Abstract
Reduction of food intake by exogenous cholecystokinin (CCK) has been demonstrated primarily for its short molecular form, CCK-8. Mounting evidence, however, implicates CCK-58 as a major physiologically active CCK form, with different neural and exocrine response profiles than CCK-8. In three studies, we compared meal-pattern effects of intraperitoneal injections CCK-8 vs. CCK-58 in undeprived male Sprague-Dawley rats consuming sweetened condensed milk. In study one, rats (N=10) received CCK-8, CCK-58 (0.45, 0.9, 1.8 and 3.6 nmole/kg) or vehicle before a 4-hour test-food presentation. At most doses, both CCK-8 and CCK-58 reduced meal size relative to vehicle. Meal-size reduction prompted a compensatory shortening of the intermeal interval (IMI) after CCK-8, but not after CCK-58, which uniquely increased the satiety ratio (IMI/size of the preceding meal). In the second study, lick patterns were monitored after administration of 0.9nmole/kg CCK-58, CCK-8 or vehicle. Lick cluster size, lick efficiency and interlick-interval distribution remained unaltered compared to vehicle, implying natural satiation, rather than illness, following both CCK forms. In study 3, threshold satiating doses of the two CCK forms were given at 5 and 30 minutes after meal termination, respectively. CCK 58, but not CCK-8 increased the intermeal interval and satiety ratio compared to vehicle. In conclusion, while CCK 58 and CCK-8 both stimulate satiation, thereby reducing meal size, CCK-58 consistently exerts a satiety effect, prolonging IMI. Given the physiological prominence of CCK-58, these results suggest that CCK’s role in food intake regulation may require reexamination.
Keywords: CCK-58, intermeal interval, satiety, meal pattern, lick microstructure
1. Introduction
Nutrient regulation of appetite, food intake and ultimately body weight occurs through a myriad of neural and hormonal signals [8, 54, 65]. Prominent short-term signals are elicited by the presence of nutrients in the small intestine [29, 32, 35]. A classical satiation signal is the peptide cholecystokinin (CCK), which is secreted during and after meals from I-cells primarily in the proximal small intestine [12, 37].
Historically, CCK was the first gastrointestinal peptide that was observed to inhibit feeding in rats after exogenous administration [20, 47]. Subsequently, physiological doses of CCK were shown to inhibit food intake in other species, including humans [3, 38]. Further studies showed that exogenous CCK-8 increased satiation primarily via capsaicin-sensitive fibers of the afferent vagus nerve, which relays signals to the hindbrain and then farther up the neural axis. A physiological role of CCK in satiation is strongly supported by the observation in several studies that food intake is increased following administration of various CCK1-receptor antagonists [4, 41, 49, 56]. Finally, sensitivity to the short-term anorexic effects of CCK-8 is tuned by long-term adiposity factors, such as leptin and insulin [12, 39, 43, 60].
Importantly, ingestive-behavior researchers have routinely focused on actions of the synthetic octapeptide form of cholecystokinin, CCK-8. Several reports suggest, however, that pre-pro CCK produced in small intestinal I-cells gives rise to many other bioactive peptide forms, all containing the defining seven amino-acid carboxyl terminus, but differing in total amino-acid length [47]. The most commonly identified forms are CCK-8, CCK-22, CCK-33, CCK-39 and CCK-58. In parallel with the further optimization of blood collection and processing protocols, CCK-58 has increasingly emerged as the major CCK form that is actually released from gastrointestinal I-cells of rats [45], dogs [15, 16], and perhaps humans [13]. A key feature of protocol improvement for blood processing, adopted in our recently developed RAPID method, was to effectively limit the ex vivo degradation of extracted peptides from blood or tissue [57]. We suggest that in earlier studies, peptide degradation during sample processing led to an overestimation of blood and intestinal levels of small CCK forms, along with a consistent underestimation of CCK-58 levels.
The above considerations suggest re-assessment of CCK’s physiology. Previously, it was assumed that all CCK forms containing the hepta amino-acid carboxyl terminal possess equal bioactivity [19, 44], but more recently, functional studies of CCK-8 and CCK-58 (both of which contain this sequence) have revealed quantitative and qualitative differences. For example, CCK-58 has a 3-fold longer plasma half life than CCK-8 in dogs [25] and in rats induces a more prolonged activation of afferent-vagal neurons [33], the primary pathway conveying CCK-8 induced satiation [49]. Importantly, CCK-8 and CCK-58 have qualitatively distinct effects on a prime physiological action of CCK, pancreatic secretion patterns. CCK-8 stimulates fluid secretion only minimally at any dose, and actually eliminates secretion when delivered at high concentrations. In contrast, CCK-58 stimulates fluid secretion dose-dependently. Overall, post-meal pancreatic responses were mimicked more closely by CCK-58 than by CCK-8 [68]. Importantly, infusion of high doses of CCK-58 did not induce the pancreatic hypertrophy or pancreatitis typically observed after identical doses of exogenous CCK-8 in rats [24, 67]. These qualitative differences led us to suggest that generalized conclusions derived from functional studies using CCK-8 or other shorter forms of cholecystokinin may need re-evaluation using a major endocrine form of the peptide, CCK-58 [24, 45].
Ultimately, energy intake depends on two parameters: meal size and meal frequency. Early studies by Gibbs, Smith et al. focused primarily on acute effects of exogenous CCK, which reduces meal size, reflecting increased satiation. A plethora of subsequent observations have confirmed CCK as a physiologically important satiation signal, considered by many to be the classic example of such a peptide, contributing along with other gut-derived signals such as gastric distention to meal termination and thus limitation of meal size [8, 49]. The issue of whether CCK also enhances satiety, i.e., prolongs the time interval until the next meal is voluntarily initiated, was examined later, with mixed results. West et al. (1984) [62] infused CCK-8 intraperitoneally at the start of each spontaneous meal in rats and observed that meal-size reduction was accompanied by a progressive increase in meal frequency (i.e., a shortening of the IMI), ultimately returning food intake to the basal level. In a subsequent study, however, the same group [64] observed that the compensatory shortening of the IMI could be prevented, at least for several days, by continued post-meal CCK infusions. In addition, Hsiao et al. [27] and Vanderweele et al. [59] observed a dose-dependent lengthening of the IMI after CCK-8 administration, contrary to the original observations by West et al. [62]. Studies with CCK1-receptor antagonists [7, 41, 56] and/or non-nutritional CCK secretagogues [7, 36] further indicate that endogenous CCK - besides reducing meal size (i.e. promoting satiation) - may also act to prolong the IMI (i.e., promoting satiety). If CCK-58 is, indeed a predominant endogenous CCK form, characterization of its impact on feeding should also include intermeal effects.
One of us (JRR) previously reported that exogenous CCK-58 and CCK-8 equipotently reduce meal size in rats [21], and that CCK-58 was furthermore associated with reduced total intake for up to two hours after administration, which could indicate that CCK-58 enhances IMI (and satiety) more than does CCK-8. To replicate and extend these observations systematically, we conducted three studies comparing the influence of CCK-8 and CCK-58. Besides meal size, we monitored within- and between-meal aspects of feeding, varying the timing of administration of the two CCK forms.
In study 1, we measured effects of 4 doses of intraperitoneally injected CCK-8 and CCK-58 on meal size and duration of the subsequent IMI during daily 4-hour regularly scheduled access to a palatable, liquid diet. Based on comparative data reviewed above, our hypothesis was that the acute reduction of meal size (i.e., a satiation effect) after CCK-58 administration would be equal to that following the (usually studied) CCK-8, but that CCK-58 would have an additional satiety-enhancing effect evidenced by a prolonged IMI.
