Abstract
Heterocellular communication in the heart is an important mechanism for matching circulatory demands with cardiac structure and function, and neuregulins (Nrgs) play an important role in transducing this signal between the hearts' vasculature and musculature. Here, we review the current knowledge regarding Nrgs, explaining their roles in transducing signals between the heart's microvasculature and cardiomyocytes. We highlight intriguing areas being investigated for developing new, Nrg-mediated strategies to heal the heart in acquired and congenital heart diseases, and note avenues for future research.
Keywords: Neuregulin, Heart, Heterocellular communication, ErbB
1. Neuregulins: structures and functions
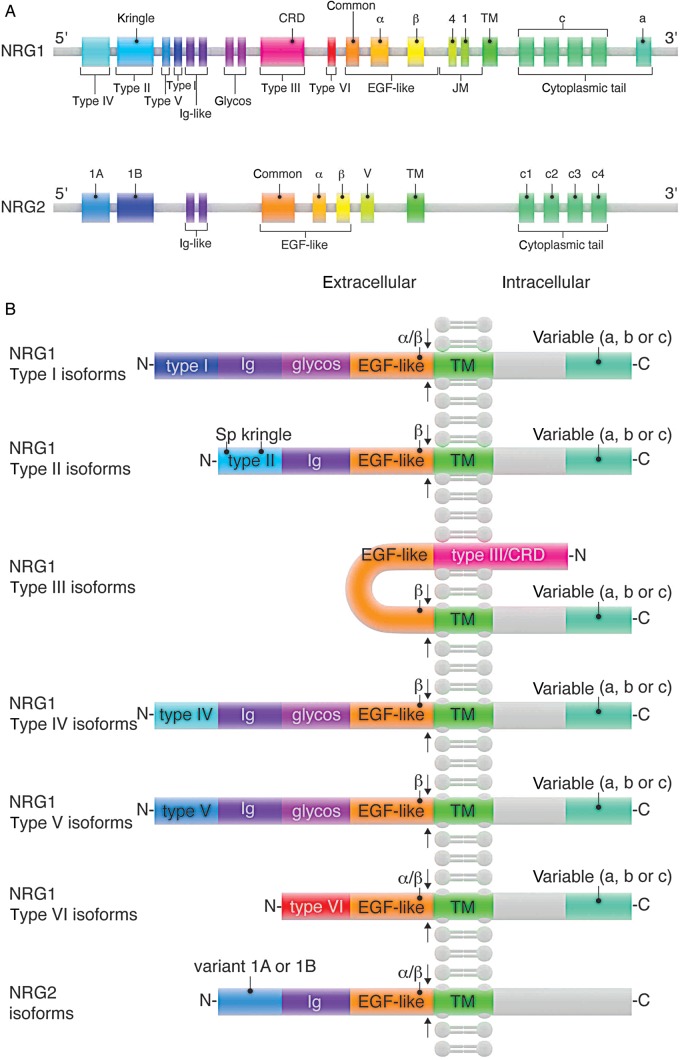
Neuregulins (Nrgs) are a family of growth factors whose genes are principally identified by the presence of an exon coding for the epidermal growth factor-like (EGF-like) domain, which mediates the interaction of Nrg proteins with the v-erb-b avian erythroblastic leukemia viral oncogene homolog (ErbB) family of receptor tyrosine kinases.1–4 Four Nrg genes are found in mammals, with partial family homology found in more distant relatives; Nrg orthologs are present in Danio rerio, Xenopus laevis, and Drosophila melanogaster genomes.5–7 Nrg was first purified from neural tissue, where it was found to promote Schwann cell proliferation, and was thus named glial growth factor (GGF).8,9 GGF was found to stimulate phosphorylation of the ErbB2 receptor tyrosine kinase, an effect linked to its mitogenic activity on Schwann cells.10 Subsequent studies identified similar phosphoErbB2-stimulating proteins, which were eventually found to be isoforms encoded by a single gene, termed NRG-1.11 The identification of three additional genes encoding similarly functioning isoforms has resulted in a variety of nomenclatures for members of the Nrg family. Nrg nomenclature includes the gene, the N-terminal sequence, the C-terminal sequence of the EGF-like domain, and finally, the cytoplasmic tail sequence. For simplicity, we use Nrgs to denote all isoforms of any of the four identified Nrg genes and specify the relevant Nrg gene and specific isoform when discussing protein products. NRG-1 is the most extensively studied gene, located on chromosome 8 in both humans and mice. NRG-1 encodes 21 exons12,13 (Figure 1A) and has been suggested to give rise to as many as 31 potential protein isoforms.14 N-terminal sequences distinguish Nrg1 isoforms as either Type I, Type II, Type III, Type IV, Type V, or Type VI13,15 (Figure 1B). Nrg-1 amino terminal regions can include a signal peptide (sp), a kringle-like domain, a cysteine-rich domain, an immunoglobulin-like (Ig) domain, and a glycosylation region (Figure 1B). NRG-2, -3, and -4 exhibit far less diversity in isoform N-terminal sequences; NRG-2 encodes two N-terminal sequence variants, Type 1A and Type 1B (Figure 1A). Consistent among all Nrgs is the EGF-like domain, which mediates receptor binding, and can be classified based on the EGF-like domain's C-terminal sequence, which varies between α and β isoforms, each of which can exist in distinct variants. C-terminal to the EGF-like domain is a juxtamembrane (JM; also called stalk) region, which serves as the proteolytic cleavage site. C-terminal to the JM region is a transmembrane (TM) domain, followed by an a-, b-, or c-type cytoplasmic tail. The structures and functions associated with each of Nrg's domains provide important clues for understanding Nrg signal specificity. Kringle domains consist of triple-looped, 3-disulfide bridges,16 are frequently found in clotting factors, and are proposed to serve as protein–protein interaction sites.17 Ig-like domains are ∼80 amino acid residues forming 7–10 β sheets and serve diverse cellular functions, including molecular transport, adhesion, morphogenic control, and cellular recognition.18 The unifying region of all Nrgs, the EGF-like domain, is a protein domain comprised of six cysteine residues, which form 3-disulfide bonds. The EGF-like domain varies at its C-terminus as either an α or β variant, based on different exon usages.19,20 In vitro studies have shown that Nrg-β isoforms are substantially more potent than Nrg-α isoforms;21–24 however, this should not suggest that Nrg-α isoforms are biologically irrelevant, as Nrg-α isoforms have been demonstrated to be critically important for breast development25 (Figure 2B).
Figure 1.
Nrg isoform structures: Nrg structures. (A) NRG-1 and NRG-2 genetic loci. (B) NRG-1 and NRG-2 protein isoform compositions. Arrows indicate putative proteolytic sites.
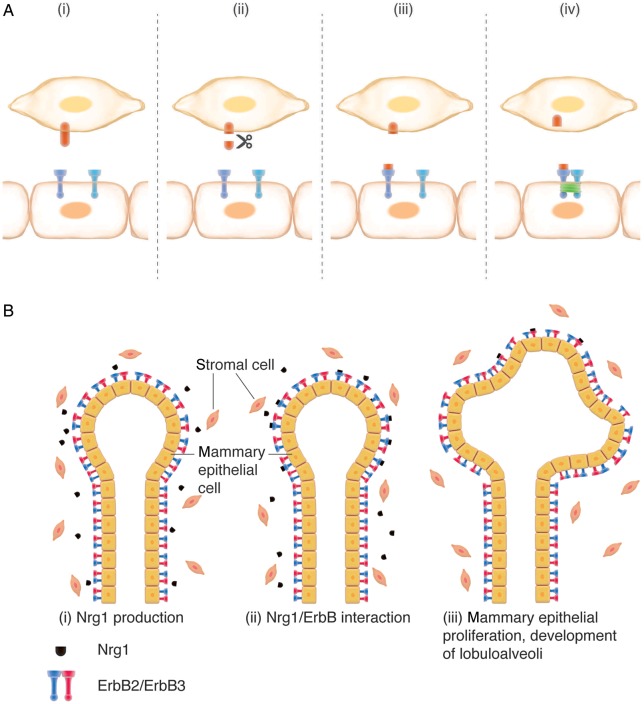
Figure 2.
