Abstract
Objective
Intimal hyperplasia (IH) continues to plague the durability of vascular interventions. Employing a validated murine model, ultrasound biomicroscopy, and speckle tracking algorithms, we tested the hypothesis that reduced cyclic arterial wall strain results in accentuated arterial wall IH.
Approach
A 9-0 suture was tied around the left mouse (n=10) common carotid artery and a 35 gauge (OD=0.14mm) blunt mandrel. We previously showed that mandrel removal results in a ~78% reduction in diameter and ~85% reduction in flow, with subsequent delayed induction of IH by day 28. Pre-op, post-op day 4 (before measurable IH) and post-op day 27 circumferential wall strains were measured in locations 1mm, 2mm and 3mm proximal to the stenosis and in the same locations on the contralateral (non-stenosed) carotid. At post-op day 28, arteries were perfusion fixed and arterial wall morphology was assessed microscopically in the same regions.
Results
Strains were the same in all locations pre-op. Wall strain was decreased in all regions proximal to the stenosis by day 4 (0.26±0.01 to 0.11±0.02; P<.001) while strains remained unchanged for the contralateral artery (P=.45). No statistical regional differences in mean strain or IH were noted at any time point for the experimental or contralateral artery. Based on the median, regions were divided into those with low strain (≤0.1) and high strain (>0.1). Average pre-op strains in both groups were the same (0.27±0.09 and 0.27±0.08). All segments in the low strain group (n=13) demonstrated significant IH formation by day 28 while only 31% of the high strain group demonstrated any detectable IH at day 28. (Mean low strain intimal thickness = 32 ± 20 m, high strain = 8.0 ± 16 m, P<.01). Changes in cross sectional area at diastole drove the reduction in strain in the low strain group, increasing significantly from pre-op to day 4 (P=.04), while lumen cross section at systole remained unchanged (P=.46). Cross sectional area at diastole and systole in the high strain group remained unchanged from pre-op to day 4 (P=.67).
Conclusions
Early reduction in arterial wall strain is associated with subsequent development of hemodynamically induced IH.
Introduction
Coronary and peripheral artery diseases afflict over 14 million Americans and remain a significant source of mortality and morbidity.1 Although a number of both open and endovascular procedures are available for treating arterial occlusive lesions, post-procedure intimal hyperplasia (IH) and pathological wall adaptation in treated arteries limit revascularization durability.2-5 Although a number of biological processes have been implicated in IH,6 a long line of evidence suggests that cellular sensing of the mechanical environment provides the cues that initiate intimal hyperplasia.7
The mechanical forces that are historically associated with vascular wall adaptations are wall shear stress, wall tensile stress and circumferential wall strain. Mechanically, stress is defined as the amount of force applied over a unit area of a surface. Wall shear stress refers to the amount of shearing force per unit area applied to the vessel lumen by the flowing blood. Similarly, wall tensile stress is defined as the force per unit cross sectional area of the vessel wall. Wall tensile stress changes throughout the cardiac cycle as the blood pressure changes from diastole to systole. As wall tensile stress increases, the vessel wall stretches and relaxes causing a strain on the wall. Circumferential wall strain is defined as the change in wall circumference normalized to the diastolic circumference of the vessel.
Low wall shear stress,8 high wall stress9 and the combination of both factors 10 may play a role in the formation of IH in the vessel wall. Others suggest that wall strain, which is closely related to wall stress, may play a more direct role in IH formation.11 Recent ex vivo studies propose that mechanical strain, the stretch of the vessel wall due to pulsing blood (in both the longitudinal and circumferential directions), works independently of the wall shear stress to cause intimal hyperplasia.12 Although a number of researchers have implicated wall shear stress as the primary factor affecting IH formation,13-16 shear stress based explanations alone fail to predict many aspects of IH.17, 18 A leading hypothesis is that the arterial wall actively maintains a homeostatic level of wall stress.19-21 This theory is supported by the observation that the number of lamellar units in any given artery is proportional to blood vessel diameter across a number of mammalian species.22 By maintaining this relationship, the vascular wall keeps the mechanical tension per lamellar unit within an amazingly narrow physiological range. Since wall stress and strain are intimately related through the mechanical properties of the vessel wall, others have suggested that wall strain, and not stress is the underlying state sensed by the cell.23 In the current study, we hypothesized that there is a direct positive relationship between reduced cyclic arterial wall strain and the formation of intimal hyperplasia. To test this hypothesis, we combined a validated murine model of intimal hyperplasia24 with a recently developed method for measuring arterial wall strains in vivo25 to unravel the relationship between wall strain and neointima formation.
