Abstract
Chronic cytomegalovirus (CMV) infection may contribute significantly to T-cell immunosenescence, chronic inflammation, and adverse health outcomes in older adults. Recent studies suggest detectable CMV DNA in peripheral monocytes as a better indicator for this persistent viral infection than anti-CMV IgG serology. Here, we conducted longitudinal comparisons of anti-CMV IgG titers, CMV DNA in the peripheral monocytes, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8+ T-cell frequencies in fifteen community-dwelling older women with twelve year follow-up. The results showed that anti-CMV IgG titers did not change over twelve years. Women with detectable CMV DNA had significantly higher IL-6 levels than those without, both at baseline (3.06+0.58 vs 1.19±0.37 pg/ml, respectively, p< .001) and at the follow-up (3.23±0.66 versus 0.98±0.37 pg/ml, respectively, p< .001). In addition, CMV pp65 (NLV)-specific CD8+ T cells were detected only in women who had CMV DNA with similar frequencies at both time points. These findings indicate that anti-CMV IgG serology is not sensitive to change nor useful for monitoring chronic CMV infection over time. They also provide a basis for further investigation into chronic CMV infection as defined by detectable CMV DNA in the peripheral monocytes and its impact on immunity and health in the elderly.
Keywords: chronic CMV infection, CMV DNA in monocytes, IL-6, CMV pp65 (NLV)-specific CD8+ T cells, longitudinal, aging
1. INTRODUCTION
A large body of literature demonstrates that chronic cytomegalovirus (CMV) infection has significant adverse impact on the immunity and health of older adults. For example, anti-CMV IgG seropositivity is a key component of the proposed “immune risk profile”, which predicts increased mortality in those 85 and above in the Swedish OCTO and NONA immune studies1–3. Data from large cohort studies of older women and Latinos have also shown significant associations of CMV seropositivity or absolute anti-CMV IgG titers with frailty, disability, and mortality4–8. It is suggested that CMV-related T-cell immunosenescence is an important mechanism for the adverse immune and health impact of this chronic viral infection which was previously thought to be harmless for immunocompetent individuals. This is supported, at least in part, by the clonal expansion of CD4+ and CD8+ T cells specific to CMV described in some CMV-seropositive older adults1;9–15. However, significant heterogeneity has been observed in the CMV-seropositive elderly population. For instance, recent large epidemiological studies have shown no association between positive anti-CMV IgG titers and frailty in a cohort of octogenarians, nor with increased disability or decreased resilience in community-dwelling older adults16;17. Critical evaluation of the literature reveals that despite CMV-seropositive older adults had an overall higher mean of CMV pp65-specific CD8+ T-cell frequencies than CMV-seronegative controls, significant number of CMV-seropositive individuals had minimal or no increase of CMV pp65-specific CD8+ T cells10;15. From an immunological point of view, positive anti-CMV IgG serology is a crude measure that merely indicates prior exposure to the virus and does not distinguish chronic (persistent) from past (resolved) infections. To begin to address this important topic, we have recently developed a highly sensitive and specific nested PCR-based assay for the detection of CMV DNA in peripheral blood monocytes, a known host cell type that harbors this virus in chronic or latent CMV infection18;19. Our data indicate that CMV DNA is present in only about 60% of CMV seropositive older adults and that CMV DNA positivity, rather than IgG titer, is associated with increased CMV pp65 (NLV)-specific CD8+ T cells20. In fact, the percentages of CMV pp65 (NLV)-specific CD8+ T cells did not overlap at all between CMV DNA+ and CMV DNA− individuals. However, little is known about any longitudinal changes of anti-CMV IgG serology, presence of CMV DNA in peripheral monocytes, and frequencies of CMV pp65 (NLV)-specific CD8+ T cells in older adults over time.
Substantial evidence suggests a key role of chronic inflammation, as marked by elevated circulating levels of IL-6 and C-reactive protein (CRP) in the development of a number of chronic conditions and adverse health in older adults. We and others have shown significant associations of elevated IL-6 and CRP levels with frailty, disability, and mortality in older adults21–25. While chronic inflammation is hypothesized as a key pathophysiological process through which chronic CMV infection contributes to adverse health outcomes in older adults, direct evidence supporting this hypothesis is scarce. Schmaltz and colleagues demonstrated synergy between CMV seropositivity and serum IL-6 levels in their associations with frailty in community-dwelling older women4. Using the nested PCR-based assay described above, we showed a significant association of chronic CMV infection, as defined by the presence of CMV DNA in the peripheral monocytes with elevated levels of neopterin, a biomarker for immune activation26. However, a direct in vivo relationship between presence of CMV DNA in peripheral monocytes and circulating IL-6 levels in older adults has yet to be investigated.