Nrg-1 heterocellular signalling: (A) Nrg heterocellular signalling between a coronary microvascular endothelial cell (top) and a cardiomyocyte (bottom). Scissor indicates proteolytic cleavage by metalloproteinases. The green box in (iv) indicates receptor phosphorylation and activation. (B) Nrg heterocellular signalling drives post-natal lobuloalveolar mammary gland changes upon pregnancy.
2. Nrg signalling specificity
Nrgs are expressed by a variety of cell types (Table 1), where they are either presented by the host cell and subject to cleavage for paracrine/juxtacrine signalling, or in the case of Nrg β-3 isoforms are instead directly secreted into the extracellular space.47 Following release into the extracellular space, Nrgs bind ErbB receptor tyrosine kinases expressed on target cells. The ErbB receptor tyrosine kinase family consists of four members: ErbB1 (also known as epidermal growth factor receptor), ErbB2, ErbB3, and ErbB4. Nrg–ErbB binding causes receptor dimerization and phosphorylation (canonical Nrg/ErbB forward signalling), or receptor cleavage and internalization (non-canonical Nrg/ErbB forward signalling).14,48–51
Table 1.
Nrg-1 and Nrg-2 isoform expression
| Gene | Isoform | Alias(es) | Receptor(s) | Prenatal tissue expression | Post-natal tissue expression | Disease overexpression | Cell types expressing |
|---|---|---|---|---|---|---|---|
| NRG-1 | Nrg1 (Type unspecified) | ErbB426 ErbB327 | Lung28,29 | Cornea30, skin31, skeletal muscle32,33, motor trigeminal nucleus2, breast34 | Gut35 | Pulmonary epithelial cells36, fibroblasts31, Golgi-II cells2, cholinergic cells of basal forebrain2 | |
| Nrg1 Type I | Acetylcholine receptor-inducing activity (ARIA)37; neu-differentiation factor (NDF)38; heregulin (HRG)39 | Hindbrain15, eye15, dorsal spinal cord15, cartilage in branchial arches15, endocardium15, trigeminal ganglion15, telencephalon15, superior/inferior colliculus15, adrenal cortex11, cortical neuroepithelium lining the lateral ventricles of the brain11, brain above the developing thalamus11, intestines11, stomach11, dorsal ganglia11, genital ridge11, liver11, dermis11 | Coronary microvasculature23 | Neurons40, astrocytes40, cardiac microvascular endothelial cells23,41 | |||
| Nrg1 Type II | Glial growth factor II (GGF2)10 | Spinal cord15, dorsal root ganglia15, skeletal muscle15, entire brain15 | Neurons40 Astrocytes40 | ||||
| Nrg1 Type III | Sensory and motor neuron-derived factor (SMDF)42 | Cranial ganglia,15 dorsal root ganglia,15 ventral column of spinal cord,15entire brain,15 olfactory epithelium15 | Neurons43, astrocytes43, motor neurons15 | ||||
| Nrg1 Type IV | Brain44 | Hippocampus44, prefrontal cortex44 | |||||
| Nrg1 Type V | |||||||
| Nrg1 Type VI | |||||||
| NRG-2 | Nrg2 | Divergent of neuregulin-1 (DON1)45; neural- and thymus-derived activator for ErbB kinases (NTAK)46 | ErbB42 ErbB32 | Telencephalon46, brain45, lung45, endocardium2 | Brain2,46, olfactory bulb2,46, cerebellum2,46, thymus46, brain45, lung45, cerebellum45, denate gyrus of hippocampus45, cerebellum45, liver2, spinal cord2, hippocampus2 | Neurons46, granule cells45, Purkinje cells2,45, granule cells2 |
Tissue and cellular expression of Nrg1 and Nrg2 isoforms. Corresponding references are indicated in superscript.
Nrg domains control the release of Nrg into the extracellular space. Nrgs can be released by being directly secreted (β-3 Nrgs) or being presented on the cell surface for ectodomain release via proteolytic cleavage.52 The JM region found between Nrg's EGF-like and TM domains serves as a proteolytic site for a disintegrin and metalloproteases (ADAMs).53,54 ADAM17, also called tumour necrosis factor-α-converting enzyme (TACE), was shown cleave Nrg in vitro.55,56 Nrgs can also be cleaved at the cytoplasmic tail by Bace1, a β-secretase, which releases Nrg's intracellular domain, which can translocate to the nucleus to promote expression of anti-apoptotic genes (reverse Nrg signalling).57,58 Interestingly, Bace1 levels in the brain are elevated in congestive heart failure,59 but it is unknown whether Bace1 levels similarly increase in the heart, which could implicate a systemic dysregulation of Nrg signalling in disease progression. Liu et al.60 showed that truncation of the C-terminal tail in Nrg1 isoforms resulted in embryonic lethality with cardiac malformations, mimicking the phenotype seen in pan-Nrg1 knockouts. C-terminal truncation of Nrg1 cytoplasmic tails blocked proteolysis of the JM region, suggesting that the Nrg cytoplasmic tail plays a crucial role in mediating ectodomain release. Further support for the concept of Nrg proteolytic control via the intracellular domain is seen by studies, showing that Nrg ectodomain release was dependent on protein kinase C phosphorylation of a cytosolic Nrg residue.61
The Nrg/ErbB interaction that initiates heterocellular signalling cascades is a fascinating example of the diverse signalling achievable by a small number of genes. Neither ErbB1 nor ErbB2 bind any of the Nrg1, 2, 3, or 4 isoforms.62,63 While ErbB3 can bind both Nrg1 and Nrg2 proteins, it is incapable of propagating the signal without heterodimerzation.64,65 Only ErbB4 can autonomously respond to Nrg binding,14 though like ErbB3 it also heterodimerizes. It has been suggested that dimer composition acts as a mechanism to regulate downstream signalling.66 This theory is supported by studies showing that ErbB2 loss results in the absence of myocardial trabeculations, suggesting that despite the ability of ErbB4/ErbB4 homodimers to form, they do not sufficiently activate ErbB2/ErbB4-induced signalling cascades.67–69 Additional signal diversification may be achieved at the ligand level, as in vitro studies have demonstrated that different Nrg's isoforms activate distinct downstream signal cascades; whether this mechanism is utilized in vivo remains to be determined.70,71
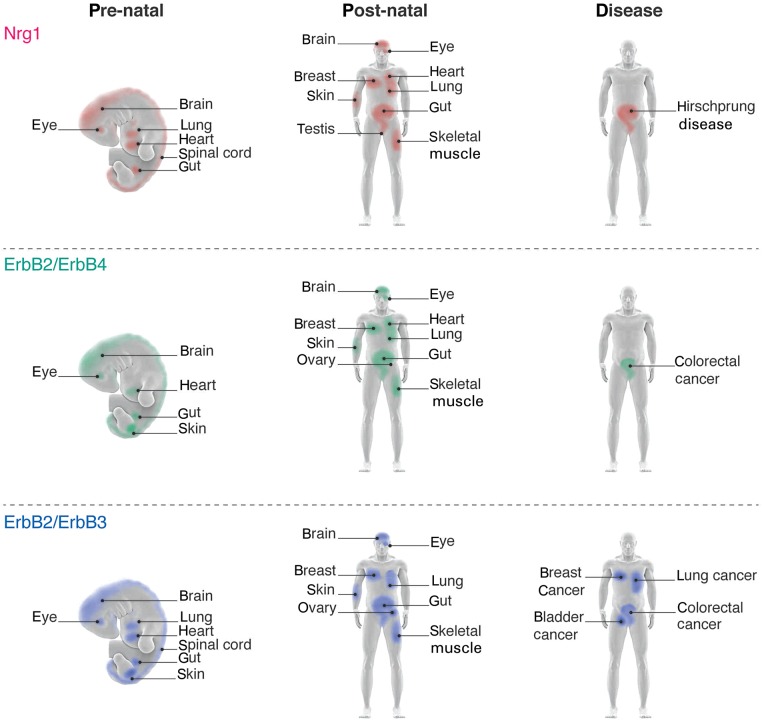
Although there are at least four identified genes encoding Nrg isoforms, only NRG-1 and NRG-2 isoforms have been shown to be expressed in the heart. Both NRG-1 and NRG-2 transcripts are detected in the embryonic endocardium, but only NRG-1 Type I isoforms continue to be expressed in the adult heart, being expressed in the endocardium and coronary microvasculature.2,15,23,41,72 Receptors for the cardiac-specific Nrgs include ErbB2, ErbB3, and ErbB4.63 Both ErbB2 and ErbB4 are expressed in the pre- and post-natal heart (Figure 3), where binding of Nrg1 to ErbB4 induces phosphorylation of itself and its co-receptor ErbB2.41 ErbB3 is expressed in the developing atrioventricular cushions, while ErbB2 and ErbB4 are found in both developing and adult ventricles41,73 (Figure 3).