Materials and Methods
Animals
All animal experimental protocols used in this study complied with the Guide for the Care and Use of Laboratory Animals26 and were approved by our local Institutional Animal Care and Use Committee. Male C57BL/6J mice (n=10) were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained on a normal chow diet.
Murine model of intimal hyperplasia
At eight weeks of age, general anesthesia was induced with 2% isoflurane gas in an induction chamber and then maintained via a nose cone with 1.0-1.5% isoflurane gas. A focal stenosis was created in the left distal common carotid artery (LCCA) as described previously.24 Briefly, the LCCA was exposed and visualized via a midline incision using a surgical microscope (OPMI-MD, Carl Zeiss, Germany). A focal stenosis was created approximately 1mm proximal to the common carotid bifurcation by tying a 9-0 nylon suture around both the artery and a blunt 35 gauge needle (external diameter of 0.14 mm, item No. NF 35 BL; World Precision Instruments, Inc., Sarasota, FL). After removal of the needle, the vessel diameter was reduced by ~78%. Our previous studies have shown this diameter reduction will produce a reduction in luminal flow of ~85%.24 After creation of the focal stenosis, the surgical incision was closed and mice were allowed to recover.
Ultrasound data acquisition and strain analyses
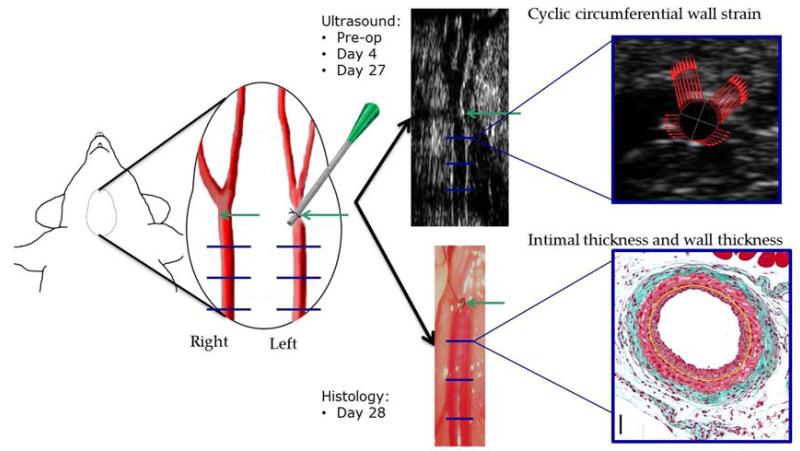
Ultrasound cine loops of both the LCCA and right common carotid artery (RCCA) were recorded preoperatively, on post-op day 4 and on post-op day 27. For ultrasound acquisition, mice were anesthetized using 1-2% isoflurane and body temperature was maintained on a moveable platform heated to 37 °C. A chemical depilatory was used to remove hair from the neck and pre-warmed Aquasonic 100H ultrasound transmission gel (Parker Laboratories, Inc., Fairfield, NJ) was applied. Using a VisualSonics Vevo 2100 Ultrasound Imaging System with MS700 50 MHz linear array transducer (FUJIFILM VisualSonics Inc., Toronto, ON, Canada), cine loops of cross sections of both the RCCA and LCCA were recorded. For the LCCA, loops were recorded 1mm, 2mm and 3mm proximal to the focal stenosis and at similar locations for the RCCA (Figure 1). Cine loops for all time points and locations were recorded at 432 frames/s with 500 consecutive frames acquired. Efforts were made to maintain depth of anesthesia with a heart rate of at least 475 beats/minute and a respiratory rate of 75-100 breaths/minute during all data collection.
Figure 1.
Study design. A focal stenosis (green arrow) was made in the left common carotid artery 1mm proximal to the carotid bifurcation. Mechanical (via ultrasound) and histological parameters were assessed 1mm, 2mm, and 3mm proximal to the focal stenosis on the left side, and in corresponding locations on the contralateral side (blue lines).