Generally, satiation or satiety actions of exogenous doses of peptides such as CCK are validated by observations that meal-size reduction is accompanied by natural prandial and post-prandial motor behaviors and the absence of signs of malaise [22, 34, 63]. Indeed, recently, one of us (JRR) reported no differences in the occurrence of grooming and locomotion in rats following administration of an anorexigenic dose of CCK-58 and vehicle [22]. In the current, second study we aimed to extend this observation by examining effects on the microstructure of licking during the first meal after CCK-58 administration. Licking in rodents is characterized by a stereotypical motor pattern, with an average of 6–7 licks per second. Subtle shifts in lick rates, the frequency and duration of pauses between licks during meals may reveal processes underlying satiation, such as the balance between positive oral and negative post-ingestive feedback signals [9, 55]. For CCK-8, lickometer measurements have been used to document that reductions in food intake reflected a natural satiation process [10], rather than impaired motor performance or lick patterns reflective of malaise/aversion that are typically observed after ingestion of toxic agents [1, 2]. We hypothesized that CCK-58, like CCK-8 would not perturb lick patterns (initial lick rates, lick cluster size and interlick interval distribution), typifying natural satiation and the absence of malaise.
In the third study, CCK-8, CCK-58 or vehicle were injected at two time points, 5 or 30 minutes after meal termination, reflecting different stages of gastric distention and food digestion[30]. Thus, contary to study one, IMI effects of the two CCK forms were evaluated without decreasing the size of the preceding meal. We hypothesized that CCK-58 would lead to stronger prolongation of the intermeal interval, with delayed onset of the next meal, compared with either CCK-8 or vehicle.
2. Materials and methods
2.1. Animals
The studies were performed with adult male Sprague-Dawley rats weighing between 350 and 500 grams (Harlan, San Diego, CA; for studies 1 and 3b and Taconic Farms, Germantown, NY for studies 2 and 3). All animal procedures followed NIH guidelines for animal care and were approved by the Institutional Animal Care and Use Committees of Cornell University Weill Medical College (studies 1 and 3A) and VA Puget Sound Health Care System (studies 2 and 3B). The animals were housed individually in polycarbonate cages on a 12-h light, 12-h dark cycle, with lights on at 0600 h. They were maintained on Purina Rat Chow 5001 (St. Louis, MO) and tap water. The experimental liquid food used was sweetened condensed milk (Eagle Brand; Borden, Columbus, OH), diluted 1:2 with tap water (energy density: circa 1.3 kcal/g or 1.44 kcal/ml; energy composition: carbohydrate 68 cal%, protein 11 cal%, fat 21 cal%).
2.2. Peptides
Rat CCK-58 was synthesized on an automatic Applied Biosystems 433A Peptide Synthesizer (Foster City, CA) using a fluoromethyl (FMOC) strategy. Blocked peptide-resin was cleaved and unblocked in 100 mg aliquots with 89% trifluoroacetate (TFA) containing 5% ethanedithiol, 3% anisol and 3% thioanisol at room temperature for 1 h. The crude peptide product was purified on a Waters reverse-phase C-18 column with a gradient of 28–42% acetonitrile containing 0.1% TFA over 45 min. Peptide purity was determined by high-performance capillary electrophoresis. Fractions above 93% purity were pooled and lyophilized. The sulfation and amino acid composition of rat sulfated CCK-58 were confirmed by mass spectral analysis. A more detailed description of the synthesis and purification of CCK-58 has been previously presented [46].
Synthetic sulfated porcine (i.e., identical to rat) CCK-8 was purchased from Bachem (Torrance, CA). Both peptides were administered intraperitoneally (ip), in a 1 ml/kg volume of phosphate buffered saline containing 0.1% w/v bovine albumin.
2.3. Lick measurements
Eight-channel lickometers (Dilog, Tallahassee, FL) were used to monitor the number and timing of individual licks with millisecond resolution. For further technical background see reference \44. Briefly, the sipper tube of each drinking bottle was inserted into the rat cage through a holder mounted onto the outside wall of the rat cage. The inside of the holder contained a metal sensor, part of an electronic circuit that relayed signals to an outside computer. During liquid-food intake, each contact of the rat tongue with the sipper tube closed the electronic circuit, generating a tiny (<60 nA) electronic signal, which was amplified and stored in a computer file together with a time stamp. The record of individual licks thus created was processed and analyzed off-line with a custom-designed software program, written in Visual Basic (Microsoft Inc., Redmond, WA) by one of the authors (J.O.). The calculated lick parameters included minute-by-minute lick rates, lick cluster size, meal onset, and distribution of licks (interlick intervals).
2.4. Procedures
Study 1: Effects of Four Equimolar Doses of CCK-8 vs. CCK-58 on Meal Size and Intermeal Interval
On each day, during a 4-week habituation period, chow was removed from the cage for 4 hours and replaced by sweetened condensed milk (with chow removed) at 1100h, i.e., 5 hours after lights on. By the end of the habituation period, rats typically ate several meals during the 4-hour milk presentation, and for each rat the size of the first milk meal varied less than 3 ml from day to day. On the five days preceding the study, rats were also habituated to daily ip injections with saline. During the experimental phase of the study, the animals were injected daily intraperitoneally with CCK-8 or CCK-58 (doses 0.45, 0.9, 1.8, or 3.6 nmol/kg) or an equal volume of vehicle immediately before presentation of sweetened condensed milk. Over the course of the study, rats received all injections in individualized, random order. Meal termination was defined by a 5-min time interval without milk intake [51]. Food intake was visually monitored by the experimenter (resolution: 1 ml) continuously until the second meal after injection, and once hourly thereafter until 4 hours after milk presentation. Upon removal of the milk, regular chow was returned to the home cage.
Study 2: Effects of IP Administration of 0.9 nmol/kg CCK-8 vs. CCK-58 on Within-Meal Lick Microstructure
Ad libitum-fed rats were habituated for 2 weeks to a daily, 1-hour food deprivation in an experimental cages with a lickometer, followed by 30-minute access to sweetened condensed milk. During the 5 days preceding the experiment, rats received daily ip injections with saline for habituation. On experimental days, they were injected ip with an equimolar, anorexic dose (0.9 nmol/kg for the given batches of CCK and rat group) of CCK-8, CCK-58 or vehicle at 3 minutes before presentation of the liquid diet. Liquid food intake was measured by weight (resolution: 0.1 g) and monitored by lickometer. Off-line, the following lick parameters were analyzed: 30-minute food intake, per-minute lick rate in 2-min intervals, cluster size (lick trains with pauses <500 ms), number of lick clusters, lick efficiency (number of licks/gram ingested milk) and distribution of interlick intervals.
Study 3
Intermeal-Interval Effects of CCK-8 and CCK-58 Administered 5 or 30 Minutes After Meal Termination. Post-prandial administration of gastrointestinal peptides at these times may differently affect the duration of the ongoing IMI, as shown for gastrin-releasing peptide1–27 [50]. The 5- and 30-minute post-prandial time points were selected to coincide with different patterns of endogenous satiation-related signaling, e.g. CCK release, gastric distention and small-intestinal presence of nutrients [30]. CCK administration could potentially induce discomfort [34] and lead to gastric retention of a large volume of ingested food especially at 5 minutes after the meal, but less so 25 minutes later [30, 50]. We considered the 5-minute, no-ingestion criterion for meal termination appropriate because - following habituation to daily liquid-food access - rats uniformly ingested a large, uninterrupted meal immediately after food presentation, followed by an IMI of at least 20 minutes.
3A. CCK Administration 5 Minutes After Meal Termination
Pre-study habituation to injections and daily milk presentation in 10 rats was imposed analogously to that described for study 1. During the experimental phase, rats received 4-hour access to sweetened condensed milk in their home cage starting at 1100h. Food intake was monitored continuously. Five minutes after termination of the first meal (a 5-min time interval without milk intake), each rat received an ip injection of 1.8 nmol/kg CCK-8, CCK-58 (the threshold anorexic doses for the CCK batches and group of rats) or isovolumic vehicle on different days, in random order. Milk intake was measured every 5 min for the remaining 4 hours of liquid-meal access. At four hours, the milk was removed and chow was returned. IMI, size of the second meal and total milk intake were also recorded.
