Abstract
Purpose
To test whether longitudinally measured health-related quality of life (HRQL) predicts transplant-related mortality (TRM) in pediatric hematopoietic stem cell transplant (HSCT).
Methods
The predictors of interest were emotional functioning, physical functioning, role functioning, and global HRQL, as rated by the parent about the child up to 6 times over 12 months of follow-up and measured by the Child-Health Ratings Inventories (CHRIs).
We used joint models, specifically shared parameter models, with time to TRM as the outcome of interest and other causes of mortality as a competing risk, via the JM software package in R. Choosing shared parameter models instead of standard survival models, such as Cox models with time-dependent covariates, enabled us to address measurement error in the HRQL trajectories and appropriately handle missing data. The non-linear trajectories for each HRQL domain were modeled by random spline functions. The survival submodels were adjusted for baseline patient, family, and transplant characteristics.
Results
Hazard ratios per one-half standard deviation difference in emotional, physical, and role functioning and global HRQL were 0.61 (95% CI: 0.46 to 0.81; p<0.001), 0.70 (0.51 to 0.96; p=0.03), 0.54 (0.34 to 0.85; p=0.007), and 0.57 (0.41 to 0.79; p<0.001), respectively.
Conclusions
HRQL trajectories were predictive of TRM in pediatric HSCT, even after adjusting the survival outcome for baseline characteristics.
Keywords: hematopoietic stem cell transplant, health-related quality of life, joint model for longitudinal and time-to-event outcomes, shared parameter model, transplant-related mortality, survival
Introduction
Hematopoietic stem cell transplant (HSCT) offers potentially life-saving therapy for children and adolescents with malignant and benign disorders of the bone marrow, immune system, and metabolism. The HSCT process typically begins with a prolonged and intensive hospitalization at a regional transplant center, which for many families is distant from home. Initially, the patient receives a conditioning regimen, which can include chemotherapy, radiation, and/or immunomodulatory drugs. This is followed by the infusion or re-infusion of healthy hematopoietic (blood forming) stem cells, which are from a healthy donor (allogeneic) or from the patient him-or herself (autologous). While most patients sustain some degree of early toxicity from the conditioning regimen regardless of the transplant type, recipients of allogeneic HSCT also face the possibility of acute graft versus host disease (aGVHD) or chronic graft versus host disease (cGVHD), caused by an immune reaction of the donor cells on the patient’s end organs.
Clinically, the first three months following HSCT are fraught with life-threatening complications, including end-organ damage, serious infection, and aGVHD, each of which can seriously compromise the recipient’s health-related quality of life (HRQL) [1–4]. Over time, care shifts back to home under the management of the local provider. Visits to the transplant center are less frequent, although monitoring continues for delayed transplant complications, including late onset infections, cGVHD, organ damage, and disease recurrence[5]. Late complications are often insidious and delays in definitive diagnosis are not uncommon. Altered HRQL may therefore serve as an early warning signal of life-threatening clinical complications, the diagnosis of which might otherwise be delayed.
Evidence of the prognostic value of both cross-sectional and longitudinally measured HRQL information has been published in Quality of Life Research, among other journals. HRQL is known to predict mortality in cancer patients[6–8] and in a range of other diseases and clinical settings including HIV[9], respiratory conditions[10], cardiac surgery[11], and intensive care[12], possessing predictive power beyond that of demographic and clinical measures alone. General health and physical functioning were the most often cited domains. Thus far, research on HRQL in pediatric patients recovering from HSCT has focused on estimating and predicting the HRQL trajectories[4,13–15]. The prognostic value of HRQL information in this population has not been previously studied. We used joint models for longitudinal and survival outcomes to investigate the ability of longitudinally observed HRQL to predict transplant-related mortality (TRM) in pediatric HSCT.
Methods
Participants
Study subjects were from two previous studies, Trajectories of Health and Adaptation after Pediatric Stem Cell Transplant (Journeys Study), which described the 12-month HRQL trajectory following HSCT[4], and HSCT-CHESS™ (Comprehensive Health Enhancement Support System) to Enhance HSCT Recovery, a randomized controlled trial of a web-based intervention designed to improve the health-related knowledge, skills, and quality of life of parents of children undergoing HSCT[16]. The study reported here was approved by the Institutional Review Board (IRB) of Tufts Medical Center.
Inclusion was limited to children whose first HRQL assessment was prior to their preparative regimen for HSCT. We used this criterion to remove variation by timing of baseline assessment[17], a nuisance variable that was controlled by design in the more recent HSCT-CHESS™ Study. The sample included 274 infants and children up to 18 years old and parent proxy raters. HRQL assessments were planned at baseline, 45 days, 3, 6, 9 (HSCT-CHESS™ only), and 12 months post-HSCT, but were permitted at any time up to the next scheduled time point. Clinical Information was collected from the medical chart at each time point, even if the family missed the HRQL assessment.