Ultrasound cine loops were loaded into VevoVasc software (Version 3.6.2.0, FUJIFILM VisualSonics Inc.) and vascular walls were traced using the automatic edge detection tool. Movement of the carotid artery wall was then tracked through at least 5 cardiac cycles and vessel area was recorded over time. From the resulting area change curves, ideal vessel diameters were computed (assuming a circular cross section) and circumferential wall strain curves (using Green’s definition27) were calculated across the 5 cardiac cycles. Average magnitude of cyclic wall strain from diastole to systole was calculated for each animal, time point and location. Additionally, mean cross sectional areas at diastole and systole cross section were computed and reported for each time point
Tissue harvest and histology
On post-op day 28, mice were again anesthetized using 1-2% isoflurane anesthesia. The chest cavity was opened and the left ventricle of the heart was punctured with a 25 gauge cannula and blood was drained through an incision in the inferior vena cava. Lactated ringer’s solution was perfused under physiological pressure (100 mmHg) for 2-3 minutes then replaced with 10% buffered formalin for 5 minutes. The LCCA and RCCA were collected and fixed in 10% buffered formalin solution overnight.
Tissue specimens were then dehydrated, cleared through several changes of ethanol and xylene and embedded in paraffin. Tissue cross sections (6-m thick) were collected along the length of the tissue. Using the carotid bifurcation as a reference for the RCCA and the focal stenosis as a reference for the LCCA, tissue cross sections were collected 1mm, 2mm and 3mm proximal to the focal stenosis and at corresponding locations on the right (the same locations at which wall strain was assessed using ultrasound). At each location, Masson’s trichrome stain was employed to visualize arterial structures via a microscope with high resolution camera (Axio A1 microscope with vision 4.7 software; Carl Zeiss). The lumen dimensions, internal elastic lamina (IEL) length and external elastic lamina (EEL) length were identified and traced. Assuming a circular cross section in vivo, the areas and radii of the lumen, IEL and EEL were determined. From these values, mean intimal thickness, wall thickness, and intima:media thickness ratio were calculated.
Statistical analyses
Data are presented as means and standard error of the mean. All statistical analyses were completed in SigmaPlot version 11 (Systat Software Inc., San Jose, CA, USA). Statistical methods employed for data comparisons included paired T-tests, One way ANOVA, and repeated measures one-way ANOVA where applicable. To determine significance in multiple comparison procedures, Tukey post-hoc analyses were used. P<.05 was considered significant.
Results
All animals survived surgery to tissue harvest. Three of the 10 mice had occluded LCCAs on harvest and their morphology data were not included in any of the analyses. Histology was available on all of the remaining animals. Due to technical difficulties related to visualizing the relatively low flow artery and edema, some day 4 and day 27 strain data was unusable and thus is missing. Strain data from all 10 animals was used in all regions in which the arterial lumen was clearly visible in the ultrasound images. The baseline data for these animals was included in the strain analyses. Table I summarizes the data available and used for the following analyses.
Table I.
Sample sizes used for strain and histological analyses on the LCCA.
| Timepoint | 2mm Strain (# of Regions) |
3mm Strain (# of Regions) |
4mm Strain (# of Regions) |
Histology (# of animals) |
|---|---|---|---|---|
| Day 0 | 10 | 10 | 10 | |
| Day 4 | 9* | 8* | 9* | |
| Day 27 | 8* | 8* | 8* | |
| Day 28 | 7† |
The lumen of some vessels were not visible via ultrasound and were therefore excluded.