The objective of this study was to investigate potential longitudinal changes of anti-CMV IgG serology and detectable CMV DNA in the peripheral monocytes, and their relationships with serum IL-6 levels and frequencies of CMV pp65 (NLV)-specific CD8+ T cells in older adults over a long period of time. We hypothesized that anti-CMV IgG serology, both CMV serostatus and absolute anti-CMV IgG titers, would not change over time and that presence of CMV DNA in monocytes instead of positive CMV serology would be associated with increased IL-6 levels and CMV pp65 (NLV)-specific CD8+ T-cell frequencies. To test these hypotheses, we took advantage of the availability of cryopreserved samples of sera and peripheral blood mononuclear cells (PBMCs) from the Women’s Health and Aging Studies (WHAS) II collected at its baseline in 1995 and again in 2007 from the same individuals and conducted repeated evaluation of the above four parameters concerning chronic CMV infection, chronic inflammation, and CMV-specific T-cell immunity, twelve years apart.
2. MATERIALS AND METHODS
2.1. Human subjects
Subjects in this study were older women who participated in the WHAS II study, which is a longitudinal cohort study of community-dwelling women aged 70 to 79 years in Baltimore, Maryland. Details of the WHAS II study design and methods have been described elsewhere27. Briefly, WHAS II participants were recruited from an age-stratified random sample of women selected from Medicare enrollees residing in 12 contiguous ZIP code areas in Baltimore, Maryland. Women were screened to identify self-reported physical disability that was categorized into four domains according to report of difficulty with tasks in mobility, upper extremity function, higher-functioning household management, and self-care. Eligible women had no disability or disability in only one domain, representing the two-thirds least-disabled women living in the community. The baseline visit of the WHAS II study was in 1994–1995, and it had longitudinal follow-up through 2007. In addition to the standardized interviews and examinations conducted by a trained registered nurse, blood samples were collected at baseline and in 2007. Sera and PBMCs were isolated and stored in −80° freezer and liquid nitrogen, respectively, using standardized protocols. The Johns Hopkins Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants.
2.2. CMV viral DNA detection in peripheral blood monocytes by nested polymerase chain reaction (PCR)
For the present study, PBMCs were retrieved from the WHAS II repository and thawed and cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Gaithersberg, MD) at 37°C in a humidified 5% CO2 incubator. Monocytes were enriched by 2 hr-incubation with removal of non-adherent cells. The number of CD14+ monocytes was assessed by flow cytometry and standardized as previously described20;26. DNA was extracted from enriched peripheral blood monocytes using a Qiagen kit (Qiagen, Valencia, CA) and quantified using a standard laboratory protocol. Nested PCR with primers targeted to the CMV UL123 gene was performed using Tapbead hot start polymerase (Promega, Madison, WI) with 1.5 mM MgCl2. The primers included a first set: forward 5’-CAATACACTTCATCTCCTCGAAAGG-3’ and reverse 5’-ATGGAGTCCTCTGCCAAGAGAAAGATGGAC-3’ and second set: forward 5’-TCTGCCAGGACATCTTTCTC-3’ and reverse 5’-GTGACCAAGGCCACGACGTT-3’) as previously reported20;26. Sample DNA (50 ng) was added to the first round PCR from which 2 µl of the product mix was added to the second round PCR with a thermal cycling program of enzyme activation for 5 min at 95°C and 40 cycles of 1 min denaturation at 94°C, 1 min annealing at 45°C, and 2 min extension at 72°C for both PCR reactions. A 167-bp CMV viral DNA fragment was visualized by gel electrophoresis and confirmed by DNA sequencing. The quality of input sample DNA was confirmed by amplification of a cellular house-keeping gene glyceraldehydes 3 phosphate dehydrogenase (GAPDH). Purified human CMV laboratory strain AD169 DNA (CMVAD169, Advanced Biotech Inc, Paterson, NJ) was employed as a positive control. In pilot experiments, CMV DNA was detected in all experiments when 2 or more copies of CMVAD169 were in the sample DNA after series of dilutions and at about half of the times when 1 copy of CMVAD169 was calculated to be present. Negative results for CMV DNA detection were confirmed by increasing the amount of input sample DNA to 500 ng.