Outcomes
The clinical outcome was time to TRM, with disease-related mortality (the only other cause of death) as competing risk. The longitudinal HRQL outcomes were emotional functioning, physical functioning, role functioning (5–18 years only), and global HRQL (5–18 only). Correlations between HRQL scales varied from 0.33 (emotional and role functioning, physical and role functioning) to 0.79 (emotional functioning and global HRQL). All children 5–18 years were assessed using the General Health module of the Child Health Ratings Inventories (CHRIs). Acceptable levels of reliability (Cronbach’s alpha 0.85–0.95), discriminant validity, and convergent validity have been published for the parent version of the CHRIs[15,18]. Children under five years who participated in the Journeys Study were assessed using the reliable (Cronbach’s alpha 0.72–0.94) and validated Infant Toddler Quality of Life Scale (ITQOL)[19,20] while those in the HSCT-CHESS Study were assessed with single global items for physical and emotional functioning from the CHRIs. Among children 5–18 years, these single global items were strongly correlated with the corresponding CHRIs domain scores at baseline (r=0.72 for physical and r=0.51 for emotional), and correlations increased over time. Responses to individual items, which were on a 5-point scale, were used to calculate summary HRQL scores according to established scoring algorithms. Although the original summary scores were on a 0–100 scale (where higher scores indicate higher functioning), scores were divided by 10 for computational reasons. The parent’s rating of the child was used for all subjects for consistency and statistical power since children under five did not rate themselves.
Joint Models
Joint models[21–26] are intended for analyses of studies with outcomes of two qualitatively different types: a longitudinal sequence of repeated measurements, and a time-to-event outcome. In the clinical literature, these outcomes are typically analyzed separately; the longitudinal data with mixed models, and the time-to-event data with survival methods. Joint models analyze the two types of outcomes together, using a single likelihood function. In this study we were interested in the time-to-event outcome (TRM) conditioned on longitudinal outcomes (domains of HRQL), and we used joint models to estimate the hazard ratios. In other applications of joint models the scientific interest may be directed more toward the longitudinal outcome, or equally to both types of outcome.
Standard survival methods, such as Cox regression with time-varying covariates, would be suboptimal for our purposes because they ignore both measurement error in the longitudinal variable and correlation between the measurement error and survival, and they impute by last-observation-carried-forward (LOCF) when there are missing data. In contrast, joint models incorporate the longitudinal measurement error. Data that are missing at random (MAR) are handled as they would be in a mixed model and even data missing not at random (MNAR) can be addressed by using time to drop-out as one of the outcomes to be analyzed. Joint models improve efficiency, reduce bias, and thus may prevent erroneous conclusions.
Estimating Joint Models in Pediatric HSCT
Joint models have two submodels, one for survival and one for longitudinal data. We estimated separate joint models for each of the four HRQL outcomes. In the longitudinal submodel, we used spline (piecewise polynomial) functions to model the relation between time and observed HRQL. The functions were selected from natural cubic and b-splines (quadratic or cubic) with 1, 2, or 3 knots. The criteria for selection were a combination of Akaike Information Criterion (AIC), visual assessment of fit, and joint model convergence. For visual assessment, we compared the spline to the trajectory of mean scores, with scores averaged at the planned assessment times (baseline, 45 days, etc.). The parameters of the splines were modeled as fixed effects plus normally distributed random effects. Thus, a random trajectory was estimated for each subject.
In the survival submodel, we used a stratified Cox model to accommodate the competing risk and we approximated the log baseline hazard by b-splines. The covariate of interest was HRQL, but rather than observed HRQL, the survival submodel used modeled HRQL (the random trajectory), which is interpreted as the latent or “true” HRQL. We assessed the bivariate relations between the following covariates and mortality (treatment-related and disease-related): child age, child gender, malignancy (y/n), disease duration in months, transplant type (autologous or related donor vs. unrelated donor), study, and parent’s emotional functioning at baseline. Variables that were associated with either mortality outcome at p<0.10 were tested in a multivariable model with backward elimination using p<0.05, and those selected by this procedure were used as covariates in the survival submodels of the joint models.
An assumption of the joint model is that observed HRQL and mortality are conditionally independent, given the latent HRQL. The parameters of the random trajectory are called “shared parameters” since they belong to both submodels. The estimated hazard ratio of the latent HRQL is the parameter of interest in this study.
