Abstract
Purpose
Recent findings suggest that combination treatment with anti-estrogen and anti-RET may offer a novel treatment strategy in a subset of breast cancer patients. We investigated the role of RET in potentiating the effects of anti-estrogen response and examined whether RET expression predicted the ability for tyrosine kinase inhibitor (TKI) to affect ERK1/2 activation in primary breast cancer.
Experimental Design
Growth response, ERK1/2 activation, Ki-67 and TUNEL were assessed in breast cancer cell lines in vitro and in xenografts with vandetanib and/or tamoxifen. Thirty tumors with matched normal breast tissue were evaluated for RET expression and response to TKI treatment.
Results
Vandetanib potentiated the inhibitory effect of tamoxifen in hormone responsive (p=0.01) and hormone insensitive (p<0.001) ERα-positive breast cancer cells. Vandetanib significantly repressed tumorigenesis of MCF-7 xenografts (p<0.001), which displayed decreased activation of ERK1/2 and AKT. Vandetanib and tamoxifen reduced the growth of established tumors with a greater effect of dual therapy compared to single agent (p=0.003), with tamoxifen reducing proliferative index and vandetanib inducing apoptosis. In primary breast cancers, RET expression correlated with the ERα-positive subtype. Relative decrease in ERK1/2 phosphorylation with TKI treatment was 42% (p<0.001) in RET-positive tumors vs. 14% (p=ns) in RET-negative tumors.
Conclusions
Vandetanib potentiated the anti-growth effects of tamoxifen in breast cancer, which was mediated through RET activation. RET predicted response to TKI therapy with minimal effects on ERK1/2 activation in RET-negative tumors. The preclinical data support evaluation of anti-estrogen in combination with TKI as a potential treatment strategy for RET-positive luminal breast cancer.
INTRODUCTION
Breast cancer has an annual incidence of 226,000 and accounts for approximately 40,000 deaths in the US, making it the second most common cause of cancer related death in women (1). Approximately 75% of breast cancers belong to the luminal subtypes, characterized by expression of the estrogen receptor alpha (ERα) (2, 3). Systemic treatment strategies for these patients rely on hormone therapy; however, patients with luminal breast cancers that are hormone insensitive have limited treatment options. Patients with luminal breast cancer have a favorable prognosis measured by rates of recurrence and disease specific long-term survival relative to other breast cancer subtypes (4, 5). However, roughly one-third of hormone receptor positive breast cancers have little response to anti-estrogen treatment or develop hormone resistance after initial response (6–8). Recently the BOLERO2 trial demonstrated improved response in women with advanced hormone receptor positive breast cancer treated with the mTOR inhibitor everolimus combined with the aromatase inhibitor exemestane, with median progression-free survival improved by 6 months compared to exemestane alone (9). Additionally, luminal breast cancers have relatively poor response to neoadjuvant chemotherapy measured by conversion to breast conserving operations, axillary clearance, and pathologic complete response, indicating an underlying lack of responsiveness to cytotoxic chemotherapies (10–12). Hormone resistant and locally advanced disease are two common clinical scenarios in which targeted molecular therapy could improve treatment options for patients with luminal breast cancer.
One marker of aggressive tumors within the luminal subtype is expression of the RET proto-oncogene (13). The RET gene encodes a receptor tyrosine kinase (RTK), constitutively activated mutants of which cause the multiple endocrine neoplasia type 2 (MEN2) syndromes and familial medullary thyroid carcinoma (14–16). Wild-type RET is expressed in breast cancer with a strong association with ERα expression (17–19); the RET gene is transcriptionally regulated by TFAP2C, which is a key transcriptional regulator of the luminal phenotype (20–23). The RET receptor is activated by glial cell line derived neurotrophic factor (GDNF), which has been shown in breast cancer models to result in activation of signal transduction pathways including ERK1/2 and AKT, leading to increased proliferation and cell survival (13, 18, 24).
Significant interaction between RET and ERα pathways has been previously described, with increased response to estrogen stimulation observed in the presence of functional RET (13, 19). RET has additionally been associated with resistance to tamoxifen and aromatase inhibitors, and increased expression has been demonstrated in hormone-resistant cell lines and primary tumors (25, 26). Previously we reported that the combination therapy with anti-estrogen and anti-RET in luminal breast cancer had a greater effect on cell growth than either therapy alone (24). Additionally, we found that antagonism of RET with a tyrosine kinase inhibitor (TKI) primarily acted to reduce growth through induction of apoptosis, while anti-ERα acted primarily through a reduction in cell proliferation, forming the biologic basis for dual treatment. On the other hand, a recent preclinical study using combination therapy with Fulvestrant and the RET inhibitor AST487 failed to demonstrate improved response with combination therapy (27). However, metastatic disease in mice with J110 tumors treated with tamoxifen demonstrated some improved response with the addition of AST487, for which the authors suggested a mechanism involving IL6 signaling. Based on these findings, we sought to further characterize the effects of anti-RET treatment using vandetanib in sensitizing luminal breast cancers to the anti-estrogen effects of tamoxifen. Furthermore, the current study was designed to provide additional pre-clinical data by assessing the effect of TKI treatment in fresh, primary breast cancer tumors in vitro and establishing a correlation between effects of TKI with RET expression.
METHODS
Cell Lines
The MCF-7 and BT-474 cell lines were obtained from the American Type Culture Collection (ATCC). Cells were grown using DMEM medium with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 degrees C and 5% CO2. The cells were not tested and authenticated by the authors and were passed for less than six months since obtaining the cells.
Chemicals and Treatment
Vandetanib was obtained from AstraZeneca Pharmaceuticals (Macclesfield, United Kingdom). For in vitro studies vandetanib was dissolved in DMSO to a final concentration of 10 μM. Z-4-hydroxytamoxifen was obtained from Sigma-Aldich (St. Louis, MO) and dissolved in ethanol to a final concentration of 10 μM for in vitro studies. Glial cell line-derived neurotrophic factor (GDNF) was purchased from Sigma-Aldrich and dissolved in PBS and dissolved to a final concentration of 2.5 ng/ml. Vandetanib and tamoxifen for xenograft studies were solubilized in 1% TWEEN 80 in deionized water.
MTT Proliferation Assay
Cells were plated on a 48 well plate at 5,000 cells per well in technical triplicate. Cells were allowed to adhere overnight and the appropriate hormone and/or drug containing media added. Samples were allowed to grow for 24–48 hours, after which they were incubated with MTT (0.5 mg/ml) for 3 hours at 37° C and crystals solubilized in 10% SDS in 0.01 M HCL for 3 hours at 37° C and read on an Infinite 200 Pro plate reader (Tecan, Switzerland) at an absorbance wavelength of 570 nm. Samples were averaged over three biologic replicates.