3B. CCK Administration 30 minutes After Meal Termination
Rats were habituated to daily access to sweetened condensed milk. The procedure of study 3A was slightly modified to equalize and moderate pre-injection meals across animals. At 1 hour before the actual experiment, rats received 4 minutes of pre-exposure to the sweetened condensed milk. Then rats were transferred to cages with lickometers and were food deprived for one hour, after which the milk was re-introduced and animals ate their first meal. Thirty minutes after meal termination, each animal was injected ip with either an anorexic dose (0.9 nmol/kg) of CCK-8 or CCK-58, or isovolumic vehicle. Lickometers were used to measure the IMI and to estimate the size of the ensuing, second meal. Five minutes after termination of the second meal, the milk was removed from the test cage.
2.5. Data Analysis
Food intake was measured by volume (ml) or weight (gram) as indicated for the different studies; all satiety ratios were defined either as IMI/volume of the previous meal. Statistical analyses were conducted using Microsoft Excel 2007 (Microsoft Inc., Redmond WA) and SYSTAT 11.0 statistical software (Systat Inc., Richmond, CA). One-way, 3-level, repeated measures ANOVA tests were used to test for differences in ingestive behaviors across the two CCK forms and vehicle. Significant ANOVA results were followed by post hoc, paired t-test comparisons of conditions using the Bonferroni criterion, with the threshold P value for significance set at 0.05/3 = 0.017.
3. Results
3.1. Study 1: Effects of four equimolar doses of CCK-8 vs. CCK-58 on meal size and intermeal interval
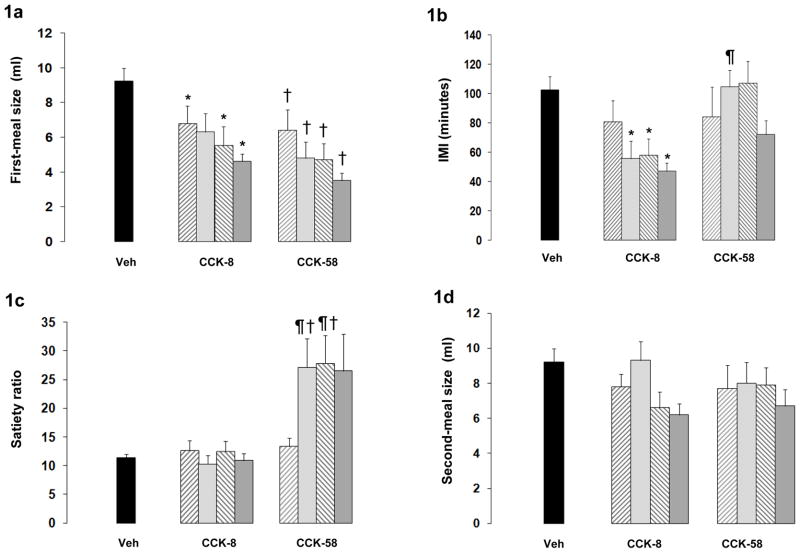
No visible behavioral abnormalities or postural changes of malaise were observed after administration of either CCK form. Significant F-test statistics for the first post-injection meal were found at all CCK doses, generally reflecting a dose-dependent reduction of meal size after CCK-8 and CCK-58 relative to vehicle, but no differences between the two peptide conditions (Fig. 1a). The ANOVA test statistics for different doses were as follows: F(2,18) = 5.5, P < 0.02 for 0.45 nmole/kg CCK, F(2,18) = 10.5, P = 0.001 for 0.9 nmole/kg CCK, F(2,18) = 7.6, P = 0.004 for 1.8 nmole/kg CCK and F(2,18) = 31.5, P < 0.001 for 3.6 nmole/kg. Results of post-hoc tests between pairs of conditions are indicated in Fig. 1a.
Figures 1a – 1e.
Results from study 1, showing meal intake patterns of rats (N=10) fed sweetened condensed milk following ip injections of four equimolar doses of CCK-58 and CCK-8 (0.45, 0.9, 1.8, and 3.6 nmole/kg) or vehicle. The criterion for meal termination in these well-habituated animals was no intake for 5 consecutive minutes. Significant between-condition effects after Bonferroni correction (P < 0.017) are indicated by: * CCK-8 vs vehicle, † CCK-58 vs vehicle, ¶ CCK-58 vs CCK-8.
(1a) Meal size (mean ± sem in ml). Both CCK forms reduced food intake significantly at all doses, with exception of the 0.9 nmole/kg CCK-8 dose; (1b) Intermeal interval (mean ± sem in minutes). Compared to vehicle, CCK-8 shortened the intermeal interval, whereas, CCK-58 had no such effect; (1c) Satiety ratio (SR) (mean ± sem in minutes/ml) for the first intermeal interval after test-food presentation. The SR is defined as the ratio of the intermeal interval and the size of the preceding meal. Compared to vehicle, CCK-58, but not CCK-8 increased SR at two middle doses (with an analogous trend at the highest doses), indicating a satiety action of the longer CCK form; (1d) Size (mean ± sem in ml)of the second meal after food presentation. The absence of significant differences between CCK-8 and CCK-58 conditions indicates that the longer IMI after CCK-58 did not result in subsequent rebound eating; (1e) Cumulating food intake (mean ± sem in ml) over the 4-hour liquid food presentation after administration of 1.8 nmole/kg CCK-8, CCK-58 or vehicle. This figure tipifies different actions of CCK-58 and CCK-8 in the following respects: (1) Similar acute reduction by CCK-8 and CCK-58 of food intake (increased satiation); (2) more sustained satiety after CCK-58 than after CCK-8 in the hours thereafter; (3) dissipation by 4 hours, of feeding effects of CCK. Analogous feeding patterns were observed for the other CCK-doses except the lowest.
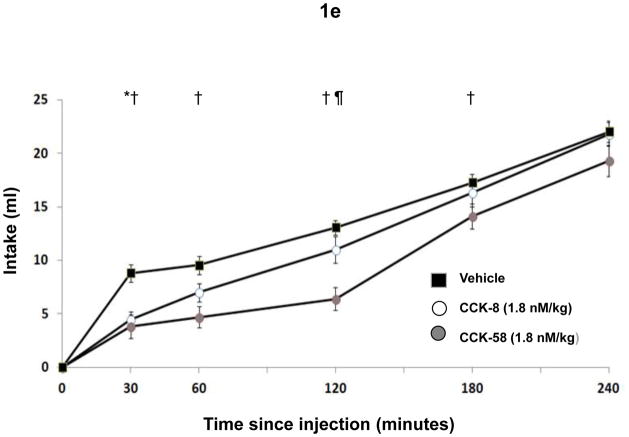
The IMI showed main effects at the three highest doses of CCK (F(2,18) = 18, P < 0.001 for 0.9 nmole/kg, F(2,18) = 5.8, P < 0.02 for 1.8 nmole/kg and F(2,18) = 10.1, P = 0.001 for the 3.6 nmole/kg CCK, respectively. As indicated in Fig. 1b, IMIs after vehicle or CCK-58 were larger than after CCK-8. The satiety ratio (the ratio of the IMI duration and size of the preceding meal, which quantifies satiety effects) differed across conditions at the three highest CCK doses ((F(2,18) = 9.9, P = 0.001 for the 0.9 nmole/kg, F(2,18) = 11.7, P = 0.001 for the 1.8 nmole/kg and F(2,18) = 4.1, P < 0.04 for the 3.6 nmole/kg CCK dose. In all three cases the mean satiety ratio was elevated after CCK-58 compared to both CCK-8 and vehicle (Fig. 1c), although due to a slightly larger within-condition variance, the Bonferroni-corrected difference of the 3.6 nmole/kg dose of CCK-58 and CCK-8 and vehicle failed to reach significance (P = 0.02). The size of the second meal did not differ in the vehicle compared with the CCK conditions, except at the highest dosis of CCK-8 (Fig. 1d), indicating that no rebound eating occurred after CCK-induced meal reduction of the first meal. Fig. 1e shows cumulative food intake data over the 4-hour period, illustrating for one of the CCK doses (1.8 nmole/kg) the different effects on total intake over time of CCK-8 (which only increased satiation) and CCK-58 (which increased both satiation and satiety).
