Abstract
Titanium (IV) and vanadium (V) complexes are highly potent anticancer agents. A challenge in their synthesis refers to their hydrolytic instability; therefore their preparation should be conducted under an inert atmosphere. Evaluation of the anticancer activity of these complexes can be achieved by the MTT assay.
The MTT assay is a colorimetric viability assay based on enzymatic reduction of the MTT molecule to formazan when it is exposed to viable cells. The outcome of the reduction is a color change of the MTT molecule. Absorbance measurements relative to a control determine the percentage of remaining viable cancer cells following their treatment with varying concentrations of a tested compound, which is translated to the compound anticancer activity and its IC50 values. The MTT assay is widely common in cytotoxicity studies due to its accuracy, rapidity, and relative simplicity.
Herein we present a detailed protocol for the synthesis of air sensitive metal based drugs and cell viability measurements, including preparation of the cell plates, incubation of the compounds with the cells, viability measurements using the MTT assay, and determination of IC50 values.
Keywords: Medicine, Issue 81, Inorganic Chemicals, Therapeutics, Metals and Metallic Materials, anticancer drugs, cell viability, cisplatin, metal complex, cytotoxicity, HT-29, metal-based drugs, MTT assay, titanium (IV), vanadium (V)
Introduction
Chemotherapy is still one of the main courses of treatments employed in the clinic for various cancer diseases, and thus vast amount of research is conducted worldwide with the aim to develop new and improved anticancer drugs. Such studies mostly begin at the chemical level, with the design and preparation of compounds, followed by biological evaluation of the cytotoxic properties in vitro. Cell viability may be assessed by various assays that provide information on cellular activity1-2.
Cisplatin is an example of a platinum complex that is widely used as a chemotherapeutic drug, which is considered an efficient treatment mainly for testicular and ovarian cancers3-4. However, its narrow activity range and severe side effects trigger studies of other potent transition metal complexes5-8. Among others, titanium (IV) and vanadium (V) complexes showed promising results of high activity and reduced toxicity9-16. Ti (IV) complexes were the first to enter clinical trials after cisplatin due to these properties; however, they have failed the trials due to formulation difficulties and hydrolytic instability. There is thus a current need to develop improved derivatives of these metal complexes that may combine high anticancer activity with water resistance15,17-21.
A challenge in the preparation of Ti (IV) and V (V) complexes refers to the hydrolytic instability of the precursor reagents; therefore, inert atmosphere should be maintained. The preparation of Ti (IV) and V (V) compounds is conducted under N2 or Ar conditions in a glove box or using Schlenk line techniques.
One common method for evaluation of the anti-cancer activity is based on the MTT (3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide) assay. This assay is a colorimetric viability assay that was presented in 1983 by Mosmann22. It is extremely well studied and characterized, and it is considered highly efficient when evaluating the effectiveness of new cytotoxic compounds due to its precision, rapidity, and its capability to be applied on variety of cell lines. This viability assay is based on the color change of the MTT molecule when it is exposed to viable cells. Measurement of the absorbance, which is proportional to the number of viable cells, and comparison to untreated controls, enables assessment of the cell growth inhibition capabilities of the compound tested.