As explained above, the only independent variable in the longitudinal submodel was a non-linear function of time. We also built joint models in which fixed effects were added to the longitudinal submodels. Mixed effects models were used to assess the relation between each HRQL outcome and the following covariates: child characteristics (child age and gender), family characteristics (parent age and gender, number of siblings, race and ethnicity, parent’s baseline emotional functioning), disease characteristics (disease duration, malignancy (y/n), prior relapse), treatment characteristics (prior transplant, location of prior care, transplant type), study, and time-varying complications (infection, aGVHD, cGVHD, and end organ toxicity). Variables that were associated with a longitudinal outcome at p<0.10 were tested in multivariable models using backward elimination with p<0.05. The selected variables (see Appendix) were used in the longitudinal submodels that incorporated covariates. For comparison with the joint models, we also ran standard Cox regression models for predicting mortality from time-varying, observed HRQL.
All analyses were done in R version 2.15.0. The joint models were estimated using the JM package in R[25] (version JM 0.9–2). The JM package used maximum likelihood estimation and the Gauss-Hermite integration rule was used to approximate the required integrals. Formulas and code are in the Appendix.
Results
Baseline Characteristics
Mean age of the children at baseline was 8.7 years (standard deviation: 5.6), and 122 (44.5%) were female. Median duration of illness was 9 months (25th to 75th percentile: 5.0 to 36.5) and 183 (66.8%) had a diagnosis of malignancy. Sixty-one (22.3%), 57 (20.8%), and 156 (56.9%) received autologous, related donor, and unrelated donor transplants, respectively (Table 1).