Figure 3.
Nrg-1 ligand and receptor localizations: (top panel) tissues expressing Nrg1 during pre and post-natal life. (Middle panel) ErbB2 and ErbB4 expression profiles. (Bottom panel) ErbB3 and ErbB4 expression profiles. Disease expression indicates tissues overexpressing Nrg (top) or its receptors (middle, bottom) concomitant with indicated diseases.
Given the high level of signal modularity of the Nrg/ErbB interaction, it is not surprising that a number of downstream targets can be activated through this interaction. Among them are the Erk1/2, PI3K/Akt, and JAK/STAT signalling cascades.74,75
3. Nrgs in cardiac development, homeostasis, and disease
In the prenatal heart, Nrg1 and Nrg2 proteins are synthesized by cardiac microvascular endothelial cells.23,41 Upon release into the extracellular space, Nrgs bind to ErbB4 receptors expressed in the myocardium. Binding of Nrg1 or Nrg2 to ErbB4 drives dimerization with ErbB2, resulting in a signal transduction cascade that promotes proliferation and differentiation of cardiomyocytes in the developing heart.76 There is a clear delineation of specificity required to drive this proliferation, both on the level of ligand and receptor. Prenatal cardiac development requires Ig-containing Nrg1 proteins, evidenced by Ig-Nrg1 knockout mice dying at embryonic day 10.5 with aberrantly thin myocardial walls, indicating that non-Ig Nrgs (i.e. Type III and Type VI) are insufficient for cardiac development.77 Interestingly, despite expression of Nrg2 proteins in the prenatal heart, they too appear to be insufficient to drive necessary levels of cardiomyocyte proliferation in the absence of Ig-Nrg1 proteins, a supposition further supported by the finding that loss of NRG-2 is not embryonically lethal, nor does it confer cardiac defects.78 At the receptor level, loss of either ErbB4 or ErbB2 results in a phenotype highly similar to Nrg1−/−, with both ErbB4−/− and ErbB2−/− mice failing to sufficiently grow and develop their myocardial walls.67,68 Specifically, the reduction in myocardial thickness seen in loss of either Nrg1−/−, ErbB4−/−, or ErbB2−/− largely stems from the failure of these developing hearts to form ventricular trabeculations.67,68,73 Levels of Nrg1 signalling in the heart must be tightly regulated, as ectopic expression of Nrg1 in the developing heart can drive hyper-trabeculation, a malformation known as ventricular non-compaction, which is associated with multiple congenital heart diseases.76,79 Surprisingly, mice knocked out for TACE1 developed enlarged, hypertrabeculated hearts.80 Why loss of TACE1 results in a phenotype dissimilar to that seen in Nrg1, ErbB2, and ErbB4 knockouts is not fully understood, though it is speculated that TACE1 loss results in hyperactivation of ErbB4, resulting in increased cardiac proliferation and trabeculation.80
ErbB3 is expressed in the developing, but not in the adult, heart and is detected in the invading mesenchyme and endocardial cardiac cushions.41,81 This distinct localization is reflected in the aberrant cardiac cushions formed in ErbB3−/− mice.81,82 In addition to driving proliferation and differentiation of working cardiomyocytes, Nrg1 proteins also promote the appropriate differentiation and recruitment of contractile cardiomyocytes to the cardiac conduction system.83
In the adult heart, Nrgs are expressed in response to physiological stress; endogenous and exogenous administration of NRG-1 has unveiled several cardioprotective and cardioregenerative functions of NRG-1, which include protection against: apoptosis, myofibrillar disarray, anthracycline-induced cardiomyopathy, and scar formation84–87 as well as proliferation of post-natal cardiomyocytes. These effects are summarized in Table 2. Interestingly, NRG-1-induced protection from myofibrillar disarray has been shown in vitro and in vivo;92,100 however, these findings contrast with the lack of myofibrillar disarray observed in ErbB2 conditional knockouts.102,103 The dichotomy seen between these gain-of-function and loss-of-function studies highlights an intriguing area for future research and suggests a potential role for ErbB4 homodimers in NRG-1-induced cardioprotection.86
Table 2.
Cardiac effects of Nrg-1 isoforms
| Neuregulin | Effects | In vitro | In vivo |
|---|---|---|---|
| Nrg1, rhNRG-1 | Improvement of myocardial function in diabetic cardiomyopathy | +88 | |
| Nrg1, rhNRG-1 | Protection against diabetic cardiomyopathy-induced cardiomyocyte apoptosis | +88 | |
| Nrg1 Type I β, rhNRG, AA 1-241 | Reduction in anthracycline-induced alterations of EC coupling | +89 | |
| Nrg1 Type II β, rhGGF2 | Cardiomyocyte proliferation | +41 | |
| Nrg1 Type II β, rhGGF2 | Cardiomyocyte survival | +41 | |
| Nrg1 Type II β, rhGGF2 | Cardiomyocyte hypertrophy | +41 | |
| Nrg1 Type II β, GGF2 | Protection of cardiac myocytes from anthracycline-induced apoptosis | +90,91 | |
| Nrg1 α | Prevents anthracycline-induced myofilament injury | −92 | |
| Nrg1 α2, EGF-like domain | Drives negative inotropic response | +93 | |
| Nrg1 β | Prevents anthracycline-induced myofilament injury | +92 | |
| Nrg1 β, rhGGF2 | Protects against anthracycline-induced cardiotoxicity | +91,94 | |
| Nrg1 β, EGF-like domain | Angiogenesis | −95 | +95 |
| Nrg1 β (extracellular domain including the Ig-domain) | Angiogenesis | −95 | |
| Nrg1 β1, AA 177-241 | Angiogenesis | +96 | |
| Nrg1 β3, EGF-like domain | Angiogenesis | +96 | +96 |
| Nrg1 β | Reduced hypertension | N/A | +97 |
| Nrg1 β, GGF2 | Improve systolic function | N/A | +98 |
| Nrg1 β (bivalent) | Attenuation of anthracycline-induced decrease in fractional shortening | N/A | +86 |
| Nrg1 β, EGF-like domain, AA176-246 | Cardiomyocyte proliferation | +87 | +87 |
| Nrg1 β, EGF-like domain, AA176-246 | Cardiomyocyte proliferation following MI | N/A | +87 |
| Nrg1 β, EGF-like domain, AA176-246 | Differentiated cardiomyocyte cell-cycle re-entry | +87 | +87 |
| Nrg1 β, EGF-like domain, AA176-246 | Improvement of cardiac structure after MI | N/A | +87 |
| Nrg1 β, EGF-like domain, AA176-246 | Improvement of cardiac function after MI | N/A | +87 |
| Nrg1 β1 | Improved Ca2+ handling | +99 | |
| Nrg1 β2a, AA Ser177-Glu237 | Prevents anthracycline-induced myofilament injury | Data not shown100 | |
| Nrg1 β2a, AA Ser177-Glu237 | Protects against anthracycline-induced cardiotoxicity | +100 | |
| Nrg1, rhNRG-1 | Prevention of Angiotensin-II-induced diastolic dysfunction | N/A | +101 |
| Nrg1, rhNRG-1 | Reduction in Angiotensin-II-induced cardiac hypertrophy | +101 | +101 |
| Nrg1, rhNRG-1 | Reduction in Angiotensin-II-induced myocardial fibrosis | +101 | +101 |
Plus indicates positive effect and minus indicates negative effect. Corresponding references are indicated insuperscript.