The MTT colorimetric assay is conducted in a 96-well plate format23. The cells may require preincubation in the wells before the addition of the tested drug. The preincubation times may vary from 0-24 hr according to the cell line properties. Cells are usually exposed to the drug for 24-96 hr depending on the drug activity. MTT solution is then added to the treated cells, where the yellow MTT is reduced to purple formazan by a variety of mitochondrial and cytosolic enzymes that are operational in viable cells (Figure 1)24. The MTT molecule is not reduced by dead cells or red blood cells (metabolically inactive cells), spleen cells (resting cells) and concanavalin A-stimulated lymphocytes (activated cells)22. After 3-4 hr of incubation with MTT the formazan precipitates. The formation of the formazan begins after 0.5 hr of incubation but for optimal results it is best to expose the cells to MTT for at least 3 hr22. Consequently, the growing medium is removed and the formazan is dissolved in an organic solvent, preferably isopropanol25, although DMSO can also be used26. The elimination of the medium is crucial for achieving accurate results since phenol red, which is widely common in growth medium, and precipitating proteins can interfere with the absorbance measurement25. When the formazan solution reaches homogeneous, the absorbance of the solution is measured using a microplate reader spectrophotometer. The absorbance at 550 nm is directly proportional to the number of cells in range of 200-50,000 cells per well, and thus very small amounts of cells can be detected22. The absorbance indicates the amount of viable cells that remained after treatment with the drug, and is compared to the absorbance of control cells that were not exposed to the drug. Analysis of the results by proper software provides the IC50 (inhibition concentration; 50%) values and their statistical errors based on several repetitions of the measurement.
The MTT assay is widely common in cytotoxicity studies for screening new anticancer compounds, due to its accuracy and relative simplicity. However, when using the MTT assay, which is depended on enzymatic reaction, one must consider that various enzyme inhibitors can affect the reduction of MTT and lead to false results27. In addition, the MTT assay does not provide any information on the molecular mechanism of the cytotoxic activity of the drug2.
Protocol
- Preparation of the V (V) complex (Figure 2);18 conduct steps 1.1-1.4 in a glove-box; if a glove-box is not available, skip to the alternative steps 2 (2.1-2.6).
- Dissolve 0.42 mmol of the ligands H2L in dry THF and add it to a stirring solution of equivalent amounts of VO(OiPr)3 in THF.
- Stir the reaction mixture at room temperature for 15 min, and remove the volatiles under vacuum.
- Add cold hexane and remove it under vacuum. A dark purple powder should be obtained in a quantitative yield.
- Weigh 8 mg of the obtained compound in an Eppendorf vial. Skip to step 3.
- Prepare the V (V) complex using a Schlenk line.
- Dissolve 0.42 mmol of the ligand H2L in dry THF in a dry Schlenk flask with a septum cap, attach to a Schlenk line and apply alternating vacuum/inert gas (N2 or Ar) environments; it is recommended to apply three such rounds and wait 2-5 min on the vacuum stages.
- Add dry THF via syringe through the septum cap.
- Add equivalent amounts of VO(OiPr)3 in a THF solution, which had been prepared similarly by hooking a proper dry Schlenk flask to the line, applying an inert environment and adding the reagents through a syringe from the vanadium solution to that of the ligand.
- Stir the reaction mixture at room temperature for 15 min and remove the volatiles under vacuum; if there is a concern of unstable products, perform Schlenk type distillation of the volatiles under inert atmosphere; use inert gas flow to attach the required glassware that had been predried.
- Add cold hexane and remove it under vacuum. A dark purple powder should be obtained in a quantitative yield.
- Weigh 8 mg of the obtained compound in an Eppendorf vial.
- Preparation of the Ti (IV) complex (Figure 2);28 conduct steps 3.1-3.3 in a glove-box; if a glove-box is not available, adjust the alternative steps discussed for the vanadium analogue (step 2) that employ a Schlenk line instead.
- Dissolve 0.35 mmol of the ligand H2L in dry THF and add it to a stirring solution of equivalent amounts of Ti(OiPr)4 in THF.
- Stir the reaction mixture at room temperature for 2 hr. Remove the volatiles under vacuum to give a yellow product in a quantitative yield.
- Weigh 8 mg of the obtained compound in Eppendorf vial.
- Preparation of HT-29 cells 96-well plate
- Culture HT-29 cells in a 75 cm2 flask with RPMI-1640 medium that contains 1% penicillin/streptomycin antibiotics, 1% L-glutamine, and 10% fetal bovine serum (FBS), at 37 °C with 5% CO2.