Table 1.
Baseline Characteristics (n=274)
| Parent age in years, mean (SD) | 38.0 (7.9) |
| Female parent, n (%) | 226 (82.5%) |
| Baseline parental emotional functioning, mean (SD) | 51.0 (20.1) |
| Child age in years, mean (SD) | 8.7 (5.6) |
| Female child, n (%) | 122 (44.5%) |
| Causal indication for transplant, malignancy, n (%) | 183 (66.8%) |
| Transplant Type, n (%) | |
| Autologous | 61 (22.3%) |
| Related allogeneic | 57 (20.8%) |
| Unrelated allogeneic | 156 (56.9%) |
| Duration of illness (months), median (25th to 75th) | 9.0 (5.0, 36.5) |
| Baseline HRQL scores, 0–10 scale, mean (SD) | |
| Emotional functioning | 7.1 (2.0) |
| Physical functioning | 6.2 (3.0) |
| Role functioning | 7.3 (2.8) |
| Global HRQL | 6.5 (2.1) |
Trajectories
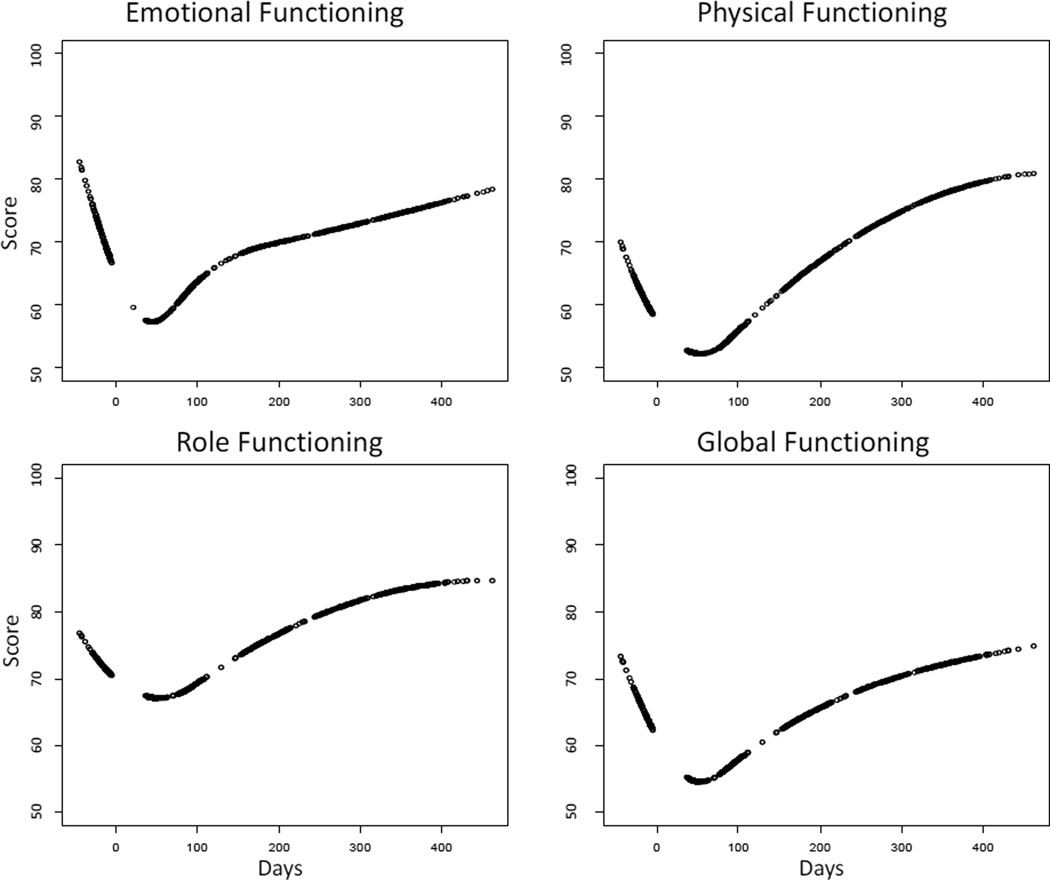
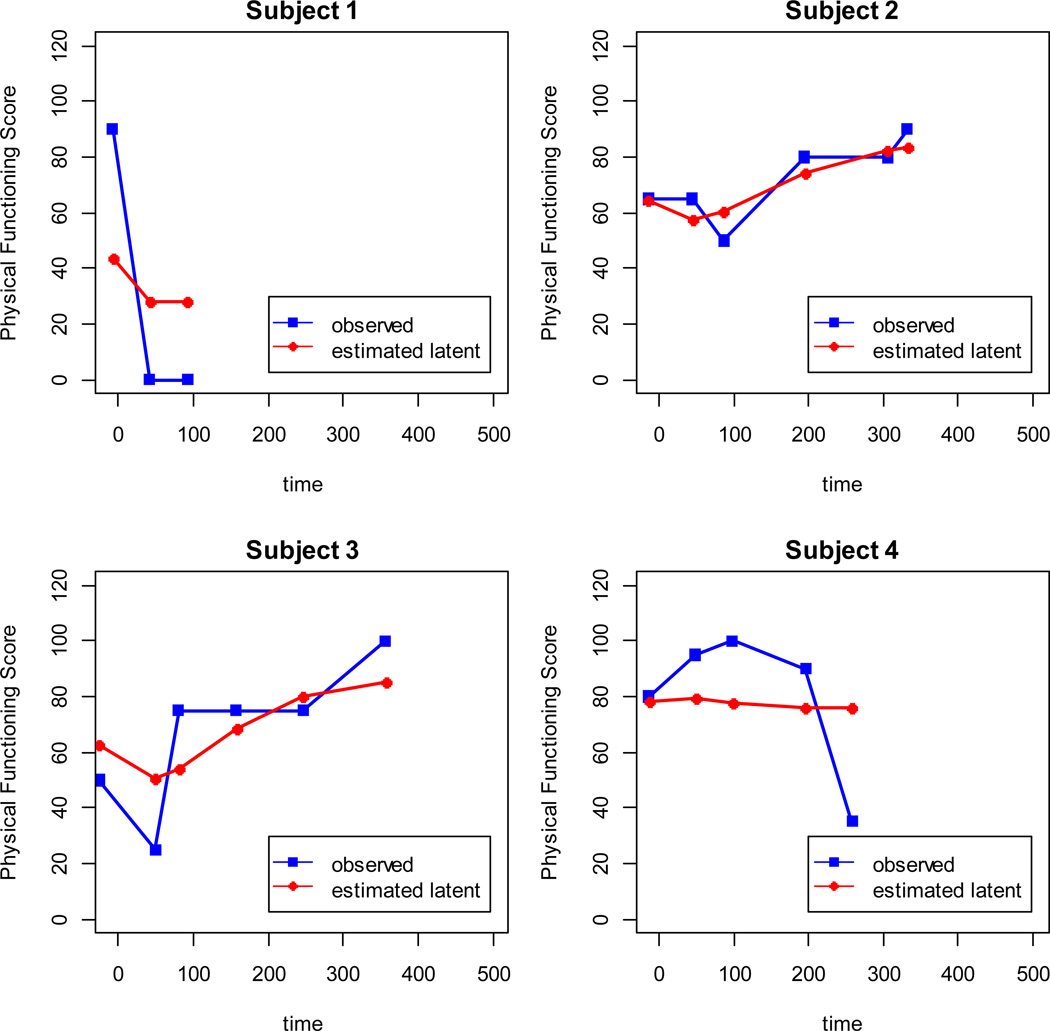
The mean trajectories of HRQL declined between the baseline assessment and 45 days posttransplant, and then increased (Figure 1). Emotional and physical functioning were evaluated for 274 subjects 0–18 years of age. Role functioning and global HRQL were evaluated for the subset of 204 subjects age 5–18. Emotional functioning and global HRQL were modeled as natural cubic splines with three knots, and physical functioning and role functioning were modeled as quadratic b-splines with one knot. Time zero is the date of transplant. Each assessment time point is associated with a point on the curve, which represents the best-fitting piecewise cubic or quadratic function. The estimated latent scores (Figure 2) vary from person to person, since the coefficients of the spline were modeled as random effects. Also, the estimates are closer to the mean than the individual’s observed values because the model accounted for measurement error.