3.2. Study 2: Effects of ip administration of 0.9 nmol/kg CCK-8 vs. CCK-58 on within-meal lick microstructure
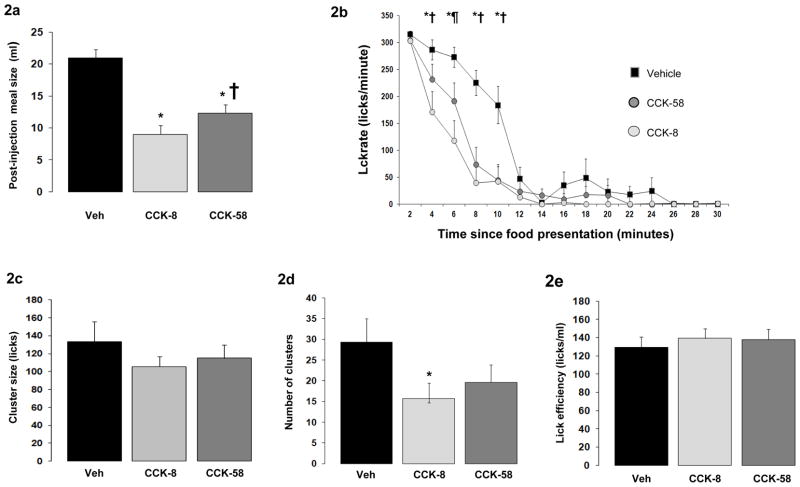
Compared to vehicle administration, CCK-8 and CCK-58 suppressed 30-minute test-diet intake significantly (overall F(2,18) = 30, P < 0.001; Fig. 2a). A significant difference occurred in meal size reduction after CCK-8 (mean: 55%) vs. CCK-58 (mean: 39%), in contrast with similar meal-size reduction in study 1. Despite this difference we considered the lick measurements to be appropriate to compare effects of equimolar anorexigenic doses of CCK-58 and CCK-8 on lick microstructure, because the used dose of CCK and observed level of meal-size reduction were clearly smaller than those inducing malaise when using CCK-8 [34, 56]. Also, modest meal-size differences are unlikely to constrain the crucial lick parameters (lick efficiency, interlick-interval distribution and lick-cluster size) considered in the current study [55].
Figures 2a – 2f.
Results from study 2, showing food intake and meal microstructure in rats (N=10) during a 30-minute presentation of sweetened condensed milk following ip injections of anorexic doses (0.9 nmole/kg) of CCK-58 and CCK-8, or vehicle. Licking was measured by electronic lickometers with millisecond resolution. Significant between-condition effects after Bonferroni correction (P < 0.017) are indicated by: * CCK-8 vs vehicle, † CCK-58 vs vehicle, ¶ CCK-58 vs CCK-8.
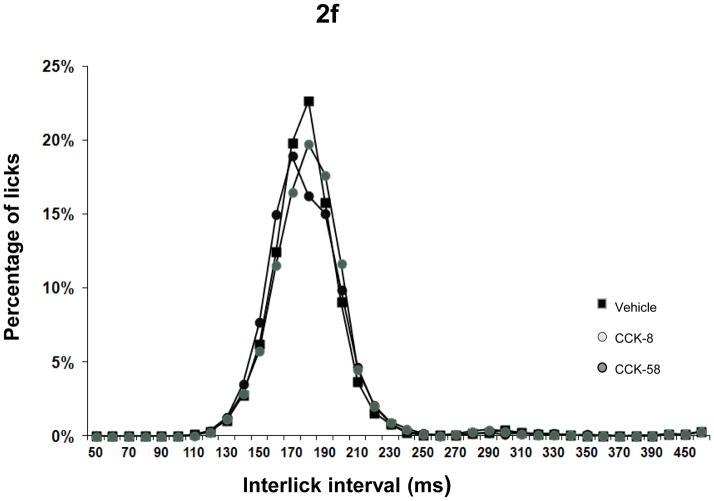
(2a) Thirty minute intake (mean ± sem in ml) of sweetened condensed milk following administration of CCK-58, CCK-8 or vehicle. Both CCK forms reduced food intake significantly; (2b) Lick rates (mean ± sem) in consecutive 2-minute intervals during the 30 minute test-food presentation period. The habituated rats all ate only one large meal starting immediately after food presentation in all conditions. Departing from an initial lick rate that equaled that of the vehicle condition, injection of both forms of CCK led to faster deceleration of lick rate. Importantly, no abrupt or irregular lick patterns occurred after administration of CCK-58; (2c) Mean ± sem of lick-cluster size. Lick cluster was defined as a uninterrupted train of licks with < 500 msec between licks. No differences were found between CCK forms and vehicle; (2d) Total number of lick clusters (mean ± sem) during 30 minute liquid presentation. The number of lick clusters, (but not of lick cluster size) was analogous to total intake in the three conditions, suggesting a similar satiation process after CCK-58, CCK-8 and vehicle; (2e) Lick efficiency (i.e. the number of licks per ml of food ingested) following ip injections of CCK-58, CCK-8 and vehicle. No differences were found between vehicle and CCK conditions, indicating unimpeded motor behavior after both forms of CCK; (2f) Distribution of interlick interval (ILI) distribution in 10-ms bins during the 30-minute presentation of liquid test food. Data are expressed as percentage of total number of licks. The similar ILI distribution after both CCK forms and vehicle administration further confirms that meal size reduction did not result from malaise or motor impediments.
Aside from food-intake reduction, no significant differences in lick microstructure were seen after CCK-8 and CCK-58. Initial 2-minute lick rate was equal across conditions, but after 2 minutes, lick rate declined more rapidly after both CCK forms than after vehicle, confirming earlier work by Davis et al. with CCK-8 [10]; Fig. 2b). Between minutes 4 and 6, lick rate was lower in the CCK-8 than in the CCK-58 condition, reflecting the smaller meal size induced by CCK-8. Lick-cluster size was equal across conditions (Fig. 2c), and - in line with overall intake data - a between-condition effect occurred for total number of clusters (F(2,18) = 9.8, P = 0.001) supported by decreased number after CCK-8 and a similar trend (Bonferroni-corrected P < 0.06) after CCK-58 administration (Fig. 2d). Lick efficiency was slightly increased in the two CCK conditions relative to the control condition (F(2,18) = 3.6, P < 0.05; Fig. 2e), and interlick interval distributions were similar after CCK-8, CCK-58 and vehicle (Fig. 2f), indicating that CCK’s feeding effects did not occur because of motor impediments or malaise.
3.3.1 Study 3A: Intermeal-interval effects of CCK-8 and CCK-58 administration 5 minutes after meal termination
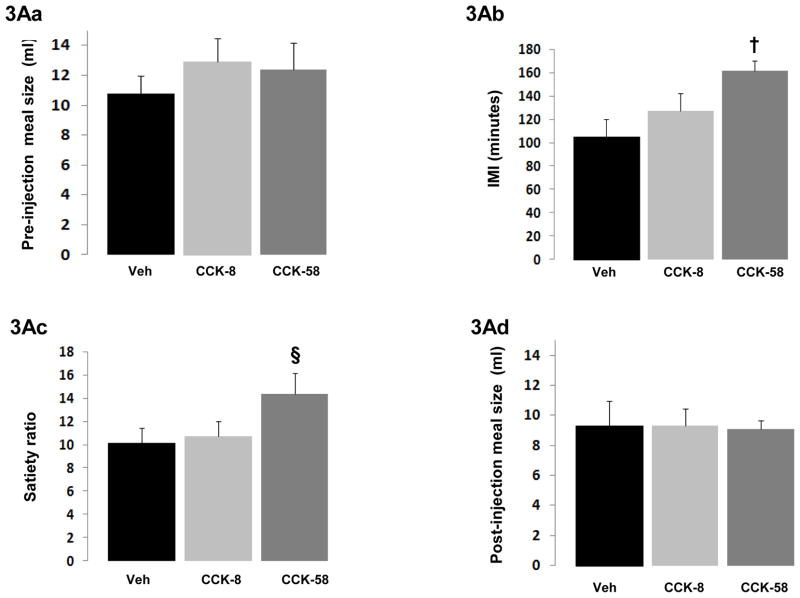
As expected, no differences were found across conditions in the size of the meal just before injection (Fig. 3Aa). A main effect of injection on IMI was found (F(2,18) = 9.3, P = 0.002), reflecting CCK-58-induced extension of IMI compared to the vehicle condition (Fig. 3Ab). Accordingly, a significant between-conditions effect was found for satiety ratio (F(2,18) = 5.2, P = 0.02); Fig. 3Ac). CCK-8 did not increase the IMI or satiety ratio. No-between-condition differences were found in the size of the second meal (Fig. 3Ad), pre- and post-injection meal combined, or cumulative 4-hour intake.