- Remove the medium when the HT-29 cells reach maximal confluency (90-100%, every 3-4 days without subculture). Wash with 1 ml of trypsin (0.25%)/EDTA (0.05%) solution and remove it.
- Add 1 ml of trypsin (0.25%)/EDTA (0.05%) solution and incubate for 5 min.
- Detach the HT-29 cells from the flask and add 10 ml of the medium to disable the trypsin activity. Transfer 5 ml of the cells mixture to a tube.
- Use pipette to transfer a few drops of the cells mixture into counter chamber. The counter chamber includes 5 x 5 squares.
- Count the cells in 5 representative squares using a microscope: the 4 squares in the corners and the one that is in the middle.
- Calculate the estimated amount of cells present. Sum the cells in the 5 representative squares and divide by 20.
- Divide 0.6 by the result of step 4.7. The received number is the amount of cell mixture required per plate. This calculation refers to a plate that contains 0.6 x 106 cells (9,000 cells per well).
- Use pipette to transfer the amount of cell mixture required per plate (as calculated in section 4.8) and add 13.2 ml of the medium (200 μl per well × 66 wells).
- Add the mixture to 96-well plate by 11-channel pipette (200 μl per well, 6 lines, 66 wells in total). One well of each line should remain empty for blank control.
- Incubate the plate at 37 °C with 5% CO2 for 24 hr to allow the cells to attach to the plate.
- Insertion of the compounds
- Add 200 μl (or different amount according to the desired concentration range) of dry THF to 8 mg of each compound weighed as described in step 1.4 (or 2.6) or 3.3. Weigh 8 mg of the ligand H2L, too.
- Dilute each compound solution further by adding 60 μl of THF in 9 different Eppendorf vials.
- Transfer with a pipette 60 μl from the mixture in section 5.1 to the first Eppendorf vial, resuspend and transfer 60 μl to the next vial, and so on until 10 different concentrations are obtained (including the parent mixture in section 5.1).
- Dilute 20 μl of each concentration in THF with 180 μl of medium.
- Prepare a control solution with THF only; 20 μl of THF with 180 μl of medium in each measurement.
- Add 10 μl from the resulting solution (including the THF control), to each well that already contains 200 μl of the aforementioned solution of cells in the medium to give final concentrations of the compound of up to 200 mg/L (the concentration range can be changed according to the compound activity).
- You may observe some precipitation at the highest concentration applied depending on the compound solubility.
- Repeat each measurement of the compound 3x (3 lines in the plate of the same compound); each line contains: cells treated with the compound in 10 different concentrations, 1 well containing cells treated with solvent only for control and 1 well without cells for blank control.
- Incubate the loaded plate for 3 days at 37 °C in 5% CO2 atmosphere.
- Cytotoxicity measurement using the MTT assay
- Prepare the MTT solution by adding 1 g of MTT powder to a 200 ml solution of RPMI-1640 medium without phenol red. The stock solution can be divided to 15 ml tubes and stored in -20 °C.
- Add 20 μl of MTT solution to each well using 11-channel pipette (except to the blank control wells without the cells from step 4.10), and incubate the plate for additional 3 hr.
- Remove the medium solution from each well.
- Add 200 μl of isopropanol to each well to dissolve formazan. Stir the plate for 0.5 hr until homogeneousness is reached.
- Measure the absorbance at 550 nm for 200 μl of the aforementioned solution by a microplate reader spectrophotometer.
- Repeat each measurement (that includes 3 repetitions, see step 5.8) on 3 different days.
- Calculate the percentage of cell viability by deducting the blank control absorbance value from the measured value, dividing the result by the absorbance of the THF solvent control value and multiplying by 100%.
- Calculate IC50 values using GraphPad Prism software (or equivalent).
- The reported IC50 is the average of all IC50 values collected on at least three different days, and the error value is the standard deviation.
Representative Results
The complexes were prepared based on established procedures18,28 and their purity may be evaluated by NMR and elemental analysis.
