Abstract
The discovery that selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine are present and bioaccumulate in aquatic ecosystems have spurred studies of fish serotonin transporters (SERTs) and changes in SSRI-sensitive behaviors as adverse outcomes relevant for risk assessment. Many SSRIs also act at serotonin 5-HT1A receptors. Since capitolizing on this action may improve treatments of clinical depression and other psychiatric disorders, novel multimodal drugs that agonize 5-HT1A and block SERT were introduced. In mammals both 5-HT1A and CB agonists, such as buspirone and WIN55,212-2, reduce anxious behaviors. Immunological and behavioral evidence suggests that 5-HT1A-like receptors may function similarly in zebrafish (Danio rerio), yet their pharmacological properties are not well characterized. Herein we compared the density of [3H] 8-hydroxy-2-di-n-propylamino tetralin (8-OH-DPAT) binding to 5-HT1A-like sites in the zebrafish brain, to that of simalarly Gαi/o-coupled cannabinoid receptors. [3H] 8-OH-DPAT specific binding was 176 ± 8, 275 ± 32, and 230 ± 36 fmol/mg protein in the hypothalamus, optic tectum, and telencephalon. [3H] WIN55,212-2 binding density was higher in those same brain regions at 6 ± 0.3, 5.5 ± 0.4 and 7.3 ± 0.3 pm/mg protein. The aquatic light-dark plus maze was used to examine behavioral effects of 5-HT1A and CB receptor agonists on zebrafish novelty-based anxiety. With acute exposure to the 5-HT1A partial-agonist buspirone (50 mg/L), or dietary exposure to WIN55,212-2 (7 μg/week) zebrafish spent more time in and/or entered white arms more often than controls (p < 0.05). Acute exposure to WIN55,212-2 at 0.5-50 mg/L, reduced mobility. These behavioral findings suggest that azipirones, like cannabinoid agonists, have anxiolytic and/or sedative properties on fish in novel environments. These observations highlight the need to consider potential ecological risks of azapirones and multimodal antidepressants in the future.
Keywords: aquatic exposure, anxiety, autoradiography, cannabinoid, Gαi/o coupled receptors, inhibition, light/dark preference, scototaxis, serotonin
1. Introduction
Following the observation of selective serotonin reuptake inhibitor (SSRI) antidepressant accumulation in fish (Brooks et al. 2005), studies of drugs in aquatic environments and their regulation have increased (Oakes et al. 2010). Though serotonin transporters (SERT) are the primary target of SSRIs such as fluoxetine or citalopram, some of their pharmacological efficacy stems from activity at serotonin 5-HT1A receptors (Lesch et al. 1991; Klimek et al. 1994; Li et al. 1996; Descarries and Riad 2012). In light of this, multimodal antidepressants targeting 5-HT1A and SERT were developed (Maurel et al., 2007; Ashby et al. 2013; Guilloux et al. 2013). Among them are vilazodone and vortioxetine which were approved in 2011 and 2013 by the U.S. FDA as antidepressants; both have high affinities for human SERT and 5-HT1A receptors (Stahl et al. 2013; Celada et al. 2013; Pehrson and Sanchez 2013). Potential environmental risks from these new drugs targeting 5-HT1A on non-target species (e.g. fish) remain unknown.
Also, 5-HT1A partial agonist azapirones such as buspirone and tandispirone remain in use as treatments for anxiety, depression and related disorders (Correa-de-Araujo et al. 2005; Ravindran and Stein 2010; Celada et al. 2013). Azapirones have not received much attention in wastewater surveys (Calisto and Esteves 2009), although one ECOSAR model study indicated that for predictive purposes buspirone falls into 3 different chemical classes (Madden et al. 2009). The persistence of the pyrimidine sub-structure of buspirone following photocatalytic degradation indicates that its derivatives are likely to remain bioactive in the environment (Radjenovic et al. 2009). Given the heightened likelihood of future introductions of 5-HT1A targeting drugs into aquatic ecosystems ushered in by new multimodal antidepressants, studies of potential target-specific effects of 5-HT1A agonists in fish are warranted.
While environmental concentrations of most pharmaceuticals are well below acute toxicity levels, chronic mode of action (MOA)-specific effects should still be of concern. However, conventional toxicological endpoints (e.g. mortality, reproduction, growth) may not capture biologically important MOA-related endpoints, including behavioral effects such as reduced locomotion, vigilance or predator avoidance, and changes in feeding, shoaling or mating (Martinovic et al. 2007; Dzieweczynski and Hebert 2012; Valenti et al. 2012). Using the adverse outcome pathway (AOP) framework, enhanced understanding of relationships among mechanistic events and subsequent responses at various levels of biological organization can improve ecological risk assessments (Ankley et al. 2010). Adult zebrafish are increasingly being used in behavior tests and they are good models for predicting or testing the effects of pharmaceuticals, toxicants or gene manipulations (Guo 2004; Norton and Bally-Cuif 2010; Kalueff et al. 2013; Norton 2013). Zebrafish assays examining anxiety, fear and related behaviors were developed, and efforts to characterize pharmaceutical effects in them continue (Maximino et al. 2010). Thus, zebrafish behaviors are ideal for studies examining MOA specific effects in the context of AOP-related endpoints.
Two serotonin 5-HT1A-like receptors have been identified in zebrafish, htr1aa, htr1ab, and their expression were mapped using in-situ hybridization (Airhart et al. 2007; Norton et al., 2008). Buspirone generally has anxiolytic and pro-social effects in vertebrates (File and Seth 2003; Bencan et al. 2009; Gould et al. 2011; 2012; Barba-Escobedo and Gould, 2012; Maaswinkel et al. 2012; 2013). However affinities of zebrafish htr1aa, htr1ab receptors are lower for 8-OH-DPAT, buspirone and WAY100635 than mammalian 5-HT1A receptors, since more 5-HT1A-like receptors and/or other binding sites are involved (Gozlan et al., 1995; Owens et al., 1997; Newman-Tancredi et al., 2001; Barba-Escobedo and Gould, 2012). Given this, we performed behavioral tests of buspirone‘s anxiolytic effects in the zebrafish light/dark plus maze, in which exploration and preference for a dark background (scototaxis) are explored in the first 5 min of introduction, when the arena is still a novel environment for the fish (Gould, 2011). We also used [3H] 8-OH-DPAT in an autoradiography study to identify high-density binding sites for this ligand in the zebrafish brain.