Tumor Xenografts
Fifty female Nu/J mice 6 weeks old (Jackson Laboratory, Bar Harbor, MN) were implanted with a 1.7-mg 30-day release estrogen pellet (Innovative Research, Sarasota, FL), which were left in place in all treatment groups for the duration of the experiment. The following day 1.5 × 106 exponentially dividing MCF-7 cells in 50% Matrigel (BD Biosciences, San Jose, CA) were injected subcutaneously into the right flank. Ten mice were randomized to receive treatment with vandetanib, 25 mg/kg by daily oral gavage, starting on the day of tumor injection. Ten mice were randomized to the control group with daily vehicle (1% TWEEN 80 in deionized water) daily gavage. The remaining 30 mice were randomized to receive tamoxifen (50 mg/kg), vandetanib (25 mg/kg), or both by daily gavage when tumors reached 0.5 cm in diameter. Four mice did not develop tumors within two weeks of injection and were not randomized to treatment or included in the analysis. In total, 9 mice were randomized to both the vandetanib and dual treatment groups and 8 mice were randomized to tamoxifen only treatment. Tumor volumes were recorded daily and calculated using the formula a*b2 where “a” represents the tumor diameter in longest dimension and “b” is the tumor length orthogonal to “a.” Mice in the vandetanib from injection group were euthanized 3 weeks following tumor injection, and all other groups were euthanized 10 days after randomization. Tumors were harvested immediately following euthanasia and placed in aliquots of formalin for immunohistochemistry and RIPA buffer for western blot analysis.
Immunohistochemistry (IHC)
Formalin fixed, paraffin embedded sections were evaluated by H&E, and IHC was performed for Ki-67 (Dako, Denmark), TUNEL (Millipore, Bilerica, MA), CD 31 (BD Biosciences) and RET (Cell Signaling Technology, Danvers, MA) with appropriate positive and negative controls. Samples were interpreted and scored by a blinded attending pathologist (RWA). RET expression was scored according to percentage of positive cells with 500 cells counted per slide. Ki-67 was scored according to percentage of positive cells with 500 nuclei counted per slide. TUNEL was quantified using positive cells per high-powered field (HPF) with 10 fields counted per sample. Microvascular density was quantified using anti-mouse CD31 with 10 HPF counted per slide and vessels only included if they exhibited a typical morphology with lumen as previously described (28).
Primary Breast Cancer Samples
Fresh tumor samples with corresponding normal breast tissue were collected from 30 patients with invasive breast cancer from the University of Iowa Breast Molecular Epidemiologic Resource (B-MER), an institutional review board approved tumor bank. Patients who received chemotherapy prior to resection were excluded. Tissue was obtained in the surgical pathology suite within 15 minutes of resection and was immediately aliquoted in RNAlater (Ambion, Carlsbad, CA) and frozen at −80° C or minced sharply and placed fresh into minimal media containing sunitinib (Sigma Aldich) at 500 nM or control media without drug. Samples were incubated for 30 minutes at 37° C and total protein collected as described below.
Western Blot
Total protein was isolated from resected xenograft and primary tumors using RIPA buffer with Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and PhosStop phosphatase inhibitor (Roche, Indianapolis, IN). The antibodies used for western blot were: RET, ERK, p-ERK, AKT and p-AKT (Cell Signaling Technologies, Danvers, MA), pRET, recognizing human phospho-RET Y905, (catalog #AF3269, R and D Systems, Inc., Minneapolis, MN), GREB-1 (Abcam, Cambridge, MA), GAPDH (Santa Cruz Biotechnologies, Santa Cruz, CA).
RNA Extraction and Real-Time Polymerase Chain Reaction
Following euthanasia, the right lower lobe of the mouse lung was resected and total RNA was isolated from the whole lung tissue using the RNeasy Mini kit (Qiagen, Valencia, CA). Primary tumor RNA was extracted using the Trizol method. Total RNA was converted to cDNA using the Superscript III kit (Invitrogen) using random hexamer primers. Quantitative PCR was performed according to standard TaqMan Fast protocol (Applied Biosystems, Carlsbad, CA). In order to quantify the tumor burden in the lung RT-PCR was performed using primers specific for human and mouse GAPDH (Applied Biosystems) as previously described (29). Primary tumor sample RNA was analyzed using TaqMan primer/probes for RET (Applied Biosystems) with 18S rRNA as endogenous control. RET expression of samples was normalized to MCF-7 expression. RT-PCR was performed in technical triplicate for each sample.
Statistical Analysis
Statistical analysis was performed using the two-sided Student’s T-test for continuous variables. Frequency association of categorical variables was performed using the Fisher’s exact test for comparisons between two groups, and using analysis of variation (ANOVA) when more than two groups were compared. All statistical calculations were performed using R (a standard statistical program). Statistical significance was defined as P < 0.05.
RESULTS
Inhibition of RET Signaling Reduces Proliferation and Increases the Anti-Proliferative Effects of Tamoxifen in Hormone Responsive and Hormone Resistant Luminal Breast Cancer
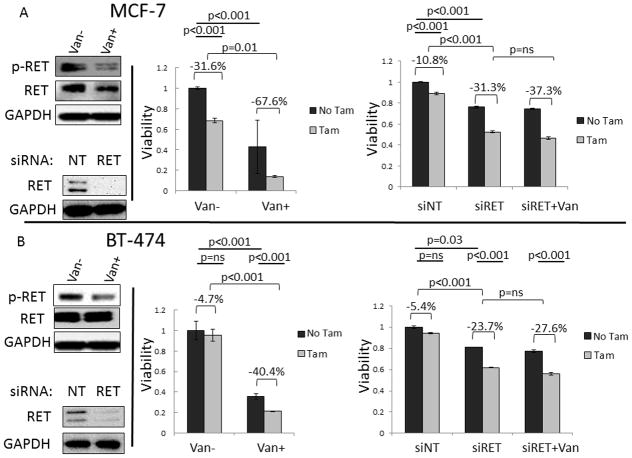
Previously we have demonstrated that inhibiting RET signaling with gene knockdown, or pharmacologic inhibition with sunitinib or vandetanib resulted in decreased proliferation in luminal breast cancer, and that effects can be combined with anti-estrogen treatment (24). To further evaluate the relationship of dual treatment we investigated the effects of tamoxifen in the presence and absence of intact RET signaling, through gene knockdown or the more RET specific TKI, vandetanib. Treatment of hormone sensitive MCF-7 cells with vandetanib resulted in a reduction in phosphylated RET without change in total RET, and reduced viability at 48 hours (p<0.001) (Figure 1). Cell viability was significantly reduced with tamoxifen alone (mean reduction 31.6%, p<0.001). In the presence of vandetanib, tamoxifen response was significantly augmented (mean reduction 67.6%, p<0.001), demonstrating a greater than 2-fold increase in the effect of tamoxifen in the presence of vandetanib, paired t-test p=0.01 (Figure 1A). Parallel experiments were performed using siRNA knockdown of RET. Knockdown of RET resulted in a significant reduction in viability (p<0.001 compared to siNT). Treatment with tamoxifen with intact RET reduced viability 10.8% (p<0.001 compared to no tamoxifen), while treatment with tamoxifen after knockdown of RET resulted in a 31.3% reduction in viability (paired t-test p<0.001). Finally, the addition of vandetanib resulted in a small, non-statistically significant reduction in growth and potentiation of tamoxifen under conditions of RET knockdown (paired t-test p=ns), demonstrating that effects of vandetanib to sensitize to tamoxifen treatment in hormone responsive MCF-7 are mediated by inhibition of RET signaling. Although tamoxifen can alter RET expression, RET expression was confirmed by western blot in all treatment groups (Supplemental Figure 1).