Figure 1.
Estimated mean HRQL. Transplant occurs on Day 0. Each point represents an assessment time point.
Figure 2.
Estimated latent physical functioning compared with observed physical functioning, for four subjects.
The JM package offers plot diagnostics for the longitudinal submodel, including (1) residuals vs. fitted and (2) Normal Q-Q. The diagnostic plots for each longitudinal model were examined and no violations were observed (data not shown).
Association Between HRQL and Mortality
There were 32 HSCT-related deaths (24 among the 5–18 year-olds) and 14 deaths from the competing risk, disease-related mortality (10 among the 5–18 year-olds). The baseline variables selected as adjusters for the survival submodel were child gender and transplant type. All four domains of HRQL were significantly associated with time to TRM. The estimated hazard ratios were 0.61 (95%CI: 0.46 to 0.81; p<0.001), 0.70 (95%CI: 0.51 to 0.96; p=0.03), 0.54 (95%CI: 0.34, 0.85; p=0.007), and 0.57 (95%CI: 0.41, 0.79; p<0.001) for emotional, physical, and role functioning and global HRQL, respectively (Table 2). None of the domains of HRQL was significantly associated with the competing risk, disease-related mortality (data not shown). The hazard ratios correspond to the modeled trajectories of the HRQL domains, such as those displayed in red diamonds in Figure 2 (random spline functions of time). In contrast, the hazard ratios in the standard Cox regressions with time-varying covariate correspond to observed trajectories of HRQL, such as those displayed in blue squares. Hazard ratios estimated by the standard approach were higher (closer to the null) than those estimated by joint modeling (Table 2). Adding covariates to the longitudinal submodel (the submodel with HRQL as the outcome) brought the estimated hazard ratios closer to the null (Table 2). Excluding child gender and transplant type from the longitudinal submodel (these were also covariates in the survival submodel) did not change the hazard ratios by much (data not shown). JM does not provide survival diagnostics for models with competing risks.
Table 2.
Association between HRQL and treatment-related mortality
| Hazard Ratio per ½ Standard Deviation in HRQL Score (95% CI, p-value) | ||||
|---|---|---|---|---|
| Method | Emotional | Physical | Role | Global |
| Joint Model | HR: 0.61 CI: 0.46 to 0.81 p<0.001 |
HR: 0.70 CI: 0.51 to 0.96 p=0.03 |
HR: 0.54 CI: 0.34 to 0.85 p=0.007 |
HR: 0.57 CI: 0.41, 0.79 p<0.001 |
| Standard Model | HR: 0.69 CI: 0.59 to 0.80 p<0.001 |
HR: 0.78 CI: 0.67 to 0.92 p=0.003 |
HR: 0.73 CI: 0.61 to 0.86 p<0.001 |
HR: 0.63 CI: 0.52 to 0.78 p<0.001 |
| Joint Model with Covariates in the Longitudinal Submodel |
HR: 0.73 CI: 0.54 to 0.98 p=0.04 |
HR: 0.77 CI: 0.56 to 1.06 p=0.14 |
HR: 0.60 CI: 0.37 to 0.98 p=0.04 |
HR: 0.75 CI: 0.54 to 1.04 p=0.08 |
Discussion
HSCT, though potentially life-saving for children, can also be highly toxic. Prognosis for such patients might be improved if their local health care teams had access to an early warning signal for serious complications of treatment. In this context, we found that emotional, physical, and role functioning, and global HRQL were highly associated with TRM with hazard ratios ranging from 0.54 to 0.70 per one-half standard deviation difference. The predictive power of the global HRQL scale[16] is particularly interesting, given the brevity of the scale (9 items). The inclusion of such a scale with other planned clinical data collection, for example in patient registries, should be considered. The information would allow for more robust analyses of the association of HRQL with other clinical outcomes, including complications of HSCT and relapse.
Research on HRQL in HSCT has focused on predicting the HRQL trajectories. In the few longitudinal studies in pediatric HSCT that have been conducted with sufficient sample size to account for the heterogeneity of outcome[1,2,4,15], HRQL scores have been found to vary by transplant type, child age, socioeconomic status, parents’ emotional functioning, and clinical complications. On average, raters (both self and proxy) perceive older children and those with allogeneic transplant from unrelated donors to have worse functioning. The Journeys Study[4] found that parent proxy raters gave lower ratings than the child during the first half of the year post-transplant, though the ratings were similar by 12 months. The disagreement between parent and child was even more pronounced in the presence of severe complications. The sensitivity of parents’ ratings to the clinical condition may partially explain the strong relationship we discovered between parents’ ratings and mortality. Our study is the first to examine the prognostic value of HRQL information in this population.