MI, myocardial infarction; EC, excitation–contraction.
NRG-1 has also been suggested to play a role in cardiac hypertrophy; however, like myofibrillar disarray, NRG-1's relationship with this cardiac effect is not fully understood. Administration of NRG-1 to cardiomyocytes in vitro has been shown to increase hypertrophy74 in neonatal rat ventricular cardiomyocytes; however, administration of NRG-1 following left anterior coronary artery (LAD) ligation was shown to decrease cardiomyocyte hypertrophy.87 Further complexity is seen in the increase of NRG-1, ErbB2, and ErbB4 expression coincident with cardiac hypertrophy, all of which rapidly decline in expression upon transition to heart failure.104 These collective findings suggest a complex relationship between NRG-1 and cardiac hypertrophy, likely involving both feed-forward and feed-back signalling pathways, and highlight another important avenue for future research.
The favourable effects which Nrg administration confers upon the heart are shared by other identified cardioprotective and cardioregenerative growth factors. Like NRG-1, administration of insulin-like growth factor 1 (IGF-1), periostin peptide, or fibroblast growth factor 1 (FGF-1) is sufficient to induce post-natal cardiomyocyte cycling.87,105–107 While FGF-1 and IGF-1 therapies result in increased cardiac hypertrophy,108 periostin peptide and NRG-1 therapies have an opposite effect, with administration of either growth factor resulting in decreased cardiomyocyte hypertrophy in adult mice or rats after experimental myocardial infarction (MI).87,106,109 Additionally, like NRG-1, IGF-1, FGF-1, FGF-2, urocortin, vascular endothelial growth factor (VEGF), transforming growth factor beta-1, and cardiotrophin therapies all are associated with decreased apoptosis in the heart.86,90,110–122 It is noteworthy that NRG-1's apoptotic protection appears to be context-dependent in animal models. For example, while NRG-1 administration following LAD ligation has no effect on apoptosis,87 both NRG-1 treatment for diabetic cardiomyopathy and bivalent NRG-1 administration following anthracycline-induced cardiotoxicity result in attenuation of cardiomyocyte apoptosis.86,88,90,110,111 Similar to NRG-1, periostin peptide administration following LAD ligation has no effect on apoptosis; it is important to note, however, that unlike NRG-1, periostin peptide was administered immediately following LAD ligation, in contrast to NRG-1 being administered 1 week following LAD ligation.106 The parallels seen between NRG-1 and other growth factors conferring cardiac protection and regeneration are likely therefore to elucidate additional therapeutic potentials for cardiac growth factors.
The importance of endogenous NRG-1 in post-natal cardiac homeostasis is further demonstrated by the resultant cardiotoxicity in patients receiving the breast cancer chemotherapeutic Herceptin, which targets the NRG-1 receptor ErbB2.123 These findings are in line with studies showing that conditional ErbB2 deletion in mice results in dilated cardiomyopathy,102,103 although the detailed cellular mechanisms of Herceptin-induced cardiomyopathy remain to be elucidated.
The importance of NRG-1 in the post-natal heart is further demonstrated by the studies showing alterations in Nrg signalling being correlated with some cardiac diseases. In a rat model of cardiac hypertrophy induced by aortic constriction, Nrg-1 transcript levels remained constant, while ErbB2 and ErbB4 transcript and protein levels significantly decreased.104 Similarly, myocardium from heart failure patients displayed normal Nrg levels, but had extremely decreased ErbB2 and ErbB4 levels.124 These findings suggest abnormalities of receptor regulation in the failing myocardium.
In contrast, heart failure induction via pacing in dogs showed a substantial increase in Nrg1, ErbB2, and ErbB4 RNA levels, with accompanying induction of the Nrg1/ErbB-activated PI3K pathway, but not Erk or Akt activation, cardioprotective pathways normally activated by Nrg1/ErbB signalling.125 Genetic screening in congenital heart disease patients revealed an association between an ERBB4 haplotype and defects of the left ventricular outflow tract.126 The dichotomy of Nrg1 expression in different models of heart failure may be a result of disease specificity, with differing maladaptive mechanisms being activated in the different diseases. Such a possibility could explain the distinct Nrg1 and ErbB expression patterns seen in a rat model for diabetic cardiomyopathy, wherein both Nrg1 and ErbB2 as well as ErbB4 are decreased, a phenotype distinct from either disease expression pattern described above.127 Similarly, Nrg is detectable in both plasma and sera from heart failure patients, although it is differentially associated with disease severity depending on the heart failure type,128,129 further supporting the concept of differential Nrg regulation based on the pathogenic mechanism.
Endogenous Nrgs may also promote cardiac function by acting on non-cardiomyocytes. NRG-1 expression promotes cardiac function following injury via induction of angiogenesis. Endothelium-derived Nrg was shown to promote angiogenesis both in vitro and in vivo, and reduction of Nrg was found to correlate with a reduction in angiogenesis following ischaemic injury, further supporting the role of Nrg as a pro-angiogenic factor.84,96,130 Intriguingly, Nrg-stimulated angiogenesis has been reported to be independent of VEGF;96 however, a different study challenges such VEGF-independence, finding that Nrg can drive angiogenesis by stimulating expression of VEGF.95
NRG-1 cardioprotection via non-cardiomyocytes may also involve actions upon cardiac fibroblasts, as NRG-1 administration following injury results in a decrease in scar size.87 Whether NRG-1 acts directly or indirectly on fibroblasts to drive this effect is unknown, and highlights another intriguing area for future mechanistic studies into how NRG-1 drives cardioprotection.
How Nrg expression is stimulated in the adult heart remains an intriguing area of investigation. Studies have found that enodthelin-1 and increased mechanical stress on the heart increase Nrg expression, whereas angiotensin-II and phenylephrine decrease Nrg expression;131 this suggests an important mechanism for regulation of Nrg signalling at the ligand level by sensing of alterations to cardiac demand. The finding that increased mechanical stress on the heart increases Nrg expression is intriguing, as integrins, a family of receptors expressed on many cells, including cardiomyocytes, act as sensors of mechanical stress as well as potentially as non-canonical receptors for Nrg1 proteins.132,133 The concept of stress driving positive regulation of Nrg expression is further supported by studies, showing that Nrg expression in the heart is increased during pregnancy, a time when haemodynamic stress increases cardiac demand.134
4. Therapeutic administration of Nrg: potentials and challenges
Given the critical requirement for Nrgs in cardiovascular development, as well as their ability to drive cardioprotective downstream signalling cascades, it is not surprising that administration of recombinant Nrg isoforms have been identified as cardiac beneficial agents with potential applications in clinical management of heart diseases. To date, ClinicalTrials.gov has listed seven clinical trials examining NRG-1 administration as a cardiac therapy. Current clinical trials for NRG-1 therapy are limited for the treatment of systolic dysfunction; it is likely that future trials may investigate the feasibility of NRG-1 therapy for the treatment of diastolic dysfunction, as animal models of diabetic cardiomyopathy- and Angiotensin-II-induced injury have shown that NRG-1 administration can attenuate diastolic dysfunction.101,110 Given the high incidence of diastolic dysfunction,135–137 future mechanistic research into possible different means and disease contexts in which NRG-1 treatment can attenuate such dysfunction presents an important area of research. Furthermore, NRG-1 has only been investigated as therapeutic treatment in dilated and hypertrophic cardiomyopathies. Whether NRG-1 treatment can improve restrictive and arrhythmogenic cardiomyopathies is unknown, and presents another intriguing area for future studies.
