Jackson ImmunoResearch) at 1:200. Alternatively sections were incubated with CD11b antibody (BD Biosciences) for 1 hour. After washing three times with PBS, sections were covered with 1% BSA in T-PBS containing goat anti-rabbit FITC conjugated antibody (Life Technologies) at 1:1,000. Antibody incubation was followed by treatment with 4′, 6-diamidino-2-phenylindole (DAPI) at 5 μg/ml for 1 hour at room temperature. Tissue sections were washed and mounted with FluorSave (Merck Biosciences). Images were acquired at 1,000X using a Nikon AX-70 fluorescent Microscope and images processed with NIS Elements software.
3. RESULTS
3.1. A20 is elevated in aged tissues
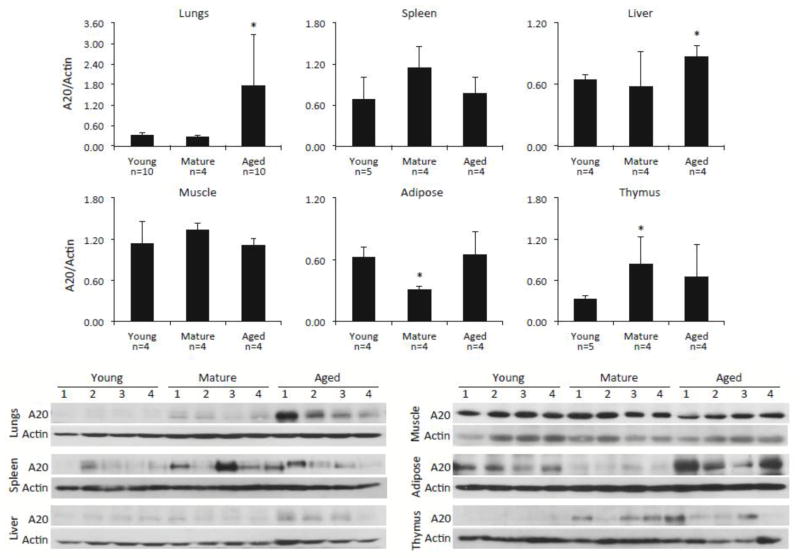
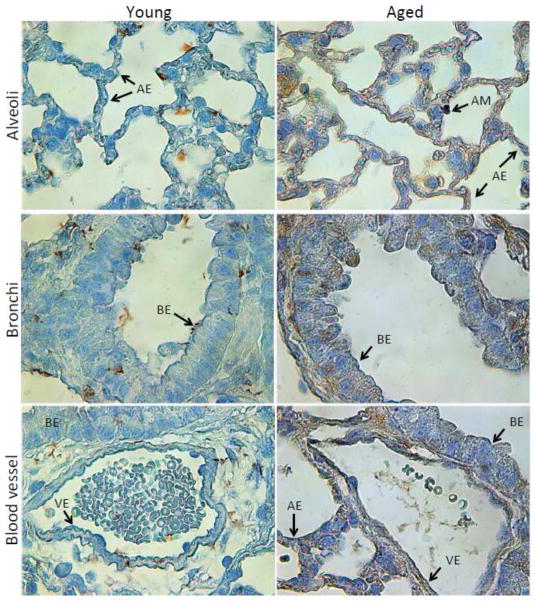
A20 has been shown to be elevated in immune cells and tissues as the result of acute injury or infection (Arvelo et al., 2002; Onose et al., 2006; Park et al., 2011). Yet, whether changes in A20 levels occurred as the result of normal aging remained unknown. Western blot analysis for A20 in different tissues from young, mature, and aged C57BL/6 mice demonstrated that aged animals had dramatically increased levels of A20 in their lungs, more moderate but significantly elevated levels of A20 in their livers, and unchanged amounts in the spleen, quadricep muscle, white adipose tissue, and thymus, versus young mice (Fig 1). For white adipose tissue and thymus, mature mice had reduced and elevated A20 levels versus young mice, respectively. Importantly, immunohistochemical staining for A20 in lung sections from young and aged mice confirmed that A20 was present in multiple cell types within the aged lungs including epithelial cells in alveoli and bronchi, vascular endothelial cells, and AM (Fig 2). Thus, A20 was not elevated in all tissues with age, but seemed to be strongly elevated within the aged lungs.
