Abstract
A review of the existing functional magnetic resonance imaging (fMRI) studies on reward anticipation in patients with attention-deficit/hyperactivity disorder (ADHD) is provided. Meta-analysis showed a significant medium effect size (Cohen’s d = 0.48–0.58) in terms of ventral–striatal (VS)-hyporesponsiveness in ADHD.
Studies on VS-responsiveness and trait impulsivity in the healthy population demonstrate the opposite relationship, i.e. impulsivity-scores positively correlated with VS activation during reward processing.
Against the background that ADHD may represent an extreme on a continuum of normal variability, the question arises as to how these contrasting findings can be integrated. We discuss three theoretical approaches, each of which integrates the opposing findings: (1) an inverted-u-shape model; (2) a (genetic) moderator model; and (3) the “unrelated model”. We conclude that at the present stage the number of existing studies in the healthy population as well as in ADHD groups is too small for a final answer. Therefore, our presented integrative approaches should be understood as an attempt to frame future research directions by generating testable hypotheses and giving practical suggestions for future studies.
Keywords: ADHD, Impulsivity, Ventral–striatal hypoactivation, Hyporesponsiveness, Ventral striatum, Reward
1. Background
Impulsivity is a multi-dimensional construct that includes cognitive, affective and motor facets (Dawe and Loxton, 2004; Moeller et al., 2001; Stanford et al., 2009; Whelan et al., 2012). It is highly relevant in both the healthy population (e.g. predicting academic achievement) and in the clinical context, because it represents a core symptom of many mental disorders, including attention-deficit/hyperactivity disorder (ADHD), Antisocial and Borderline Personality Disorders, suicidal and aggressive behaviours, pathological gambling and diverse forms of addiction (American Psychiatric Association, 2000).
Impulsivity has been assessed by specifically developed self-report questionnaires such as the Barrett Impulsiveness Scale (BIS; Patton et al., 1995) or the Eysenck Impulsivity Scale (I7; Eysenck et al., 1985). Additionally, impulsivity has been assessed with behavioural measures which can be broadly divided into (a) reward-choice tasks (Green and Myerson, 2004; Mischel, 1974) and (b) rapid-response tasks that assess the ability to inhibit a prepotent motor response: go/no-go tasks (Conners et al., 2003; Epstein et al., 2003) and stop tasks (Logan, 1994). Of note, alternative yet related constructs exist, including Sensation Seeking (Zuckerman, 1983) and the behavioural inhibition–behavioural activation scale (BIS/BAS; Carver and White, 1994). Both have been found to be positively correlated with impulsivity. However, the correlations are often only small to moderate, putatively a consequence of the multi-dimensional nature of the trait and the different focuses of the various impulsivity measures (Stanford et al., 2009).
Despite this heterogeneity, a recent review by Kennis et al. (2013) demonstrated that functional magnetic resonance imaging (fMRI) studies using reward processing tasks consistently show a positive relationship between impulsivity-related measures and brain activity within the ventral-striatum (VS) measured with blood oxygenation level dependent (BOLD) fMRI, which is one core structure of the neural reward circuit (Haber and Knutson, 2010). This circuit includes the VS with the nucleus accumbens (NAcc) as a pivotal structure that is innervated by mesostriatal dopamine and linked to anterior cingulate cortex (ACC), supplementary motor area (SMA), insula and thalamus (Kirsch et al., 2003; Knutson et al., 2001a,b; Knutson and Cooper, 2005; Liu et al., 2011). Anticipatory VS/NAcc activation has been shown to be a reliable fMRI assay within and across subjects (Plichta et al., 2012), sensitive to genetic variants (Kirsch et al., 2006; Forbes et al., 2007; Hahn et al., 2011) and linked to impulsivity in clinical and non-clinical populations (Kirsch et al., 2006; Scheres et al., 2007; Hahn et al., 2009; Plichta et al., 2009; Forbes et al., 2009; Juckel et al., 2012). Studies that investigated impulsivity and neural activation during reward processing in healthy participants are listed in Table 1. We expanded the list of references that were found in Kennis et al. (2013) by three additional clinical studies that included a healthy control group. Out of eight studies that focused on VS BOLD response during reward anticipation (with a total N of ~200), six found significantly positive correlations with impulsivity-related assays and there is only one non-replication, which actually reported the opposite finding (Yau et al., 2012). Of note, this latter study is different from the others because it correlated impulsivity scores assessed in childhood with brain activation assessed during adulthood. Against the background that neural reward-processing changes across development (Galvan, 2010; Geier and Luna, 2009; Somerville and Casey, 2010), additional longitudinal studies are needed.