Figure 3.
Figures 3Aa – 3Ad Results from study 3A, showing feeding effects on rats (N=10) receiving ip injections of 1.8 nmole/kg CCK-58, CCK-8, or vehicle at 5 minutes after termination of an ad libitum meal of sweetened condensed milk. CCK dose was administered at the threshold dose for acute meal size reduction in the batch of rats and peptides tested. The criterion for meal termination was absent intake for 5 consecutive minutes. Significant between-condition effects after Bonferroni correction (P < 0.017) are indicated by: * CCK-8 vs vehicle, † CCK-58 vs vehicle, ¶ CCK-58 vs CCK-8.
( 3Aa) Pre-injection meal size (mean ± sem in ml) before 5-minute, post-meal injections of CCK-58, CCK-8 or vehicle. Meal sizes were equal in all conditions, providing a suitable baseline against which to assess the post-meal injections of CCK forms; (3Ab) Intermeal interval (IMI; mean ± sem in minutes) following the 5-minute, post-meal injection of 1.8 nmole/kg CCK-58, CCK-8 or vehicle. CCK-58, but not CCK-8 extended the IMI, suggesting increased satiety after CCK-58; (3Ac) Satiety ratio (SR) (mean ± sem in minutes/ml) for the IMI after injection of CCK-58, CCK-8 or vehicle. The SR after CCK-58 compared to vehicle trended toward enlargement (§ indicates P = 0.02 for CCK-58 vs vehicle; Bonferroni-corrected threshold for significance 0.017); (3Ad) Post-injection meal size (mean ± sem in ml) after CCK-58, CCK-8 or vehicle administration. Despite the longer preceding IMI for CCK-58, no differences in meal size were observed, indicating an absence of rebound overeating following the prolonged IMI after CCK-58;
Figures 3Ba – 3Bd. Results from study 3B, showing feeding effects on rats (N=10) receiving ip injections of 0.9 nmole/kg CCK-58, CCK-8, or vehicle at 5 minutes after termination of an ad libitum meal of sweetened condensed milk. CCK was administered at the threshold dose for acute meal size reduction in the batch of rats and peptides tested. The criterion for meal termination was absent intake for 5 consecutive minutes. Significant between-condition effects after Bonferroni correction (P < 0.017) are indicated by: * CCK-8 vs vehicle, † CCK-58 vs vehicle, ¶ CCK-58 vs CCK-8.
(3Ba) Pre-injection meal size (mean ± sem in ml) before post-meal injections of CCK-58, CCK-8 or vehicle. Meal sizes were equal in all conditions, providing a suitable baseline against which to assess the post-meal injections of CCK forms; (3Bb) Intermeal interval (IMI; mean ± sem in minutes) following post-meal injection of CCK-58, CCK-8 or vehicle. CCK-58, but not CCK-8 extended the IMI, confirming increased satiety after CCK-58. The significant within–subjects effects after Bonferroni correction (P < 0.017) is indicated: † CCK-58 vs vehicle; (3Bc) Satiety ratio (SR) (mean ± sem in minutes/ml) for the IMI after post-meal injection of CCK-58, CCK-8 or vehicle. Only CCK-58 had a higher SR than vehicle; (3Bd) Post-injection meal size (mean ± sem in ml) after CCK-58, CCK-8 or vehicle administration. Despite the longer preceding IMI for CCK-58, no differences were observed, indicating an absence of rebound over-eating following the prolonged IMI after CCK-58
3.3.2 Study 3B: Intermeal-interval effects of CCK-8 and CCK-58 edministration 30 minutes after meal termination
No differences were found across conditions in the size of the meal immediately preceding injection (Fig. 3Ba). A main effect of injection on IMI was found (F(2,22) = 5.0, P < 0.02), showing extension of IMI by CCK-58 compared to vehicle (Fig. 3Bb). Satiety ratio data showed a similar pattern (F(2,22) = 5,6, P < 0.02; Fig. 3Bc). As in study 3A, where injections where given immediately after meal termination (i.e., when meal-induced stomach distension presumably was near maximum), CCK-8 administered 30 minutes post-meal (when a significant proportion of stomach contents had presumably emptied into the small intestine) failed to increase the IMI or satiety ratio. No between-condition differences were found for the size of the second meal (Fig. 3Bd) or combined intake of meals 1 and 2.
4. Discussion
Although CCK-58 is the dominant circulating form of cholecystokinin, knowledge of its effects on food intake is scant. Thus far, researchers have focused on effects of a shorter form of the peptide, CCK-8, which induces short-term reduction of meal size. Here, we report for the first time the results of a direct comparison of effects of CCK-58 and CCK-8 on intra- and intermeal aspects of feeding in rats during daily presentations of a mixed-macronutrient test food (sweetened condensed milk). In study 1, pre-meal ip injections of CCK-58 or equimolar CCK-8 similarly reduced meal size (indicating enhanced satiation) at the four doses tested (ranging from 0.45 to 3.6 nmole/kg). Detailed lickometer measurements showed an early decline of intra-meal lick rates for both CCK forms compared to vehicle, without signs of motor impairment or malaise. Importantly, CCK-58 and CCK-8 differently affected the duration of the intermeal interval, indicating different satiety. CCK-8 induced meal-size reduction was followed by a compensatory shortening of IMI compared to vehicle, as described in the literature [62]. In contrast, meal-size reduction after CCK-58 maintained or enhanced the subsequent IMI. Accordingly, the satiety ratio (i.e., the quotient of IMI and size of the preceding meal) after CCK-58 was significantly enlarged relative to that after CCK-8 and vehicle. The satiety effect of CCK-58 bolus injection was restricted in duration; by 4 hours, total energy intake had returned to equal in both types of CCK and vehicle conditions.
The different effects on IMI of CCK-8 and CCK-58 were confirmed in study 3, in which the peptides were administered at 5 and 30 minutes post-prandially. Again, CCK-58, but not CCK-8 increased IMI durations and/or satiety ratio relative to the vehicle condition, without altering the size of the subsequent meal. Of note, the statistically significant differences in IMI and satiety ratio in study 3 were found only between the CCK-58 and vehicle. IMI differences between CCK-8 and CCK-58 were not found to be significant at the given study size, presumably because CCK-8 induced a (non-significant) prolongation of IMI relative to vehicle. Combined, the results of the current studies support a dual anorexigenic action of CCK-58. Besides inducing satiation like CCK-8, CCK-58 more clearly induces satiety, as reflected by maintenance, or lengthening of the IMI, depending on the pre- or post-prandial timing of injection. In contrast, CCK-8 reduces the IMI when administered before meals.
Besides the expected similar meal-size reduction by CCK-58 and CCK-8, the maintenance of the subsequent IMI by CCK-58 but not by CCK-8 at the three highest doses (study 1), was the most salient of our results. This robust observation, made during a scheduled feeding regimen is in line with the earlier reported IMI extension by CCK-58 (but not by CCK-8) during ad-libitum chow feeding [22]. Additionally, CCK-58’s actions are consistent with earlier reported IMI effects of endogenous CCK signaling [7, 36, 41], and stronger reductions of food intake by larger CCK forms than by CCK-8 [61].