Figure 1. A20 is elevated in some but not all aged tissues.
Representative Western blots for A20 in designated tissues from young, mature and aged C57BL/6 male mice. Following detection of A20, membranes were stripped and probed for total actin. Relative levels of protein were determined by densitometry with A20 values divided by those for actin. Comparison between groups was done using a two-tailed Student’s t-test. Asterisks denote a statistically significant difference (P<0.05). The number of biological samples measured by densitometry is indicated below the X-axis.
Figure 2. Immunohistochemistry of A20 in the lungs.
Representative images of lung sections from young (n=3) and aged (n=3) C57BL/6 mice following immunohistochemistry for A20. Images were captured using light microscopy. Abbreviations and arrows denote different cell types: AE = alveolar epithelial, AM = alveolar macrophage, BE = bronchial epithelial, VE = vascular endothelial.
3.2 A20 is elevated in alveolar macrophages and is associated with reduced TRAF6 polyubiquitination
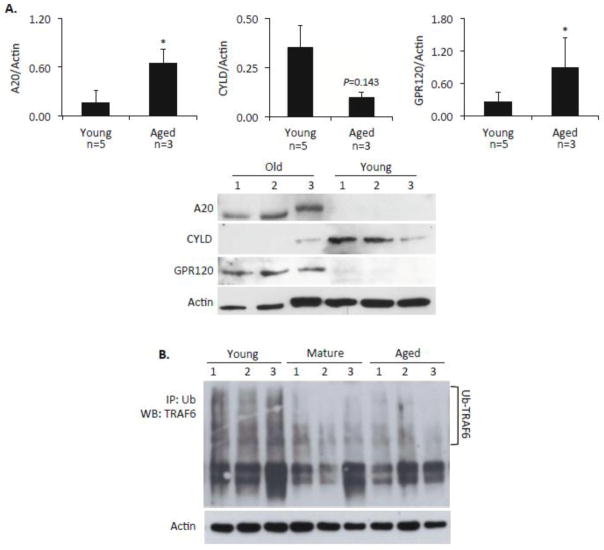
Because AM are the first innate immune cell encountered by aspirated respiratory tract pathogens and are essential for protection against pneumococcal pneumonia (Knapp et al., 2003), we next examined A20 levels in isolated AM and observed a statistically significant increase in A20 levels for those from aged versus young mice (Fig 3A). This coincided with decreased TRAF6 polyubiquitination of TRAF6 by aged AM following a 20-minute exposure to live S. pneumoniae (Fig 3B). These important results are consistent with and potentially explain our published findings that showed AM from aged mice do not fully activate NFκB or the MAPK p38 and JNK and do not produce robust amounts of IL-6 or TNFα following exposure to S. pneumoniae (Boyd et al., 2012).
Figure 3. A20 is elevated in alveolar macrophages and is associated with poor TRAF6 polyubiquitination.
A) Western blot and the corresponding densitometric analysis for A20, CYLD, and GPR120 in isolated alveolar macrophages from healthy young and aged C57BL/6 mice. Comparison between groups was done using a two-tailed Student’s t-test. Asterisks denote a statistically significant difference (P<0.05). The number of biological samples measured by densitometry is indicated below the X-axis. B) Western blot for polyubiquitinated A20 in alveolar macrophages from 3 young, 3 mature, and 3 aged mice following a 20-minute exposure to S. pneumoniae. Proteins levels prior to immunoprecipitation were confirmed to be equal by performing a Western blot for actin. Due to the limited protein volume that can be obtained from each mouse, following immunoprecipitation with antibodies to ubiquitin, the entire protein sample was loaded onto the gel.