In mammals, 5-HT1A and endocannabinoid CB1 and CB2 receptors are coupled to Gαi/o proteins that inhibit adenylate cyclase and CAMP; drugs acting as partial-agonists at these receptors can suppress anxiety by this common mechanism (Dumuis et al. 1988; Prather et al. 2000; Papoucheva et al. 2004; File and Seth 2003; Chevaleyre et al. 2007; Naderi et al. 2008). In zebrafish CB receptors have been cloned, mapped and pharmacologically characterized. One zebrafish CB1-like and two CB2-like receptors can be distinguished by CP-55,940, HU-210 and WIN55,212-2 binding properties (Lam et al., 2006; Rodriguez-Martin et al. 2007a,b). Since zebrafish CB pharmacological properties are better characterized than their 5-HT1A –like receptors, yet buspirone has been more extensively used in zebrafish behavior studies (e.g. Bencan et al. 2009; Maaswinkel et al. 2012), our research objectives were to compare: 1) high-density binding sites of [3H] WIN55,212-2 and [3H] 8-OH-DPAT, and 2) behavioral properties of WIN55,212-2 and busprione in the context of novelty-based anxiety. We hypothesized that zebrafish 5-HT1A receptors would produce similar MOA-specific effects to the mammalian receptor – and that these effects would be similar to the CB1 receptors, given their common Gαi/o-coupling.
2. Methods
2.1 Fish husbandry
Wild-type (Tropical 5D) fish were used for the acute drug treatment experiments conducted at Baylor University (Waco, TX, USA). Adult wild-type zebrafish (Petco, Wayne, NJ, USA) were used for the autoradiography and gelatin diet treatment experiments conducted at William Paterson University (Wayne, NJ, USA). Fish were maintained on 16:8 (Waco) or 14:10 (Wayne) photoperiod in recirculating systems containing deionized water supplemented with ~200 mg/L Instant Ocean salts (Aquatic Ecosystems, Apopka, FL, USA) to raise the conductivity to approximately 500 μS/cm and a pH of 7.0. Fish were fed Artemia supplemented with flake food (Aquatic Ecosystems, Apopka, FL, USA) twice daily. All procedures were conducted according to approved IACUC protocols.
2.2 Quantitative Autoradiography
Brain tissue preparation: Adult male zebrafish (n = 4) were sacrificed by decapitation without anesthesia, and the head was frozen on powdered dry ice and stored at −80°C. In preparation for cryostat sectioning, the lower jaw and fins were cut away, and the zebrafish head was mounted on a cryostat chuck with mounting medium (TissueTek, Torrence, CA, USA). The brain in skull was sectioned coronally in 20 μm slices using a −18°C cryostat (Leica, Bannockburn, IL, USA), and sections were thaw-mounted onto chilled gelatin coated microslides. Brain tissue was collected according to the zebrafish brain atlas (Wullimann et al. 1996) from plates 92 for dorsal telencephlon, 127 for ventral thalamic nucleus, and 168-179 for the optic tectum and caudal zone of the periventricular hypothalamus. Tissue sections on slides were vacuum desiccated for 18 h at 4°C, and then frozen at −80°C until use in autoradiography assays. Sections on slides were desiccated as they defrosted for 1 h at 4°C before use in binding assays.
[3H] 8-OH-DPAT binding
Sections on slides were pre-incubated in 170 mM Tris HCl buffer, pH 7.6, for 30 min at room temperature (25°C). They were transferred to slide mailers to incubate for 1 hr in 10 ml of buffer containing 2 nM [3H] 8-OH-DPAT (Perkin-Elmer, Boston, MA, USA). Non-specific binding was defined by adding 10 μM WAY 100635 (Sigma, St Lois, MO, USA) to buffer in a separate slide mailer in which a duplicate serial set of sections was incubated. Incubation was terminated by two 5 min washes in 170 mM Tris–HCl buffer, pH 7.6, at 4°C followed by a 5 sec dip in 4°C de-ionized water.
[3H] WIN55,212-2 binding
Sections on slides were pre-incubated for 30 min in 50 mM Tris 120 mM HCl, pH 7.4 buffer at 25°C, then incubated in the same buffer containing 3% bovine serum albumin and 10 nM [3H] WIN55,212-2 (Amersham/GE Healthcare, Boston, MA) for 2 hours. Nonspecific binding was defined using 1 mM of CB1 antagonist AM251 (Sigma. St. Louis, MO). Brain sections were washed for 4 h in 50 mM Tris–HCl 0.3% BSA buffer, pH 7.4, at 4 °C, then dipped for 5 sec in 4°C deionized water.
Quantitative autoradiography
Sections were dried on a slide warmer and exposed to Kodak BioMax MR Film (VWR, Sugar Land, TX, USA) for 10 weeks for [3H] 8-OH-DPAT, or 4 weeks for [3H] WIN55,212-2, along with tritiated standards (American Radiolabled Chemicals, St Louis, MO, USA) calibrated to brain mash (Geary et al. 1985). Autoradiograms were captured with a digital imaging system: Nikon lens, Kaiser copy stand, “Northern Lights” illuminator (InterFocus Imaging Ltd., Linton, England), monochrome digital camera and frame grabber card (Scion Corporation, Frederick, MD, USA). Digital brain images were calibrated from grayscale to units of fmol/mg protein, and measured using NIH Image, version 1.47 (NIH, Bethesda, MD, USA) on Macintosh OS 9.2.
2.3 Exposure scenarios
Acute aqueous exposure of zebrafish was accomplished by immersing individual zebrafish (N= 8-10) for 10 min. WIN55,212-2 (Ascent Scientific, Cambridge, MA) dissolved in DMSO at 0.1%, or buspirone (Sigma, St Louis MO) was dissolved into culture water at concentrations of 0.5, 5, or 50 mg /L. These concentrations were based on behavioral effects seen in Braida et al. (2007) and Bencan et al. (2009), and the duration based on the finding in Sackerman et al. (2010) that SSRI uptake into brain via bath exposure was evident after 3-5 min. Fish were then allowed to depurate in clean culture water for 15 min before being transferred into the light/dark cross maze.
In addition to acute aqueous exposures, dietary exposures were also completed with WIN55212-2. Spiked-food was prepared by mixing 1 mg of WIN55,212-2 with 7 g dry gelatin food powder (Aquatic-Eco Systems, Apopka, FL, USA) generally following our previously reported approach (Gould et al. 2007). Deionized water (3 ml) was then added and the slurry was mixed thoroughly to make 10 g of spiked-food. WIN55,212-2 spiked gelatin-based food was spread out on a plastic weigh-boat and refrigerated until solid; the dry food was then crumbled. A drug-free control batch of diet (without WIN55,212-2) was prepared similarly. On a daily basis for 1 week, 60 mg of food was slowly added to each tank of 6 fish so that all food was consumed within 5 min of floating on the surface. This dietary regimen culminated in a nominal consumption rate for each fish of 1 μg WIN55,212-2/day.