Results from studies 3A and 3B, in which post-meal CCK-58 injections increased the IMI appear to contrast with the commonly held view (based upon the much-cited classic studies with CCK-8 by West et al. [62, 64]) that the anorexigenic effects of brief infusions of CCK are restricted to an acute reduction of meal size, compensated for by reduced IMI and therefore not affecting overall food intake. It should be noted, however, that West et al. [64] and Hsiao et al. [27] reported IMI-sustaining effects even of exogenous CCK-8, provided it is administered post-prandially In our studies, hints of IMI extension effects after post-meal CCK-8 were discernible but statistically insignificant when rigorous statistical testing was applied. The observation that CCK-58 prolonged the IMI more clearly at two post-prandial time points, reflecting different physiological states (e.g., endogenous CCK levels, level of gastric distention and small-intestinal presence of nutrients) indicates the robustness of CCK-58’s satiety effects. Notably, the effects of CCK differ from those of another gastrointestinal peptide, GRP, tested by one of us (JG). GRP-induced prolongation of IMI was seen only after administration at 5 or 15, but not at 30 minutes post-prandially, possibly due to increased gastric retention of meal contents [50].
Given CCK-58’s likely role as a dominant circulating and physiologically active CCK form in rats and other mammalian species, the current results suggest that a re-qualification of CCK’s anorexigenic effects to include satiety as well as satiation may be appropriate. It would be implied that previous feeding studies relying on exogenous CCK-8 may have underestimated CCK’s role in meal patterning. Parenthetically, the notion of multiple forms of CCK having different sites and modes of action is not unique in the gastrointestinal-peptide realm: other illustrations of this phenomenon include somatostatin [58], gastrin [48] and PYY [23].
The robust behavioral observations of more pronounced satiety effects by CCK-58 than by CCK-8 highlights the importance of further studies to identify the underlying physiological mechanisms. Based on current insight, different efficacy of the two CCK forms at the CCK-1 receptor (CCK-1R) does not seem to be a key factor. In case of equal receptor occupancy, the two CCK forms trigger equal intracellular calcium responses and membrane-receptor dynamics. In fact, receptor affinity of CCK-58 appears to be less than that of CCK-8 [66]. In our view, CCK-58’s satiety actions are more likely related to the diverging kinetics of short and long CCK forms after their absorption in blood and lymph along with related central effects. Immediately after ip administration, both forms of CCK can be expected to reach the CCK-1R expressed on afferent fibers of the vagus nerve in the gastrointestinal laminae propria at a comparably rapid rate (see [52]), which could explain similar acute satiation effects and meal-size reduction. At the next distinguishing stage, part of the CCK enters the hepatic portal vein blood and undergoes obligatory hepatic processing, which affects CCK-8 and CCK-58 differently. In the liver, CCK-8, is extracted, sequestered and intracellularly metabolized by a mechanism that selectively processes peptides with fewer than 10 amino acids [28, 40]. In contrast, blood-borne CCK-58 traverses the liver and accesses the general circulation. Additionally, longer CCK forms may stimulate receptors in the liver, possibly inducing satiety-related activity in the hepatic branch of the afferent vagus nerve (as shown for CCK-33 vs. CCK-8; [14]). The unrestricted hepatic passage of CCK-58 results in rapid systemic distribution with potentially pleiotropic effects, supported by a three-fold plasma half-life for CCK-58 relative to that of CCK-8 (i.e., 4.4 vs 1.3 minutes [25]). Aside from portal-vein entry, a second, slower conduit for ip-injected (as well as endogenous) CCK might be provided by lymph ducts draining the abdominal cavity and small-intestinal wall [31, 52]. Radiolabeled, ip-administered CCK-8 can be traced in lymph within 15 minutes, with maximum concentrations occurring after 45 minutes. Lymph-borne gastrointestinal peptides are thought to be relatively protected from peptidase breakdown, permitting post-meal concentrations in lymph to reach levels several-fold higher than in plasma [31]. One could speculate that CCK-58, which is more lipophilic than CCK-8, is taken up preferentially in lymph ducts, where it is protected from proteolysis, and exerts late satiety effects, either from within lymph ducts, or after ejection into the blood stream.
Clearly, CCK both from exogenous and somatic origin contributed to results in our current study. The identity of hepatic, post-hepatic, and, possibly, post-lymphatic sites of action mediating the satiety effects of CCK-58 merits further investigation. CCK-58 has shown qualitatively unique actions on pancreatic secretion [68], and prolonged activation of vagal afferent neurons [33], which might be relevant. Moreover, activation of sympathetic afferents [6] and circumventricular sites at CNS hindbrain level [18, 43] could be involved in the satiety actions by CCK-58.
Any empirical claim regarding satiation and satiety actions of exogenous compounds should consider the potential role of malaise or motor impediments in decreased food intake. Meal size reduction by 50–70% following CCK administration, as found in the current studies, has been established as non-aversive [56]. Our lickometer data confirmed that CCK-58’s acute satiation effect was not related to aversion, motor impairment or malaise. First, the decreased meal size after pre-meal injections of CCK-8 or CCK-58 occurred in conjunction with a reduced number, but not size, of eating bouts, suggesting a natural, non-aversive satiation process [2]. Secondly, inter-lick interval distribution was unperturbed after CCK-58 (see [1, 2] for illustrations of perturbed lick patterns). Finally, lick efficiency, i.e., the number of licks per ingested volume was not altered by anorectic doses of either form of CCK. The earlier finding by Davis et al. [10] of reduced lick efficiency after CCK-8 administration was not replicated, probably because of the approximately 8-times larger dose used in the former study. In sum, these data do not indicate that meal size reduction induced by CCK-58 did not involve any motor impediments or malaise.
A possible contribution of aversion induced by post-meal administration of CCK seems less remote. According to Kulkosky [34], aversion might occur if large doses CCK are administered immediately after large meals. This would be relevant primarily for studies 3a and 3b in which CCK was given 5 or 30 minutes post-prandially. However, to our knowledge, Conditioned Taste Aversion (CTA) has been shown only with pharmacological doses larger than the 0.9 and 1.8 nmole/kg used in the current studies [11, 34]. If some aversive mechanism might have occurred through synergy of gastrointestinal distension and exogenous CCK-induced afferent vagal signals [42, 53], it is unclear why such an effect would occur after CCK-58 but not after CCK-8 and be capable of extending the IMI, in view of the considerable gastric emptying rate of liquid foods [30] and nutrient removal by intestinal absorption in rats.
Some limitations in scope of the current results should be noted. As with all studies using exogenous CCK, ours probably resulted in supra-physiological CCK levels at some sites immediately after peptide administration and/or meals, although we did not monitor systemic levels of CCK. Arguably, however, ip CCK injections may reasonably mimic the most prominent signal transduction process underlying endogenous satiation by endogenous CCK, i.e., the paracrine exposure of vagal afferent neurons to CCK released from duodenal I-cells [49]. Furthermore, we monitored food intake under specific circumstances, i.e., scheduled, brief presentation of highly palatable foods during the light phase. This lends special significance to the earlier finding by one of us (JRR; ref [22]), that CCK-58 administered during the early dark phase delayed the onset of feeding by rats on chow, a finding that also implies an IMI-extending effect of this peptide form.