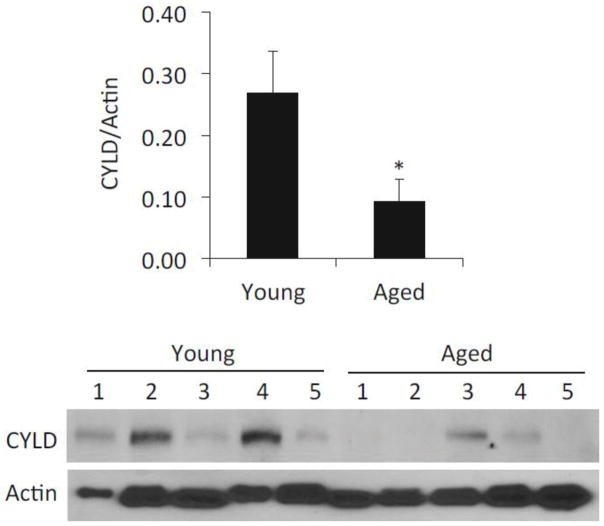
Of note, the deubiquitinase CYLD is also a negative regulator of NFκB signaling and plays an important role in the negative regulation of inflammation by macrophages (Kovalenko et al., 2003; Nishanth et al., 2013). To test if age-dependent changes in CYLD might also contribute to ADMD phenotype we compared basal CYLD levels in samples collected from healthy young and aged C57BL/6 mice. In sharp contrast to the results observed for A20, lung levels of CYLD were significantly lower in aged mice (Fig 4) as well as in isolated AM (Fig 3A). Thus, ADMD is not the result of elevated CYLD.
Figure 4. CYLD levels are lower in the aged lungs.
Western blot for CYLD in homogenized whole lungs from healthy young and aged C57BL/6 male mice (n=5 per cohort). Membranes were stripped and probed for total actin. Relative levels of protein were determined by densitometry with CYLD values divided by those for actin. Comparison between groups was done using a two-tailed Student’s t-test. Asterisks denote a statistically significant difference (P<0.05).
3.3 Expression of A20 dampens the cytokine response but not phagocytosis
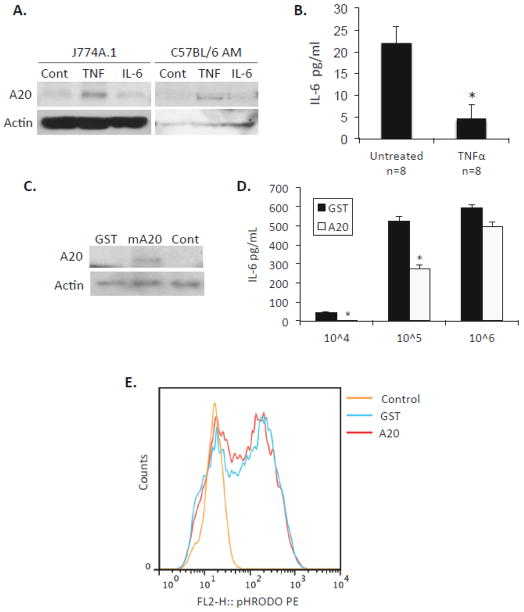
TNFα and IL-6 are the two pro-inflammatory cytokines most frequently associated with inflamm-aging. What is more, elevated levels of both have been associated with increased susceptibility to community-acquired pneumonia (Yende et al., 2005). To determine which cytokine was involved in enhanced A20 levels we exposed both J774A.1 and primary AM from young mice to 10 ng/ml of TNFα or IL-6 overnight and measured A20 levels (Fig 5A). In both instances, cells exposed to TNFα had increased A20, whereas those exposed to IL-6 were unchanged versus untreated controls. Importantly, exposure of isolated AM from young mice to TNFα was sufficient to suppress the production of IL-6 following exposure to S. pneumoniae (Fig 5B). To determine if increased A20 was alone sufficient to abrogate macrophage function, we induced expression of myc-A20 or GST in J774A.1 macrophages using retroviral particles (Fig 5C) and tested these cells for their ability to respond to ethanol killed S. pneumoniae with de novo IL-6 production. J774A.1 macrophages expressing A20 produced significantly less IL-6 than those induced with GST when challenged with 105 CFU equivalents (Fig 5D). Interestingly, this muted response was overcome when the amount of bacteria the macrophages were exposed too was increased 10-fold. We also tested the ability of these cells to phagocytize S. pneumoniae. We found that over expression of A20 had no effect on the phagocytic capacity of macrophages (Fig 5E). Thus increased A20 was sufficient to induce a muted IL-6 response, but this could be overcome with greater bacterial stimulation and enhanced A20 had no impact on the ability of macrophages to take up bacteria.