3 arteries were fully occluded at day 28 and therefore were not used for histological analysis
Wall Strain Analysis
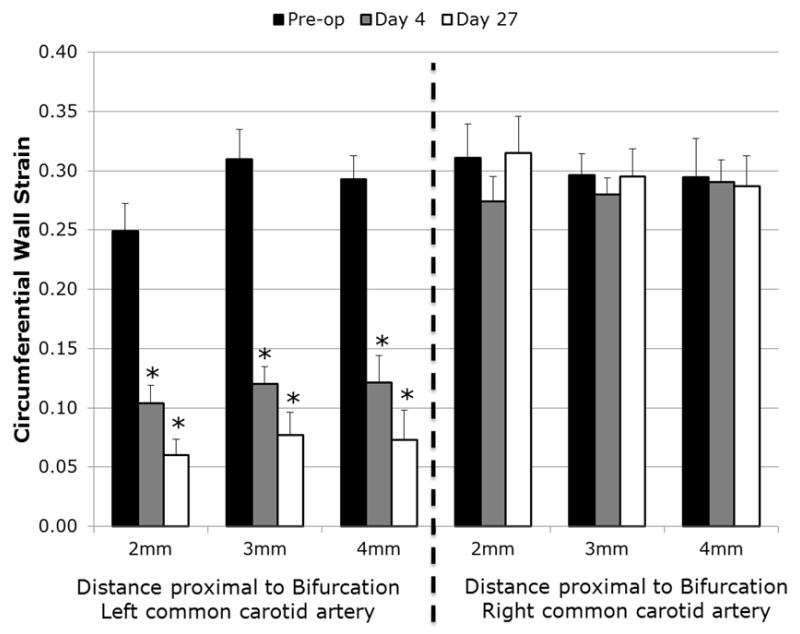
Wall strains at baseline (pre-op) were consistent in all regions of both the left and right common carotid artery with no significant differences (Figure 2; P=.56). At day 4, all regions on the focal stenosis (left) side were significantly reduced compared to pre-op strains (P<.001) while no changes in strain occurred on the contralateral (right) side (P=.90). At day 27, strains remained reduced on the left side compared to pre-op measurements but did not significantly reduce further compared to strains at day 4 (P=.2). Strains on the contralateral artery remained unchanged compared to pre-op strains at day 27. No regional differences in strain were seen amongst either the stenosis or contralateral locations at any time point.
Figure 2.
Regional differences in strain. Wall strains were assessed on the left (partially stenosed) and right (unaltered) common carotid artery in the regions shown in Figure 1. Average for each side indicates the average of all regions. No differences amongst spatial locations were found on either side at any time point. * indicates P<.05 compared to pre-op values.
Histological analysis of wall adaptations
Histological analysis of tissues collected at day 28 was completed in all animals with a patent LCCA (n=7). Intimal thickness, wall thickness, and intima:media thickness ratio were computed at 2, 3 and 4mm proximal to the carotid bifurcation (regions which correspond to those where ultrasound data was acquired). IH occurred near the focal stenosis of the LCCA, but none was observed in the contralateral RCCA (Figure 3A). No regional differences were found in any calculated parameter amongst the three spatial regions. Mean wall thickness of the LCCA tended to be higher than that of RCCA, with a significantly 56% thicker wall 3mm proximal to the bifurcation (P=.012) (Figure 3B).
Figure 3.
Morphological analysis of day-28 carotid arteries. (A) The intimal thickness of LCCA was significantly higher than that of RCCA at the same location. (B) LCCA tends to have higher wall thickness than its counterpart RCCA. (C) Representative histology for each of the three regions on the LCCA and RCCA. † indicates P < .05 compared to RCCA value at the same cross sectional location.
Temporal progression of arterial lumen cross sectional areas
Arterial cross sectional areas at both diastole and systole were assessed pre-op, and at post-op day 4 and day 27. Since no differences among spatial positions of either the LCCA or RCCA were noted, strain values for all regions were analyzed together. Cross sectional area at diastole in all spatial regions (2, 3 and 4mm proximal to the carotid bifurcation) on the focal stenosis side remained unchanged from pre-op to post-op day 4 (0.10±0.01mm2 [n=30] vs. 0.12±0.01mm2 [n=26]; P=.19). However, cross sectional area at diastole was decreased at post-op day 27 compared to both pre-op area and post-op day 4 area (0.07±0.01mm2[n=24]; P<.001). On the contralateral (right) side, cross sectional area at diastole increased from pre-op to post-op day 4 (0.10±0.01 mm2 [n=30] vs. 0.13±0.01 mm2 [n=26]; P=0.009) and remained increased at post-op day 27 compared to pre-op (0.14±0.01 mm2 [n=25]; P<.001). Cross sectional area at diastole were statistically the same for the focal stenosis and contralateral arteries at pre-op and day 4, but contralateral cross section was larger at post-op day 27 (P<.001).