2.3. Measurements of serum anti-CMV IgG antibody titers and IL-6 levels
Anti-CMV IgG titers were determined in frozen sera collected at both time points from the same individuals using a commercially available enzyme-linked immunosorbent assay (ELISA, GenWay Biotech Inc., San Diego, CA) with an interassay coefficient of variance (CV) of 5.2%; a titer of 1.2 ELISA Units (EU)/ml or greater was pre-determined by the manufacturer as CMV-seropositive. Data on IL-6 and CRP levels of the study subjects at WHAS II baseline visit are available in the WHAS II dataset, which was measured in duplicate using commercially available ELISA from frozen serum samples (High-Sensitivity IL-6 or CRP Quantikine Kit, respectively, R&D Systems, Minneapolis, MN). In the present study, the same High-Sensitivity IL-6 Quantikine kit from the same manufacturer was employed to measure IL-6 levels in frozen serum samples collected in 2007, which has the sensitivity of 0.02 pg/ml and interassay CV of 3.9%.
2. 4. Determination of HLA-A2 status and frequencies of CMV pp65 (NLV)-specific CD8+ T-cells by tetramer analysis
HLA-A2 status (positive/negative) was determined by PCR as described28. CMV pp65 (NLV)-specific CD8+ T cells were identified using an HLA-A2 Class I tetramer containing the CMV pp65495–503 (NLVPMVATV) peptide (Beckman Coulter, Inc. Miami, FL). This CMV pp65-tetramer was conjugated to allophycocyanin (APC) and was used with conjugated antibodies (Becton Dickinson) to CD4 (Pacific Blue), and CD8 (APC-Cy7), and analyzed on a Canto II flow cytometer (Becton Dickinson). The percentages of tetramer binding CD4+ T cells and CD8dim T cells were subtracted from the percentage of CMV pp65 (NLV)-specific tetramer binding.
2. 5. Statistical analysis
We compared baseline demographic and health characteristics between the eight CMV DNA (−) women and the seven CMV DNA (+) women using the two-sided two-sample t-test with unequal variances for continuous variables and the Fisher’s exact test for categorical variables. The type I error rate was set to 0.05 for all tests performed. Stata/SE software, version 12.1 was used for hypothesis testing (Stata Corporation, College Station, TX).
3. RESULTS
3.1. Baseline demographic and clinical characteristics of the study participants
Fifteen WHAS II participants completed the study. Participant selection was based on the availability of sufficient sera and PBMCs collected at baseline visit in 1994–1995 and follow-up visit in 2007. Table 1 summarizes major demographic and clinical characteristics of the study participants at WHAS II baseline. The mean age of the participants was 72.3 years. The majority were Caucasians with an education level of high school or above. Participants with detectable CMV DNA and those without were similar in age, race, sex, education, BMI, number of diseases, self-reported health status, and smoking status. Participants with detectable CMV DNA had a trend to higher serum CRP level than those without. The difference did not reach statistical significance.
Table 1.
Selected demographic and clinical characteristics of the study participants by CMV DNA detection status at WHAS II baseline in 1994–1995
| Characteristics | Total participan ts (n=15) |
CMV DNA (−) (n=8) |
CMV DNA (+) (n=7) |
P Value |
|---|---|---|---|---|
| Age (yrs): mean (SD) | 72.3 (2.4) | 72.0 (2.6) | 72.7 (2.2) | .57 |
| Education (yrs): mean (SD) | 15.5 (2.6) | 14.0 (2.9) | 16.1 (2.2) | .35 |
| Race (White), % | 73.3 | 62.5 | 85.7 | .57 |
| BMI (kg/m2), mean (SD) | 26.3 (4.0) | 26.0 (4.6) | 26.7 (3.4) | .74 |
| Smoking status (%) | .45 | |||
| Never smoker | 40.0 | 25.0 | 57.1 | |
| Former smoker | 53.3 | 62.5 | 42.9 | |
| Current smoker | 6.7 | 12.5 | 0 | |
| Number of clinical diagnoses: mean (SD) | 1.5 (1.0) | 1.4 (1.2) | 1.6 (0.8) | .71 |
| Self-reported health (%) | .72 | |||
| Excellent | 20.0 | 12.5 | 28.6 | |
| Very good | 40.0 | 50.0 | 28.6 | |
| Good | 33.3 | 25.0 | 42.9 | |
| Fair | 6.7 | 12.5 | 0 | |
| Serum IL6 (pg/ml): mean (SD) | 2.07 (1.08) | 1.19 (0.37) | 3.07 (0.62) | < .001 |
| Serum CRP (pg/ml): mean (SD) | 2.57 (1.23) | 2.24 (0.67) | 2.96 (1.63) | .31 |
Note: P values were from two-sample t-tests between CMV DNA (+) and (−) study groups; WBC= white blood cell; IL-6=interleukin-6; SD=standard deviation.