Figure 5. TNFα but not IL-6 induces A20 production and this suppresses the macrophage cytokine response to S. pneumoniae.
A) Western blot for A20 following overnight exposure of J774A.1 macrophages and primary AM from young mice with either 10 ng/ml of TNFα, IL-6, or left untreated. B) AM from young mice were exposed to TNFα or left untreated overnight then exposed to 105 CFU equivalent of ethanol killed S. pneumoniae for 4 hours (n=8 per cohort). Intracellular IL-6 was measured by ELISA following cell lysis. C) Western blot for A20 following exposure of J774A.1 macrophages to retrovirus particles that induce expression of either GST or myc-A20. D) IL-6 production by GST and myc-A20 transduced J774A.1 macrophages following their exposure to 104, 105 and 106 CFU equivalents of ethanol killed S. pneumoniae for 4 hours. E) J774A.1 cells with induced expression of GST or myc-A20 were co-incubated for 30 minutes with S. pneumoniae labeled with the pH sensitive dye phRODO. Relative levels of phagocytosis were determined by flow cytometry. Because phRODO is only fluorescent under acidic conditions only macrophages with bacteria within a phagolysosome are fluorescent.
3.5 Anti-inflammatory fish oil reduces A20 and protects aged mice from pneumonia
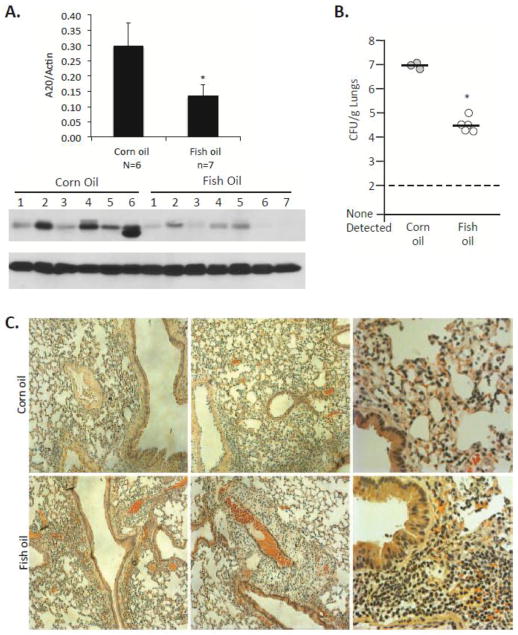
If ADMD is the result of inflamm-aging, then pharmacological reduction in inflamm-aging should reduce A20 levels and protect against bacterial challenge. Macrophages express high levels of GPR120, the receptor for anti-inflammatory n-3 polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid (Oh et al., 2010). Importantly, GPR120 was elevated in AM isolated from aged mice (Fig 3A). We therefore tested if aged mice fed chow supplemented to 4% with purified fish oil containing 18% docosahexaenoic acid and 12% eicosapentaenoic acid for 2 months (i.e. fish oil diet), had lower A20 levels in their lungs versus those receiving n-6 polyunsaturated fatty acid linoleic acid (i.e. corn oil) supplemented diets or normal chow. As shown in Fig 6A, this was shown to be the case. More importantly, aged mice that received fish oil had a 100-fold reduction in the number of bacteria in their lungs 24 hours after intranasal challenge with S. pneumoniae versus the corn oil fed controls (Fig 6B). Pathological examination of aged mouse lungs after 24 hours, suggested that those receiving fish oil had greater numbers of immune cells in their lungs (Fig 6C), which may have facilitated clearance of bacteria. This was subsequently confirmed by immunofluorescent microscopy, which detected greater numbers of macrophages (F4/80+, CD11b+) and neutrophils (Ly6G+, CD11b+) in the lungs of mice that had received the fish oil diet (Supplemental Fig 1). These results suggest that ADMD is potentially reversible.