Table 1.
FMRI studies focusing on reward processing and impulsivity-related traits in healthy subjects.
| Study | Sample characteristics |
N | Sex | Impulsivity assaya | Paradigm | Finding in VS | Reward phase |
|---|---|---|---|---|---|---|---|
| Beaver et al. (2006) | Adults (22.0 ± 8.1) | 14 | M/F | BAS | Food rewards | Positively correlated | Anticipationb |
| Hahn et al. (2009) | Adults (29.4 ± 7.6) | 24 | M/F | SPSRQ | MID | Positively correlated | Anticipation |
| Simon et al. (2010) | Adults (24.8 ± 3.2) | 24 | M/F | BAS | MID | Positively correlated | Receiptc |
| Cohen et al. (2005) | Not reported | 17 | Not reported | Extraversion | Gambling task | Positively correlated | Receiptc |
| Abler et al. (2006) | Adults (22–36) | 11 | M | SSS/NS | MID | Positively correlated | Anticipation |
| Forbes et al. (2009) | Adults (43.8 ± 6.5) | 89 | M/F | BIS | Card guessing | Positively correlated | Receiptd |
| Hariri et al. (2006) | Adults (43.5 + 6.5) | 45 | DD | Card guessing | Positively correlated | Receiptd | |
| Galvan et al. (2007) | Adults (25.3); adolescents (16.0); children (9.8) | 12/12/13 | M/F | BRPM | MID | Positively correlated | Anticipation |
| Cservenka et al. (2013) | Adolescents (age not reported) | 94 | M/F | SSS | WOF | No correlation | Anticipation |
| Bjork et al. (2008) | Adolescentse (13.8 ± 0.4) | 13 | M/F | SSS | MID | Positively correlated | Anticipation |
| Hoogman et al. (2011) | Adults (38.0 ± 11.2)e | 49 | M/F | DD | MID | Positively correlated | Anticipation |
| Yau et al. (2012) | Adults (20.1 ± 1.3)e | 20 | M/F | YSR-E | MID | Negatively correlated | Anticipation |
BAS: behavioural apporach system; SPSRQ: Sensitivity to Punishment and Sensitivity to Reward Questionnaire; SSS/NS: Sensation Seeking Scale/Novelty Seeking; BIS: Barratt Impulsiveness Scale; DD: delay discounting; BRPM: Benthin Risk Perception Measure; YSR-E: Youth Self-Report (externalizing behaviour); WOF: wheel of fortune task.
Passive viewing of food cues.
No correlation found during anticipation.
Paradigm does not include an anticipation phase.
Representing a healthy control group in a study with a clinical group.
Reflecting the multidimensional character of impulsivity (Dawe and Loxton, 2004; Moeller et al., 2001), the studies listed in Table 1 used a wide range of personality trait markers that are all positively correlated (Meda et al., 2009) and have been found to be related to ADHD (Downey et al., 1997; Luman et al., 2012; Martel et al., 2008; Zuckerman, 1983). Taken together, the existing fMRI studies in the healthy population as listed in Table 1 suggest that there is a positive relationship between impulsivity-related traits and VS-response during reward processing including anticipation of reward.
2. BOLD fMRI studies on reward processing in ADHD
Current models of ADHD (Sagvolden et al., 2005; Sonuga-Barke, 2011; Tripp and Wickens, 2008) suggest abnormal reward sensitivity to be an important etiological factor. The Dynamic Developmental Theory (Sagvolden et al., 2005) proposes that low levels of tonic dopamine lead to steeper and shorter delay-of-reinforcement gradients in individuals with ADHD. Consequently, a reinforcer looses its value relatively quickly and as a result, those with ADHD should demonstrate relatively strong preferences for immediate rewards. The Dopamine Transfer Deficit Theory (Tripp and Wickens, 2008, 2009) hypothesizes that altered activity of the phasic DA response to reinforcement characterizes those with ADHD. Specifically, it hypothesizes that anticipatory firing of dopamine cells to cues that predict reinforcement is disturbed in ADHD, leading to problems with the anticipation of rewards. Finally, the Delay Aversion Theory (Sonuga Barke et al., 1992a,b, 1996) proposes that individuals with ADHD are characterized by a unique motivational style. Specifically, those with ADHD are thought to be motivated to escape or avoid waiting times. This would lead to a relatively strong preference for an immediate outcome when a choice is offered, and to the allocation of attention to non-temporal stimuli when there is no choice and the delay is inescapable. It is assumed that this motivational style is, in part, the natural result of negative emotions experienced during waiting. These models agree on a hypofunctioning dopamine system that may result in unique reward processing in ADHD and lead to increased levels of impulsivity. In sum, the predictions of these models include that individuals with ADHD show (1) relatively strong preferences for smaller-but-sooner over larger-but-later rewards and (2) reduced striatal dopamine release during reward anticipation.