3.2. Comparison of CMV DNA detection and anti-CMV IgG titers between 1995 and 2007
As shown in Table 2, most WHAS II baseline samples were collected in 1995 except for two collected in late 1994 (subjects #1 and #8). Among the fifteen samples tested, twelve were CMV seropositive and three seronegative. Subject #1 had a borderline positive anti-CMV IgG titer, 1.73 EU/ml. When tested for the presence of CMV viral DNA in peripheral monocytes, seven CMV-seropositive subjects had detectable CMV DNA. The remaining five seropositives, including subject #1, and the three seronegative individuals had no detectable viral DNA in the peripheral monocytes. Importantly, when these women were tested again in 2007 (now in their 80s), their CMV serostatus and absolute anti-CMV IgG titers remained essentially the same (Table 2). Subject #1 became borderline seronegative in 2007, but her absolute anti-CMV IgG titer changed very little (1.12 EU/ml). All seven women who had detectable CMV DNA in the peripheral monocytes at baseline remained CMV DNA positive in 2007, and all eight women who did not have CMV DNA at baseline remained CMV DNA negative twelve years later. Of note, while subject #1 was converted to borderline CMV seropositive to seronegative, she were CMV DNA negative at both time points. These results indicate little longitudinal change in anti-CMV IgG serology (serostatus or absolute titers) or CMV DNA status from 1994–1995 to 2007.
Table 2.
Summary of the four study parameters and dates of sample collection
| Sample collected at baseline visit (1994–5) | Sample collected in 2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Date of sample collection |
Anti- CMV IgG titers* (EU/ml) |
CMV DNA |
IL-6 (pg/ml) |
CMV pp65 (NLV) - CD8+ T cells (%) |
Date of sample collection |
Anti- CMV IgG titers (EU/ml) |
CMV DNA |
IL-6 (pg/ml) |
CMV pp65 (NLV) - CD8+ T cells (%) |
| 1 | 11/21/1994 | 1.73 | − | 1.91 | 11/07/2007 | -** | − | 1.04 | ||
| 2 | 10/10/1995 | - | − | 1.14 | 11/06/2007 | - | − | 0.95 | ||
| 3 | 08/10/1995 | - | − | 1.04 | 10/30/2007 | - | − | 0.93 | ||
| 4 | 07/03/1995 | - | − | 1.22 | 0.04 | 11/20/2007 | - | − | 1.51 | 0.02 |
| 5 | 06/13/1995 | 14.05 | − | 1.28 | 0.02 | 11/21/2007 | 15.51 | − | 0.70 | 0.02 |
| 6 | 01/25/1995 | 7.35 | − | 0.55 | 11/16/2007 | 9.76 | − | 0.38 | ||
| 7 | 09/13/1995 | 9.79 | − | 1.24 | 12/19/2007 | 11.13 | − | 0.89 | ||
| 8 | 12/22/1994 | 11.23 | − | 1.14 | 0.02 | 10/29/2007 | 10.91 | − | 1.44 | 0.01 |
| 9 | 12/05/1995 | 10.16 | + | 3.38 | 11/20/2007 | 12.88 | + | 3.88 | ||
| 10 | 03/16/1995 | 8.15 | + | 2.51 | 11/27/2007 | 9.24 | + | 2.36 | ||
| 11 | 12/04/1995 | 11.48 | + | 3.53 | 0.85 | 11/20/2007 | 11.01 | + | 3.43 | 0.88 |
| 12 | 09/27/1995 | 17.16 | + | 4.11 | 0.64 | 11/21/2007 | 17.48 | + | 3.43 | 0.83 |
| 13 | 06/21/1995 | 7.54 | + | 2.58 | 11/20/2007 | 7.753 | + | 2.41 | ||
| 14 | 10/19/1995 | 16.53 | + | 2.5 | 10/22/2007 | 16.31 | + | 2.89 | ||
| 15 | 12/11/1995 | 3.18 | + | 2.84 | 12/07/2007 | 5.16 | + | 4.24 | ||
Values below the predetermined cut-off value for CMV-seropositivity (1.2 EU/ml) were recorded (−);
The absolute anti-CMV IgG titer value was 1.12 EU/ml.