Figure 6. Fish oil lowers A20 levels and improves resistance to bacterial infection.
A) Western blot and the corresponding densitometric analysis for A20 in the lungs of aged C57BL/6 mice that had received chow supplemented with fish oil and corn oil, respectively, for 2 months. Comparison between groups was done using a two-tailed Student’s t-test. Asterisks denote a statistically significant difference (P<0.05). The number of biological samples measured by densitometry is indicated below the X-axis. B) The number of bacteria in the lungs of mice on these supplemented diets 24 hours after intranasal challenge with S. pneumoniae. Each circle denotes an individual mouse. Asterisks denote a statistically significant difference (P<0.05). C) Representative images captured using light microscopy of Hematoxylin & Eosin stained lung sections from young C57BL/6 mice that were on supplemented diets 24 hours after infection with S. pneumoniae.
4. DISCUSSION
Increased susceptibility to infection with aging is due to multiple defects in both the innate and adaptive arms of the immune system. For tissue macrophages, this includes a reduced pro-inflammatory cytokine response to Toll-like receptor ligands, diminished phagocytosis and bacterial killing ability, and impaired macrophage polarization (Boehmer et al., 2004; Brubaker et al., 2011; Mahbub et al., 2012). Importantly, in an aged animal ADMD coincides with inflamm-aging. What is more, elevated levels of IL-6 and TNFα have been directly linked to enhanced susceptibility to lung infection (Yende et al., 2005). Herein our experimental studies suggest that A20 is basally elevated in lung cells as a result of increased TNFα levels with aging. We also show that elevated A20 impairs the ability of macrophages to respond to bacteria, such as with S. pneumoniae, with a robust cytokine response. Our studies therefore provide a molecular explanation for one important aspect of ADMD. Importantly, inflamm-aging may be contributing to the enhanced susceptibility to infection in many ways. Previously we have shown that the pro-inflammatory phenotype of senescent cells promotes bacterial adhesion to lung cells through increased expression of NFκB-regulated host proteins that are co-opted by respiratory tract pathogens for adhesion (Shivshankar et al., 2011).
Interestingly, A20 levels were starkly elevated only within the lungs of C57BL/6 mice. Why this would be the case is not clear as studies by Spencer et al. have shown that other tissues from aged C57BL/6 mice have greater levels of basally elevated NFκB activation (i.e. inflamm-aging) versus young controls (Spencer et al., 1997). Yet studies suggest that the lungs are particularly affected during advanced age. Aged lungs of humans and mice have been documented to have increased levels of senescent cells, elevated levels of pro-inflammatory cytokines, and an increased presence of neutrophils despite the absence of infection (Kreiling et al., 2011; Meyer et al., 1998; Shivshankar et al., 2011). We speculate that this sterile inflammation in the aged lungs, and concomitant with this the elevated A20 levels, is because of the daily and lifetime accumulation of insults (e.g. infectious agents, allergens, chemicals and toxins), that are experienced by this organ. Indeed cellular senescence and with it lung inflammation is well documented to occur in the lungs as a result of smoking (Tsuji et al., 2010). Regardless, of the cause, immunohistochemistry and Western blot data clearly demonstrated that A20 levels were starkly elevated for epithelial cells within alveoli and the bronchial lining as well as vascular endothelial cells. While unexplored, this finding strongly implies that the chemokine response by these cells to bacteria would also be impaired and this would also contribute towards the susceptibility to pneumonia.
Similar to A20, CYLD negative regulates NFκB activation with A20 and CYLD deletion both having been shown to result in an unregulated, excessive and damaging pro-inflammatory response (Lee et al., 2000; Nishanth et al., 2013). Interestingly A20 but not CYLD was elevated as the result of aging. In fact, CYLD was lower in the samples obtained from aged mice. This suggests that these ubiquitin editing enzymes are differentially regulated and have separate roles during normal aging. Along such lines, A20 and CYLD have been shown to have separate roles during B-cell activation with Toll-like receptor ligands (Chu et al., 2012). While in this study we implicate elevated A20 as a cause of ADMD, obtaining a more comprehensive understanding of how these deubiquitinases are regulated, their overlapping/distinct roles, and clarity in our understanding of their role during aging is important to understand how the innate immune system is altered during aging.