Nrg's cardiac benefits in vivo include its ability to decrease scar size and improve cardiac function following MI.87 A complete picture of the ways Nrg drives such cardiac benefits is still being elucidated; our laboratory has found that Nrg increases cardiomyocyte number, rather than size, following infarct; however, it is likely that this is not the sole mechanism responsible for the functional improvements by Nrg in acute heart failure. Gu et al.138 demonstrated that increased cardiac myosin light chain kinase (cMLCK) expression and regulatory light chain phosphorylation accompanied post-Nrg improvement of function in hearts; however, Chang et al.139 found that suppression of the cMLCK expression did not mitigate Nrg's cardiac benefits, suggesting alternative pathways utilized.
A critical question to answer for the advancement of Nrg therapy is how, when, and in what disease types, Nrg should be administered. Most studies have examined Nrg in a cardiac beneficial role with administration following injury. Pre-treatment with Nrg under ex vivo conditions was found to increase scar formation; this, however, is in contrast with an in vitro study showing Nrg promoting cardiomyocyte survival.84,140
Nrg has also been investigated as a therapeutic to be co-administered to cancer patients receiving anthracycline therapy. In vivo studies have shown that administration of Nrg prior to and during anthracycline treatment helps protect against anthracycline-induced cardiotoxicity,86,91,94,100 suggesting promise for Nrg as a co-therapeutic for cancer patients receiving anthracycline therapy. Engineered bivalent Nrg has been shown to drive protection from anthracycline-induced cardiotoxicity,86 and it presents another means with which the diverse Nrgs can be utilized to protect the injured heart.
The promise of Nrg in clinical management of heart failure is potentially mitigated by one of Nrg's own great strengths—its mitogenic potential. Although ErbB2 does not directly bind to Nrgs, ERBB2 gene amplification leading to overexpression is a key feature of many cancers, the best documented of which is breast cancer (reviewed in141). The finding that expression of one component of the Nrg signalling system is increased in some cancers has led to the supposition that therapeutic application of recombinant NRG-1 for cardiac therapy may unintentionally stimulate proliferation of non-cardiac cells. Close examination of ErbB2's function in promoting tumorgenicity reveals that ErbB2-assosciated tumorgenicity is often Nrg-independent. Studies have shown that overexpressed ErbB2 found in tumours is frequently a mutant version, which is constitutively active in the absence of a ligand.142,143 Additionally, silencing of the NRG-1 locus is a hallmark of many breast cancers,144 though a genetic NRG-1 translocation that produces secreted Nrg1 is associated with other breast cancers,145–149 suggesting that the capacity for Nrg to induce and progress malignancy is tightly tied to biological context. Direct binding partners for Nrgs, ErbB3 and ErbB4, have also been associated with cancer via incidence of their up-regulation in cancers; however, assignment of causality may be premature, as overexpression of either receptor is associated with both improved150 and reduced151 cancer prognosis.
Another concern for clinical use of NRG-1 for cardioprotection is whether NRG-1 therapy will specifically activate its intended cardiac receptors. As NRG-1 is administered systemically, it is possible that its post-natal receptors throughout the body may be activated and promotes adverse reactions. Data from clinical trials revealed two incidences of skin cancer, as well as complaints of nausea;152 while another trial reported no significant side effects.153 It is important to note that the two incidences of skin cancer were concluded to have been pre-existing conditions. It is likely that the relationship between NRG-1 isoform specificity and receptor activation accounts for the minimal adverse side effects reported; however, publication of results from other clinical trials will be important in assessing the potential of NRG-1 systemic administration. If NRG-1 systemic administration is determined to be unfeasible in certain patient populations, alternative means of delivery would present important therpautic alternatives. Our laboratory has demonstrated that interpedicardial gel-foam delivery of periostin peptide allows for cardioprotection without systemic administration;154 similar drug delivery of NRG-1 could be utilized in potential patient populations restricted for systemic NRG-1 adminstration.
Reduced Nrg levels are also associated with an increased risk for the development of schizophrenia;155 however, as the link between Nrg and schizophrenia is based on reduction in basal Nrg levels, administration of exogenous Nrg for cardiac dysfunction is not expected to raise risk for schizophrenia. Despite this, given the fine modulation of signalling in both cardiac and neuronal systems, as well as the ability for some Nrgs to cross the blood–brain barrier,43,156,157 clinical studies evaluating Nrg as a cardiac therapy should probably include cognitive and behavioural monitoring.
Inappropriate Nrg signalling is also linked to Hirschsprung disease, a congenital disorder characterized by a regional lack of innervation in the gut, which prevents gut motility. Increased Nrg, both at the transcript and protein levels, was found in the intestines of Hirschsprung disease patients,35 suggesting that inappropriate Nrg signalling is a causal factor in this disease.
5. Conclusions
The genetic architecture of Nrgs and their receptors allows for diverse isoforms to be expressed. The diverse isoforms have both overlapping and distinct requirements, reflecting the complexity of the Nrg/ErbB signalling system. The diverse effects achievable by Nrgs are beginning to be harnessed for clinical therapies, with the Nrg's multiple cardiac effects showing great promise for treating heart failure. Continued elucidation of Nrg signalling shows great promise for not only clinical therapies, but also for better understanding of the pathogenesis and progression of heart failure.
Conflict of interest: none declared.
Funding
Our research was supported by the Department of Cardiology and the Translational Investigator Program (Boston Children's Hospital); National Heart, Lung, and Blood Institute (grant nos K08-HL-085143 and R01-HL-106302); The Children's Cardiomyopathy Foundation; and the Harold S. Geneen Charitable Trust Award Program.