Some investigators have used a non-time varying change variable for prognostication. For example, change in HRQL between cancer diagnosis and 12 months post diagnosis was used in one study to predict subsequent mortality[8]. However, that analysis limited the authors to only two of the five time-points that were measured and only those patients who survived to 12 months. Other studies utilize all time points, analyzing HRQL as a time-varying covariate in a standard Cox model[7]. That approach can be affected by bias since it ignores measurement error and employs LOCF imputation. When compared to standard Cox regression with time-varying covariate, the joint models in our study estimated hazard ratios that differed by 0.08 to 0.19. Similarly, Ibrahim et al uncovered bias when comparing joint models to standard Cox regression in an analysis of HRQL and survival in patients treated for metastatic breast cancer[21].
Our joint models that incorporated covariates in the longitudinal submodels estimated attenuated hazard ratios as compared to models using the random non-linear function of time only. The additional covariates were fixed effects, the coefficients of which represent aggregate effects of the variables. Hence their inclusion removed some of the random variation inherent in the individual trajectories. Exclusion of the covariates that were also in the survival submodel did not change the hazard ratios for HRQL by much. Thus, the attenuation was likely not due to inclusion of some of the same variables in both submodels.
We used the original, validated sum scores that were calculated in the two studies that contributed subjects to our analyses. Another modeling option would be a joint model that incorporates item response theory [27]. Such a model could preserve the direct relationships between the test items and the latent constructs, detect inconsistencies in response patterns, handle floor and ceiling effects, and retain other advantages of IRT models. However, a larger sample size would be required.
One of the limitations of our analysis was that the JM package limits the user to one continuous, longitudinal outcome. Therefore, the HRQL outcomes were tested one at a time, using information from a single rater. For the same reason, time-varying clinical complications could not be included in the models. Future research is needed to assess the added prognostic value of each discrete domain of HRQL and the relative value of parent proxy ratings as compared with child self-ratings. Methods for assessing the value of HRQL over and above information on time-varying clinical outcomes are also needed. An unavoidable limitation is the use of different scales for different age groups (infant to adolescent).
In conclusion, we found that emotional, physical, and role functioning, and global HRQL were highly associated with treatment-related mortality during the first year post-transplant in pediatric HSCT patients. Altered HRQL may serve as an early warning signal of life-threatening, insidious clinical complications in this population.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), Grant Number UL1 TR000073 through Tufts Clinical and Translational Science Institute (CTSI), and the NIH National Cancer Institute grant R21 CA152628 through the Institute for Clinical Research and Health Policy Studies (ICRHPS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
Model building for the longitudinal submodels
Appendix Table 1 shows which covariates were considered for each longitudinal submodels. Variables that were associated with a longitudinal outcome at p<0.10 were tested in multivariable models using backward elimination with p<0.05.
Appendix Table 1.
| Covariate | Emotional | Physical | Role | Global |
|---|---|---|---|---|
| Baseline variables | ||||
| Child age | M | M | NS | M |
| Child gender | NS | D | NS | NS |
| Malignancy | M | NS | D | M |
| HSCT type | M | M | M | M |
| Log duration of illness | D | D | M | D |
| Prior HSCT | NS | NS | NS | NS |
| Prior relapse | NS | NS | NS | NS |
| Location prior care | NS | D | NS | NS |
| Parent age | D | D | NS | D |
| Parent gender | NS | NS | NS | NS |
| Baseline parent emotional functioning | M | M | M | M |
| Siblings | NS | D | NS | NS |
| Parent white race | M | NS | NS | D |
| Parent Hispanic | NS | NS | NS | M |
| Study | D | M | NS | M |
| Indicator for item vs. scale | D | NS | n/a | n/a |
| Time-dependent variables | ||||
| Infection | M | M | M | M |
| AGVHD | M | D | M | M |
| CGVHD | M | M | NS | NS |
| Bearman | M | M | D | M |
NS=variable not associated with the longitudinal outcome; D=variable associated with longitudinal outcome at p<0.10, but dropped from the multivariable model during backward elimination; M=included in the multivariable model.
The joint model
The notation is defined in Appendix Table 2. The longitudinal submodel for the ith individual at time t is
where, in the case of emotional functioning for example, ηi (t ) is the sum of a natural cubic spline (ns) with fixed effects coefficients and a ns with random effects coefficients. The random effects have multivariate normal distribution with mean zero. The errors are independent and normally distributed with mean zero and are also independent of the random effects.