In previous studies we have determined that aged mice challenged with S. pneumoniae failed to respond to pneumococci following intratracheal challenge during early infection. This contributed to a 100-fold greater difference in the number of S. pneumoniae in the lungs of aged versus young mice 24 hours after challenge, greater tissue damage in the lungs of aged mice, and 90% versus 20% mortality, respectively (Boyd et al., 2012; Hinojosa et al., 2009). Basally elevated A20 levels in the lungs of aged mice pre-infection and reduced TRAF6 polyubiquitination following infection is a potential explanation for enhanced susceptibility, as it explains why we also observed reduced NFkB activation and reduced activation of the MAPK p38 and JNK in AM from aged mice. Importantly, we observed no defect in opsonophagocytosis for J774A.1 cells transfected with A20. This suggests that the inability of macrophages to take up bacteria is through different mechanisms. Interestingly, in the same cells, the blunted de novo IL-6 response was overcome when we increased the amount of ethanol-killed S. pneumoniae. Thus, suggesting that enhanced TLR stimulation is one method to overcome the anergizing effects of basally elevated A20. Since vaccine efficacy is dependent on appropriate cytokine production by antigen presenting cells such as resident macrophages and dendritic cells, this finding supports current strategies to overcome the weak vaccine response in the elderly by including TLR adjuvants (Dulthie et al., 2011).
A20 could be induced in a cell line and primary macrophages with overnight treatment of TNFα but not IL-6. This was consistent with the fact that A20 is known to be upregulated following GS3K activation by TNFα and directly implicates this inflamm-aging cytokine as a cause of ADMD. Along such lines, diet supplementation of aged mice with n-3 PUFA, an anti-inflammatory agent, reduced A20 levels and dramatically improved resistance of aged mice to pneumococcal infection. These findings support the counterintuitive notion that a mild anti-inflammatory drug can be used to lower basal A20 levels and restore the acute pro-inflammatory response to infection. n-3 PUFA most likely has other effects that were unexplored such as lowering the levels of NFκB-regulated bacterial ligands. One important caveat to our in vitro and ex vivo A20 induction with TNFα results is that we employed high concentrations of cytokines (10 ng/ml). Yet these results serve as proof that exposure to TNFα, but not IL-6, can suppress primary AM function. Importantly, the effects of long-term exposure of AM to low-levels of TNFα remain unknown. How elevated A20 levels impacts other parameters of AM function such as chemotaxis, antigen presentation, or even wound repair following lung injury also remains unknown and these topics warrant study.
In summary, the ADMD phenotype is recognized to contribute to the increased susceptibility of the elderly to infection. Past studies have shown that this is due in part to reduced NFkB and MAPK activation. Herein we show for the first time that normal aging is associated with increased A20 levels in lung cells and macrophages, presumably due to inflammaging and increased TNFα levels. This in turn contributed to the inability of AM to respond to bacterial stimulation with a pro-inflammatory cytokine response. Importantly, this phenotype could be ameliorated with a mild anti-inflammatory agent.
Supplementary Material
HIGHLIGHTS.
A20 is elevated in lungs and alveolar macrophages from healthy aged mice
TRAF6 polyubiquitination is inhibited in alveolar macrophages from aged mice
The aged macrophage phenotype can be induced by expressing A20 or TNFα treatment
TNFα but not IL-6 induces A20
Dietary fish oil lowers A20 levels and protects aged mice from S. pneumoniae
Acknowledgments
We thank David E. Harrison and Kevin Flurkey at The Jackson Laboratory for their gift of aged mice. Financial support for CJO came from the Morrison Trust, The Glenn Foundation, and NIH AG033274.