Cross sectional area at systole was the same for the LCCA and RCCA at pre-op (0.15±0.01 mm2 [n=30] vs. 0.15±0.01 mm2 [n=30]; P=.50). Cross sectional area at systole in the LCCA was unchanged compared to pre-op at post-op day 4 (0.14±0.01 mm2 [n=26]; P=.64) but was decreased at post-op day 27 (0.08±0.01 mm2 [n=25]; P<.001). In the RCCA, cross sectional area at systole increased compared to pre-op at day 4 (0.17±0.01 mm2 [n=30]; P=.004) and continued to increase at post-op day 27 (0.20±0.01 mm2 [n=30]; P=.01) (Figure 4).
Figure 4.
Changes in lumen area at (A) diastole and (B) systole. *Indicates P<.05 compared to pre-op values
Relationship between acute strain and subsequent intimal thickness
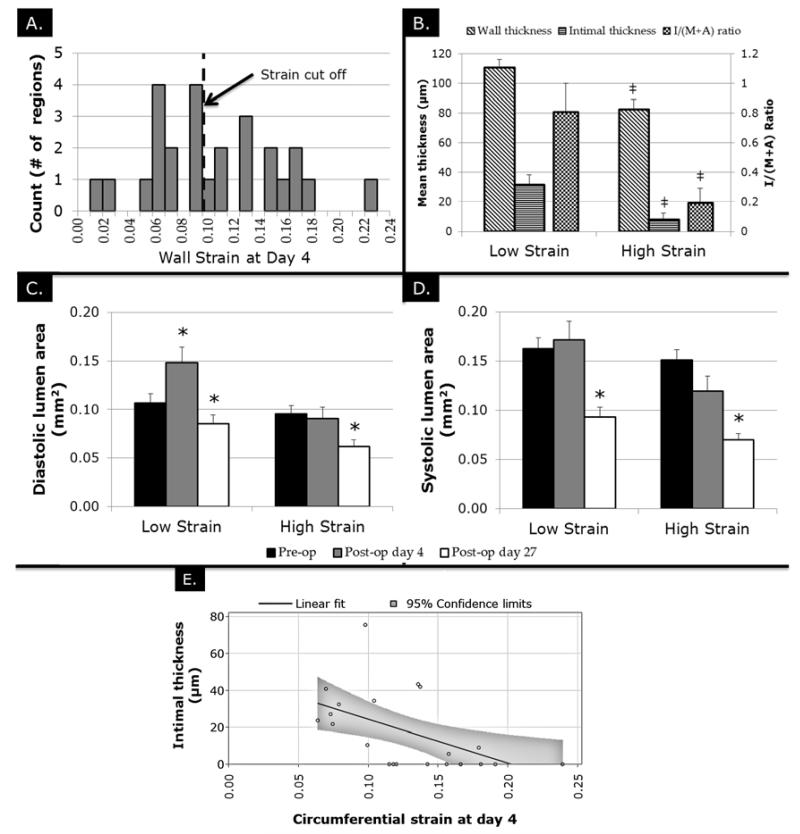
Analysis of the limited number of wall strain values in all regions at post-op day 4 using a histogram demonstrated a range of results (Figure 5A). To further analyze the acute changes in wall strain produced by this model, the LCCA data were divided into regions with strain >0.10 (n=13) and those with strain ≤0.10 (n=13). Given that strain data was available for 26 out of 30 total regions at day 4, these regions were used and the median was utilized as the threshold for the two groups. Of those regions with strains ≤0.10 at post-op day 4, all regions went on to form IH or to completely occlude by day 28. Of those regions with strain >0.1 at post-op day 4, only 31% (4 regions) went on to form any detectable IH by day 28.
Figure 5.
Differences in LCCA regions with low (<0.1) and high (>0.1) day 4 strain values. (A) Histogram analysis showing the distribution of strain values amongst regions with cut off value. (B) Histological outcome of LCCA regions with low and high strain. ‡ indicates P<.05 vs. low strain values. (C) Changes in LCCA area at diastole over time in low and high strain groups. (D) Changes in LCCA area at systole over time in low and high strain groups. (E) Linear fit between wall strain at day 4 and intimal thickness at day 28 * indicates P<.05 compared to pre-op values.