The survival submodel for the ith individual is defined by the cause-specific hazards for TRM
and disease-related mortality
The log baseline hazard is approximated by b-splines.
The joint distribution of HRQL (one domain at a time), TRM, and disease-related mortality was modeled as
The joint model assumes that observed HRQL and mortality are conditionally independent, given the latent HRQL.
Appendix Table 2.
Model notation
| Symbol | Meaning |
|---|---|
| T | The time-to-event outcomes: HSCT-related mortality and competing risk disease-related mortality |
| Y | Observed HRQL |
| [T, Y] | The joint probability distribution of T and Y |
| η | The random trajectory, interpreted as the latent “true” HRQL |
| [T | η] | The conditional distribution of T given eta |
| λ | Cause-specific hazard |
| λ0 | Baseline hazard |
| γ, α | Coefficients of the survival submodel |
| ε | Error term of the longitudinal submodel |
R Code for Emotional Functioning Joint Model
#### Cox PH Competing Risk Model ####
#Data for Cox PH (before competing risk format) data_surv
id event eventtime childfemale hscttype
141 1 76 1 1
143 0 447 0 0
145 0 360 0 0
148 0 374 1 1
152 0 378 0 0
154 1 12 1 0
155 0 388 0 0
156 0 466 1 1
158 0 465 1 0
160 0 399 0 0
:
#create competing risk dataset
data_surv_cr<-crLong(data_surv, "event", "0")
data_surv_cr
id event eventtime childfemale hscttype strata status2
141 141 1 76 1 1 1 1
141.1 141 1 76 1 1 2 0
143 143 0 447 0 0 1 0
143.1 143 0 447 0 0 2 0
145 145 0 360 0 0 1 0
145.1 145 0 360 0 0 2 0
148 148 0 374 1 1 1 0
148.1 148 0 374 1 1 2 0
152 152 0 378 0 0 1 0
152.1 152 0 378 0 0 2 0
#Fit Cox PH competing risk model
fitCoxef_cr <-coxph(Surv(eventtime, status2)~ (childfemale + hscttype)*strata + strata(strata), data=data_surv_cr, x=T)
#### Code for LME Model ####
#Data for LME model
data_long
id time childEF
141 −7 0.000
141 42 5.000
143 −25 5.714
143 107 8.571
143 155 8.929
143 360 8.929
143 416 8.571
145 −18 6.429
145 45 4.643
145 88 5.714
:
#Fit LME model
fitLMEef<-lme(childEF~ns(time, df=4),
random=list(id = pdDiag(form = ~ ns(time, df=4))), na.action=na.omit, data=data_long)
#### Code for JM Model ####
fitJMef <-jointModel(fitLMEef, fitCoxef_cr, timeVar="time", method="spline-PH-aGH", interFact=list(value=~strata, data=data_surv_cr), CompRisk=T)
Reference
- 1.Phipps S, Dunavant M, Garvie PA, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: I. Descriptive outcomes. Bone Marrow Transplant. 2002;29:425–434. doi: 10.1038/sj.bmt.1703377. [DOI] [PubMed] [Google Scholar]
- 2.Phipps S, Dunavant M, Lensing S, Rai SN. Acute health-related quality of life in children undergoing stem cell transplant: II. Medical and demographic determinants. Bone Marrow Transplant. 2002;29:435–442. doi: 10.1038/sj.bmt.1703376. [DOI] [PubMed] [Google Scholar]
- 3.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18:162–171. doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons SK, Tighiouart H, Terrin N. Assessment of health-related quality of life in pediatric hematopoietic stem cell transplant recipients: progress, challenges and future directions. Expert Rev Pharmacoecon Outcomes Res. 2013;13:217–225. doi: 10.1586/erp.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MZ, Ley C, Tarzian AJ. Isolation in blood and marrow transplantation. West J Nurs Res. 2001;23:592–609. doi: 10.1177/019394590102300605. [DOI] [PubMed] [Google Scholar]
- 6.Bonnetain F, Paoletti X, Collette S, Doffoel M, Bouche O, Raoul JL, Rougier P, Masskouri F, Barbare JC, Bedenne L. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res. 2008;17:831–843. doi: 10.1007/s11136-008-9365-y. [DOI] [PubMed] [Google Scholar]
- 7.Sadetsky N, Hubbard A, Carroll PR, Satariano W. Predictive value of serial measurements of quality of life on all-cause mortality in prostate cancer patients: data from CaPSURE (cancer of the prostate strategic urologic research endeavor) database. Qual Life Res. 2009;18:1019–1027. doi: 10.1007/s11136-009-9526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai WL, Chien CY, Huang HY, Liao KC, Fang FM. Prognostic value of quality of life measured after treatment on subsequent survival in patients with nasopharyngeal carcinoma. Qual Life Res. 2013;22:715–723. doi: 10.1007/s11136-012-0213-8. [DOI] [PubMed] [Google Scholar]