2.4 Light/Dark Cross Maze
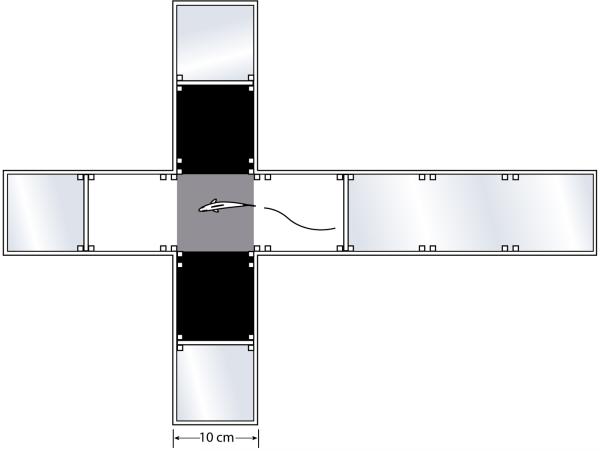
The protocol described in (Gould 2011) was followed for the light/dark preference testing in a testing arena that is novel to the fish. Clear, acrylic offset cross mazes (http://www.ezrascientific.com) were configured with the 3 side arms off the center square blocked at 10 cm and one side arm blocked at 20 cm. The arms were lined with black or white poly squares (Figure 1). A camera and 60 W light were hung above each maze. The maze was filled with habitat water to a depth of 4 cm. Fish naïve to the maze were introduced by dip net into the center square and behavior was recorded over 5 min. The number of crosses into each square, time spent in white arms, and latency to move from the middle square was quantified. Mazes were rinsed and water was refreshed between each trial.
Figure 1.
Top view of light/dark aquatic plus maze. Protocols for test are provided in Gould (2011).
2.5 Statistical analysis
One-way ANOVAs or t-tests were used for statistical comparisons of the recorded observational data among exposure concentrations (Sigma Plot 11.0, Systat Software and/or Statistica, Tulsa, OK, USA). Pairwise post-hoc comparisons were made using a Tukey‘s post hoc test.
3. Results
3.1. Autoradiography
Cannabinoid receptor binding to [3H] WIN55,212-2 was significantly higher than 5-HT1A binding to [3H] 8-OH-DPAT, as shown by representative autoradiograms in Figure 2. The lowest density region was the ventral thalamus, while the dorsal telencephlon had the highest density for both receptors (Table 1). Both ligands had relatively high binding density in the caudal zone of the periventricular hypothalamus, indicating the possible presence of regulatory 5-HT1A and CB receptors in this region.
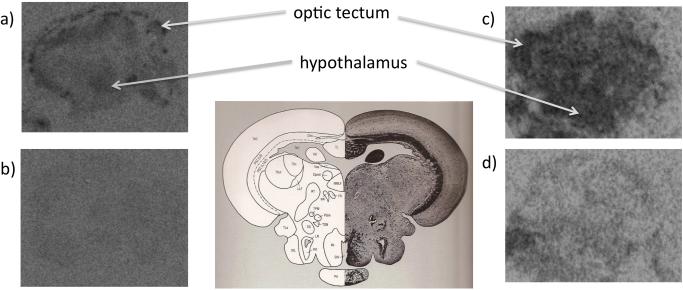
Figure 2.
Representative autoradiograms from coronal sections of the zebrafish brain. a) Total and b) nonspecific binding to putative zebrafish 5-HT1A and receptors, and c) total and d) non-specific binding to CB receptors between plates 162 and 168 in the zebrafish brain atlas Wullimann et al. (1996), plate 162 is shown. The optic tectum and periventricular hypothalamus are indicated by arrows.
Table 1.
[3H] 8-OH-DPAT specific binding to 5-HT1A and [3H] WIN55,212-2 specific binding to CB receptors in zebrafish brain. Data are mean ± S.E.M. in units of fmol/mg protein, N = 4 fish.
| Receptor | Hypothalamus | Optic Tectum | Ventral Thalamus | Dorsal Telencephlon |
|---|---|---|---|---|
| 5-HT1A | 176 ± 8 | 275 ± 32 | 112 ± 32 | 230 ± 36 |
| CB1 and 2 | 5207 ± 416 | 5760 ± 252 | 3660 ± 412 | 7265 ± 400 |
3.2 Behavioral effects of buspirone and WIN55,212-2
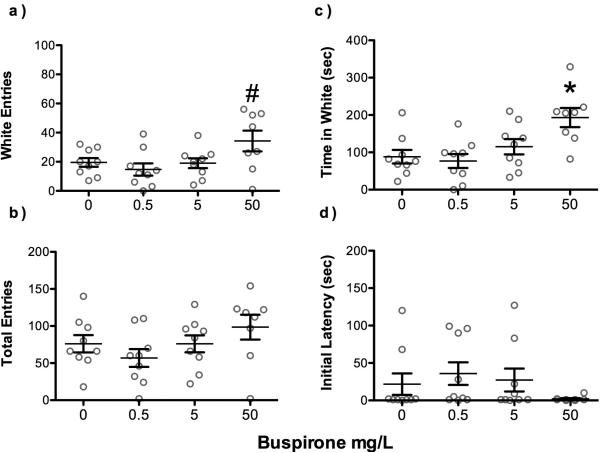
Zebrafish exposed to 50 mg/L busprirone entered white arms more frequently than those exposed to 0.5 mg/L (p<0.05; Figure 3a). Zebrafish acutely exposed to buspirone at 50 mg/L spent significantly more time in the white arms of the behavioral plus maze than controls (F(3,31)=6, p < 0.05; Figure 3b). No significant differences were observed for the total number of arm entries, number of entries into white arms or latency time to leave the middle chamber as shown in Figure 3c and d, respectively. One fish in the 50 mg/L buspirone group was an outlier, as it did not leave the center box until after 300 sec, so it was excluded from the analysis. Otherwise there were 9 fish per treatment level.
Figure 3.
Dose-dependent influence of acute agonism of putative 5-HT1A receptors on zebrafish light/dark plus maze behavior. a) Zebrafish administered 50 mg/L serotonin 5-HT1A agonist buspirone entered white arms more frequently (# p<0.05) than those administered 0.5 mg/L, and b) also spent significantly more time on white than controls (* p<0.05). c) Total arm entries did not differ among buspirone doses, and d) all fish had similar latency to leave the middle box of the maze to begin exploring the arms.