Cross sectional area at diastole remained unchanged in the strain >0.1 group from pre-op to day 4 (0.10±0.01 mm2 vs. 0.09±0.01; n=13; P=.93) but were significantly reduced compared to pre-op at post-op day 27 (0.06±0.01 mm2; n=13; P=.04). Cross sectional area at diastole in the strain ≤0.1 group were increased from pre-op to post-op day 4 (0.10±0.01 mm2 vs. 0.15±0.02 mm2) but returned to their pre-op levels at day 27 (0.09±0.01 mm2; n=13; P=.40 compared to pre-op; Figure 5B). Cross sectional area at systole, however, remained unchanged from pre-op to day 4 in both >0.1 (0.15±0.01 mm2 vs. 0.12±0.02 mm2; n=13; P=.13) and ≤0.1 (0.16±0.01 mm2 vs. 0.17±0.02 mm2; P=.89) groups and both decreased below pre-op baseline by day 27 (P=.005 and P=.01, respectively; Figure 5C). The strain >0.1 group had significantly smaller intimal thickness than the strain ≤0.1 group (8±4 μm vs. 32±7 μm; P=.006). Similarly, the strain >0.1 group had both a smaller wall thickness (82±7 μm vs. 111±6 μm; P=.009) and intima:media area ratio (0.19±0.10 vs. 0.81±0.19; P=.006; Figure 5D). The relationship between circumferential wall strain and intimal thickness at day 28 was further analyzed using linear regression. Figure 5E shows the linear fit along with 95% confidence limits.
Discussion
Vascular cells are equipped to sense and respond to the luminal hemodynamic environment. Several surface receptors (e.g. integrins, VEGFR), ion channels (e.g. K+ and Ca++), and the cell cytoskeleton may be specialized in mechano-sensing and mechano-transduction.7 Endothelial cells sense physiologic shear force, releasing mediators such as NO and KLF2 to maintain a quiescent state for smooth muscle cells and homeostasis of the whole vessel wall.28 Both clinical and experimental observations demonstrate that disturbed flow and/or low wall shear stress accelerate IH development.15, 16 Laminar, high blood flow (uniform high shear stress) generally exhibits an opposing effect on intimal growth29, 30 Although generally protective, shear may be deleterious when reaching an extremely high level or becoming disordered,31 and shear based explanations fail to account for many phenomenon associated with IH.
Compared to the well-established effect of shear stress, the impact of wall strain on the development of IH is much less defined. Evidence in vivo in vein grafts suggests that wall strain correlates positively with intimal thickening.32 In the in vitro setting, mechanical stretch activates several pathways capable of regulating smooth muscle cell phenotype, but biologic information in the arterial in vivo setting is limited. This dearth of mechanistic knowledge into links between strain and IH is largely the result of limited capabilities in the longitudinal measurement of strain in the intact animal. Herein, we find that vessels with early (four days after acute hemodynamic perturbation) low wall strain consistently develop IH 28 days after creation of an outflow focal stenosis. Furthermore, only 31% of arteries that demonstrated early high strain went on to develop a measurable amount of IH. Such an analytical approach stands as a reasonable strategy to understand this small dataset. However, via multiple linear regression (intimal thickness as a dependent variable, wall strain and location proximal to the focal stenosis as independent variables), IH can be modeled as a linear combination of wall strain and distance proximal to the stenosis. Wall strain appears to account for the ability to predict IH (P=.007), while location does not (P=.343). Again, application of multiple linear regression to these data is limited by the small sample size and goodness of fit was suboptimal (R2=.36).
Wall strains measured herein used cross sectional area at diastole as a baseline, meaning that strain from diastole to systole was measured. This baseline was chosen because the zero strain state (with zero luminal pressure) cannot be measured in the living mouse. In order to better understand how wall strains are reduced in the short-term, cross sectional area at diastole and systole was assessed. In the low strain group, a short-term reduction in lumen area at diastole was shown while no change in lumen area at systole was found. In the high strain group, no changes in lumen area at diastole or systole was observed. Our results demonstrate a decrease in LCCA vessel diameter in all mice by day 27 post-op, a finding that was previously reported at 2 weeks post-operatively in a similar flow model in rabbits.33 However, we reveal at 4 days that diameter at diastole increases significantly in animals that will eventually form IH, a phenomenon that has not been previously reported.