3.3. Comparison of longitudinal relationships between CMV DNA detection, anti-CMV IgG serology, and serum IL-6 levels between 1995 and 2007
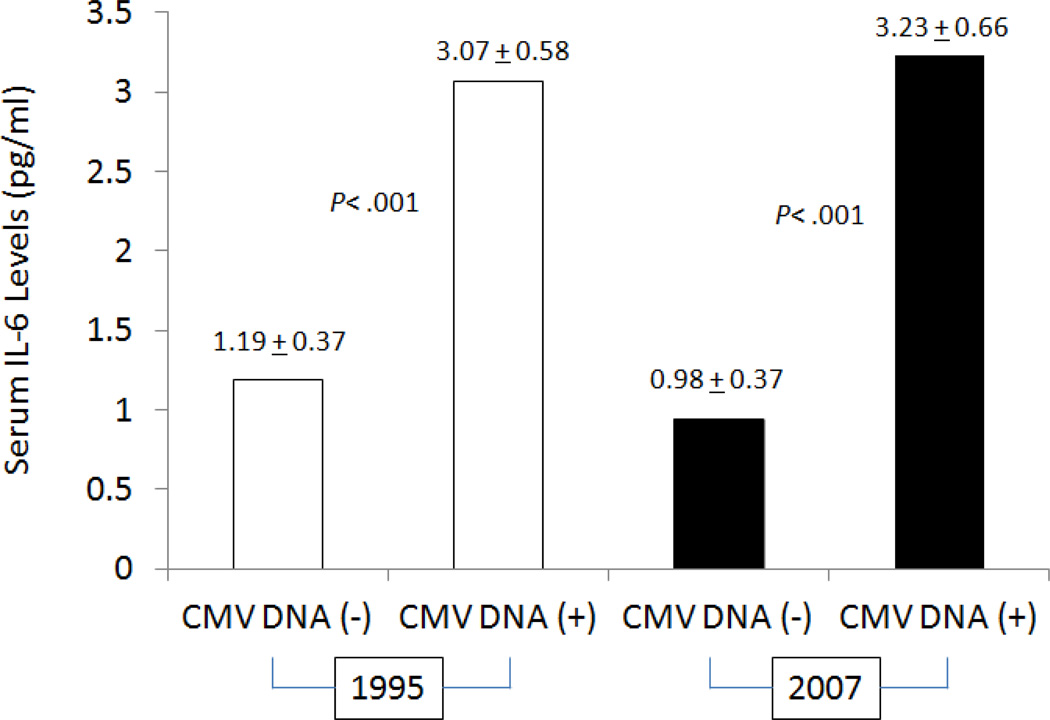
Next, we evaluated whether CMV DNA detection in the peripheral monocytes and anti-CMV IgG serology were associated with serum IL-6 levels at the two time points tested. As shown in Fig. 1, participants with detectable CMV DNA in the peripheral monocytes had significantly higher serum IL-6 levels than those without in both 1995 (3.06+0.58 versus 1.19±0.37 pg/ml, respectively, p< .001) and 2007 (3.23±0.66 versus 0.98±0.37 pg/ml, respectively, p< .001). Among the eight women who had no detectable viral DNA in the peripheral monocytes at both times, serum IL-6 levels were very similar at baseline and follow-up visits. This was true for the four individuals who were CMV seronegative (including subject #1) and the remaining four who were seropositive with high absolute anti-CMV IgG titers (Table 2). All seven women who had detectable CMV DNA in the peripheral monocytes were also CMV seropositive with high absolute anti-CMV IgG titers. It was those who had higher serum IL-6 levels in both 1995 and 2007 (Table 2). These data suggest that the presence of CMV DNA in the peripheral monocytes rather than anti-CMV serology is associated with elevated serum IL-6 levels.
Figure 1.