The data received from the MTT assay is analyzed to evaluate the cytotoxicity of the compound18,21. First, subtraction of the blank control absorbance value from all the other values is performed. Second, the THF solvent control value is set to 100% viability, as no growth inhibiting compound was added. All other values are transformed into percentage of viability according to the control. The concentrations applied and the relative viability percentage calculated are then inserted into the GraphPad Prism software (or equivalent) to determine the IC50 values based on a nonlinear regression of a variable slope (four parameters) model (Figure 4, Table 1)29.
The measurements should be performed on a range of concentrations that will produce a graph displaying an even point distribution: at least three points on each plateau and three points to define the slope (Figure 3, graph A). Insufficient measurements at high concentrations or low concentrations (Figure 3, graph B) will impair the nonlinear regression fit of the curve required for determination of IC50 values, and as a result, diminish their accuracy.
Relative IC50 values, as determined by the nonlinear regression fit29, correspond to the concentrations leading to 50% of the cell growth inhibition possible by the compound tested, which is calculated as the middle point between the maximal and minimal cell viability measured (not necessarily 100% and 0%, respectively, see Figure 3, graph C, a). For instance, if a compound inhibits only 60% of cell growth relative to the control (and 0% at low concentrations), its relative IC50 is the concentration leading to 30% inhibition relative to the control. The error values should reflect the deviation of the results obtained for different repetitions from the average IC50 value reported, taken as the STDs. In addition, maximal cell growth inhibition (%) values should be reported, as calculated by subtracting from 100% the minimal viability measured.
An alternative analysis may include determination of absolute IC50 values. These correspond to the concentrations leading to 50% cell growth inhibition relative to the 100% control, regardless of the maximal and minimal cell viability measured. Such values can also be derived by a non-linear regression model. As these values are absolute, reporting the maximal cell growth inhibition values is optional. As depicted in Figure 3, graph C, a compound may have a relatively low activity in terms of maximal cell growth inhibition properties (curve a), and still exhibit a relative IC50 value that is lower not only than its absolute one, but also than that of a compound that reaches close to 100% growth inhibition (curve b). It is thus extremely valuable to present the curve along with the reported IC50 values of any kind.
When inspecting the results of the experiments described in the protocol (Figure 4, Table 1), it is clear that cisplatin and complexes LTi(OiPr)2 and VO(OiPr) are highly active. It is also noticeable that the closer the maximal inhibition value of a given compound is to 100%, the closer the relative IC50 value is to the absolute one. When comparing the IC50 values among the different compounds, the Ti (IV) complex has the highest cytotoxicity and its IC50 values are lower than those of cisplatin and the V (V) complex. However, the free ligand exhibits markedly reduced activity, since following addition of the ligand at different concentrations, the cell viability does not drop as significantly as it does for its complexes. This assures that the high anticancer activity of the complexes relies on the metal centers. Since the free ligand barely reaches 50% cell growth inhibition, an absolute IC50 value cannot be accurately derived. In fact, a relative value also could not have been adequately calculated by the software due to the minor activity and corresponding high error values. Nevertheless, it is clear that even if a relative IC50 value could have been derived for H2L, it would not have reflected an appreciable activity, thus rendering the report of the maximal inhibition value along with relative IC50 values (and the depiction of the curve) essential.
| Relative IC50 (μM) | Absolute IC50 (μM) | Maximal inhibition (%) | |
| LVO(OiPr) | 13±3 | 19±8 | 81% |
| LTi(OiPr)2 | 3±1 | 5±3 | 87% |
| H2L | - | - | 47% |
| Cisplatin | 14±5 | 13±6 | 91% |
Table 1. Cytotoxicity results for the compounds tested: Relative and absolute IC50 (μM) and maximum cell growth inhibition values toward HT-29 cells following an incubation period of three days with the V (V) complex LVO(OiPr), Ti (IV) complex LTi(OiPr)2, the free ligand H2L and cisplatin;18,21 IC50 values are given as average of at least three times three repetitions with error values as the STDs.