FIGURE 1. Anti-RET Reduces Luminal Breast Cancer Viability and Sensitizes to Anti-ERα.
A. Treatment of MCF-7 resulted in reduced phosphorylated RET with no change in total RET. Treatment of hormone responsive MCF-7 with vandetanib or tamoxifen reduces cell viability after 48 hours (p<0.001). Treatment with vandetanib sensitizes hormone sensitive MCF-7 to tamoxifen treatment such that treatment with tamoxifen results in a larger reduction in viability in the presence of vandetanib (−67.6%) then with control treatment (−31.6%), p=0.01 using the paired t-test. Knockdown of RET causes a reduction in viability compared to non-targeting (NT) siRNA (p<0.001) and results in an increase in tamoxifen sensitivity (−10.8% to −31.3%, paired t-test p=0.01) which is not increased with both RET knockdown and vandetanib treatment (−37.3%, paired t-test p=ns), confirming that the effects are mediated by RET. B. Treatment of hormone resistant luminal breast cancer cells, BT-474 with vandetanib reduces phosphorylated RET with no effect on total RET expression. Vandetanib reduces cell viability (p<0.001) with no significant effect with tamoxifen alone (p=ns). Similar to MCF-7, treatment with vandetanib increases sensitivity to tamoxifen, −40.4% vs −4.7%, p<0.001 with paired t-test. Knockdown of RET reduces viability compared to NT siRNA (p=0.03) and sensitizes hormone resistant BT-474 to tamoxifen treatment (−23.7% compared to −5.4%, paired t-test p<0.001). Treatment with vandetanib under conditions of RET knockdown did not result in a significant reduction in viability or change in tamoxifen sensitivity (−23.7% vs −27.6%, paired t-test p=ns). All westerns were performed in triplicate with representative blots shown. All MTT experiments were performed in technical and biologic triplicate with mean and standard deviation reported. P values were calculated using unpaired (straight lines) and paired (brackets) student’s t-test where appropriate.
Similar to MCF-7, vandetanib treatment of the hormone resistant luminal breast cancer cell line BT-474 resulted in a reduction of phosphorylated RET without change in total RET expression (Figure 1B). Treatment of BT-474 cells with tamoxifen alone failed to result in a significant reduction in viability (p=ns). However, in the presence of vandetanib, tamoxifen resulted in a significant reduction in viability (mean reduction 40.4%, p<0.001), Figure 1B. Hence, the addition of vandetanib resulted in a 8-fold augmentation in the cell viability effects of tamoxifen (−4.7% vs. −40.4%, paired t-test p<0.001). Parallel experiments demonstrated that knockdown of RET reduced cell viability compared to non-targeting siRNA (p=0.03). Similar to vandetanib treatment, compared to NT transfection, knockdown of RET increased the effects of tamoxifen treatment (−5.4% vs. −23.7%, paired t-test p<0.001). Vandetanib treatment with or without tamoxifen under conditions with knockdown of RET failed to demonstrate any additional effects on cell viability compared to RET knockdown alone (paired t-test p=ns). Hence, the data supports a model where the ability of vandetanib to augment tamoxifen effects on cell viability are mediate through RET. These results demonstrate that inhibition of RET signaling can reduce proliferation in two ways—through direct effects and through increasing sensitivity to anti-estrogen. Of particular clinical significance, anti-RET treatment increased anti-estrogen effects in both hormone sensitive and hormone resistant luminal breast cancer.
Vandetanib Reduces MCF-7 Xenograft Tumorigenesis and Metastatic Potential
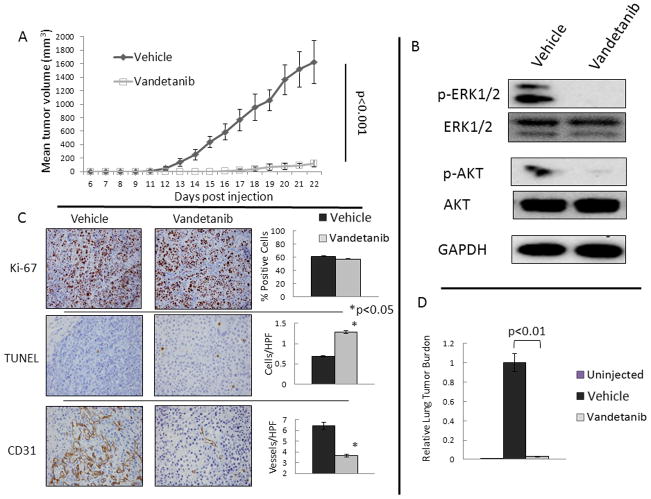
To test the observed effects of vandetanib in vivo, we studied tumorigenesis using a xenograft model. Athymic mice injected with MCF-7 cells had a decreased rate of tumor formation when treated with daily gavage of vandetanib from the time of tumor cell injection (Figure 2A). Mice treated with Vandetanib were not noted to have obvious weight loss compared to the control treated mice, and no drug specific toxicity including diarrhea or visible rash were observed. To investigate the effects of vandetanib treatment on RET downstream pathways, protein from xenograft tumors was evaluated using phosphorylation specific western blot for ERK1/2 and AKT. Tumors from animals treated with vandetanib had a reduction in activation of ERK1/2 and AKT, which demonstrates that vandetanib acted to abrogate RET signaling in the xenograft tumors (Figure 2B). Immunohistochemistry demonstrated no significant change in mean Ki-67 proliferative index in the vandetanib treated tumors (Figure 2C). Staining for TUNEL showed a significantly higher number of apoptotic cells per high powered field in the vandetanib treated group compared to the vehicle treated group (1.28 vs. 0.69 cells/HPF, p<0.001). Evaluation of microvascular density using CD31 staining demonstrated a significant reduction in vessel formation in tumors from the vandetanib treated animals compared to the control (3.7 vs. 6.45 Vessels/HPF, p=0.008). These results demonstrate that vandetanib reduced tumor growth through decreased activation of RET downstream mediators and a subsequent increase in apoptosis with effects on angiogenesis also noted.