References
- 1.Peles E, Yarden Y. Neu and its ligands: from an oncogene to neural factors. BioEssays News Rev Mol Cell Dev Biol. 1993;15:815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- 2.Carraway KL, III, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, et al. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, et al. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, et al. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo A, Kinrade EF, Georgiou M. The Drosophila neuregulin vein maintains glial survival during axon guidance in the CNS. Dev Cell. 2001;1:679–690. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- 6.Honjo Y, Kniss J, Eisen JS. Neuregulin-mediated ErbB3 signaling is required for formation of zebrafish dorsal root ganglion neurons. Dev Camb Engl. 2008;135:2615–2625. doi: 10.1242/dev.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JF, Zhou H, Pun S, Ip NY, Peng HB, Tsim KW. Cloning of cDNAs encoding xenopus neuregulin: expression in myotomal muscle during embryo development. Brain Res Mol Brain Res. 1998;58:59–73. doi: 10.1016/s0169-328x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 8.Brockes JP. Glial growth factor—a new component of the brain and pituitary. Birth Defects Orig Artic Ser. 1983;19:277–285. [PubMed] [Google Scholar]
- 9.Lemke GE, Brockes JP. Identification and purification of glial growth factor. J Neurosci Off J Soc Neurosci. 1984;4:75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 11.Orr-Urtreger A, Trakhtenbrot L, Ben-Levy R, Wen D, Rechavi G, Lonai P, et al. Neural expression and chromosomal mapping of Neu differentiation factor to 8p12-p21. Proc Natl Acad Sci USA. 1993;90:1867–1871. doi: 10.1073/pnas.90.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, et al. Isolation of the neu/HER-2 stimulatory ligand: a 44 kDa glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 13.Steinthorsdottir V, Stefansson H, Ghosh S, Birgisdottir B, Bjornsdottir S, Fasquel AC, et al. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Mei L, Xiong W-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, et al. Isoform-specific expression and function of neuregulin. Dev Camb Engl. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson S, Petersen TE, Sottrup-Jensen L, Claeys H. Proteases and Biological Control. Reich E, Rifkin DB, Shaw E, eds. 123–149 (Cold Spring Harbor Laboratory, New York, 1975) [Google Scholar]
- 17.Patthy L, Trexler M, Váli Z, Bányai L, Váradi A. Kringles: modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases. FEBS Lett. 1984;171:131–136. doi: 10.1016/0014-5793(84)80473-1. [DOI] [PubMed] [Google Scholar]
- 18.Halaby DM, Poupon A, Mornon J. The immunoglobulin fold family: sequence analysis and 3D structure comparisons. Protein Eng. 1999;12:563–571. doi: 10.1093/protein/12.7.563. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen NE, Abadi N, Sliwkowski MX, Reilly D, Skelton NJ, Fairbrother WJ. High-resolution solution structure of the EGF-like domain of heregulin-alpha. Biochemistry (Mosc) 1996;35:3402–3417. doi: 10.1021/bi952626l. [DOI] [PubMed] [Google Scholar]
- 20.Rosnack KJ, Stroh JG, Singleton DH, Guarino BC, Andrews GC. Use of capillary electrophoresis-electrospray ionization mass spectrometry in the analysis of synthetic peptides. J Chromatogr A. 1994;675:219–225. doi: 10.1016/0021-9673(94)85275-8. [DOI] [PubMed] [Google Scholar]
- 21.Lu HS, Chang D, Philo JS, Zhang K, Narhi LO, Liu N, et al. Studies on the structure and function of glycosylated and nonglycosylated neu differentiation factors. Similarities and differences of the alpha and beta isoforms. J Biol Chem. 1995;270:4784–4791. doi: 10.1074/jbc.270.9.4784. [DOI] [PubMed] [Google Scholar]
- 22.Marikovsky M, Lavi S, Pinkas-Kramarski R, Karunagaran D, Liu N, Wen D, et al. ErbB-3 mediates differential mitogenic effects of NDF/heregulin isoforms on mouse keratinocytes. Oncogene. 1995;10:1403–1411. [PubMed] [Google Scholar]
- 23.Cote GM, Miller TA, LeBrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1α and β isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Hobbs SS, Coffing SL, Le AT, Cameron EM, Williams EE, Andrew M, et al. Neuregulin isoforms exhibit distinct patterns of ErbB family receptor activation. Oncogene. 2002;21:8442–8452. doi: 10.1038/sj.onc.1205960. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Cleary S, Mandarano MA, Long W, Birchmeier C, Jones FE, et al. The breast proto-oncogene, HRGalpha regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002;21:4900–4907. doi: 10.1038/sj.onc.1205634. [DOI] [PubMed] [Google Scholar]
- 26.Plowman GD, Green JM, Culouscou JM, Carlton GW, Rothwell VM, Buckley S, et al. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 27.Carraway KL, III, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, et al. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- 28.Dammann CEL, Nielsen HC, Carraway KL., III Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167:1711–1716. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Nethery D, Kern JA. Neuregulin-1 induces branching morphogenesis in the developing lung through a P13K signal pathway. Exp Lung Res. 2004;30:465–478. doi: 10.1080/01902140490476454. [DOI] [PubMed] [Google Scholar]
- 30.Brown DJ, Lin B, Holguin B. Expression of neuregulin 1, a member of the epidermal growth factor family, is expressed as multiple splice variants in the adult human cornea. Invest Ophthalmol Vis Sci. 2004;45:3021–3029. doi: 10.1167/iovs.04-0229. [DOI] [PubMed] [Google Scholar]
- 31.Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C. et al. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci. 2010;123:3102–3111. doi: 10.1242/jcs.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebrasseur NK, Coté GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–C1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 33.LeBrasseur NK, Mizer KC, Parkington JD, Sawyer DB, Fielding RA. The expression of neuregulin and erbB receptors in human skeletal muscle: effects of progressive resistance training. Eur J Appl Physiol. 2005;94:371–375. doi: 10.1007/s00421-005-1333-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G. et al. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang CS, Ngan ES, Tang WK, So MT, Cheng G, Miao XP. et al. Mutations in the NRG1 gene are associated with Hirschsprung disease. Hum Genet. 2012;131:67–76. doi: 10.1007/s00439-011-1035-4. [DOI] [PubMed] [Google Scholar]
- 36.Patel NV, Acarregui MJ, Snyder JM, Klein JM, Sliwkowski MX, Kern JA. Neuregulin-1 and human epidermal growth factor receptors 2 and 3 play a role in human lung development in vitro. Am J Respir Cell Mol Biol. 2000;22:432–440. doi: 10.1165/ajrcmb.22.4.3854. [DOI] [PubMed] [Google Scholar]
- 37.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 38.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y. et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 39.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, et al. Specific regulation of NRG1 isoform expression by neuronal activity. J Neurosci Off J Soc Neurosci. 2011;31:8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 42.Ho WH, Armanini MP, Nuijens A, Phillips HS, Osheroff PL. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J Biol Chem. 1995;270:14523–14532. doi: 10.1074/jbc.270.24.14523. [DOI] [PubMed] [Google Scholar]
- 43.Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. J Neurosci Off J Soc Neurosci. 2011;31:5699–5709. doi: 10.1523/JNEUROSCI.3477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ, et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J Biol Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- 45.Busfield SJ, Michnick DA, Chickering TW, Revett TL, Ma J, Woolf EA, et al. Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Mol Cell Biol. 1997;17:4007–4014. doi: 10.1128/mcb.17.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higashiyama S, Horikawa M, Yamada K, Ichino N, Nakano N, Nakagawa T, et al. A novel brain-derived member of the epidermal growth factor family that interacts with ErbB3 and ErbB4. J Biochem (Tokyo) 1997;122:675–680. doi: 10.1093/oxfordjournals.jbchem.a021806. [DOI] [PubMed] [Google Scholar]
- 47.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 48.Ni C-Y, Murphy MP, Golde TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 49.Hatzimanolis A, McGrath JA, Wang R, Li T, Wong PC, Nestadt G, et al. Multiple variants aggregate in the neuregulin signaling pathway in a subset of schizophrenia patients. Transl Psychiatry. 2013;3:e264. doi: 10.1038/tp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedrique SP, Fazzari P. Nrg1 reverse signaling in cortical pyramidal neurons. J Neurosci Off J Soc Neurosci. 2010;30:15005–15006. doi: 10.1523/JNEUROSCI.4669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 52.Burgess TL, Ross SL, Qian YX, Brankow D, Hu S. Biosynthetic processing of neu differentiation factor. Glycosylation trafficking, and regulated cleavage from the cell surface. J Biol Chem. 1995;270:19188–19196. doi: 10.1074/jbc.270.32.19188. [DOI] [PubMed] [Google Scholar]