Scheres et al. (2007) published the first BOLD fMRI study investigating the neural reward systems’ response of adolescents with ADHD. They administered the monetary incentive delay (MID) task (Knutson et al., 2000, 2001b), a widely used fMRI task that has high test–retest reliability (Plichta et al., 2012). This task includes three distinctive phases: (1) anticipation period (i.e. a delay); (2) target; and (3) feedback. The anticipation phase starts with the presentation of a cue (an abstract symbol) signalling the possibility of winning (or avoiding to lose) money followed by a variable delay of some seconds. At the end of this variable delay, participants see a brief flashing target stimulus. In order to win (or avoid loosing) money, the participant has to respond quickly enough with a simple button press when the target stimulus is presented. The response-time window is adaptive and has a range of ~160–260 ms. After the participant’s response, feedback about the actual performance is presented. The task is designed such that participants win (or avoid loosing) on about 67% of trials by dynamically modifying the length of the response-time window: When the subject was too slow, the RT window is enlarged by some milliseconds in the succeeding trial. When the subject was fast enough the RT window will be shortened. The earned money is typically paid to the participant at some time after the scanning procedure in cash (Scheres et al., 2007) or via bank-transfer (Hoogman et al., 2011).
The MID task allows the investigation of two important facets of neural reward processing: (1) reward anticipation, i.e. the anticipation of a potential win (or avoiding a potential loss) which occurs between cue and target presentation and (2) feedback processing during the presentation of the performance feedback (including the monetary outcome). The major finding of Scheres et al. (2007) was that adolescents with ADHD showed a relative VS-hypoactivation during reward anticipation when compared to matched typically developing controls. Notably, the symptom domain of hyperactivity–impulsivity, but not inattention, was negatively correlated with VS-response, suggesting that the difference in VS activation between ADHD and control participants was mainly due to the symptoms of hyperactivity–impulsivity. Additionally, no significant difference in neural activation occurred during feedback presentation. Together with reward related behavioural differences in ADHD which are reviewed elsewhere (Luman et al., 2005) the finding of a relative VS-hypoactivation fits well into the predictions of major motivational ADHD theories (Sagvolden et al., 2005; Sonuga-Barke et al., 2010; Tripp and Wickens, 2008).
Strohle et al. (2008) investigated adults with ADHD and also administered the MID task. They replicated VS-hyporesponsiveness in adult patients with ADHD during reward anticipation. A year later, Plichta et al. (2009) applied a reward delay discounting (DD) task (McClure et al., 2004) that includes the presentation of monetary rewards which are available to the participant either immediately (i.e. right after the fMRI scan) or after a certain delay of time (e.g. 2 weeks). Again, VS-hyporesponsiveness was evident during the presentation of the monetary reward options. Furthermore, patients with ADHD showed relative hyperactivation during delayed reward processing in dorsal parts of the striatum and the amygdalae, underpinning the predictions of the delay aversion hypothesis (Sonuga Barke, 2002, 2003, 2005; Sonuga Barke et al., 1992a,b, 1996).
Subsequently two further fMRI studies were published that both replicated VS-hyporesponsiveness during reward anticipation in adults with ADHD (Carmona et al., 2012; Hoogman et al., 2011). These studies are particularly important because they have much larger sample sizes (up to >100 subjects) as compared to the former studies and further substantiated VS-hypoactivation effects in ADHD. Furthermore, the study of Carmona et al. (2012) controlled for potential anatomical abnormalities (volumetric and shape alterations) in the basal ganglia (Hoekzema et al., 2012) Stoy et al. (2011) reported neural hyporesponsiveness within the putamen when comparing ADHD non-remitters to healthy controls. Furthermore, the direction of the VS-effect was consistent with the other reports, i.e. VS-response in ADHD < healthy controls (personal communication, 12-11-2012). In 2013, there is one study that replicated VS-hyporesponsiveness in individuals with ADHD of the inattentive subtype (Edel et al., 2013).