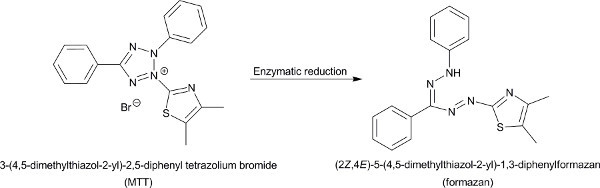 Figure 1. The reduction of MTT by mitochondrial and cytosolic enzymes to formazan. Click here to view larger figure.
Figure 1. The reduction of MTT by mitochondrial and cytosolic enzymes to formazan. Click here to view larger figure.
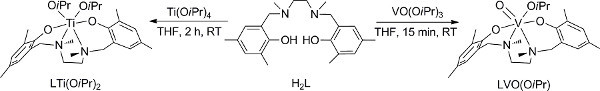 Figure 2. Preparation of V (V) and Ti (IV) complexes18,28. Click here to view larger figure.
Figure 2. Preparation of V (V) and Ti (IV) complexes18,28. Click here to view larger figure.
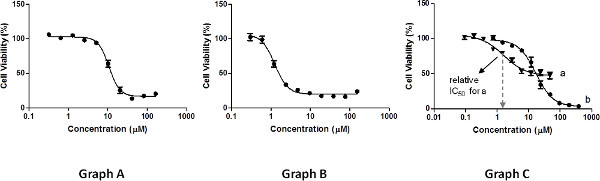 Figure 3. Examples of results of cell viability measurements. Click here to view larger figure.
Figure 3. Examples of results of cell viability measurements. Click here to view larger figure.
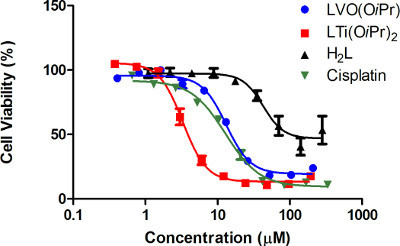 Figure 4. Dose dependent cytotoxicity curves for the compounds tested: Dependence of HT-29 cell viability on administered concentration of the V (V) complex LVO(OiPr) (blue), the Ti (IV) complex LTi(OiPr)2 (red), free ligand H2L (black) and cisplatin (green), following an incubation period of three days, as obtained from at least three times three repetitions as reflected by the error values18,21.
Figure 4. Dose dependent cytotoxicity curves for the compounds tested: Dependence of HT-29 cell viability on administered concentration of the V (V) complex LVO(OiPr) (blue), the Ti (IV) complex LTi(OiPr)2 (red), free ligand H2L (black) and cisplatin (green), following an incubation period of three days, as obtained from at least three times three repetitions as reflected by the error values18,21.
Discussion
The method described in this manuscript combines chemical synthesis with biological cell viability assay. As with any biological methods concerning viable cells, it is vital to work in a laminar hood, and maintain sterile conditions, including the use of sterile organic solvents. Also, preparation and storage of hydrolytically unstable metal based drugs should be carried out under inert conditions, for which glove box or Schlenk line techniques should be utilized.
The selection of the technique to work under inert environment is mostly dependent on the availability of a glove-box. If one is available, all syntheses that require room temperature conditions are extremely convenient to perform therein. Syntheses that require heating are normally taken out of the box in a Schlenk flask and are attached to the Schlenk line. Where a glove-box is not available, all manipulations can be carried out with carefully selected glassware on a Schlenk line, while using the vacuum/inert gas (N2 or Ar) manipulations to maintain dry atmosphere.