ABBREVIATIONS
- ADMD
age-dependent macrophage dysfunction
- BALF
bronchoalveolar lavage fluid
- BSA
bovine serum albumin
- DAPI
4′, 6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle medium
- GST
glutathione S-transferase
- H&E
hematoxylin & eosin
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection, NFκB, nuclear factor kappa B
- NIA
National Institute for Aging Research
- PBS
phosphate-buffered saline
- PUFA
polyunsaturated fatty acids
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. CITATIONS
- Arvelo MB, Cooper JT, Longo C, Daniel S, Grey ST, Mahiou J, Czismadia E, Abu-Jawdeh G, Ferran C. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology. 2002;35:535–543. doi: 10.1053/jhep.2002.31309. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011;2:487–500. [PMC free article] [PubMed] [Google Scholar]
- Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging Dis. 2011;2:346–360. [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Soberon V, Glockner L, Beyaert R, Massoumi R, van Loo G, Krappmann D, Schmidt-Supprian M. A20 and CYLD do not share significant overlapping functions during B cell development and activation. J Immunol. 2012;189:4437–4443. doi: 10.4049/jimmunol.1200396. [DOI] [PubMed] [Google Scholar]
- Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–2152. [PubMed] [Google Scholar]
- Doyle SE, O’Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, Lane TF, Cheng G. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulthie MS, Plessner Windish H, Reed SG. Use of defined TLR lignads as adjuvants with human vaccines. Immunol Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini M, Sammicheli C, Margarit I, Maione D, Grandi G, Giuliani MM, Mori E, Nuti S. A new flow-cytometry-based opsonophagocytosis assay for the rapid measurement of functional antibody levels against Group B Streptococcus. J Immunol Methods. 2012;378:11–19. doi: 10.1016/j.jim.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kelly C, Shields MD, Elborn JS, Schock BC. A20 regulation of nuclear factor-kappaB: perspectives for inflammatory lung disease. Am J Respir Cell Mol Biol. 2011;44:743–748. doi: 10.1165/rcmb.2010-0339TR. [DOI] [PubMed] [Google Scholar]
- Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Resp Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Kreiling JA, Tamamori-Adachi M, Sexton AN, Jeyapalan JC, Munoz-Najar U, Peterson AL, Manivannan J, Rogers ES, Pchelintsev NA, Adams PD, Sedivy JM. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10:292–304. doi: 10.1111/j.1474-9726.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–589. doi: 10.1016/j.biocel.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, Hadler J, Cieslak PR, Whitney CG. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interf Cyt Res. 2012;32:9. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Dixit VM. A20 edits ubiquitin and autoimmune paradigms. Nat Genet. 2011;43:822–823. doi: 10.1038/ng.916. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, Heldin CH, Landstrom M. TRAF6 ubiquitinates TGFbeta type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naucler P, Darenberg J, Morfeldt E, Ortqvist A, Henriques Normark B. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax. 2013;68:571–579. doi: 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- Nishanth G, Deckert M, Wex K, Massoumi R, Schweitzer K, Naumann M, Schluter D. CYLD enhances severe listeriosis by impairing IL-6/STAT3-dependent fibrin production. PLoS Pathog. 2013;9:e1003455. doi: 10.1371/journal.ppat.1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onose A, Hashimoto S, Hayashi S, Maruoka S, Kumasawa F, Mizumura K, Jibiki I, Matsumoto K, Gon Y, Kobayashi T, Takahashi N, Shibata Y, Abiko Y, Shibata T, Shimizu K, Horie T. An inhibitory effect of A20 on NF-kappaB activation in airway epithelium upon influenza virus infection. Eur J Pharmacol. 2006;541:198–204. doi: 10.1016/j.ejphar.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Park SH, Park-Min KH, Chen J, Hu X, Ivashkiv LB. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat Immunol. 2011;12:607–615. doi: 10.1038/ni.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, Centers for Disease Control. Streptococcus pneumoniae, 2011. 2013. Active Bacterial Core Surveillance Report, Emerging Infections Program Network. [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011;10:798–806. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NF, Poynter ME, Im SY, Daynes RA. Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol. 1997;9:1581–1588. doi: 10.1093/intimm/9.10.1581. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, de Rekeneire N, Kritchevsky SB. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ting AT, Marcu KB, Bliska JB. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.