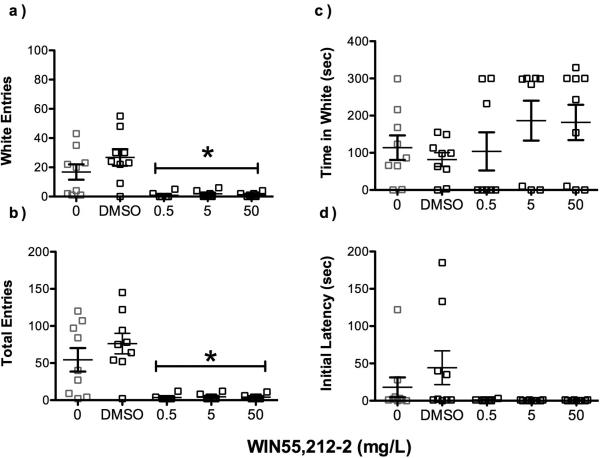
Acute aqueous exposure to WIN55,212-2 resulted in altered behavioral anxiolytic responses (Figure 4). Zebrafish exposed to 0.5, 5 and 50 mg/L WIN55,212-2 had significantly fewer white arm entries and total arm entries than either unexposed or vehicle controls (F(4,37) > 10, p <0.05; Figure 4a). A general trend towards increased time in white zones with higher concentrations of WIN55,212-2 was evident, but this was not significant due to the large variation in both the control and experimental groups (Figure 4b). It may be important to note that latency to leave the center box decreased with all concentrations of WIN55,212-2, though not significantly different from controls (p = 0.07). Two fish, one in concentration 5 mg/L and another in 50 mg/L, displayed aberrant behavior in this assay by completing 8-10 times more total arm crosses and 5-10 times more white arm entries than the other zebrafish within its concentration exposure level. A third fish in the 0.5 mg/L group did not move from the center box until after 300 sec. As such, these fish were removed from the analysis, resulting in a total 9 fish for both unexposed and 0.1% DMSO treated groups, and 8 fish in the WIN55,212-2 group at each treatment level.
Figure 4.
Concentration dependent influence of acute agonism of cannabinoid binding sites on zebrafish light/dark plus maze behavior. Zebrafish administered WIN55,212-2 in 0.1% DMSO vehicle a) entered white arms less than controls, but b) dwelling time in white arms was lower, though not significantly different from controls. Treated fish also c) made fewer arm entries overall, irrespective of the dose of WIN55,212-2 (N=8-9, p<0.05), and d.) there was a trend toward WIN55,212-2 fish having reduced latency to exit the middle chamber as compared to vehicle-treated controls.
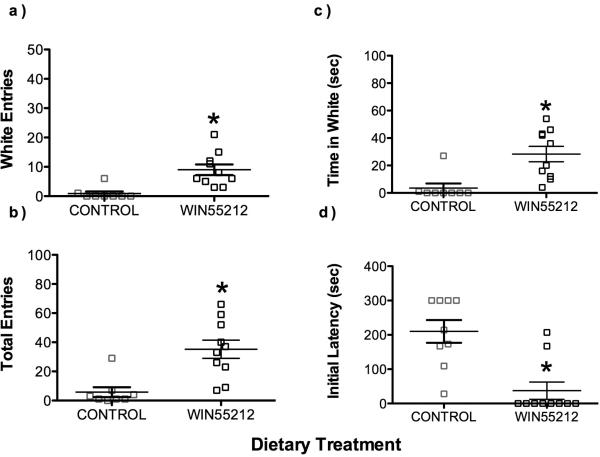
Zebrafish administered 1 μg/d of the cannabinoid agonist WIN55,212-2 in spiked diet for 1 week entered white arms significantly more (F(1,16) = 36.8, p < 0.01), and entered more arms overall (F(1,16) = 14.7, p< 0.01) as compared to controls fed unspiked gelatin food. WIN55,212-2-fed zebrafish also spent significantly more time exploring white arms (F(1,16) = 12.8, p <0.05), and less time frozen in the middle than controls (F(1,16) = 27.6, p < 0.001), as shown in Figure 5.
Figure 5.
Influence of sustained cannabinoid receptor agonism on behavior in light/dark plus maze. Zebrafish administered 1 ug/d of WIN55,212-2 in spiked diet for 1 week a) entered a higher percentage of white arms (p < 0.01), b) more arms (p< 0.01), c) spent more time exploring white arms (p <0.05), and d) spent significantly less time frozen in the middle, than zebrafish fed control diet (p < 0.001). There were 8 controls and 10 WIN55,212-2-treated fish.
4. Discussion
Leveraging mammalian pharmacology and toxicology data appears quite promising to identify drugs presenting risks to fish and other wildlife (Berninger and Brooks 2010; Boxall et al. 2012). The use of therapeutic target conservation and predictions of internal plasma concentration, dubbed the ‘read across hypothesis’, to predict effects in non-target species is gaining traction (Rand-Weaver et al. 2013); with the strongest support for this hypothesis coming from the study examining the behavioral effects of the SSRI sertraline in fathead minnows (Valenti et al. 2012). This has lent credence to the idea that mammalian pharmacodynamic information may be used to develop predictions for substances presenting potential environmental hazards to aquatic life (Huggett et al. 2003; Gunnarsson et al. 2008; Brooks et al. 2009; Berninger and Brooks 2010). By characterizing the ligand-binding properties of receptors in fish, this information may assist in compound prioritization for future read-across efforts.
However, findings from mammalian studies cannot always be generalized back to fish or other aquatic organisms for predictive purposes without further investigation. While pharmacological properties of active receptor binding sites are largely conserved among fish and mammals (Ringholm et al., 2002; Huggett et al., 2003; Schiöth et al. 2003; Ton et al. 2006; Gunnarson et al., 2008; Mueller and Wullimann 2009; Panula et al. 2010), this is not always the case. There are duplicate copies for many receptor genes in fish, and some of them acquired mutations altering sister protein ligand binding properties (Winberg and Nilsson 1996; Boehmler et al., 2007; Boehmler et al., 2009; Brunet et al. 2006; Norton et al., 2008). For example, differences in relative SSRI affinity, and higher affinities of the norepinephrine reuptake inhibitor desipramine for SERT binding sites occur in fish as compared to rats (Gould et al., 2007). This is due in part to the fact that there are two homologs to the mammalian SERT, “sert a” and “sert b” in zebrafish that differ in their binding properties (Wang et al. 2006; Norton et al., 2008). Higher affinity of zebrafish SERTs for desipramine was found to be due to only three amino acid residues at active binding sites that differed from humans and other mammals (Severinsen et al. 2008). Yet behavioral and binding properties of most drugs in fish remain uncharacterized.