In the cytotoxicity evaluation process, it is necessary to examine the appearance of the cells throughout the experiment. Before the beginning of the experiment, the growth medium of healthy adherent cells should be clear. A contaminated culture will appear turbid. Also, after the incubation of the cells with trypsin-EDTA, the cells should detach from the flask and immerse into the medium. If most of the cells remain adhered to the flask, the flask should be further incubated for several minutes.
The length of one such measurement is five days. We allow one day for the adherent cells to attach and acclimate to the cell plate, and three days for the incubation of the cells with the tested drugs in order to enable the cells to proceed to apoptosis. An additional day is required for incubation with MTT. This entire measurement should be repeated at least two more times on different days starting from different cell cultures, in order for the measurements to be independent.
The technique as described herein is applied on adherent cell types. In order to apply this method on nonadherent cell types several adjustments should be made. It is no longer required to dedicate one day for the cells to attach and acclimate to the cell plate, and therefore the preparation of the plate and the supplement of the tested drugs can be accomplished on the same day. In addition, in the final step of the measurement, just after the incubation of the cells with MTT, the solution should be centrifuged before the removal of medium and addition of isopropanol.
It is also possible to replace the utilization of MTT as the marker of vital cells with an alternative assay for cell growth inhibition, in which the incubation time of the cells with the marker is shorter than that with MTT30. Another factor to consider when choosing the marker is the possible mechanism of action of the tested drug27. The MTT assay provides information on mitochondria metabolic activity, and since it is dependent on enzymatic reactions, it can be influenced by enzyme inhibitors.
The combination of drug preparation with the evaluation of its cytotoxicity through the MTT assay represents an effective method for the development of new metal-based cytotoxic drugs. Nevertheless, this method does not provide any information regarding the cell death mechanism, the phase in the cell cycle that is affected by the drug and its possible biological targets. For these purposes additional methods, such as Propidium Iodide (PI) Uptake Assay and Cell Cycle Analysis should be performed31.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
Funding was received from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013) / ERC Grant agreement no [239603]
References
- Niles AL, Moravec RA, Riss TL. Update on in vitro cytotoxicity assays for drug development. Expert Opinion on Drug Discovery. 2008;3:655–669. doi: 10.1517/17460441.3.6.655. [DOI] [PubMed] [Google Scholar]
- Hatok J, et al. In vitro assays for the evaluation of drug resistance in tumor cells. Clinical and Experimental Medicine. 2009;9:1–7. doi: 10.1007/s10238-008-0011-3. [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature Reviews Drug Discovery. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Muggia F. Platinum compounds 30 years after the introduction of cisplatin: Implications for the treatment of ovarian cancer. Gynecologic Oncology. 2009;112:275–281. doi: 10.1016/j.ygyno.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Bruijnincx PC, Sadler PJ. New trends for metal complexes with anticancer activity. Current Opinion in Chemical Biology. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Archiv der Pharmazie (Weinheim. 2007;340:117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- Desoize B. Metals and metal compounds in cancer treatment. Anticancer Research. 2004;24:1529–1544. [PubMed] [Google Scholar]
- Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Antitumour metal compounds: more than theme and variations. Dalton Transactions. 2008. pp. 183–194. [DOI] [PubMed]
- Meléndez E. Titanium complexes in cancer treatment. Critical Reviews in Oncology/Hematology. 2002;42:309–315. doi: 10.1016/s1040-8428(01)00224-4. [DOI] [PubMed] [Google Scholar]
- Evangelou AM. Vanadium in cancer treatment. Critical Reviews in Oncology/Hematology. 2002;42:249–265. doi: 10.1016/s1040-8428(01)00221-9. [DOI] [PubMed] [Google Scholar]
- Djordjevic C. Antitumor activity of vanadium compounds. Metal ions in biological systems. 1995;31:595–616. [PubMed] [Google Scholar]
- Caruso F, Rossi M, Pettinari C. Anticancer titanium agents. Expert Opinion on Therapeutic Patents. 2001;11:969–979. [Google Scholar]
- Caruso F, Rossi M. Antitumor titanium compounds. Mini-Review in Medicinal Chemistry. 2004;4:49–60. doi: 10.2174/1389557043487565. [DOI] [PubMed] [Google Scholar]
- Kostova I. Titanium and vanadium complexes as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry. 2009;9:827–842. doi: 10.2174/187152009789124646. [DOI] [PubMed] [Google Scholar]
- Tshuva EY, Ashenhurst JA. Cytotoxic Titanium(IV) Complexes: Renaissance. European Journal of Inorganic Chemistry. 2009. pp. 2203–2218.