Serum IL-6 concentrations were determined in samples collected from eight women with detectable CMV DNA (CMV DNA+) and seven women without (CMV DNA−) in both 1995 and 2007, and they were compared between the two study groups in 1995 (open bars) and 2007 (solid bars). P values for the group differences were determined using two-sided two-sample t-test with unequal variances with log transformed IL-6 values
3.4.Comparison of longitudinal relationships between CMV DNA detection, anti-CMV IgG serology, and CMV pp65 (NLV)-specific CD8+ T cells between 1995 and 2007
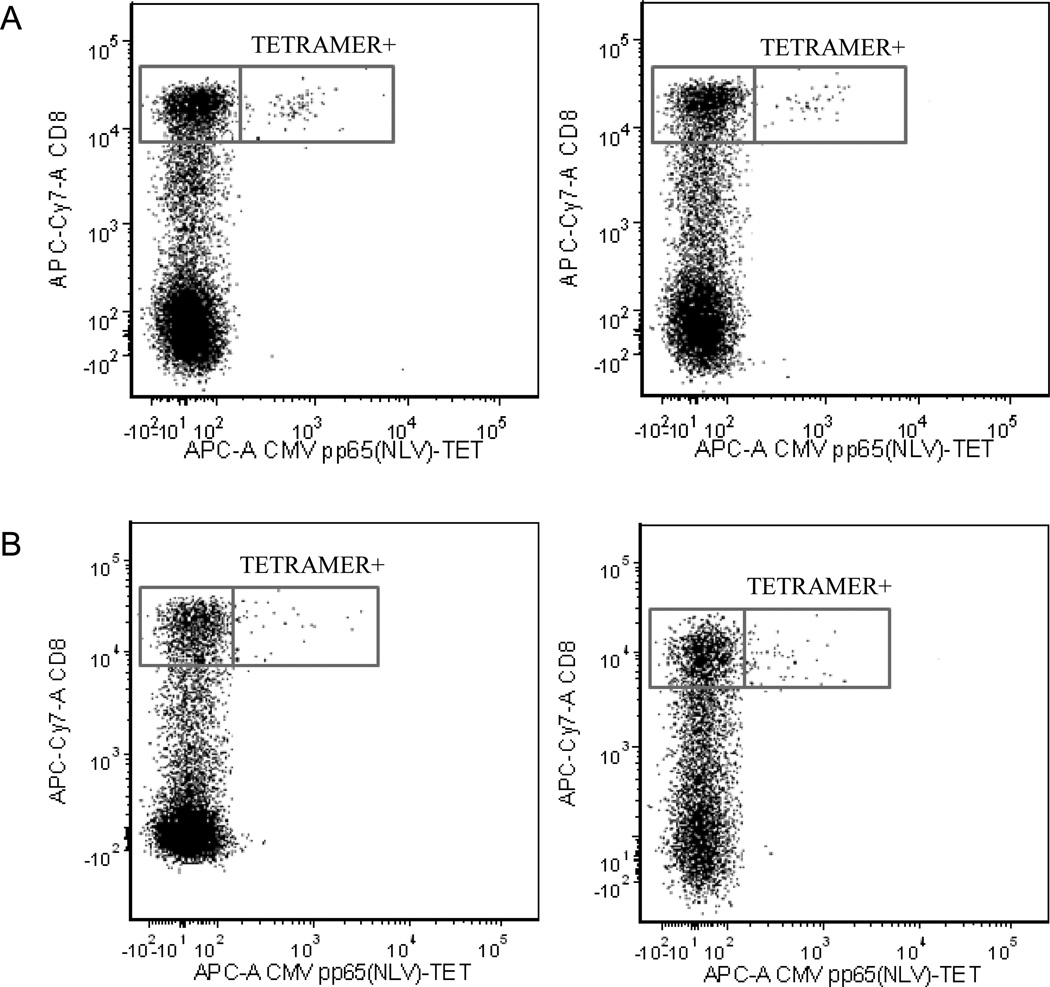
We previously showed that presence of CMV DNA in the peripheral monocytes rather than positive anti-CMV IgG serology was associated with increased frequencies of CMV pp65 (NLV)-specific CD8+ T cells among CMV-seropositive older adults who had the HLA-A2 allele20. In this study, five women had HLA-A2 allele: three without detectable CMV DNA in the peripheral monocytes and two with. The three women with no detectable CMV DNA in the peripheral monocytes (one woman was CMV-seronegative) had minimal or no detectable CMV pp65 (NLV)-specific CD8+ T cells at both baseline and follow-up visits. In contrast, the two women with viral DNA in the peripheral monocytes had detectable CMV pp65 (NLV)-specific CD8+ T cells and the percentages of these CMV pp65 (NLV)-tetramer positive CD8+ T cells were similar between baseline and follow-up visits (Table 2). Fig. 2 is a dot plot from flow cytometry analysis of PBMC samples from subjects #11 and #12 demonstrating percentages of CMV pp65 (NLV)-tetramer specific CD8bright T cells at both times. These results are consistent with our previous observations20 and further indicate that CMV pp65 (NLV)-specific CD8+ T-cell frequencies were maintained in the two elderly women who had viral DNA in the peripheral monocytes at two time points twelve years apart.