- 53.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci Off J Soc Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montero JC, Yuste L, Díaz-Rodríguez E, Esparís-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci. 2000;16:631–648. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- 55.Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A. Roles of Meltrin beta/ADAM19 in the processing of neuregulin. J Biol Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- 56.Horiuchi K, Zhou H-M, Kelly K, Manova K, Blobel CP. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev Biol. 2005;283:459–471. doi: 10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Luo X, Prior M, He W, Hu X, Tang X, Shen W, et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem. 2011;286:23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 59.Nural-Guvener HF, Mutlu N, Gaballa MA. BACE1 levels are elevated in congestive heart failure. Neurosci Lett. 2013;532:7–11. doi: 10.1016/j.neulet.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Hwang H, Cao L, Buckland M, Cunningham A, Chen J, et al. Domain-specific gene disruption reveals critical regulation of neuregulin signaling by its cytoplasmic tail. Proc Natl Acad Sci USA. 1998;95:13024–13029. doi: 10.1073/pnas.95.22.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang M, Dubbin K, D'Aiello A, Hartmann M, Lodish H, Herrlich A, et al. Epidermal growth factor (EGF) ligand release by substrate-specific a disintegrin and metalloproteases (ADAMs) involves different protein kinase C (PKC) isoenzymes depending on the stimulus. J Biol Chem. 2011;286:17704–17713. doi: 10.1074/jbc.M110.187823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 64.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., III Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004;569:332–336. doi: 10.1016/j.febslet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Sweeney C, Carraway KL., III Ligand discrimination by ErbB receptors: differential signaling through differential phosphorylation site usage. Oncogene. 2000;19:5568–5573. doi: 10.1038/sj.onc.1203913. [DOI] [PubMed] [Google Scholar]
- 67.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 68.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, et al. A dual role for ErbB2 signaling in cardiac trabeculation. Dev Camb Engl. 2010;137:3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sweeney C, Lai C, Riese DJ, II, Diamonti AJ, Cantley LC, Carraway KL., III Ligand discrimination in signaling through an ErbB4 receptor homodimer. J Biol Chem. 2000;275:19803–19807. doi: 10.1074/jbc.C901015199. [DOI] [PubMed] [Google Scholar]
- 71.Crovello CS, Lai C, Cantley LC, Carraway KL., III Differential signaling by the epidermal growth factor-like growth factors neuregulin-1 and neuregulin-2. J Biol Chem. 1998;273:26954–26961. doi: 10.1074/jbc.273.41.26954. [DOI] [PubMed] [Google Scholar]
- 72.Yamada K, Ichino N, Nishii K, Sawada H, Higashiyama S, Ishiguro H, et al. Characterization of the human NTAK gene structure and distribution of the isoforms for rat NTAK mRNA. Gene. 2000;255:15–24. doi: 10.1016/s0378-1119(00)00309-7. [DOI] [PubMed] [Google Scholar]
- 73.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 74.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 75.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 2002;27:306–313. doi: 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- 76.Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- 77.Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci USA. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Britto JM, Lukehurst S, Weller R, Fraser C, Qiu Y, Hertzog P, et al. Generation and characterization of neuregulin-2-deficient mice. Mol Cell Biol. 2004;24:8221–8226. doi: 10.1128/MCB.24.18.8221-8226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stöllberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2004;17:91–100. [Google Scholar]
- 80.Shi W, Chen H, Sun J, Buckley S, Zhao J, Anderson KD, et al. TACE is required for fetal murine cardiac development and modeling. Dev Biol. 2003;261:371–380. doi: 10.1016/s0012-1606(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 81.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 82.Erickson SL, O'Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Dev Camb Engl. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 83.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol. 2008;44:831–854. doi: 10.1016/j.yjmcc.2008.02.278. [DOI] [PubMed] [Google Scholar]
- 86.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, et al. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation. 2013;128:152–161. doi: 10.1161/CIRCULATIONAHA.113.002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 88.Li B, Zheng Z, Wei Y, Wang M, Peng J, Kang T, et al. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol. 2011;10:69. doi: 10.1186/1475-2840-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, et al. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, et al. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Bian Y, Sun M, Silver M, Ho KK, Marchionni MA, Caggiano AO, et al. Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am J Physiol Heart Circ Physiol. 2009;297:H1974–H1983. doi: 10.1152/ajpheart.01010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 93.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 94.Bian Y, Silver M, Ho KKL, Marchionni MA, Kang PM, Goukassian DA, et al. Neuregulin1 improved cardiac function in doxorubicin-treated mice with cardiomyocyte-specific over expression of a dominant-negative PI3Kp110α. J Cardiovasc Dis Diagn. 2013;01:120. [Google Scholar]
- 95.Iivanainen E, Paatero I, Heikkinen SM, Junttila TT, Cao R, Klint P, et al. Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res. 2007;313:2896–2909. doi: 10.1016/j.yexcr.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 96.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–H2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 97.Matsukawa R, Hirooka Y, Nishihara M, Ito K, Sunagawa K. Neuregulin-1/ErbB signaling in rostral ventrolateral medulla is involved in blood pressure regulation as an antihypertensive system. J Hypertens. 2011;29:1735–1742. doi: 10.1097/HJH.0b013e32834937d6. [DOI] [PubMed] [Google Scholar]
- 98.Hill MF, Patel AV, Murphy A, Smith HM, Galindo CL, Pentassuglia L. et al. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PloS ONE. 2013;8:e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brero A, Ramella R, Fitou A, Dati C, Alloatti G, Gallo MP, et al. Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res. 2010;88:443–452. doi: 10.1093/cvr/cvq238. [DOI] [PubMed] [Google Scholar]
- 100.Liu X, Gu X, Li Z, Li X, Li H, Chang J, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 101.Hervent A-S, Vandekerckhove L, Keulenaer GWD. Neuregulin-1 antagonizes myocardial fibrosis and diastolic dysfunction in angiotensin-II treated mice. Eur Heart J. 2013;34:P2434. [Google Scholar]
- 102.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 103.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hübner N, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, et al. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 105.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, et al. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 107.Engel FB, Hsieh PCH, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cittadini A, Strömer H, Katz SE, Clark R, Moses AC, Morgan JP, et al. Differential cardiac effects of growth hormone and insulin-like growth factor1 in the rat. A combined in vivo and in vitro evaluation. Circulation. 1996;93:800–809. doi: 10.1161/01.cir.93.4.800. [DOI] [PubMed] [Google Scholar]
- 109.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li B, Xiao J, Li Y, Zhang J, Zeng M. Gene transfer of human neuregulin-1 attenuates ventricular remodeling in diabetic cardiomyopathy rats. Exp Ther Med. 2013;6:1105–1112. doi: 10.3892/etm.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.An T, Zhang Y, Huang Y, Zhang R, Yin S, Guo X, et al. Neuregulin-1 protects against doxorubicin-induced apoptosis in cardiomyocytes through an Akt-dependent pathway. Physiol Res Acad Sci Bohemoslov. 2013;62:379–385. doi: 10.33549/physiolres.932516. [DOI] [PubMed] [Google Scholar]
- 112.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM, et al. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci. 1995;92:8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cuevas P, Reimers D, Carceller F, Martinez-Coso V, Redondo-Horcajo M, Saenz de Tejada I, et al. Fibroblast growth factor-1 prevents myocardial apoptosis triggered by ischemia reperfusion injury. Eur J Med Res. 1997;2:465–468. [PubMed] [Google Scholar]