It should also be noted that the observed effect of single doses of CCK-58 on cumulative food intake was transient and had dissipated by 4 hours. Clearly, it seems appropriate to class CCK-58 as a short-term satiety factor, with limited potential as a regulator of long-term energy homeostasis. Indeed, previous studies in which the actions of CCK were prolonged artificially, i.e., by protracted continuous or meal-contingent infusions or by chemical modification to sustain CCK’s bioactivity, have repeatedly shown at most, only transient effects of food intake and body. This is further supported by a recent meal pattern analysis of selective CCK-1R knockout rats with a non-obesogenic genetic background, which displayed neither hyperphagic nor obese phenotypes [5]. Nevertheless, the satiety effect of CCK-58 raises the interesting issue of how effectively repeated or continuous administration of this peptide could stave off the compensatory increases in meal frequency that are usually seen with CCK-8 induced meal size reduction. As an early prelude to such studies, Hsiao et al. [26] showed that long-term infusion of a larger CCK form (CCK-33) consistently lengthened IMI and reduced total food intake over a period of 2 days. Also, possible modulation of CCK’s satiety actions by adiposity-derived signals (e.g. leptin; insulin) [39, 43, 60] - if confirmed - would contribute to the understanding of energy homeostasis.
The data reported here add satiety to the list of CCK-induced actions that differ qualitatively between CCK-58 and CCK-8, actions that also include stimulation of pancreatic fluid secretion, induction of pancreatitis, activation of vagal afferent neurons and stimulation of gallbladder contraction. Thus, CCK-58 deviates in most of its essential peripheral actions from CCK-8, the form that has been researched the most. The central actions of exogenous CCK-58 related to feeding have not yet been evaluated, but its wider systemic distribution and the fact that CCK-58 is a major form in the central nervous system [17] impel such studies. Interestingly, CCK-58 causes less hypertrophy/damage than CCK-8 to pancreas and - likely- other tissues [67], and an improved safety profile would certainly support applicability of the longer peptide form. In conclusion, additional studies of CCK-58, once it becomes more broadly available to the research community, may open new perspectives about the role of CCK in the physiological and pharmacological control of food intake.
Highlights.
CCK-58 is a prominent endogenous, but scantily studied form of cholecystokinin
Meal pattern effects of CCK-58 were compared with those of CCK-8 in rats
Four equimolar doses of CCK-58 and CCK-8 similarly reduced meal size
CCK-58, but not CCK-8 increased satiety, by enhancing the intermeal interval
CCK-58’s satiety effects may suggest re-examination of CCK’s role in feeding
Acknowledgments
Grant support
The studies in this paper were supported by the following National Institute of Health grants: NIH DK33580 and DK083449 (JRR,Jr), DK33248 (JG) and NIDDK RO1 DK61516 (DEC).
Footnotes
Authors’ contributions to this manuscript
JO and JRR Jr. drafted and finalized the manuscript. JG and DEC contributed to the finalization of the manuscript. All authors contributed to the concept and design of the studies. JO performed the rat studies and data analysis.
Disclosures
The authors declare no financial or other interest related to the contents of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1613–9. doi: 10.1152/ajpregu.2001.280.6.R1613. [DOI] [PubMed] [Google Scholar]
- 2.Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological post-prandial plasma concentrations. Clin Sci (Lond) 1995;89:375–81. doi: 10.1042/cs0890375. [DOI] [PubMed] [Google Scholar]
- 4.Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, et al. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. Am J Physiol Regul Integr Comp Physiol. 2009;296:R476–84. doi: 10.1152/ajpregu.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blevins JE, Overduin J, Fuller JM, Cummings DE, Matsumoto K, Moralejo DH. Normal feeding and body weight in Fischer 344 rats lacking the cholecystokinin-1 receptor gene. Brain Res. 2009;1255:98–112. doi: 10.1016/j.brainres.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TA, Washington MC, Metcalf SA, Sayegh AI. The feeding responses evoked by cholecystokinin are mediated by vagus and splanchnic nerves. Peptides. 2011;32:1581–6. doi: 10.1016/j.peptides.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol. 1999;276:R429–34. doi: 10.1152/ajpregu.1999.276.2.R429. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JD. Some new developments in the understanding of oropharyngeal and postingestional controls of meal size. Nutrition. 1999;15:32–9. doi: 10.1016/s0899-9007(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 10.Davis JD, Smith GP, Kung TM. Cholecystokinin changes the duration but not the rate of licking in vagotomized rats. Behav Neurosci. 1995;109:991–6. doi: 10.1037//0735-7044.109.5.991. [DOI] [PubMed] [Google Scholar]
- 11.Deupree D, Hsiao S. Cholecystokinin octapeptide, proglumide, and conditioned taste avoidance in rats. Physiol Behav. 1987;41:125–8. doi: 10.1016/0031-9384(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 12.Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8–12. doi: 10.1097/MED.0b013e32834eb77d. [DOI] [PubMed] [Google Scholar]
- 13.Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, Reeve JR., Jr Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol. 1987;253:G477–82. doi: 10.1152/ajpgi.1987.253.4.G477. [DOI] [PubMed] [Google Scholar]
- 14.Eisen S, Phillips RJ, Geary N, Baronowsky EA, Powley TL, Smith GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R456–R62. doi: 10.1152/ajpregu.00062.2005. [DOI] [PubMed] [Google Scholar]
- 15.Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem. 1987;262:214–7. [PubMed] [Google Scholar]
- 16.Eysselein VE, Reeve JR, Jr, Shively JE, Hawke D, Walsh JH. Partial structure of a large canine cholecystokinin (CCK58): amino acid sequence. Peptides. 1982;3:687–91. doi: 10.1016/0196-9781(82)90171-1. [DOI] [PubMed] [Google Scholar]
- 17.Eysselein VE, Reeve JR, Jr, Shively JE, Miller C, Walsh JH. Isolation of a large cholecystokinin precursor from canine brain. Proc Natl Acad Sci U S A. 1984;81:6565–8. doi: 10.1073/pnas.81.21.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry M, Hoyda TD, Ferguson AV. Making sense of it: roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp Biol Med (Maywood) 2007;232:14–26. [PubMed] [Google Scholar]
- 19.Gardner JD, Conlon TP, Kleveman HL, Adams TD, Ondetti MA. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975;56:366–75. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–95. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 21.Glatzle J, Raybould HE, Kueper MA, Reeve JR, Jr, Zittel TT. Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr Neurosci. 2008;11:69–74. doi: 10.1179/147683008X301432. [DOI] [PubMed] [Google Scholar]
- 22.Goebel-Stengel M, Stengel A, Wang L, Ohning G, Tache Y, Reeve JR., Jr CCK-8 and CCK-58 differ in their effects on nocturnal solid meal pattern in undisturbed rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R850–60. doi: 10.1152/ajpregu.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandt D, Schimiczek M, Struk K, Shively J, Eysselein VE, Goebell H, et al. Characterization of two forms of peptide YY, PYY(1–36) and PYY(3–36), in the rabbit. Peptides. 1994;15:815–20. doi: 10.1016/0196-9781(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 24.Green GM, Reeve JR., Jr Unique activities of cholecystokinin-58; physiological and pathological relevance. Curr Opin Endocrinol Diabetes Obes. 2008;15:48–53. doi: 10.1097/MED.0b013e3282f3d92b. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann P, Eberlein GA, Reeve JR, Jr, Bunte RH, Grandt D, Goebell H, et al. Comparison of clearance and metabolism of infused cholecystokinins 8 and 58 in dogs. Gastroenterology. 1993;105:1732–6. doi: 10.1016/0016-5085(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao S, Wang CH. Continuous infusion of cholecystokinin and meal pattern in the rat. Peptides. 1983;4:15–7. doi: 10.1016/0196-9781(83)90158-4. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao S, Wang CH, Schallert T. Cholecystokinin, meal pattern, and the intermeal interval: can eating be stopped before it starts? Physiol Behav. 1979;23:909–14. doi: 10.1016/0031-9384(79)90199-9. [DOI] [PubMed] [Google Scholar]