To date, one study has been published that did not replicate VS-hyporesponsiveness in individuals with ADHD during reward anticipation (Paloyelis et al., 2012). This study used a modified MID with a probability schedule that was different as compared to the other MID studies which might explain the non-replication: As described above, the probability of winning money after responding to the target during win-trials is ~67% in the classical MID. Paloyelis et al. (2012) used a probability of ~80%. This might be a crucial difference because Fiorillo et al. (2003) demonstrated that sustained dopamine signals between cue and reward are highest when reward outcomes occur under conditions of uncertainty (i.e., when P = 0.5 and lowest for P = 0 and P = 1). Therefore, a probability of P ~ 0.8 as used in Paloyelis et al. most likely lead to a relatively lower VS-responses in the healthy control group as compared to studies using the classical MID (P ~ 0.67). One might speculate that patients with ADHD benefit from higher predictability because it may increase a blunted VS response, e.g. by increasing attention (Sikstrom and Soderlund, 2007), and as a result, differences between ADHD and healthy controls may become smaller. Future studies on VS-responsiveness in ADHD using the MID with different probabilities could clarify this important question.
For the sake of completeness, two additional published fMRI studies should be mentioned, although they focused on different aspects of reward/motivational processing (Lemiere et al., 2012; Wilbertz et al., 2012). The study by Wilbertz et al. exclusively focused on reward delivery rather than reward anticipation and found no differences in the VS, replicating the finding of Scheres et al. (2007). The pilot study by Lemiere et al. found VS-hyperactivity in the ADHD group when contrasting trials of inescapable vs. escapable delay. Of note, there was no explicit reward associated with the performance of the participants. The authors interpreted the activation to represent an enhanced salience signal (i.e., inescapable delays were thought to be more aversive as compared to escapable delays). Finally, there is an analogue study that assessed ADHD-symptoms in a sample of healthy volunteers with self-report questionnaires. Consistent with the patient studies, Stark et al. (2011) found the ADHD-symptom scores to be negatively correlated with VS-response during reward anticipation.
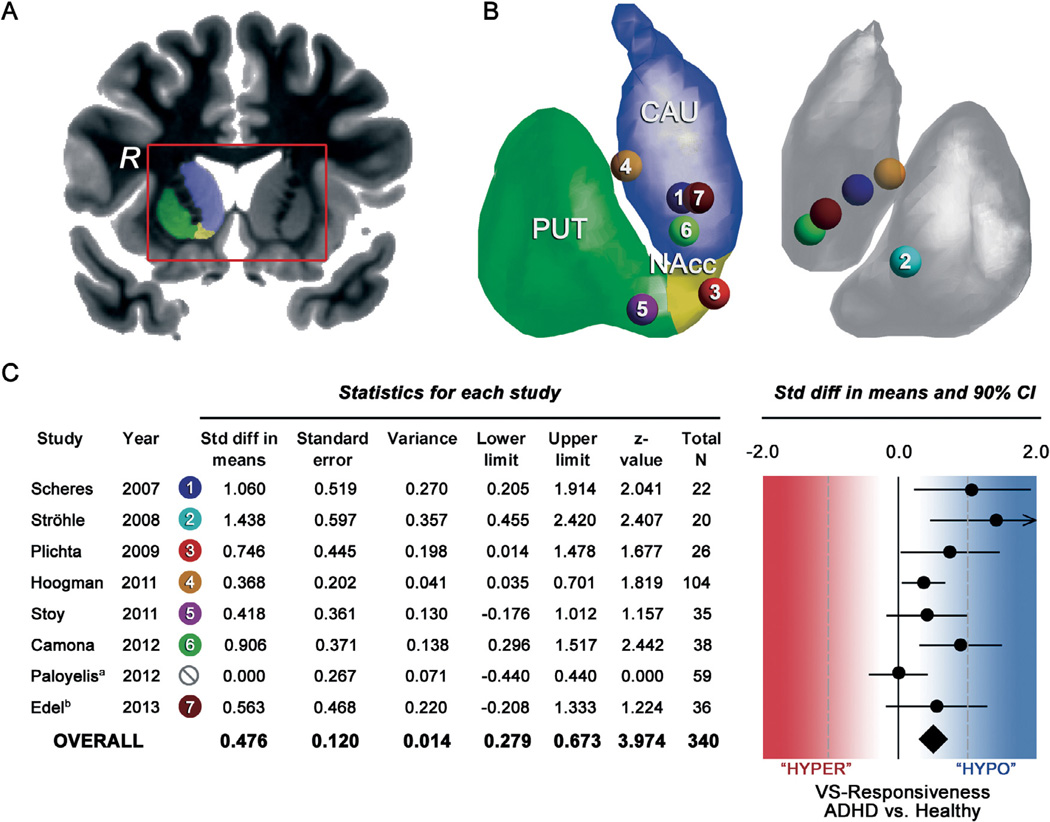