- 114.Iwai-Kanai E, Hasegawa K, Fujita M, Araki M, Yanazume T, Adachi S, et al. Basic fibroblast growth factor protects cardiac myocytes from iNOS-mediated apoptosis. J Cell Physiol. 2002;190:54–62. doi: 10.1002/jcp.10036. [DOI] [PubMed] [Google Scholar]
- 115.Zhang GW, Wen T, Gu TX, Li-Ling J, Wang C, Zhao Y, et al. Transmyocardial drilling revascularization combined with heparinized bFGF-incorporating stent activates resident cardiac stem cells via SDF-1/CXCR4 axis. Exp Cell Res. 2012;318:391–399. doi: 10.1016/j.yexcr.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Z, Rivkees SA. Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev Dyn Off Publ Am Assoc Anat. 2000;217:388–400. doi: 10.1002/(SICI)1097-0177(200004)217:4<388::AID-DVDY6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 117.Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, et al. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- 118.Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ, et al. Vascular endothelial growth factor prevents apoptosis and preserves contractile function in hypertrophied infant heart. Circulation. 2006;114:I290–I295. doi: 10.1161/CIRCULATIONAHA.105.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang BC, Zander DS, Mehta JL. Hypoxia-reoxygenation-induced apoptosis in cultured adult rat myocytes and the protective effect of platelets and transforming growth factor-β1. J Pharmacol Exp Ther. 1999;291:733–738. [PubMed] [Google Scholar]
- 120.Baxter GF, Mocanu MM, Brar BK, Latchman DS, Yellon DM. Cardioprotective effects of transforming growth factor-β1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol. 2001;38:930–939. doi: 10.1097/00005344-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 121.Chen H, Li D, Saldeen T, Mehta JL. TGF-β1 modulates NOS expression and phosphorylation of Akt/PKB in rat myocytes exposed to hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2001;281:H1035–H1039. doi: 10.1152/ajpheart.2001.281.3.H1035. [DOI] [PubMed] [Google Scholar]
- 122.Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272:5783–5791. doi: 10.1074/jbc.272.9.5783. [DOI] [PubMed] [Google Scholar]
- 123.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast Edinb Scotl. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Rohrbach S, Niemann B, Silber R-E, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium—depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 125.Doggen K, Ray L, Mathieu M, Mc Entee K, Lemmens K, De Keulenaer GW. Ventricular ErbB2/ErbB4 activation and downstream signaling in pacing-induced heart failure. J Mol Cell Cardiol. 2009;46:33–38. doi: 10.1016/j.yjmcc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 126.McBride KL, Zender GA, Fitzgerald-Butt SM, Seagraves NJ, Fernbach SD, Zapata G, et al. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birt Defects Res A Clin Mol Teratol. 2011;91:162–168. doi: 10.1002/bdra.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gui C, Zhu L, Hu M, Lei L, Long Q. Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2012;21:414–420. doi: 10.1016/j.carpath.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 128.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120:310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geisberg CA, Wang G, Safa RN, Smith HM, Anderson B, Peng XY, et al. Circulating neuregulin-1β levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron Artery Dis. 2011;22:577–582. doi: 10.1097/MCA.0b013e32834d3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hedhli N, Dobrucki LW, Kalinowski A, Zhuang ZW, Wu X, Russell RR, III, et al. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res. 2012;93:516–524. doi: 10.1093/cvr/cvr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemmens K, Segers VFM, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281:19469–19477. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 132.Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 133.Ieguchi K, Fujita M, Ma Z, Davari P, Taniguchi Y, Sekiguchi K, et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin-1/ErbB signaling. J Biol Chem. 2010;285:31388–31398. doi: 10.1074/jbc.M110.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–H942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 135.Kloch-Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound. 2012;10:10. doi: 10.1186/1476-7120-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Desai CS, Colangelo LA, Liu K, Jacobs DR, Jr, Cook NL, Lloyd-Jones DM, et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. 2013;177:20–32. doi: 10.1093/aje/kws224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 138.Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88:334–343. doi: 10.1093/cvr/cvq223. [DOI] [PubMed] [Google Scholar]
- 139.Chang AN, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull JT. The effects of neuregulin on cardiac myosin light chain kinase gene-ablated hearts. PloS ONE. 2013;8:e66720. doi: 10.1371/journal.pone.0066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ebner B, Lange SA, Eckert T, Wischniowski C, Ebner A, Braun-Dullaeus RC, et al. Uncoupled eNOS annihilates neuregulin-1β-induced cardioprotection: a novel mechanism in pharmacological postconditioning in myocardial infarction. Mol Cell Biochem. 2013;373:115–123. doi: 10.1007/s11010-012-1480-y. [DOI] [PubMed] [Google Scholar]
- 141.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 142.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S. et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 143.Alajati A, Sausgruber N, Aceto N, Duss S, Sarret S, Voshol H, et al. Mammary tumor formation and metastasis evoked by a HER2 splice variant. Cancer Res. 2013;73:5320–5327. doi: 10.1158/0008-5472.CAN-12-3186. [DOI] [PubMed] [Google Scholar]
- 144.Chua YL, Ito Y, Pole JC, Newman S, Chin SF, Stein RC. et al. The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene. Oncogene. 2009;28:4041–4052. doi: 10.1038/onc.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schaefer G, Fitzpatrick VD, Sliwkowski MX. Gamma-heregulin: a novel heregulin isoform that is an autocrine growth factor for the human breast cancer cell line, MDA-MB-175. Oncogene. 1997;15:1385–1394. doi: 10.1038/sj.onc.1201317. [DOI] [PubMed] [Google Scholar]
- 146.Wang XZ, Jolicoeur EM, Conte N, Chaffanet M, Zhang Y, Mozziconacci MJ. et al. gamma-Heregulin is the product of a chromosomal translocation fusing the DOC4 and HGL/NRG1 genes in the MDA-MB-175 breast cancer cell line. Oncogene. 1999;18:5718–5721. doi: 10.1038/sj.onc.1202950. [DOI] [PubMed] [Google Scholar]
- 147.Adélaïde J, Huang HE, Murati A, Alsop AE, Orsetti B, Mozziconacci MJ, et al. A recurrent chromosome translocation breakpoint in breast and pancreatic cancer cell lines targets the neuregulin/NRG1 gene. Genes Chromosomes Cancer. 2003;37:333–345. doi: 10.1002/gcc.10218. [DOI] [PubMed] [Google Scholar]
- 148.Huang HE, Chin SF, Ginestier C, Bardou VJ, Adélaïde J, Iyer NG, et al. A recurrent chromosome breakpoint in breast cancer at the NRG1/neuregulin 1/heregulin gene. Cancer Res. 2004;64:6840–6844. doi: 10.1158/0008-5472.CAN-04-1762. [DOI] [PubMed] [Google Scholar]
- 149.Prentice LM, Shadeo A, Lestou VS, Miller MA, deLeeuw RJ, Makretsov N, et al. NRG1 gene rearrangements in clinical breast cancer: identification of an adjacent novel amplicon associated with poor prognosis. Oncogene. 2005;24:7281–7289. doi: 10.1038/sj.onc.1208892. [DOI] [PubMed] [Google Scholar]
- 150.Mittra I, Redkar AA, Badwe RA. Prognosis of breast cancer: evidence for interaction between c-erbB-2 overexpression and number of involved axillary lymph nodes. J Surg Oncol. 1995;60:106–111. doi: 10.1002/jso.2930600208. [DOI] [PubMed] [Google Scholar]
- 151.Agrup M, Stål O, Olsen K, Wingren S. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat. 2000;63:23–29. doi: 10.1023/a:1006498721508. [DOI] [PubMed] [Google Scholar]
- 152.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 153.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 154.Polizzotti BD, Arab S, Kühn B. Intrapericardial delivery of gelfoam enables the targeted delivery of periostin peptide after myocardial infarction by inducing fibrin clot formation. PLoS ONE. 2012;7:e36788. doi: 10.1371/journal.pone.0036788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rösler TW, Depboylu C, Arias-Carrión O, Wozny W, Carlsson T, Höllerhage M. et al. Biodistribution and brain permeability of the extracellular domain of neuregulin-1-β1. Neuropharmacology. 2011;61:1413–1418. doi: 10.1016/j.neuropharm.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 157.Kastin AJ, Akerstrom V, Pan W. Neuregulin-1-beta1 enters brain and spinal cord by receptor-mediated transport. J Neurochem. 2004;88:965–970. doi: 10.1046/j.1471-4159.2003.02224.x. [DOI] [PubMed] [Google Scholar]