Figure 2.
Flow cytometric dot plots from CMV pp65 (NLV)495–504 tetramer analyses of two participants demonstrating tetramer binding to CD8bright+ T cells, but minimal binding to CD8dim or CD8− T cells. A: participant #11, left panel: sample obtained in 1995, right panel: sample obtained in 2007; B. participant #12, left panel: sample obtained in 1995, right panel: sample obtained in 2007.
4. DISCUSSION
To our knowledge, this study is the first evaluating longitudinal relationships between presence of CMV DNA in the peripheral monocytes, anti-CMV IgG serology, circulating IL-6 levels, and frequencies of CMV pp65 (NLV)-tetramer specific CD8+ T cells in older women. The results, obtained from samples collected at two time points over a decade apart, demonstrate that anti-CMV IgG serology did not change over this long period of time and that it was the presence of CMV DNA in the peripheral monocytes that was associated with increased IL-6 levels and CMV pp65 (NLV)-specific CD8+ T cells at both time points.
Most previous studies of chronic CMV infection in older adults have been cross-sectional measuring anti-CMV IgG titers at one time point. A key finding from this study is that anti-CMV IgG serology stayed essentially the same over a decade, suggesting that neither CMV serostatus nor absolute antibody titers are sensitive to change or useful for monitoring chronic CMV infection over time in older adults. Detectable CMV DNA in the peripheral monocytes provides initial physical evidence of the presence of the virus in the immune system. Consistent with our previous data20;26, none of the CMV seronegative subjects had detectable viral DNA in the peripheral monocytes. CMV DNA status also remained unchanged at both times, likely because nested PCR-based detection of CMV DNA is currently a qualitative assay. Further investigations including quantification of CMV viral load in the peripheral monocytes are needed.
Another key finding of this study is that participants with detectable CMV DNA in the peripheral monocytes had higher serum IL-6 levels than those without CMV DNA in the peripheral monocytes at baseline and in 2007. The literature on the relationship between anti-CMV IgG serology and IL-6 levels has been inconsistent. For example, Trzonkowski and colleagues observed significant association between anti-CMV IgG titers and IL-6 levels in a cross-sectional analysis29. In contrary, data from the Hertfordshire Ageing Study at baseline and 10-year follow-up visits showed no association between CMV seropositivty and IL-6 or CRP levels30. In addition, Bennett et al reported that while high anti-Epstein-Bar virus antibody titers were related to high levels of IL-6 and CRP in older persons with high anti-CMV antibody titers, there were no significant associations between anti-CMV antibody titers and IL-6 or CRP levels 31. Results from the present study provide evidence supporting the link between chronic CMV infection as defined by presence of CMV DNA in the peripheral monocytes and elevated IL-6 levels in older women over a 12-year follow-up. This study coupled with our previous reports20;26 suggest a possible explanation for the contradictory results described in the literature. That is, CMV IgG serology (serostatus or absolute anti-CMV IgG titer) may not be a reliable indicator for chronic/persistent CMV infection in older adults. However, it is also likely that mechanisms other than chronic CMV infection may contribute to elevated IL-6 levels and age-related chronic inflammation. Regarding serum CRP levels, while no data was available for the 2007 visit in this study, available data at the baseline visit indicate that participants with detectable CMV DNA had a trend to higher CRP levels than those without albeit this trend was not statistically significant (Table 1).Unlike IL-6 which is a monokine, CRP is predominantly produced by the liver and its production may be less directly influenced by chronic CMV infection32.
Longitudinal data on CMV-specific CD8+ T-cell frequencies are limited except for one 2-yr study in 12 subjects and reported 6-yr follow-up data at 3 time points in one person15;33. The present study report data on CMV pp65 (NLV)-specific CD8+ T-cell frequencies obtained in two older women with detectable CMV DNA in the peripheral monocytes at two time points over a decade apart, suggesting relative stability of CMV T-cell immunity specific to pp65 (NLV) epitope over time.