We speculate that the lower level of strain early is likely associated with altered vessel wall properties (biologic and mechanical). Though they have high heart rates, mice are known to have blood pressures similar to humans. Assuming a systolic pressure of 120 mmHg and a diastolic pressure of 80 mmHg, the 28 day wall modulus of elasticity (using a thin wall model based on the Law of Laplace and assuming linear vessel properties) yielded 152±27 kPa for the low strain group and 100±17 kPa in the high strain cohort. These computed elasticities fall in the same order of magnitude as those computed using more accurate (pressure based) measurements in previous studies.34 Although there was a trend toward higher modulus of elasticities in the low strain group, there is no statistical difference between the two groups (P=.10)
The results also support high frequency ultrasound as an emerging tool for quantifying arterial wall strains noninvasively in vivo. With recent advances in the spatial resolution of ultrasound systems and improved speckle tracking algorithms, it is now possible to consistently measure strains on the murine arterial wall with little inter or intra-operator variability.35 We have previously presented a method to measure cyclic wall strains on the murine aortic wall to assess the changes in wall strain during aneurysm formation.25 In this study, we translated our previously reported methods to assess strain in a murine model of IH.
Limitations are acknowledged. Wall shear descriptions in this model have been previously described.24 However, in neither that report nor the current were direct pressure measurements obtained due to technical limitations. Luminal flow and wall shear stresses were not computed in the present study. However, care was taken to create as technically identical as possible reductions in wall shear stress and thus shear stress reductions are assumed to be equivalent among animals. Also, certainly future studies need to verify these biomicroscopy based determinations by way of direct longitudinal interrogations of the mechanical qualities of the arterial wall tissues.
In conclusion, we describe a positive relationship between early reduction in cyclic arterial wall strain and the eventual formation of IH in a validated murine model. Use of high-frequency ultrasound and speckle tracking appears to provide a useful approach for measuring mechanical strains in this setting. The approaches described offer a potentially powerful tool to unravel the temporal relationships between hemodynamic perturbations, mechanical forces, and the subsequent arterial wall adaptations.
Clinical Significance.
Intimal hyperplasia limits the durability of vascular interventions such as angioplasties, bypasses, dialysis access grafts, and endarterectomies. Utilizing high frequency ultrasound to determine mechanical wall strain in mice at the very earliest stages of intimal hyperplasia in a pre-clinical model, we find that low strain precedes the development of intimal hyperplastic lesions. Additionally, we demonstrate that decreases in wall strain are due primarily to early increases in the diastolic area of the vessel lumen, while systolic areas remain unchanged. Manipulation of wall strain stands as a potential approach to limit the development of intimal hyperplasia.
Acknowledgements
The authors would like to thank Dr. Ronglih Liao and the Brigham and Women’s Hospital Cardiovascular Physiology Core for their technical assistance and support.
Sources of Funding
Supported by the National Heart, Lung, and Blood Institute (T32HL007734), the American Heart Association (12GRNT9510001, 12GRNT1207025, 12PRE9020036), the Lea Carpenter du Pont Vascular Surgery Fund, the National Natural Science Foundation of China (No: 30801123, 81201234) and the Reserve Talents of Universities Overseas Research Program of Heilongjiang
Footnotes
Publisher's Disclaimer: This is a PDF fil of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its fina citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
VevoVasc software (Version 3.6.2.0, FUJIFILM VisualSonics, Inc.) was provided by FUJIFILM VisualSonics, Inc.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Namini H, Seely L. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg. 2005;42(3):456–64. doi: 10.1016/j.jvs.2005.05.001. discussion 64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012;11(9):755–63. doi: 10.1016/S1474-4422(12)70159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morice MC, Serruys PW, Barragan P, Bode C, Van Es GA, Stoll HP, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50(14):1299–304. doi: 10.1016/j.jacc.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. JVascIntervRadiol. 2005;16(3):331–8. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 6.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190(3):300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J InternMed. 2006;259(4):381–92. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 8.Holmes DR, Jr., Savage M, LaBlanche JM, Grip L, Serruys PW, Fitzgerald P, et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2002;106(10):1243–50. doi: 10.1161/01.cir.0000028335.31300.da. [DOI] [PubMed] [Google Scholar]