Table 2 summarizes all existing BOLD fMRI studies from 2007 to 2013 on reward processing in ADHD that investigated VS-responsiveness. Fig. 1 illustrates the peak-voxel effects within the VS and results of a formal meta-analysis (using Comprehensive Meta-Analysis software (Version 2; Biostat, Englewood, New Jersey)) that included all but the three latter studies (Lemiere et al., 2012; Stark et al., 2011; Wilbertz et al., 2012). Of note, the included studies with a total N = 340 are rather homogeneous with regards to the applied paradigm (6 out of 8 studies used the MID task) and the outcome parameter (VS-response) which is important for meta-analysis in the given situation of relatively few studies (Valentine et al., 2010).
Table 2.
FMRI studies focusing on reward processing in ADHD.
| Study | Sample characteristicsa | N (P/HC) | Sex | Paradigm | Reward anticipation | VS Finding | Correlation with ADHD symptoms |
|---|---|---|---|---|---|---|---|
| Scheres et al. (2007) | Adolescents (14.3 ± 1.6) | 11/11 | M | MID | Yes | VS ↓ | Negative (CPRS-HI)g |
| Strohle et al. (2008) | Adults (32.4 ± 8.1) | 10/10 | M | MID | Yes | VS ↓ | Negative (DSM-IV symptom score) |
| Plichta et al. (2009) | Adults (23.3 ± 5.2) | 14/12 | M | DD | Yesc | VS ↓ | n.s. (ASRS) |
| Hoogman et al. (2011) | Adults (38.3 ± 11.3) | 63/41 | M | MID | Yes | VS ↓ | Negativef DD |
| Stoy et al. (2011) | Adults (27.3 ± 3.8) | 12/11/12b | M | MID | Yes | VS ↓e | n.s. (Conners Adult ADHD Rating Scale) |
| Carmona et al. (2012) | Adults (33.6 ± 10.3) | 19/19 | M | MID | Yes | VS ↓ | Negative (CAARS-HI)g |
| Wilbertz et al. (2012) | Adults (37.1 ± 9.13) | 28/28 | M + F | CGT | No | – | n.a. |
| Lemiere et al. (2012) | Adolescents (14.7 ± 1.5) | 10/10 | M + F | EDI | No | VS ↑ | n.a. |
| Paloyelis et al. (2012) | Adolescents (15.5 ± 2.2) | 29/30 | M | MILT | Yes | No VS effect; interaction | n.s. (Conners’ DSM-IV parent ratings) |
| Edel et al. (2013) | Adults (33.1 ± 10.7) | 12/12/12d | M | MID | YES | VS ↓ | n.a. |
ADHD: attention-deficit/hyperactivity disorder; P: patients; HC: healthy controls; M: male; F: female; MID: monetary incentive delay task; CPRS: Conners’ Parent Rating Scale; DD: delay discounting task; CGT: card guessing task; EDI: Escape Delay Incentive; MILT: Motivated Incidental Learning Task; VS: ventral striatum activity; DD = delay discounting parameter; n.a. = not applicable; ASRS = Adult ADHD Self-Report Scale.
Mean age followed by standard deviation.
First number: drug-naïve patients; second number: patients treated with methylphenidate; third number: healthy controls.
No real time elapse but imaginative.
First number: ADHD-combined type; second number: ADHD-inattentive type; third number: healthy controls.
Not significant.
The correlation between VS activity and impulsivity in healthy comparison subjects (positive slope) was significantly different from the correlation in ADHD patients (negative slope).
Correlations between VS activity and clinical measures are only significant when analyzing the sample as a whole (ADHD and control subjects together).