The primary goal of this manuscript was to highlight the importance of understanding how drugs targeting piscine 5-HT1A–like receptors might alter fish behavior, especially since they will become more concentrated in aquatic environments with the introduction of new multimodal antidepressants such as vilazodone and vortioxetine. We hypothesized that if the population of htr1aa, htr1ab receptors in the zebrafish brain (Norton et al., 2008) had a conserved pharmacological response with mammalian 5-HT1A, then their agonism by buspirone should illicit similar behavioral responses to the activation of cannabinoid receptors by WIN55,212-2. This is because both receptor types are Gαi/o-coupled in mammals, and CB receptors are likewise in zebrafish (Dumuis et al., 1988; Chevalreyre et al., 2007; Rodriguez-Martin et al., 2007a). Autoradiographic data was collected to compare the binding distribution of [3H] 8-OH-DPAT and [3H] WIN55212 in zebrafish, as these ligands have been extensively used to describe 5-HT1A and CB1&2 receptors in mammalian studies. We predicted that zebrafish 5-HT1A and CB-like receptors would have similar binding density in key regions shaping emotionality, and their agonism would produce similar MOA-specific effects as their mammalian equivalents. This was based on our reasoning that similarities in their novelty-based behavioral response would reveal the potential for an evolutionarily conserved sharing of Gαi/o-coupled downstream effects of their agonism.
Our binding results are consistent with the previously described distribution of the HT1aa and HT1ab receptors in the zebrafish brain as characterized by in situ hybridization (Norton et al. 2008). We found high levels of [3H] 8-OH-DPAT binding in the caudal zone of the periventricular hypothalamus relative to other thalamic regions in zebrafish brain sections, as shown in Figure 2 and table 1. As compared to specific [3H] 8-OH-DPAT binding in the ventromedial nucleus of the mouse hypothalamus performed using the same protocol, their densities (130-200 fmol/mg protein in mice vs. 176 ± 8 fmol/mg protein in zebrafish) are comparable (Gould et al., 2012; 2014). CB receptor binding properties appear to have be largely evolutionarily conserved across vertebrate species (Meccariello et al. 2007; Rodriguez-Martin et al., 2007b; Soderstrom 2009; Klee et al. 2011). In zebrafish, synthetic and endocannabinoid binding properties of CB1 and CB2-like receptors were characterized (Rodriguez-Martin et al. 2007a; Rodriguez-Martin et al. 2007b). The density of zebrafish [3H] WIN55,212-2 binding tended to be slightly higher (3.6 – 7.3 pm/mg protein) than observed in a prior study in rat brain using the same ligand (Bmax = 1.2 – 6.1 pm/mg protein (Brevogel et al. 1997). [3H] WIN55,212-2 binding sites were consistent with prior reports (Rodriguez-Martin et al., 2007a), with high density in the dorsal telencephlon (Table 1).
In light/dark maze tests we saw that only buspirone exposure at a concentration of 50 mg/L significantly increased zebrafish dwelling times and crosses into white maze arms (Figure 3). Light/dark preference was used to characterize anxiolysis by buspirone in shoaling groups of zebrafish (Gebauer et al. 2011), and also when this compound was injected into fish (Maximino et al. 2011). In Gebauer et al. (2011) zebrafish were exposed to 3 mg/L buspirone and shoaling group behaviors were digitally quantified in dive tank and light/dark preference tests. Buspirone-exposed zebrafish shoals dwelled higher in the water column, and spend more time in white zones (Gebauer et al. 2011). Increased zebrafish exploration of higher parts of a dive tank appears to be concentration dependent > 6 mg/L with buspirone (Bencan et al. 2009; Connors et al., 2011; Maximino et al. 2013). Injections of buspirone also increased the time fish spent in white compartments in similar tests, while not changing the total number of crosses, showing no locomotive impairment (Maximino et al. 2011). Taken together, this suggests buspirone exhibits anxiolytic properties in zebrafish at a range of concentrations > 3 mg/L.
Similarly, exposure of zebrafish to 100 mg/L of citalopram (an SSRI) increased exploration of higher portions of the dive tank, but did not increase the time or proportion of entries into white arms in the light/dark plus maze (Sackerman et al. 2010) What remains unclear is if similar alterations in fish behavior will have adverse consequences on survival and reproduction in fish and other aquatic organisms inadvertently exposed to azapirones or multimodal antidepressants if exposed at lower concentrations for extended periods, and what the effects of mixtures will be. Studies in zebrafish embryos demonstrate that aqueous fluoxetine exposure changed not only SERT but also 5-HT1A receptor function with detrimental effects on locomotion and the nervous system (Airhart et al. 2007; Airhart et al. 2012). This area deserves more attention, given the advent of multimodal SERT and 5-HT1A targeting drugs.
Cannabinoids also mediate anxiolytic effects via cannabinoid receptors, with more recent evidence that CB2 receptors may play a major role in rodent emotionality assays such as the elevated plus maze (Akinshola et al. 1999; Onaivi et al. 2006; Onaivi et al. 2008). In mice, the cannabinoid agonist WIN55212-2 has anxiolytic properties at a dose of 0.25-2 mg/kg in the elevated plus maze (Naderi et al. 2008). However, in contrast to buspirone, very few behavioral tests investigating the actions of cannabinoid compounds have been run in zebrafish. Of those behavioral investigations, one study found that salvinorin A acted via cannabinoid receptors to produce rewarding effects in a conditioned place preference test at low doses, but produced a trance-like effect at higher doses (Braida et al. 2007).
It is unlikely that cannabinoids represent aquatic contaminants of grave potential concern, since few are water-soluble. However, in this study we used them as reference Gαi/o compounds to compare the effects of buspirone. Since they have far greater affinity for CB receptors than native compounds WIN55,212-2 and other synthetic CB agonists have mixed dose-dependent effects on mammalian anxiety, with anxiolytic actions only at lower doses (Ashton 2008). Bath exposure to WIN55,212-2 at 0.5-50 mg/L reduced zebrafish locomotor activity (Figure 4). In a related study, a similar exposure to WIN55,212-2 at 1 mg/L increased shoalmate-seeking in a preference test (Barba-Escobedo and Gould 2012). However, the increased shoal seeking was modest in comparison to that produced by buspirone exposure at 10 mg/L. Also, in the present study 1-week dietary exposure to WIN55,212-2 promoted zebrafish exploration, as most spent more time in white arms (Figure 5). One limitation to dietary WIN 55,212-2 exposure is that individual fish in groups may consume different amounts of spiked food, resulting from social dominance hierarchies (Dahlbom et al., 2012). Nonetheless, the behavior of most fish exposed by diet to WIN55,212-2 was similar to that of fish with 50 mg/L buspirone exposures.
In sum, pharmacological approaches including receptor binding assays and basic behavior studies can lead to ecologically relevant data and be used as tools to strengthen comparative pharmacodynamic predictions between species. This information may ultimately be used to develop and strengthen connections within AOPs, which support more robust ecological risk assessments. The main findings of our study were similar densities and distributions of 5-HT1A-like and CB receptors in zebrafish, and similar anxiolotic-to-sedative behavioral effects of acute exposures to buspirone and 1 week exposures to WIN55,212. Hence in read-across-studies these receptors may possibly be explored as toxicological targets for SSRI and the next generation of antidepressant exposures in fish and other aquatic vertebrates.