- 9.Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, et al. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(4):665–71. doi: 10.1161/ATVBAHA.107.158030. [DOI] [PubMed] [Google Scholar]
- 10.Newby AC. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol. 2012;56(5-6):232–44. doi: 10.1016/j.vph.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Kuzuya M, Kanda S, Sasaki T, Tamaya-Mori N, Cheng XW, Itoh T, et al. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003;108(11):1375–81. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- 12.Ballyk PD, Walsh C, Butany J, Ojha M. Compliance mismatch may promote graft — artery intimal hyperplasia by altering suture-line stresses. Annals of Vascular Surgery. 1998;31:229–37. doi: 10.1016/s0197-3975(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 13.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 14.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91(9):845–51. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 15.Wentzel JJ, Krams R, Schuurbiers JC, Oomen JA, Kloet J, Der Giessen WJ, et al. Relationship between neointimal thickness and shear stress after Wallstent implantation in human coronary arteries. Circulation. 2001;103(13):1740–5. doi: 10.1161/01.cir.103.13.1740. [DOI] [PubMed] [Google Scholar]
- 16.Richter Y, Groothuis A, Seifert P, Edelman ER. Dynamic flow alterations dictate leukocyte adhesion and response to endovascular interventions. JClinInvest. 2004;113(11):1607–14. doi: 10.1172/JCI21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryer EJ, Hom RP, Sakakibara K, Nakayama KI, Nakayama K, Faries PL, et al. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(4):780–6. doi: 10.1161/01.ATV.0000209517.00220.cd. [DOI] [PubMed] [Google Scholar]
- 18.Lemson MS, Tordoir JH, Daemen MJ, Kitslaar PJ. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000;19(4):336–50. doi: 10.1053/ejvs.1999.1040. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekar B, Nelson JF, Colston JT, Freeman GL, Macdonald L, Radler M, et al. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. 2011 [Google Scholar]
- 20.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–93. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia - association with tangential stress. J Vasc Surg. 1987;5(1):126–36. [PubMed] [Google Scholar]
- 22.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20(1):99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Zhao JB, Wang GR, Gregersen H, Kassab GS. Remodeling of the zero-stress state of femoral arteries in response to flow overload Remodeling of the zero-stress state of femoral arteries in response to flow overload. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280:H1547–H59. doi: 10.1152/ajpheart.2001.280.4.H1547. [DOI] [PubMed] [Google Scholar]
- 24.Tao M, Mauro CR, Yu P, Favreau JT, Nguyen B, Gaudette GR, et al. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. Am J Pathol. 2013;182(1):277–87. doi: 10.1016/j.ajpath.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favreau JT, Nguyen BT, Gao I, Yu P, Tao M, Schneiderman J, et al. Murine ultrasound imaging for circumferential strain analyses in the angiotensin II abdominal aortic aneurysm model. J Vasc Surg. 2012;56(2):462–9. doi: 10.1016/j.jvs.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Public Health Service Policy on Humane Care and Use of Laboratory Animals. Office of Laboratory Animal Welfare, National Institutes of Health; Bethesda, MD: 2002. [Google Scholar]
- 27.Fung Y-C. Biomechanics: Mechanical Properties of Living Tissues. 1993. [Google Scholar]
- 28.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117(8):1082–9. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS, et al. A novel vein graft model: adaptation to differential flow environments. American journal of physiology Heart and circulatory physiology. 2004;286(1):H240–5. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- 30.Mattsson EJ, Kohler TR, Vergel SM, Clowes AW. Increased blood flow induces regression of intimal hyperplasia. ArteriosclerThrombVascBiol. 1997;17(10):2245–9. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- 31.Paszkowiak JJ, Dardik A. Arterial wall shear stress: observations from the bench to the bedside. VascEndovascularSurg. 2003;37(1):47–57. doi: 10.1177/153857440303700107. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz LB, O’Donohoe MK, Purut CM, Mikat EM, Hagen PO, McCann RL. Myointimal thickening in experimental vein grafts is dependent on wall tension. J Vasc Surg. 1992;15(1):176–86. doi: 10.1067/mva.1992.33805. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Makarova N, Tsukahara R. Lysophosphatidic acid-induced arterial wall remodeling: Requirement of PPARÎ3 but not LPA 1 or LPA 2 GPCR. Cellular signalling. 2009;21:1874–84. doi: 10.1016/j.cellsig.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological Reviews. 2011:327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg. 2006;44(2):372–6. doi: 10.1016/j.jvs.2006.04.047. [DOI] [PubMed] [Google Scholar]