- 9.de Boer-van der Kolk IM, Sprangers MA, Prins JM, Smit C, de WF, Nieuwkerk PT. Health-related quality of life and survival among HIV-infected patients receiving highly active antiretroviral therapy: a study of patients in the AIDS Therapy Evaluation in the Netherlands (ATHENA) Cohort. Clin Infect Dis. 2010;50:255–263. doi: 10.1086/649216. [DOI] [PubMed] [Google Scholar]
- 10.Olofson J, Dellborg C, Sullivan M, Midgren B, Caro O, Bergman B. Qualify of life and palliation predict survival in patients with chronic alveolar hypoventilation and nocturnal ventilatory support. Qual Life Res. 2009;18:273–280. doi: 10.1007/s11136-009-9445-7. [DOI] [PubMed] [Google Scholar]
- 11.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, Shroyer AL, VillaNueva CB, O'Brien M, Moritz TE, Henderson WG, Grover FL, Sethi GK, Hammermeister KE. Healthrelated quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281:1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 12.Khouli H, Astua A, Dombrowski W, Ahmad F, Homel P, Shapiro J, Singh J, Nallamothu R, Mahbub H, Eden E, Delfiner J. Changes in health-related quality of life and factors predicting long-term outcomes in older adults admitted to intensive care units. Crit Care Med. 2011;39:731–737. doi: 10.1097/CCM.0b013e318208edf8. [DOI] [PubMed] [Google Scholar]
- 13.Felder-Puig R, diGallo A, Waldenmair M, Norden P, Winter A, Gadner H, Topf R. Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: results of a longitudinal, multi-center study. Bone Marrow Transplant. 2006;38:119–126. doi: 10.1038/sj.bmt.1705417. [DOI] [PubMed] [Google Scholar]
- 14.Nuss SL, Wilson ME. Health-related quality of life following hematopoietic stem cell transplant during childhood. J Pediatr Oncol Nurs. 2007;24:106–115. doi: 10.1177/1043454206296033. [DOI] [PubMed] [Google Scholar]
- 15.Parsons SK, Shih MC, DuHamel KN, Ostroff J, Mayer DK, Austin J, Martini DR, Williams SE, Mee L, Sexson S, Kaplan SH, Redd WH, Manne S. Maternal perspectives on children's health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol. 2006;31:1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]
- 16.Rodday AM, Terrin N, Parsons SK. Measuring global health-related quality of life in children undergoing hematopoietic stem cell transplant: a longitudinal study. Health and Quality of Life Outcomes. 2013;11:26. doi: 10.1186/1477-7525-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons SK, Shih MC, Ratichek S, Recklitis CJ Chang G for the Journeys to Recovery Study. Establishing the Baseline in Longitudinal Evaluation of Health-Related Quality of Life (HRQL): The Pediatric Hematopoietic Stem Cell Transplantation (HSCT) Example. Presentation at the Patient-Reported Outcomes Assessment in Cancer Trials, National Cancer Institute, September 2006. 2006 [Google Scholar]
- 18.Parsons SK, Shih MC, Mayer DK, Barlow SE, Supran SE, Levy SL, Greenfield S, Kaplan SH. Preliminary psychometric evaluation of the Child Health Ratings Inventories (CHRIs) and Disease-Specific Impairment Inventory-HSCT (DSII-HSCT) in parents and children. Qual Life Res. 2005;14:1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- 19.Landgraf JM, Abetz L. Final Report to Schering-Plough Laboratories and Health Technology Associates. Boston: New England Medical Center; 1994. The Infant/toddler quality of life questionnaire: Conceptual framework, logic, content, and preliminary psychometric results. [Google Scholar]
- 20.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–460. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. PMID: 12933568. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Zeger S. Joint analysis of longitudinal data comprising repeated measures and times to events. App Stat. 2001;50:375–387. [Google Scholar]
- 24.Tsiatis AA, Davidian M. Joint modeling of longitudinal and time-to-event data: an overview. Statistica Sinica. 2004;14:809–834. [Google Scholar]
- 25.Rizopoulos D. JM: An R package for the joint modelling of longitudinal and time-toevent data. Journal of Statistical Software. 2010;35(9):1–33. [Google Scholar]
- 26.Rizopoulos D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R. 1st ed. CRC Press; 2012. [Google Scholar]
- 27.Klein Entink RH, Fox JP, van den HA. A mixture model for the joint analysis of latent developmental trajectories and survival. Stat Med. 2011;30:2310–2325. doi: 10.1002/sim.4266. [DOI] [PubMed] [Google Scholar]