FIGURE 2. Vandetanib Inhibits MCF-7 Xenograft formation.
A. Ten mice were treated with vandetanib, 25 mg/kg daily by oral gavage from the time of tumor injection, and had reduced tumor formation and growth compared to ten vehicle gavaged animals. Mean tumor volume is shown with error bars representing the standard deviation. B. Protein from xenograft tumors demonstrates decreased activation of the RET pathway downstream targets ERK1/2 and AKT in vandetanib treated animals compared to control treated. Three tumors from each group were analyzed by western blot and representative blots are shown. C. Immunohistochemistry (IHC) was performed on all 20 tumors with representative slides shown and quantified with graphs representing the mean and standard deviation of the spread of data for the treatment and vehicle groups. IHC of tumors demonstrates no change in proliferative index (Ki-67) with vandetanib treatment. Vandetanib caused a significant induction of apoptosis measured by TUNEL positive cells, and resulted in a reduction in microvessel density measured by CD31. Stars indicate p<0.05. D. Treatment with vandetanib reduces detectable tumor cells in the lung. The p values were calculated using the Student’s t-test for continuous variable data.
Due to the observed effects of growth reduction in primary MCF-7 xenograft tumor sites by systemic therapy with vandetanib, we investigated the effects of systemic treatment on disease burden in the mouse lung. RNA from the lungs of mice that were not injected with human tumor cells did not demonstrate amplification of the quantitative PCR probe for human GAPDH (Figure 2D). Treatment with vandetanib in lung tissue evaluated at 22 days post-inoculation resulted in 97% reduction in tumor cells in the lung compared to vehicle-treated animals, p<0.01. These results show decreased tumor cells in the lung of treated mice, which could represent decreased tumor in the lung parenchyma, a reduction in micrometastases or a reduction in the number of circulating tumor cells.
Vandetanib Reduces Growth of Established MCF-7 Xenografts and Potentiates the Effects of Tamoxifen
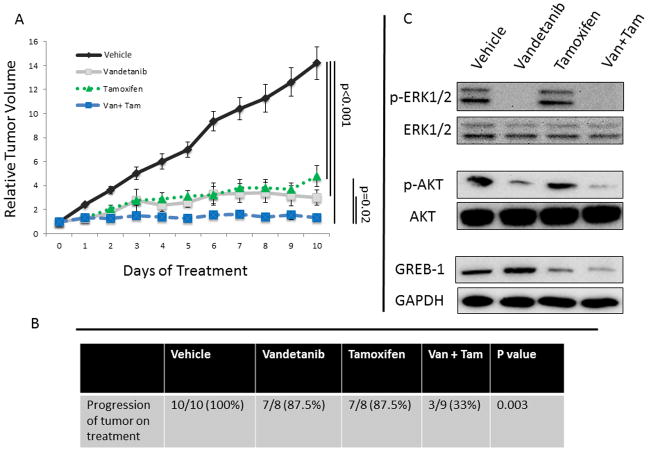
To test the potential synergistic effects of anti-RET and anti-estrogen treatment in vivo, we investigated the effects of treatment with vandetanib, tamoxifen, or dual treatment on established MCF-7 xenografts. Treatment with single agent of either vandetanib or tamoxifen resulted in a significant reduction in the growth of established xenografts, p<0.001 (Figure 3A). Combination therapy with both vandetanib and tamoxifen resulted in significantly reduced mean tumor growth compared to either agent alone (p=0.02). All of the mice in the vehicle-treated group had progression (defined as a tumor volume on day 10 greater than tumor volume on randomization). By comparison, 87.5% of mice in each of the single drug treatment groups progressed; however, only 33% of animals in the dual treatment group had tumor progression, p=0.003 by ANOVA (Figure 3B).
FIGURE 3. Dual Treatment with Vandetanib and Tamoxifen Reduces Xenograft Tumor Growth Greater Than Either Agent Alone.
A. Daily gavage with either vandetanib (9 mice) or tamoxifen (8 mice) resulted in a significant reduction in tumor growth in established xenografts compared to vehicle gavage (10 mice). Animals treated with a combination of both vandetanib and tamoxifen (9 mice) had significantly reduced tumor growth compared to either agent alone (p=0.02). Mean relative tumor volume (normalized to the volume at the time of randomization to treatment) is reported with error bars representing the standard deviation. The p values were calculated using the Student’s t-test to compare treatment groups. B. Mice in the dual treatment group were significantly less likely to have progression of the primary tumor site than single agent or control treated animals, p=0.003. The p value was calculated using analysis of variation (ANOVA). C. Protein from xenograft tumors demonstrates that treatment with vandetanib reduces phosphorylation of ERK1/2 and AKT which are downstream signalers in the RET pathway. Treatment with tamoxifen reduced expression of the estrogen response gene GREB-1. Dual treatment (Van + Tam) resulted in decreased activation of RET and ERα pathways. Three tumors were analyzed from each treatment group and representative western blots are shown.
Protein was harvested from xenograft tumors and effects of single and dual treatment on downstream markers of RET and ERα activation was assessed by western blot. Tumors from mice treated with vandetanib had reduced activation of ERK1/2 and AKT, Figure 3C. Tumors from mice treated with tamoxifen had decreased expression of GREB-1, an estrogen response gene (30), which is necessary for estrogen dependent growth (31). These results demonstrate the efficacy of systemic treatment to reduce RET and ERα downstream activation and highlight the distinct pathways of RET and ERα signaling, informing the rationale for use of dual receptor therapy.