Compounds such as azapirones and the newer multimodal antidepressants could readily be screened for in municipal wastewaters using HPLC/MS/MS (Calza et al. 2004). If they are persistent in aquatic environments, their long term effects on a larger set of aquatic organisms could be characterized in future studies. Based on these findings, if necessary, cost-effective mechanisms to remove them from affected wastewaters, such as advanced oxidative processes could be employed to remedy the situation (Klavarioti et al. 2009; Radjenovic et al. 2009). Alternatively, if the newer multimodal antidepressants are not only more effective, but also have reduced tendency to persist or biomagnify, they could instead reduce the burden of pharmaceuticals and their metabolites in surface waters.
Highlights.
Concentrations of 5-HT1A receptor-targeting drugs may rise in aquatic ecosystems
Mammalian 5-HT1A and CB are Gαi/o coupled, and their agonism is anxiolytic
Zebrafish 5-HT1A–binding sites shared some high density areas with CB receptors
Fish behavior following drug exposure shows if mechanisms of action are conserved
Anxiolytic or sedative properties occurred with either agonist type in zebrafish
Acknowledgements
We thank Krista Prosser and the Brooks lab group for assistance with zebrafish behavioral studies, and Dr. Lynette Daws and Dr. Alan Frazer for the use of their laboratory facilities for autoradiography studies. This project was supported by Grant No. MH086708 (to GG) and DA032890 (to EO) from the National Institutes of Health, a sub-award from Grant No. T42/CCT610417 from the National Institute for Occupational and Environmental Health (NIOSH)/Centers for Disease Control and Prevention (CDC) to the Southwest Center for Occupational and Environmental Health (SWCOEH), a NIOSH Education and Research Center (to GG), and by the Glasscock Fund for Excellence in Environmental Science (to KC), and Department of Environmental Science at Baylor University (to BB). Undergraduate student involvement was supported by funds from the office of Dean Sandra DeYoung at William Paterson University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC). Neurotoxicol Teratol. 2007 doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG, Monaco PJ. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol Teratol. 2012;34:152–160. doi: 10.1016/j.ntt.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Akinshola BE, Chakrabarti A, Onaivi ES. In-vitro and in-vivo action of cannabinoids. Neurochem Res. 1999;24:1233–1240. doi: 10.1023/a:1020968922151. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornugn MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. Adverse Outcome Pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Kehne JH, Bartoszyk GD, Renda MJ, Athanasiou M, Pierz KA, Seyfried CA. Electrophysiological evidence for rapid 5-HT(1A) autoreceptor inhibition by vilazodone, a 5-HT(1A) receptor partial agonist and 5-HT reuptake inhibitor. Eur J Pharmacol. 2013;714:359–365. doi: 10.1016/j.ejphar.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Ashton J. Pro-drugs for indirect cannabinoids as therapeutic agents. Curr Drug Deliv. 2008;5:243–247. doi: 10.2174/156720108785915050. [DOI] [PubMed] [Google Scholar]
- Barba-Escobedo PA, Gould GG. Visual social preferences of lone zebrafish in a novel environment: strain and anxiolytic effects. Genes Brain Behav. 2012;11:366–373. doi: 10.1111/j.1601-183X.2012.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology, Biochemistry and Behavior. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger JP, Brooks BW. Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicology Letters. 2010;193:69–78. doi: 10.1016/j.toxlet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R. D4 Dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007;6:155–166. doi: 10.1111/j.1601-183X.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Boehmler W, Petko J, Woll M, Frey C, Thisse B, Thisse C, Canfield VA, Levenson R. Identification of zebrafish A2 adenosine receptors and expression in developing embryos. Gene Expr Patterns. 2009;9:144–151. doi: 10.1016/j.gep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall ABA, Rudd M, Brooks BW, Caldwell D, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley J, Verslycke T, Ankley GT, Beazley K, Belanger S, Berninger JP, Carriquiriborde P, Coors A, DeLeo P, Dyer S, Ericson J, Gagne F, Giesy JP, Gouin T, Hallstrom L, Karlsson M, Larsson DGJ, Lazorchak J, Mastrococco F, McLaughlin A, McMaster M, Meyerhoff R, Moore R, Parrott J, Snape J, Murray-Smith R, Servos M, Sibley PK, Straub JO, Szabo N, Tetrault G, Topp E, Trudeau VL, van der Kraak G. Pharmaceuticals and Personal Care Products in the environment: What are the big questions? Environmental Health Perspectives. 2012;120:1221–1229. doi: 10.1289/ehp.1104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology. 2007;190:441–448. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Russell JL. Determination of select antidepressants in fish from an effluent-dominated stream. Environmental Toxicology and Chemistry. 2005;24:464–469. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Foran CM, Richards SM, Weston J, Turner PK, Stanley JK, Solomon KR, Slattery M, La Point TW. Aquatic ecotoxicology of fluoxetine. Toxicology Letters. 2003;142:169–183. doi: 10.1016/s0378-4274(03)00066-3. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Huggett D, Boxall A. Pharmaceuticals and Personal Care Products in the environment: Research needs for the next decade. Environmental Toxicology and Chemistry. 2009;28:2469–2472. doi: 10.1897/09-325.1. [DOI] [PubMed] [Google Scholar]
- Brunet FG, Crollius HR, Paris M, Aury JM, Gibert P. Gene loss and evolutionary rates following whole-genome duplication in teleost fishes. Mol Biol Evol. 2006;23:1808–1816. doi: 10.1093/molbev/msl049. [DOI] [PubMed] [Google Scholar]
- Calisto V, Esteves VI. Psychiatric pharmaceuticals in the environment. Chemosphere. 2009;77:1257–1274. doi: 10.1016/j.chemosphere.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A Receptors as Targets for Agents to Treat Psychiatric Disorders: Rationale and Current Status of Research. CNS Drugs. 2013;27:703–716. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors KA, Vallenti TW, Prosser KN, Brooks BW, Onaivi ES, Gould GG. Effects of 5-HT1A or endocannabinoid receptor agonists on zebrafish behavior in novel environments. The Toxicologist. 2011;120:PS 964. [Google Scholar]
- Correa-de-Araujo R, Miller GE, Banthin JS, Trinh Y. Gender differences in drug use and expenditures in a privately insured population of older adults. J Womens Health. 2005;14:73–81. doi: 10.1089/jwh.2005.14.73. [DOI] [PubMed] [Google Scholar]
- Dahlbom SJ, Backström T, Lundstedt-Enkel K, Winberg S. Aggression and monoamines: effects of sex and social rank in zebrafish (Danio rerio). Behav Brain Res. 2012;228:333–8. doi: 10.1016/j.bbr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Descarries L, Riad M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos Trans R Soc Lond B Biol Sci. 2012;1601:2416–2425. doi: 10.1098/rstb.2011.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Bockaert J. Pharmacology of 5- hydroxytryptamine1A receptors which inhibit cAMP production in hippocampal and cortical neurons in primary culture. Mol. Pharmacol. 1988;33:178–186. [PubMed] [Google Scholar]