Fig. 1.
Panel A shows the anatomical area of interest, i.e. the ventral-striatum (VS) including nucleus caudate (CAU), putamen (PUT) and the nucleus accumbens (NAcc). The right hemisphere is indicated by an “R”. Panel B is a 3-D representation of the striatum showing the peak-voxels by means of coloured dots of each single study on reward anticipation in ADHD. Panel C shows the data and results of the meta-analysis including a forest plot of effect sizes for VS-responsiveness during reward anticipation in ADHD. The diamond symbol represents the pooled estimate across all studies and indicates a medium effect (ES = 0.48; P < 0.001) in terms of VS-hyporesponsiveness in ADHD. Notes: aEffect size estimates were set to zero because these studies did not find significant VS effects and therefore did not report exact t/P-values. bThe averaged effect-size was calculated from the separate effect-size estimates (ES = 0 for ADHD-combined type; ES = 1.126 for ADHD-inattentive type). Panel B was visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Summarizing the results of the meta-analysis, the overall effect size expressed as standard mean difference (Cohen’s d) between groups is 0.48 and is significantly different from zero (Z = 3.97; P < 0.0001). Therefore, across all published studies there is a medium (according to Cohen’s rule of thumb) effect of VS-hyporesponsiveness in ADHD during reward anticipation. Underpinning the validity of this finding is the fact that the effect was found in adolescents and adults with ADHD. Furthermore, the effect was consistent across two distinct paradigms (MID and DD) arguing for a task independent dysfunction, as long as anticipatory processes are involved. Including only MID task studies increased the overall effect size to 0.58 (Z = 4.13; P < 0.001). One study did not replicate significant VS-hyporesponsiveness, which may be partially explained by sample characteristics or specific design issues. In the following section, potential reasons and explanations for non-replications and caveats for the interpretation of VS responsiveness will be discussed.
3. Three integrative approaches
So far, we have shown that VS-hyporesponsiveness during reward anticipation is evident in patients suffering from ADHD, while studies in the healthy population demonstrate the opposite relationship. Against the background that ADHD may be better viewed as representing the extreme of a continuum of normal variability than as a latent category (Frazier et al., 2007; Haslam et al., 2006; Hudziak et al., 2007), the question arises as to how these contrasting findings can be integrated.
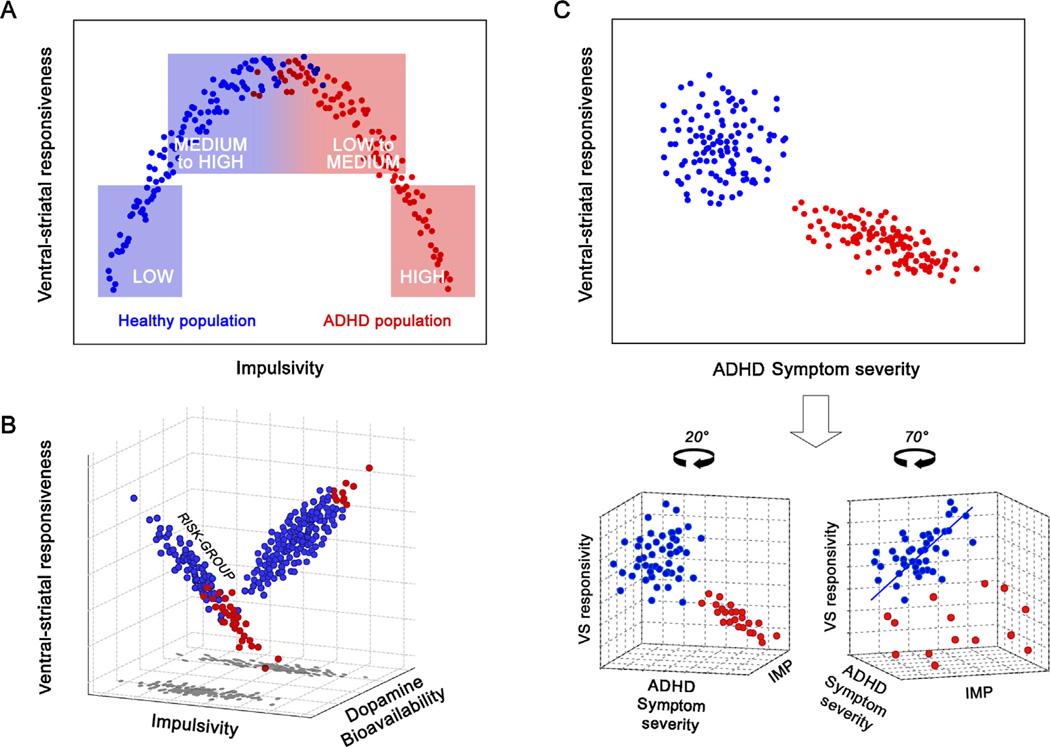
At least three different approaches that integrate the opposite findings can be considered: (a) an inverted u-shape model; (b) a “moderator model” which assumes that patients and healthy controls stem from different sub-populations that differ with regards to, e.g. genetic background impacting the relationship of impulsivity and VS responsivity and, finally, (c) the “unrelated model” that assumes other facets than classical impulsivity factors to additionally account for the VS-hyporesponsiveness in ADHD (see Fig. 2 for a graphical representation of the three models). Strengths and weaknesses of these three theoretical approaches are discussed in the following sections.