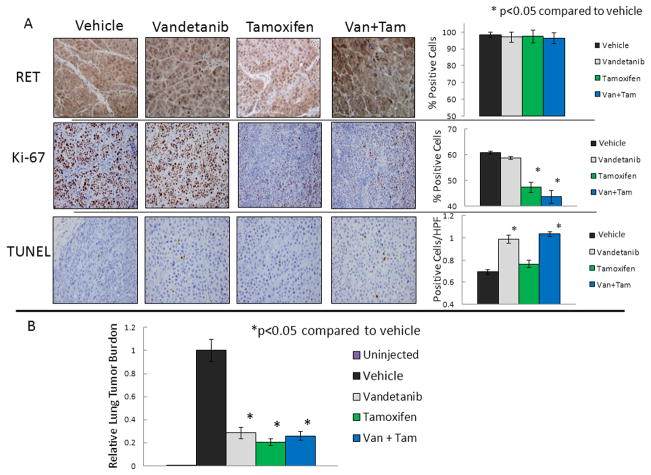
Prior studies have shown that RET is an estrogen response gene, so we tested the effect of vandetanib, tamoxifen, or dual treatment on RET expression. IHC for RET expression in the xenografts demonstrated no significant change in RET expression in any of the treatment groups (Figure 4). Similar to the tumorigenesis experiment, analysis of tumor tissue from mice with established tumors treated with vandetanib showed no significant differences in Ki-67 positivity on IHC compared to tumors from control animals. However, tumors from vandetanib treated animals demonstrated a significant induction in TUNEL positive apoptotic cells compared to control treated animals (0.99 cells/HPF vs. 0.69 cells per HPF, p=0.03), Figure 4A. Systemic treatment with tamoxifen resulted in a similar reduction in growth as vandetanib; however, IHC demonstrated that tamoxifen treatment resulted in a significant reduction in proliferative index compared to vehicle-treated animals (47% vs. 61%, p=0.02), without changes in apoptosis determined by TUNEL. Dual treatment with tamoxifen and vandetanib resulted in a larger reduction in tumor growth rate (Figure 3A), decreased progression compared to single treatment (Figure 3B) and both a reduction in Ki-67 (via anti-estrogen) and an induction of apoptosis (via anti-RET), p<0.05 (Figure 4A). These results provide further evidence that ERα and RET drive cell growth through distinct pathways which can be simultaneously targeted by a dual treatment strategy. Treatment of mice with established xenografts with tamoxifen, vandetanib, or both, similarly resulted in significant reduction of lung tumor burden, p<0.05; however, there was no increased effect seen with dual treatment, Figure 4B.
FIGURE 4. Vandetanib Induces Apoptosis, Tamoxifen Reduces Proliferative Index and Both Effects in Dual Treated Tumors.
A. Immunohistochemistry (IHC) demonstrates that RET expression is not changed by vandetanib, tamoxifen or dual (Van + Tam) treatment. Vandetanib treatment induces TUNEL, tamoxifen treatment reduces Ki-67 positivity, and both effects are seen with dual treatment. IHC was performed on slides from all xenograft tumors with representative slides shown and graphs represent the mean and standard deviation for each group. B. Animals treated with vandetanib, tamoxifen, or dual treated had reduced tumor burden in the lung. The p values were calculated using the Student’s t-test for continuous variables.
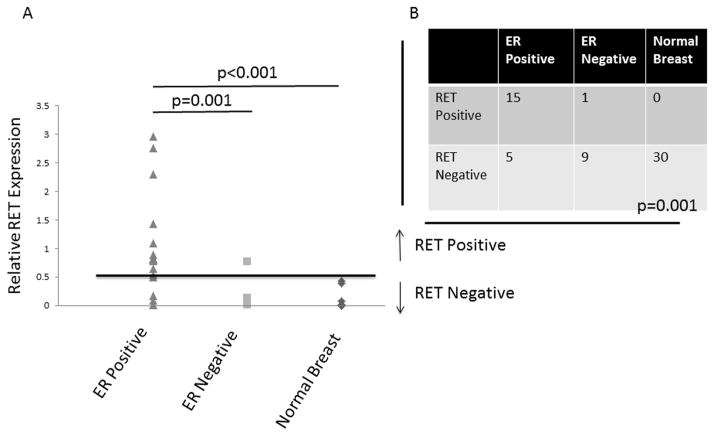
RET Expression is Increased in ER-Positive Compared to ER-Negative Breast Cancer and Normal Breast Tissue
Previously it has been reported that RET is expressed in breast cancer in association with the ERα gene expression cluster (19, 32). To further characterize RET expression in breast cancers and normal breast tissue we obtained samples from 20 ERα-positive tumors, 10 ERα-negative tumors with 30 patient matched normal breast tissue samples and analyzed RET expression by quantitative RT-PCR. Mean RET expression in ERα-positive tumors was 8 times higher than expression in ERα-negative tumors, and 20 times higher than mean expression in corresponding normal breast tissue (Figure 5A, p<0.001). Using a threshold of 0.5 fold expression relative to MCF-7, 15/20 (75%) of ERα-positive tumors were RET positive, 1/10 (10%) of ERα-negative tumors were RET positive, and 0/30 normal breast tissue samples were positive for RET, p=0.001, Figure 5B. These results demonstrate that RET is more highly expressed in ERα-positive tumors, and that it is relatively poorly expressed in normal breast, thus making RET an ideal molecule for targeted therapy.
FIGURE 5. RET Expression is Associated with ERα Positive Breast Cancers.
A. RNA extracted from primary human breast cancers and patient matched normal breast tissue demonstrates significantly higher mean RET expression in ERα-positive tumors compared to ERα-negative tumors and normal breast tissue. The data represent values from 20 ERα-positive, 10 ERα-negative tumors, and the 30 samples of matched normal breast tissue, which are overlaid for some data points. The p values were calculated using the Student’s t-test. B. When a cutoff of 0.5 fold RET expression relative to MCF-7 is used, significantly more ERα-positive tumors are RET positive compared to ERα-negative and normal breast tissue, p=0.001. The p value was calculated using the analysis of variation (ANOVA).
Response to in Vitro TKI Correlates with RET Expression in Primary Breast Cancers
Previously we have reported that activation of ERK1/2 is a marker of RET pathway activation and that in vitro inhibition or gene knockdown of RET resulted in decreased phosphorylated ERK1/2 (24). To examine the response to TKI treatment across a spectrum of breast cancer, primary tumors were obtained fresh from resection specimens and treated in vitro with sunitinib or vehicle, in parallel. Tumor tissue was assessed for RET expression by RT-PCR, IHC and western blot and samples were analyzed for phosphorylated ERK1/2 and AKT by western blot, Figure 6A and B. Primary breast tumors demonstrated a significant mean reduction in phosphorylated ERK1/2 compared to control treated (29% reduction, p<0.001), whereas, normal breast tissue treated with sunitinib did not show decreased activation of ERK1/2, Figure 6C. The data were stratified by RET expression and compared for RET-negative and RET-positive tumors. Where they could be compared, expression of RET protein by western blot was confirmed in samples with RET RNA expression (Supplemental Figure 2). The RET-negative tumors demonstrated no significant effect of ERK1/2 activation with TKI treatment. By contrast, the RET-positive tumors demonstrated a significant reduction in ERK1/2 activation with sunitinib, p<0.001. Comparing tumor samples with differential RET expression determined by quantitative RT-PCR, there was a significantly greater effect in the reduction in mean ERK1/2 activation with sunitinib treatment in RET-positive compared to RET-negative tumors (42% vs. 14%, p=0.01), Figure 6D. Although sunitinib has activity against multiple RTKs, response measured by activation of ERK1/2 was highly correlated with RET expression. Samples were also measured for activated AKT by phosphorylation specific western blot. Treatment with sunitinib did not significantly reduce activated AKT in all tumors (mean reduction 9%, p=0.43) or significantly reduce activated AKT in RET positive tumors (10% reduction) relative to RET negative tumors (8% reduction, p=0.62). These results could indicate that the observed effects are independent of the PI3K/AKT/mTOR pathway, or that our 30-minute treatment was insufficient to induce reduced activation of this pathway in primary tumors.