- 28.Hunter EB, Powers SP, Kost LJ, Pinon DI, Miller LJ, LaRusso NF. Physicochemical determinants in hepatic extraction of small peptides. Hepatology. 1990;12:76–82. doi: 10.1002/hep.1840120113. [DOI] [PubMed] [Google Scholar]
- 29.Jordan HA, Moses H, 3rd, MacFayden BV, Jr, Dudrick SJ. Hunger and satiety in humans during parenteral hyperalimentation. Psychosom Med. 1974;36:144–55. doi: 10.1097/00006842-197403000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol. 1992;263:R813–9. doi: 10.1152/ajpregu.1992.263.4.R813. [DOI] [PubMed] [Google Scholar]
- 31.Kohan AB, Yoder SM, Tso P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol Behav. 2011;105:82–8. doi: 10.1016/j.physbeh.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koopmans HS, Walls EK, Willing AE. Does the integrated level of all plasma nutrients control daily food intake? Brain Res Bull. 1991;27:429–34. doi: 10.1016/0361-9230(91)90137-9. [DOI] [PubMed] [Google Scholar]
- 33.Kreis ME, Zittel TT, Raybould HE, Reeve JR, Jr, Grundy D. Prolonged intestinal afferent nerve discharge in response to cholecystokinin-58 compared to cholecystokinin-8 in rats. Neuroscience Letter. 1997;230:89– 92. doi: 10.1016/s0304-3940(97)00483-7. [DOI] [PubMed] [Google Scholar]
- 34.Kulkosky PJ. Conditioned food aversions and satiety signals. Ann N Y Acad Sci. 1985;443:330–47. doi: 10.1111/j.1749-6632.1985.tb27083.x. [DOI] [PubMed] [Google Scholar]
- 35.Langhans W. Fatty acid oxidation in the energostatic control of eating--a new idea. Appetite. 2008;51:446–51. doi: 10.1016/j.appet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Lateef DM, Washington MC, Sayegh AI. The short term satiety peptide cholecystokinin reduces meal size and prolongs intermeal interval. Peptides. 2011;32:1289–95. doi: 10.1016/j.peptides.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Liddle RA. Cholecystokinin cells. Annu Rev Physiol. 1997;59:221–42. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 38.Lieverse RJ, Jansen JB, Masclee AM, Lamers CB. Satiety effects of cholecystokinin in humans. Gastroenterology. 1994;106:1451–4. doi: 10.1016/0016-5085(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 39.Matson CA, Wiater MF, Kuijper JL, Weigle DS. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides. 1997;18:1275–8. doi: 10.1016/s0196-9781(97)00138-1. [DOI] [PubMed] [Google Scholar]
- 40.Meijer DKF, Ziegler K. Mechanisms for the hepatic clearance of oligopeptides and proteins. In: Audus KL, Raub TJ, editors. Biological barriers to protein delivery. New York & London: Plenum Press; 1993. pp. 339–407. [Google Scholar]
- 41.Miesner J, Smith GP, Gibbs J, Tyrka A. Intravenous infusion of CCKA-receptor antagonist increases food intake in rats. Am J Physiol. 1992;262:R216–9. doi: 10.1152/ajpregu.1992.262.2.R216. [DOI] [PubMed] [Google Scholar]
- 42.Moran TH, McHugh PR. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol. 1982;242:R491–7. doi: 10.1152/ajpregu.1982.242.5.R491. [DOI] [PubMed] [Google Scholar]
- 43.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–10. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ondetti MA, Rubin B, Engel SL, Pluscec J, Sheehan JT. Cholecystokinin-pancreozymin: recent developments. Am J Dig Dis. 1970;15:149–56. doi: 10.1007/BF02235646. [DOI] [PubMed] [Google Scholar]
- 45.Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G255–65. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- 46.Reeve JR, Jr, Keire DA, Coskun T, Green GM, Evans C, Ho FJ, et al. Synthesis of biologically active canine CCK-58. Regul Pept. 2003;113:71–7. doi: 10.1016/s0167-0115(02)00301-4. [DOI] [PubMed] [Google Scholar]
- 47.Rehfeld JF. Cholecystokinin. Best Practice & Research Clinical Endocrinology & Metabolism. 2004;18:569– 86. doi: 10.1016/j.beem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Rehfeld JF, Bardram L, Hilsted L, Poitras P, Goetze JP. Pitfalls in diagnostic gastrin measurements. Clin Chem. 2012;58:831–6. doi: 10.1373/clinchem.2011.179929. [DOI] [PubMed] [Google Scholar]
- 49.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–73. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Rushing PA, Gibbs J. Prolongation of intermeal interval by gastrin-releasing peptide depends upon time of delivery. Peptides. 1998;19:1439–42. doi: 10.1016/s0196-9781(98)00097-7. [DOI] [PubMed] [Google Scholar]
- 51.Rushing PA, Houpt TA, Henderson RP, Gibbs J. High lick rate is maintained throughout spontaneous liquid meals in freely feeding rats. Physiol Behav. 1997;62:1185–8. doi: 10.1016/s0031-9384(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 52.Schade DS, Eaton RP. The peritoneum--a potential insulin delivery route for a mechanical pancreas. Diabetes Care. 1980;3:229–34. doi: 10.2337/diacare.3.2.229. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz GJ, Moran TH. Sub-diaphragmatic vagal afferent integration of meal-related gastrointestinal signals. Neurosci Biobehav Rev. 1996;20:47–56. doi: 10.1016/0149-7634(95)00039-h. [DOI] [PubMed] [Google Scholar]
- 54.Smith GP. The controls of eating: brain meanings of food stimuli. Prog Brain Res. 2000;122:173–86. doi: 10.1016/s0079-6123(08)62137-8. [DOI] [PubMed] [Google Scholar]
- 55.Smith GP. John Davis and the meanings of licking. Appetite. 2001;36:84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- 56.Smith GP, Gibbs J. The development and proof of the CCK hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD, Iversen LL, editors. Multiple Cholecystokinin Receptors in the CNS. Oxford: Oxford University Press; 1992. pp. 166–82. [Google Scholar]
- 57.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Tache Y, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–8. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tannenbaum GS, Ling N, Brazeau P. Somatostatin-28 is longer acting and more selective than somatostatin-14 on pituitary and pancreatic hormone release. Endocrinology. 1982;111:101–7. doi: 10.1210/endo-111-1-101. [DOI] [PubMed] [Google Scholar]
- 59.Vanderweele DA, Granja JA, Deems DA. Discomfort or satiety: the spontaneous meal pattern may serve as a predictor. In: Hoebel DG, Novin D, editors. The Neural Basis of Feeding and Reward. Brunswick, ME: Haer Institute; 1982. pp. 167–73. [Google Scholar]
- 60.Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 61.Washington MC, Coggeshall J, Sayegh AI. Cholecystokinin-33 inhibits meal size and prolongs the subsequent intermeal interval. Peptides. 2011;32:971–7. doi: 10.1016/j.peptides.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 62.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984;246:R776–87. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- 63.West DB, Greenwood MR, Marshall KA, Woods SC. Lithium chloride, cholecystokinin and meal patterns: evidence that cholecystokinin suppresses meal size in rats without causing malaise. Appetite. 1987;8:221–7. doi: 10.1016/0195-6663(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 64.West DB, Greenwood MR, Sullivan AC, Prescod L, Marzullo LR, Triscari J. Infusion of cholecystokinin between meals into free-feeding rats fails to prolong the intermeal interval. Physiol Behav. 1987;39:111–5. doi: 10.1016/0031-9384(87)90407-0. [DOI] [PubMed] [Google Scholar]
- 65.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9:489–98. doi: 10.1016/j.cmet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SV, Harikumar KG, Burgess RJ, Reeve JR, Jr, Miller LJ. Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G641–7. doi: 10.1152/ajpgi.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto M, Reeve JR, Jr, Green GM. Supramaximal CCK-58 does not induce pancreatitis in the rat: role of pancreatic water secretion. Am J Physiol Gastrointest Liver Physiol. 2007;292:G964–74. doi: 10.1152/ajpgi.00338.2004. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto M, Reeve JR, Jr, Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol. 2005;288:G866–79. doi: 10.1152/ajpgi.00389.2003. [DOI] [PubMed] [Google Scholar]