There are several limitations of the study. First, the small sample size requires expanding our analysis to include larger cohorts of older adults. Secondly, the longitudinal evaluation was conducted in samples collected at only two time points and 12-year follow-up was not long relative to participants’ age at baseline. Further evaluation including additional WHAS II visits is currently under way. In addition, our analysis was restricted to older women, which requires validation of our findings in other older adult populations, particularly older men. Despite these limitations, results from this study support our original hypotheses and provide initial evidence suggesting that anti-CMV IgG serology does not change meaningfully over time and that presence of CMV DNA in the peripheral monocytes rather than positive anti-CMV IgG titers is longitudinally associated with increased IL-6 levels and CMV pp65 (NLV)-specific CD8+ T cells. These findings also provide a basis for further investigations into the role and mechanisms of chronic CMV infection, as defined by the presence of CMV DNA in the peripheral monocytes, in the development of chronic inflammation and its associated chronic conditions in the elderly.
Research highlights.
-
◆
Longitudinal comparison of CMV serology, CMV DNA, IL-6, and CMV pp65 CD8+ T cells
-
◆
No meaningful changes of anti-CMV IgG serology in older women over 12-yr follow-up
-
◆
Higher serum IL-6 levels in subjects with CMV DNA in monocytes than those without
-
◆
Stable CMV pp65 CD8+ T cells in older women with CMV DNA in monocytes over 12-yrs
Acknowledgments
Funding acknowledgement:
This work is supported in part by NIH grant R21-AG-043874 (PI: Dr. Sean X. Leng) and funding from the Irma and Paul Milstein Program for Senior Health of the Milstein Medical Asian American Partnership (MMAAP) Foundation (www.mmaapf.org) (to Dr. Sean X. Leng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 2.Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson BO, Ernerudh J, Johansson B, et al. Morbidity does not influence the T-cell immune risk phenotype in the elderly: findings in the Swedish NONA Immune Study using sample selection protocols. Mech Ageing Dev. 2003;124:469–476. doi: 10.1016/s0047-6374(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 4.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strandberg TE, Pitkala KH, Tilvis RS. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. JAMA. 2009;301:380–382. doi: 10.1001/jama.2009.4. [DOI] [PubMed] [Google Scholar]
- 9.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Hislop A, Gudgeon N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 11.Pita-Lopez ML, Gayoso I, DelaRosa O, et al. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun Ageing. 2009;6:11. doi: 10.1186/1742-4933-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vescovini R, Biasini C, Fagnoni FF, et al. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- 14.Vescovini R, Biasini C, Telera AR, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 15.Hadrup SR, Strindhall J, Kollgaard T, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 16.Mathei C, Vaes B, Wallemacq P, Degryse J. Is CMV infection protective for frailty in the oldest old? Associations between CMV infection and, functinal impairment and frailty in the Belfrail (BFc80+) cohort of octogenarians. Journal of the American Geriatrics Society. 2011;59:2201–2208. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 17.Vallejo AN, Hamel DL, Jr, Mueller RG, et al. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6:e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 19.Taylor-Wiedeman J, Hayhurst GP, Sissons JG, Sinclair JH. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. J Gen Virol. 1993;74(Pt 2):265–268. doi: 10.1099/0022-1317-74-2-265. [DOI] [PubMed] [Google Scholar]
- 20.Leng SX, Qu T, Semba RD, et al. Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp65(495–503)-specific CD8 (+) T cells in older adults. Age (Dordr) 2011;33:607–614. doi: 10.1007/s11357-011-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 22.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 23.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 24.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng SX, Li H, Xue QL, et al. Association of detectable cytomegalovirus (CMV) DNA in monocytes rather than positive CMV IgG serology with elevated neopterin levels in community-dwelling older adults. Exp Gerontol. 2011;46:679–684. doi: 10.1016/j.exger.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 28.Liang B, Zhu L, Liang Z, et al. A simplified PCR-SSP method for HLA-A2 subtype in a population of Wuhan, China. Cell Mol Immunol. 2006;3:453–458. [PubMed] [Google Scholar]
- 29.Trzonkowski P, Mysliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett DB, Firth CM, Phillips AC, et al. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11:912–915. doi: 10.1111/j.1474-9726.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 31.Bennett JM, Glaser R, Malarkey WB, Beversdorf DQ, Peng J, Kiecolt-Glaser JK. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav Immun. 2012;26:739–746. doi: 10.1016/j.bbi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol. 2010;26(Suppl A):41A–44A. doi: 10.1016/s0828-282x(10)71061-8. [DOI] [PubMed] [Google Scholar]
- 33.Wallace DL, Masters JE, De Lara CM, et al. Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects. Immunology. 2011;132:27–38. doi: 10.1111/j.1365-2567.2010.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]