Figure 6. TKI Treatment Reduces ERK1/2 Activation in Primary Breast Tumors and Response is Associated with RET Expression.

A. Immunohistochemistry of resected tumors demonstrating a representative RET-positive and RET-negative sample. B. Representative western blots showing a greater reduction in ERK1/2 activation and no effect on AKT activation with sunitinib treatment in a RET-positive tumor compared to a RET-negative tumor. Western blots for ERK1/2, p-ERK1/2, AKT, and p-AKT were performed on all 30 tumors with representative blots shown. C. Fresh primary human breast cancer tissue treated in vitro with sunitinib had a significant reduction in mean ERK1/2 activation relative to control treated tumor tissue. Patient matched normal breast tissue did not have reduced ERK1/2 activation with sunitinib treatment. ERK1/2 activation was quantified for all 30 tumors and matched normal breast tissue. D. The reduction in ERK1/2 activation in response to sunitinib treatment was significantly greater in RET expressing tumors. The p values calculated in C and D was performed using the Student’s t-test.
DISCUSSION
There are a subset of patients with advanced luminal breast cancer that have limited treatment options due to poor response to cytotoxic chemotherapy or the development of hormone resistance. Developing successful treatment options for these patients will rely on a better understanding of molecular determinants of tumor growth, progression and hormone resistance. Investigation of these factors will allow identification of patients with more aggressive tumors predicted to have worse disease related outcomes, as well as inform subsequent development of targeted therapies. Recent investigation has demonstrated that expression of the RET proto-oncogene in luminal breast cancer is involved in proliferation and survival of tumor cells, and overexpression and activation has been implicated in the development of hormone resistance (25, 26). Previously we have reported there are independent pathways regulated by RET and ERα which can be targeted independently with dual receptor-targeted therapy to reduce in vitro growth in luminal breast cancer (24). Herein, the effect of dual treatment is further clarified in hormone sensitive and hormone resistant luminal breast cancer lines and shown in a xenograft model to increase efficacy over single agent treatment to prevent tumor growth and progression. Additionally, analysis of primary tumors demonstrated that response to the TKI, sunitinib, is associated with RET expression and that RET is both a target and a marker for patient selection for this type of treatment.
Previously TKIs have been investigated as systemic therapy in patients with breast cancer. Clinical trials in breast cancer with TKIs have focused directed therapy based on anti-VEGF or anti-EGFR activity. One TKI with activity against RET, sunitinib, has been examined in a limited number of clinical trials in breast cancer focused on unresectable metastatic disease. Some response has been demonstrated in these highly selected patients. Most investigators have suggested that combination therapy be considered as mono-therapy failed to demonstrate improved response compared to more conventional agents (33–37). Vandetanib has been examined in small series of breast cancer patients with refractory metastatic disease. In one trial of 46 patients with previously treated breast cancer, doses of 100 mg or 300 mg were well tolerated but demonstrated limited activity as monotherapy (38). In a recent trial, 35 patients were treated with combination therapy using vandetanib in combination with docetaxel, but vandetanib was not shown to improve the response rate (39). However, the small number of patients limited the ability to determine whether or not there was a response to treatment attributable to vandetanib. In a phase I trial using vandetanib in combination with cyclophosphamide and methotrexate, vandetanib at a daily dose of 200 mg was well tolerated and objective response was reported, though determining the efficacy of vandetanib was not possible due to the study design (40). Several factors are important when considering these limited earlier studies. First, these studies did not consider RET as the molecular target and combining tumors with and without RET expression would likely mask effects without subgroup analysis. Secondly, the studies were limited to highly selected patients with aggressive chemoresistant cancer. RET expression is strongly linked to ERα-positive breast cancers, which is a group that would likely be underrepresented in this cohort. Additionally, the prior studies with vandetanib have been uniformly underpowered and have not utilized RET expression as a data stratification factor which could mask a quantifiable response to treatment in RET expressing tumors. Finally, utilization of RET specific endpoints, such as ERK1/2 or AKT activation, or tumor apoptotic rate, which we have shown to be associated with xenograft response, could be more informative biomarkers of response to vandetanib treatment in luminal breast cancer.
Demonstration of systemic TKI therapy as an efficacious treatment in breast cancer relies on the identification and selection of patients most likely to have a response. RET expression levels have been shown in one study to be predictive of induction of apoptosis with sunitinib treatment in liver cancer xenografts (41). Previously we have shown that although the available molecules with anti-RET activity have activity against multiple receptors, in vitro activity of the TKIs sunitinib and vandetanib are significantly reduced in the absence of functional RET (24). In this manuscript, we further these investigations by showing that blocking RET potentiated the effect of anti-estrogen therapy in hormone responsive and hormone insensitive ERα-positive breast cancer. Furthermore, we established RET as a molecular marker for response to TKI treatment by demonstrating that only RET-positive breast tumors reduced ERK1/2 activation in response to TKI treatment. Although further characterization is needed to confirm RET as a marker of cellular TKI response, these data indicate that RET expressing breast cancers could be most likely to respond to treatment and that results of future investigation should be stratified according to RET expression. In this study we identify 53% of tumors and 75% of ERα-positive tumors as RET-positive, which is within the published range of 25–100% (18, 19, 25, 27). Additionally, we have identified that vandetanib reduced microvessel formation in luminal breast cancer xenografts. The mechanism leading to reduced microvasculature with vandetanib treatment, which could be mediated at least in part by VEGF family receptors, as well as the relative contribution of this effect on the observed reduction in tumor growth, require further study. Similar findings have previously been shown in models of glioblastoma and hepatocellular carcinoma (42, 43). This effect is believed to be mediated by VEGF family receptors and indicates that anti-tumor results seen in luminal breast cancer xenografts with vandetanib treatment could be due to antagonism of multiple receptors. Vandetanib has additionally been shown to have anti-tumor activity in non-small cell lung cancer (NSCLC), which is thought to be mediated by EGFR, which is expressed in luminal breast cancer and normal breast epithelium (44, 45). Interestingly, the effects of vandetanib in NSCLC were increased in dual treatment with anti-estrogen, indicating some interaction between ERα and RTK (RET, EGFR, or others) pathways in multiple cancer types. More recently, the effects of dual EGFR and ERα antagonism were investigated in breast cancer, and the efficacy of EGFR inhibition with lapatinib when added to letrozole has been shown in ERα-positive, HER-2 negative metastatic breast cancer, with effects most pronounced in low ERα expressing ERα-positive tumors (46). Additionally, in lung cancer cell lines, a recent study showed benefit of combining anti-EGFR and anti-estrogen (47). Although multiple studies have shown interaction between EGFR and ERα pathways (48, 49), further study is needed to elucidate the role of RET, EGFR, and VEGFRs in determining growth characteristics, interaction with hormone signaling pathways, and mediating the anti-tumor effects of vandetanib in luminal breast cancer.