- Dzieweczynski TL, Hebert OL. Fluoxetine alters behavioral consistency of aggression and courtship in male Siamese fighting fish, Betta splendens. Physiology & Behavior. 2012;107:92–97. doi: 10.1016/j.physbeh.2012.06.007. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Filippi A, Mahler J, Schweitzer J, Driever W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J Comp Neurol. 2010;518 doi: 10.1002/cne.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrecki KM, Klaine SJ. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquatic Toxicology. 2008;88:207–213. doi: 10.1016/j.aquatox.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Geary WA, Toga AW, Wooten GF. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985;337:99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, Lara DR. Effects of anxiolytics in zebrafish: simialrities and differences between benzodiazepines, buspirone and ethanol. Pharmacology Biochemistry and Behavior. 2011;99:480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Giltrow E, Eccles PD, Winter MJ, McCormack PJ, Rand-Weaver M, Hutchinson TH, Sumpter JP. Chronic effects assessment and plasma concentrations of the beta-blocker propranolol in fathead minnows (Pimephales promelas). Aquatic Toxicology. 2009;95:195–202. doi: 10.1016/j.aquatox.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Thibault S, Laporte AM, Lima L, Hamon M. The selective 5-HT1A antagonist radioligand [3H]WAY 100635 labels both G-protein-coupled and free 5-HT1A receptors in rat brain membranes. Eur J Pharmacol. 1995;288:173–86. doi: 10.1016/0922-4106(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gould G, Kalueff AV, Cachat JM. Zebrafish Neurobehavioral Protocols. Springer Protocols; 2011. Aquatic light/dark plus maze novel environment for assessing anxious vs. exploratory behavior in zebrafish (Danio rerio) and other small teleost fish. pp. 99–108. [Google Scholar]
- Gould GG, Brooks BW, Frazer A. [3H] Citalopram binding to serotonin transporter sites in minnow brains. Basic & clinical pharmacology & toxicology. 2007;101:203–210. doi: 10.1111/j.1742-7843.2007.00100.x. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Seillier A, Weiss G, Giuffrida A, Burke TF, Hensler JG, Rock C, Tristan A, McMahon LR, Salazar A, O'Connor JC, Satsangi N, Satsangi RK, Gu TT, Treat K, Smolik C, Schultz ST. Acetaminophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:260–269. doi: 10.1016/j.pnpbp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Burke TF, Osorio MD, Smolik CM, Zhang WQ, Onaivi ES, Gu TT, Desilva MN, Hensler JG. Enhanced novelty-induced corticosterone spike and upregulated serotonin 5-HT(1A) and cannabinoid CB(1) receptors in adolescent BTBR mice. Psychoneuroendocrinology. 2014;39:158–169. doi: 10.1016/j.psyneuen.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Repérant C, Orvoën S, Gardier AM, Hen R, Ebert B, Miller S, Sanchez C, David DJ. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology. 2013;73:147–159. doi: 10.1016/j.neuropharm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Gunnarsson L, Jauhiainen A, Kristiansson E, Nerman O, Larsson DGJ. Evolutionary conservation of human drug targets in organisms used for environmental risk assessment. Environmental Science & Technology. 2008;42:5807–5813. doi: 10.1021/es8005173. [DOI] [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Huggett DB, Cook JC, Ericson JF, Williams RT. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Human and Ecological Risk Assessment. 2003;9:1789–1799. [Google Scholar]
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10. 2013 doi: 10.1089/zeb.2012.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavarioti M, Mantzavinos D, Kassinos D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int. 2009;35:402–17. doi: 10.1016/j.envint.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Klee EW, Schneider H, Clark KJ, Cousin MA, Ebbert JO, Hooten WM, Karpyak VM, Warner DO, Ekker SC. Zebrafish: a model for the study of addiction genetics. Hum Genet. 2011;131:977–1008. doi: 10.1007/s00439-011-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Zak-Knapik J, Mackowiak M. Effects of repeated treatment with fluoxetine and citalopram, 5-HT uptake inhibitors, on 5-HT1A and 5-HT2 receptors in the rat brain. J Psychiatry Neurosci. 1994;19:63–67. [PMC free article] [PubMed] [Google Scholar]
- Lam CS, Rastegar S, Strähle U. Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience. 2006;138:83–95. doi: 10.1016/j.neuroscience.2005.10.069. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Hoh A, Schulte HM, Osterheider M, Müller T. Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive-compulsive disorder. Psychopharmacology. 1991;105:415–420. doi: 10.1007/BF02244438. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- Maaswinkel H, Le N, He L, Zhu L, Weng W. Dissociating the effects of habituation, black walls, buspirone and ethanol on anxiety-like behavioral responses in shoaling zebrafish. A 3D approach to social behavior. Pharmacol Biochem Behav. 2013;108:16–27. doi: 10.1016/j.pbb.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Zhu L, Weng W. The immediate and the delayed effects of buspirone on zebrafish (Danio rerio) in an open field test: a 3-D approach. Behavioural Brain Research. 2012;234:365–374. doi: 10.1016/j.bbr.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Madden JC, Enoch SJ, Hewitt M, Cronin MT. Pharmaceuticals in the environment: good practice in predicting acute ecotoxicological effects. Toxicol Lett. 2009;185:85–101. doi: 10.1016/j.toxlet.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Martinovic D, Hogarth WT, Jones RE, Sorensen PW. Exposure to environmental estrogens suppresses the hormones, behavior and competitive reproductive fitness of adult male fathead. Environmental Toxicology and Chemistry. 2007;26:271–278. doi: 10.1897/06-065r.1. [DOI] [PubMed] [Google Scholar]
- Maximino C, da Silva AWB, Gouveia A, Jr, Herculano AM. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:624–631. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, da Silva AWB, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: a critical review. Behavioural Brain Research. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Maximino C, Puty B, Benzecry R, Araújo J, Lima MG, de Jesus Oliveira Batista E, Renata de Matos Oliveira K, Crespo-Lopez ME, Herculano AM. Role of serotonin in zebrafish (Danio rerio) anxiety: relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology. 2013;71:83–97. doi: 10.1016/j.neuropharm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Meccariello R, Chianese R, Cobellis G, Pierantoni R, Fasano S. Cloning of type 1 cannabinoid receptor in Rana esculenta reveals differences between genomic sequence and cDNA. FEBS J. 2007;274:2909–2920. doi: 10.1111/j.1742-4658.2007.05824.x. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL. Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J Toxicol Environ Health B. 2011;14:387–412. doi: 10.1080/10937404.2011.578559. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav Evol. 2009;74:30–42. doi: 10.1159/000229011. [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh AM, Khani A, Motamedi F. Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacol Biochem Behav. 2008;89:64–75. doi: 10.1016/j.pbb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Verrièle L, Millan MJ. Differential modulation by GTPgammaS of agonist and inverse agonist binding to h5-HT(1A) receptors revealed by [3H]-WAY100,635. Br J Pharmacol. 2001;132:518–24. doi: 10.1038/sj.bjp.0703832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WH, Folchert A, Bally-Cuif L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Comp Neurol. 2008;511:521–542. doi: 10.1002/cne.21831. [DOI] [PubMed] [Google Scholar]