Fig. 2.
Three hypothetical approaches that integrate the findings on correlations between impulsivity and VS-activity in healthy individuals and patients with ADHD. Panel A shows the inverted u-shape model. Low-medium to high impulsive groups in the healthy population (blue boxes) and patients with ADHD (red boxes) are highlighted to show that VS group differences can depend on sample selection. Panel B shows the moderator model which assumes a third background variable determining the correlation direction (e.g. dopamine bioavailability). Panel C represents the “unrelated model” which states that it is ADHD-specific symptoms and not impulsivity that is negatively related to VS-response. See text for further details.
4. Evaluation of the approaches
4.1. Model I – the inverted u-shape model
The inverted u-shape model (see Fig. 2A) is an elegant account and has proven its explanatory value in many other research areas (Cools and D’Esposito, 2011; Seamans and Yang, 2004). A classical example is the Yerkes–Dodson law describing an inverted u-shape relationship between arousal and performance. According to this law, optimal performance is achieved at a medium arousal level. As for impulsivity and its relation to VS-response, the model would predict a maximum of VS-response at a medium impulsivity level and decrements of VS-response at both ends, i.e. in extremely high and extremely low impulsive individuals.
However, empirical evidence for the validity of an inverted u-shape relationship in the context of impulsivity and VS-response is as of yet missing. In order to test this model, samples stratified for impulsivity should be acquired, i.e. low and high impulsive healthy subjects should be compared to mildly and severely affected patients with ADHD. Validity of the inverted-u-model would be indicated by two findings: (1) pooled group data should be best explained by a quadratic regression and (2) the overlap of the two groups’ impulsivity scores is small, with the healthy subjects on the left side and the ADHD subjects on the right side of the distribution (see Fig. 2A). In the case of separate group analyses, positive linear relation should be existent in the healthy group, whereas a negative linear relation should be existent in the ADHD group.
In summary, the inverted u-shape model is an elegant and hypothesis-generating account with some potential to explain non-replications. However, empirical evidence is missing.
4.2. Model II – the moderator model
Recently, it has been shown that strength and direction of the relationship between impulsivity and VS responsivity is not homogeneous even across healthy individuals (Hahn et al., 2011). In fact, this relationship has been shown to be genetically modulated by a polymorphism in the DAT1 gene. The DAT1 gene codes for the dopamine transporter protein that is expressed abundantly in the striatum. Synaptic dopamine (DA) bioavailability in the VS is mainly regulated by the dopamine transporter, which is abundantly expressed in this region (Ciliax et al., 1999). A variable number of tandem repeats (VNTR) polymorphism exists in the DAT1 gene and this VNTR element is repeated between 3 and 13 times but occurs with highest frequency in the 9- and 10-repeat forms (9R and 10R). Different levels of DAT expression have been associated with the different repeat forms (Mill et al., 2002) and the DAT 9R variant has been recently described as a risk factor for persisting ADHD (Brown et al., 2011; Franke et al., 2010). However, until now it is not fully clear whether in vivo an increased or a decreased tonic level of available dopamine is resulting. A detailed discussion of this polymorphisms’ functionality can be found in Hahn et al. (2011) and Hoogman et al. (2013).