Based on these findings vandetanib should be investigated in breast cancer using RET as a marker for selection and stratification. The majority of RET expressing breast cancers belong to the luminal subtype, and selection of patients in this subtype allows for combination with anti-estrogen, which could allow for enhancement of anti-proliferative effects with dual treatment. Systemic TKI therapy in breast cancer with RET as the therapeutic target has not been previously investigated in combination with anti-estrogen, which could demonstrate increased efficacy compared to single agent therapy. Establishment of a new treatment paradigm in luminal breast cancer based on anti-RET and ant-estrogen could improve outcomes for a large number of patients with limited treatment options.
Supplementary Material
Statement of Translational Relevance.
Patients with advanced luminal breast cancer have limited treatment options due to poor response to cytotoxic chemotherapy and the potential for hormone resistance. Our preclinical data indicates that the tyrosine kinase inhibitor (TKI) vandetanib is synergistic with anti-estrogen therapy, thus increasing the efficacy of hormonal therapy with dual treatment in both hormone responsive and hormone resistant luminal breast cancer. In primary breast tumors, we demonstrate that RET expression predicts response to TKI treatment. This study provides the preclinical evidence that inhibition of RET with vandetanib should be investigated in conjunction with anti-estrogen therapy in luminal breast cancer. Establishing a targeted molecular therapy to increase hormone sensitivity and improve tumor response to systemic therapy would provide an important treatment option for many patients with luminal breast cancer.
Acknowledgments
The authors would like to thank AstraZeneca, LC for providing vandetanib for this work. This work was supported by the National Institutes of Health grants R01CA109294 (PI: R.J. Weigel), T32CA148062 (PI: R. J. Weigel) and by a generous gift from the Kristen Olewine Milke Breast Cancer Research Fund. PMS and JPD were supported by the NIH grant T32CA148062.
Footnotes
All authors have no conflict of interest with this manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. P Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA-J Am Med Assoc. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Redmond C, Wolmark N, Wieand HS. Disease-free survival at intervals during and following completion of adjuvant chemotherapy: the NSABP experience from three breast cancer protocols. Cancer. 1981;48:1273–80. doi: 10.1002/1097-0142(19810915)48:6<1273::aid-cncr2820480602>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Redmond CK, Wickerham DL, Rockette HE, Brown A, Allegra J, et al. Relation of estrogen and/or progesterone receptor content of breast cancer to patient outcome following adjuvant chemotherapy. Breast Cancer Res Treat. 1983;3:355–64. doi: 10.1007/BF01807588. [DOI] [PubMed] [Google Scholar]
- 6.Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. Journal of Clinical Oncology. 2000;18:3758–67. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 7.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. Journal of Clinical Oncology. 2003;21:2101–9. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 8.Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. Journal of Clinical Oncology. 2012;30:2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spanheimer PM, Carr JC, Thomas A, Sugg SL, Scott-Conner CE, Liao J, et al. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg. 2013;206:2–7. doi: 10.1016/j.amjsurg.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooe A, Takahara S, Sumiyoshi K, Yamamoto H, Kawai J, Shiba E. Relationship between intrinsic subtypes and tumor responses to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Breast Dis. 2012;34:9–17. doi: 10.3233/BD-130345. [DOI] [PubMed] [Google Scholar]
- 12.Kim SI, Sohn J, Koo JS, Park SH, Park HS, Park BW. Molecular subtypes and tumor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Oncology. 2010;79:324–30. doi: 10.1159/000322192. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Mayer JA, Mazumdar A, Brown PH. The rearranged during transfection/papillary thyroid carcinoma tyrosine kinase is an estrogen-dependent gene required for the growth of estrogen receptor positive breast cancer cells. Breast Cancer Res Treat. 2012;133:487–500. doi: 10.1007/s10549-011-1775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lairmore TC, Dou SS, Howe JR, Chi D, Carlson K, Veile R, et al. A 1.5-megabase yeast artificial chromosome contig from human-chromosome 10q11.2 connecting 3 genetic-loci (Ret, D10s94, and D10s102) closely linked to the Men2a locus. P Natl Acad Sci USA. 1993;90:492–6. doi: 10.1073/pnas.90.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cote GJ, Wohllk N, Evans D, Goepfert H, Gagel RF. Ret Protooncogene Mutations in Multiple Endocrine Neoplasia Type-2 and Medullary-Thyroid Carcinoma. Bailliere Clin Endoc. 1995;9:609–30. doi: 10.1016/s0950-351x(95)80638-5. [DOI] [PubMed] [Google Scholar]
- 16.Jhiang SM, Fithian L, Weghorst CM, Clark OH, Falko JM, ODorisio TM, et al. RET mutation screening in MEN2 patients and discovery of a novel mutation in a sporadic medullary thyroid carcinoma. Thyroid. 1996;6:115–21. doi: 10.1089/thy.1996.6.115. [DOI] [PubMed] [Google Scholar]
- 17.Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, et al. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr-Relat Cancer. 2006;13:1109–20. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 18.Esseghir S, Todd SK, Hunt T, Poulsom R, Plaza-Menacho I, Reis-Filho JS, et al. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor up-regulation in breast cancer. Cancer Res. 2007;67:11732–41. doi: 10.1158/0008-5472.CAN-07-2343. [DOI] [PubMed] [Google Scholar]
- 19.Boulay A, Breuleux M, Stephan C, Fux C, Brisken C, Fiche M, et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008;68:3743–51. doi: 10.1158/0008-5472.CAN-07-5100. [DOI] [PubMed] [Google Scholar]