- Norton WHJ. Toward developmental models of psychiatric disorders in zebrafish. Front Neural Circuits. 2013;7:79. doi: 10.3389/fncir.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes KD, Coors A, Escher BI, Fenner K, Garric J, Gust M, Knacker T, Kuster A, Kussatz C, Metcalfe CD, Monteiro S, Moon TW, Mennigen JA, Parrott J, Pery ARR, Ramil M, Roenefahrt I, Tarazona JV, Sanchez-Arguello P, Ternes TA, Boucard T, Trudeau VL, Van Der Kraak GJ, Servos MR. Environmental risk assessment for the serotonin reuptake inhibitor fluoxetine: Case study using the European risk assessment framework. Integrated Environmental Assessment and Management. 2010;6:524–539. doi: 10.1002/ieam.77. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3:1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Owen SF, Huggett DB, Hutchinson TH, Hetheridge MJ, Kinter LB, Ericson JF, Sumpter JP. Uptake of propranolol, a cardiovascular pharmaceutical, from water into fish plasma and its effects on growth and organ biometry. Aquatic Toxicology. 2009;93:217–224. doi: 10.1016/j.aquatox.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–22. [PubMed] [Google Scholar]
- Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environmental Toxicology and Chemistry. 2009;28:2677–2684. doi: 10.1897/08-556.1. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Papoucheva E, Dumuis A, Sebben M, Richter DW, Ponimaskin EG. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J Biol Chem. 2004;279:3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2013;1:1–13. doi: 10.1017/S1092852913000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather PL, Martin NA, Breivogel CS, Childers SR. Activation of cannabinoid receptors in rat brain by WIN 55212-2 produces coupling to multiple G protein alpha-subunits with different potencies. Mol Pharmacol. 2000;57:1000–10. [PubMed] [Google Scholar]
- Radjenovic J, Petrovic M, Barcelo D. Complementary mass spectrometry and bioassays for evaluating pharmaceutical-transformation products in treatment of drinking water and wastewater. Trends in Analytical Chemistry. 2009;28:562–580. [Google Scholar]
- Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environmental Science & Technology. 2013;47:11384–11395. doi: 10.1021/es402065a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;71:839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- Ringholm A, Fredriksson R, Poliakova N, Yan YL, Postlethwait JH, Larhammar D, Schiöth HB. One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J Neurochem. 2002;82:6–18. doi: 10.1046/j.1471-4159.2002.00934.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin I, de Velasco EM, Rodriguez RE. Characterization of cannabinoid-binding sites in zebrafish brain. Neurosci Lett. 2007a;413:249–254. doi: 10.1016/j.neulet.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin I, Herrero-Turrion MJ, Marron Fdez de Velasco E, Gonzalez-Sarmiento R, Rodriguez RE. Characterization of two duplicate zebrafish Cb2-like cannabinoid receptors. Gene. 2007b;389:36–44. doi: 10.1016/j.gene.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- Schiöth HB, Lagerström MC, Watanobe H, Jonsson L, Vergoni AV, Ringholm A, Skarphedinsson JO, Skuladottir GV, Klovins J, Fredriksson R. Functional role, structure, and evolution of the melanocortin-4 receptor. Ann N Y Acad Sci. 2003;994:74–83. doi: 10.1111/j.1749-6632.2003.tb03164.x. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM. Antidepressant pharmaceuticals in two U. S. effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environmental Science & Technology. 2010;44:1918–1925. doi: 10.1021/es9022706. [DOI] [PubMed] [Google Scholar]
- Severinsen K, Sinning S, Müller HK, Wiborg O. Characterisation of the zebrafish serotonin transporter functionally links TM10 to the ligand binding site. J Neurochem. 2008;105:1794–1805. doi: 10.1111/j.1471-4159.2008.05285.x. [DOI] [PubMed] [Google Scholar]
- Soderstrom K. Lessons from nonmammalian species. Curr Top Behav Neurosci. 2009;1:173–198. doi: 10.1007/978-3-540-88955-7_7. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Lee-Zimmerman C, Cartwright S, Morrissette DA. Serotonergic drugs for depression and beyond. Curr Drug Targets. 2013;14:578–585. doi: 10.2174/1389450111314050007. [DOI] [PubMed] [Google Scholar]
- Stanley JK, Ramirez AJ, Chambliss CK, Brooks BW. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere. 2007;69:9–16. doi: 10.1016/j.chemosphere.2007.04.080. [DOI] [PubMed] [Google Scholar]
- Ton C, Lin Y, Willett C. Zebrafish as a model for developmental neurotoxicity testing. Birth Defects Res A Clin Mol Teratol. 2006;76:553–567. doi: 10.1002/bdra.20281. [DOI] [PubMed] [Google Scholar]
- Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW. Human therapeutic plasma levels of the Selective Serotonin Reuptake Inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environmental Science & Technology. 2012;46:2427–2435. doi: 10.1021/es204164b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti TW, Perez-Hurtado P, Chambliss CK, Brooks BW. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environmental Toxicology and Chemistry. 2009;28:2685–2694. doi: 10.1897/08-546.1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Takai R, Yoshioka H, Shirabe K. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J Exp Med. 2006;208:267–274. doi: 10.1620/tjem.208.267. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson G. Multiple high-affinity binding sites for [3H] serotonin in the brain of a telost fish, the Arctic charr (Salvelinus alpinus). J Exp Biol. 1996;199:2429–2435. doi: 10.1242/jeb.199.11.2429. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Mukai M. Endocrine disruption in the context of life cycles: perception and transduction of environmental cues. General and Comparative Endocrinology. 2009;163:92–96. doi: 10.1016/j.ygcen.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Wong RY, Oxendine SE, Godwin J. Behavioral and neurogenomic transcriptome changes in wild-derived zebrafish with fluoxetine treatment. BMC Genomics. 2013;14 doi: 10.1186/1471-2164-14-348. doi:10.1186/1471-2164-1114-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhäuser; Basel, Switzerland: 1996. [Google Scholar]