- Buettner KM, Valentine AM. Bioinorganic Chemistry of Titanium. Chemical Reviews. 2012;112:1863–1881. doi: 10.1021/cr1002886. [DOI] [PubMed] [Google Scholar]
- Meker S, Margulis-Goshen K, Weiss E, Magdassi S, Tshuva EY. High antitumor activity of highly resistant salan-titanium(IV) complexes in nanoparticles: an identified active species. Angewandte Chemie International Edition in English. 2012;51:10515–10517. doi: 10.1002/anie.201205973. [DOI] [PubMed] [Google Scholar]
- Reytman L, Braitbard O, Tshuva EY. Highly cytotoxic vanadium(V) complexes of salan ligands; insights on the role of hydrolysis. Dalton Transactions. 2012;41:5241–5247. doi: 10.1039/c2dt11514j. [DOI] [PubMed] [Google Scholar]
- Tzubery A, Tshuva EY. Cytotoxicity and Hydrolysis of trans-Ti(IV) Complexes of Salen Ligands: Structure-Activity Relationship Studies. Inorganic Chemistry. 2012;51:1796–1804. doi: 10.1021/ic202092u. [DOI] [PubMed] [Google Scholar]
- Tshuva EY, Peri D. Modern cytotoxic titanium(IV) complexes; Insights on the enigmatic involvement of hydrolysis. Coordination Chemistry Reviews. 2009;253:2098–2115. [Google Scholar]
- Shavit M, Peri D, Manna CM, Alexander JS, Tshuva EY. Active cytotoxic reagents based on non-metallocene non-diketonato well-defined C-2-symmetrical titanium complexes of tetradentate bis(phenolato) ligands. Journal of the American Chemical Society. 2007;129:12098–12099. doi: 10.1021/ja0753086. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival - Application to Proliferation and Cyto-Toxicity Assays. Journal of Immunological Methods. 1983;65(83):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ahmadian S, Barar J, Saei AA, Fakhree MAA, Omidi Y. Cellular Toxicity of Nanogenomedicine in MCF-7 Cell Line: MTT assay. Journal of Visualized Experiments. 2009. p. e1191. [DOI] [PMC free article] [PubMed]
- Gonzalez RJ, Tarloff JB. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicology in Vitro. 2001;15:257–259. doi: 10.1016/s0887-2333(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid Colorimetric Assay for Cell-Growth and Survival - Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. Journal of Immunological Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Alley MC, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Research. 1988;48:589–601. [PubMed] [Google Scholar]
- Weyermann J, Lochmann D, Zimmer A. A practical note on the use of cytotoxicity assays. International Journal of Pharmaceutics. 2005;288:369–376. doi: 10.1016/j.ijpharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Chmura AJ, et al. Group 4 complexes with aminebisphenolate ligands and their application for the ring opening polymerization of cyclic esters. Macromolecules. 2006;39:7250–7257. [Google Scholar]
- Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharmaceutical Statistics. 2011;10:128–134. doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- Yung WKA. In vitro Chemosensitivity Testing and its Clinical-Application in Human Gliomas. Neurosurgical Review. 1989;12:197–203. doi: 10.1007/BF01743984. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Evan GI. Current Topics in Developmental Biology. 1997;36:259–278. doi: 10.1016/s0070-2153(08)60507-4. [DOI] [PubMed] [Google Scholar]