- 20.Woodfield GW, Chen Y, Bair TB, Domann FE, Weigel RJ. Identification of primary gene targets of TFAP2C in hormone responsive breast carcinoma cells. Genes Chromosomes Cancer. 2010;49:948–62. doi: 10.1002/gcc.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stine ZE, McGaughey DM, Bessling SL, Li S, McCallion AS. Steroid hormone modulation of RET through two estrogen responsive enhancers in breast cancer. Hum Mol Genet. 2011;20:3746–56. doi: 10.1093/hmg/ddr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanheimer PM, Woodfield GW, Cyr AR, Kulak MV, White-Baer LS, Bair TB, et al. Expression of the RET proto-oncogene is regulated by TFAP2C in breast cancer independent of the estrogen receptor. Annals of Surgical Oncology. 2013;20:2204–12. doi: 10.1245/s10434-012-2570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cyr A, Kulak MV, Park JM, Bogachek MV, Spanheimer PM, Woodfield GW, White-Baer L, O’Malley YQ, Sugg SL, Olivier AK, Zhang W, Domann FE, Weigel RJ. TFAP2C governs the luminal epithelial phenotype in mammary development and carcinogenesis. Oncogene. 2013 doi: 10.1038/onc.2013.569. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spanheimer PM, Cyr AR, Gillum MP, Woodfield GW, Askeland RW, Weigel RJ. Distinct pathways regulated by RET and estrogen receptor in luminal breast cancer demonstrate the biological basis for combination therapy. Annals of Surgery. 2013 doi: 10.1097/SLA.0b013e3182a6f552. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaza-Menacho I, Morandi A, Robertson D, Pancholi S, Drury S, Dowsett M, et al. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene. 2010;29:4648–57. doi: 10.1038/onc.2010.209. [DOI] [PubMed] [Google Scholar]
- 26.Morandi A, Martin LA, Gao Q, Pancholi S, Mackay A, Robertson D, et al. GDNF-RET Signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Research. 2013;73:3783–95. doi: 10.1158/0008-5472.CAN-12-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattelli A, Nalvarte I, Boulay A, Roloff TC, Schreiber M, Carragher N, et al. Ret inhibition decreases growth and metastatic potential of estrogen receptor positive breast cancer cells. EMBO Mol Med. 2013;5:1335–50. doi: 10.1002/emmm.201302625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura L, Carvalho FM, Alves BG, Bacchi CE, Goes JC, Calil MA, et al. Association between intratumoral lymphatic microvessel density (LMVD) and clinicopathologic features in endometrial cancer: a retrospective cohort study. World Journal of Surgical Oncology. 2010;8:89. doi: 10.1186/1477-7819-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Research. 2000;60:6367–75. [PubMed] [Google Scholar]
- 31.Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–9. doi: 10.1007/s10549-005-1483-4. [DOI] [PubMed] [Google Scholar]
- 32.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yardley DA, Dees EC, Myers SD, Li S, Healey P, Wang Z, et al. Phase II open-label study of sunitinib in patients with advanced breast cancer. Breast Cancer Research and Treatment. 2012;136:759–67. doi: 10.1007/s10549-012-2285-0. [DOI] [PubMed] [Google Scholar]
- 34.Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Research and Treatment. 2010;121:121–31. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. Journal of Clinical Oncology. 2012;30:921–9. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 37.Kozloff M, Chuang E, Starr A, Gowland PA, Cataruozolo PE, Collier M, et al. An exploratory study of sunitinib plus paclitaxel as first-line treatment for patients with advanced breast cancer. Annals of Oncology. 2010;21:1436–41. doi: 10.1093/annonc/mdp565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller KD, Trigo JM, Wheeler C, Barge A, Rowbottom J, Sledge G, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clinical Cancer Research. 2005;11:3369–76. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 39.Boer K, Lang I, Llombart-Cussac A, Andreasson I, Vivanco GL, Sanders N, et al. Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized Phase II study. Invest New Drugs. 2012;30:681–7. doi: 10.1007/s10637-010-9538-8. [DOI] [PubMed] [Google Scholar]
- 40.Mayer EL, Isakoff SJ, Klement G, Downing SR, Chen WY, Hannagan K, et al. Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Research and Treatment. 2012;136:169–78. doi: 10.1007/s10549-012-2256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bousquet G, Varna M, Ferreira I, Wang L, Mongiat-Artus P, Leboeuf C, et al. Differential regulation of sunitinib targets predicts its tumor-type-specific effect on endothelial and/or tumor cell apoptosis. Cancer Chemother Pharmacol. 2013;72:1183–93. doi: 10.1007/s00280-013-2300-0. [DOI] [PubMed] [Google Scholar]
- 42.Jo MY, Kim YG, Kim Y, Lee SJ, Kim MH, Joo KM, et al. Combined therapy of temozolomide and ZD6474 (vandetanib) effectively reduces glioblastoma tumor volume through anti-angiogenic and anti-proliferative mechanisms. Mol Med Rep. 2012;6:88–92. doi: 10.3892/mmr.2012.868. [DOI] [PubMed] [Google Scholar]
- 43.Inoue K, Torimura T, Nakamura T, Iwamoto H, Masuda H, Abe M, et al. Vandetanib, an inhibitor of VEGF receptor-2 and EGF receptor, suppresses tumor development and improves prognosis of liver cancer in mice. Clinical Cancer Research. 2012;18:3924–33. doi: 10.1158/1078-0432.CCR-11-2041. [DOI] [PubMed] [Google Scholar]
- 44.Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. Journal of Thoracic Oncology. 2012;7:485–95. doi: 10.1097/JTO.0b013e31824177ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar S, Mazumdar A, Dash R, Sarkar D, Fisher PB, Mandal M. ZD6474, a dual tyrosine kinase inhibitor of EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces apoptosis in breast cancer cells. Cancer Biol Ther. 2010;9:592–603. doi: 10.4161/cbt.9.8.11103. [DOI] [PubMed] [Google Scholar]
- 46.Finn RS, Press MF, Dering J, O’Rourke L, Florance A, Ellis CE, et al. Quantitative ER and PgR assessment as predictors of benefit from lapatinib in postmenopausal women with hormone receptor-positive, HER-2 negative metastatic breast cancer. Clinical Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-13-1260. in press. [DOI] [PubMed] [Google Scholar]
- 47.Garon EB, Pietras RJ, Finn RS, Kamranpour N, Pitts S, Marquez-Garban DC, et al. Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer. Journal of Thoracic Oncology. 2013;8:270–8. doi: 10.1097/JTO.0b013e31827d525c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Diaz MR, Yee D. Fulvestrant regulates epidermal growth factor (EGF) family ligands to activate EGF receptor (EGFR) signaling in breast cancer cells. Breast Cancer Research and Treatment. 2013;139:351–60. doi: 10.1007/s10549-013-2541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emde A, Mahlknecht G, Maslak K, Ribba B, Sela M, Possinger K, et al. Simultaneous inhibition of estrogen receptor and the HER2 pathway in breast cancer: effects of HER2 abundance. Transl Oncol. 2011;4:293–300. doi: 10.1593/tlo.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.