Empirically, a strong positive relationship between impulsivity and VS response in homozygote carriers of the 10/10 repeat allele of the DAT1 has been shown. In sharp contrast, no such relationship in 9-repeat allele carriers was existent (Hahn et al., 2011). That means, two genetically determined subpopulations exist that show differential trait-brain correlations. Such moderating effects of genetic polymorphism on links between personality dimensions (including impulsivity) and neural activation have come into focus only recently (Hahn et al., 2011; Hariri et al., 2009). Importantly, such moderating effects suggest a more complex model of the relationship between impulsivity and VS responsivity as shown in Fig. 2B.
The moderator model assumes differential relationships between impulsivity and VS responsivity depending on, e.g. dopamine (DA) bioavailability (i.e. is the amount of dopamine in the synaptic cleft), which in turn impacts on the fMRI BOLD response (Knutson and Gibbs, 2007). Of note, DA bioavailability might not be the only variable that has a moderating impact on the bivariate relation. One can easily think of environmental factors as well, e.g. parenting style and the development of delay aversion (Sonuga Barke, 2005; Sonuga Barke et al., 1992a,b, 1996). The moderator model makes further assumptions that fit well with empirical findings: it assumes that a relatively large subpopulation exists in which the occurrence of ADHD is relatively rare, and a smaller subpopulation with a relatively large proportion of ADHD. This assumption fits with the findings that specific dopamine transporter (DAT) gene variants (or other genetic variants) can represent a disease risk factor (Asherson et al., 2007; Gizer et al., 2009; Yang et al., 2007). I.e., here it is also assumed that there is a large subpopulation that does not carry a genetic risk-variant and has a low prevalence for ADHD, while a smaller subpopulation exists that carries the risk-variant and has a higher prevalence for ADHD. Interestingly, a recent study confirmed the impact of the DAT 9R variant as a risk factor for persisting ADHD (Brown et al., 2011; Franke et al., 2010), providing a link between the findings in healthy controls and patients with ADHD.
If such distinct subpopulations indeed exist, this could also explain the relative over-sampling of patients with a particular risk variant in the negative relationship group, while the majority of the healthy individuals stem from the positive relationship cohort or are (with a high likelihood) at the middle of the bivariate distribution of the negative relationship cohort. The model also explains heterogeneity and high variance in clinical samples, which is, according to this model, a consequence of sampling from two different subpopulations.
In summary, the moderator model is interesting because it explicitly includes the genetic dimension that has been shown to be of great importance in ADHD (Faraone and Mick, 2010). Furthermore and in contrast to model I, this model is attractive because empirical evidence is existent (Hahn et al., 2011; Paloyelis et al., 2012).
4.3. Model III – the “unrelated model”
Although the assumption of a close link between ADHD-symptom severity and trait impulsivity has high face validity, it is possible that the blunted VS response as observed in individuals with ADHD is related to additional disease-related factors and not trait impulsivity per se (see Fig. 2C). This model integrates the clinical and the healthy population findings as reviewed above by assuming that
a negative correlation between VS response and ADHD symptom severity exists in affected subjects,
a spurious correlation between VS response and symptom severity exists when correlating pooled data, i.e. healthy individuals and patients with ADHD, due to a significant group difference in VS-response,
a positive correlation between VS response and impulsivity (not ADHD scores) exists in healthy individuals which is a distinct – but correlated – dimension.
Interestingly, nearly all studies on clinical samples with high-impulsivity including ADHD, addiction (Beck et al., 2009; Peters et al., 2011) and pathological gambling (Reuter et al., 2005) show VS-hyporesponsiveness to be related to symptom severity and not to an impulsivity score per se. Although impulsivity is a core symptom of these diseases, symptom severity includes many more facets. These can be either disease-unspecific (e.g. length of disease, treatment history, co-morbidity) or disease-specific (e.g. hyperactivity, inattention). Symptom severity may also include consequences arising from high impulsivity (e.g. excessive legal and illegal drug consumption). This, in turn, could mean that the reported association between impulsivity scores and VS-responsiveness in healthy individuals cannot directly be extrapolated to patient populations but additional processes must be taken into account. The basic idea is that high trait impulsivity and its associated behaviours (e.g. drug intake) in an initially healthy person can represent a risk factor for chronic overstimulation of the reward system and subsequently occurring neural changes (Chang et al., 2007; Shin et al., 2013; Volkow et al., 2007). Repeated over-stimulation of the reward system during adolescents, a highly sensitive time window (Galvan, 2010; Spear, 2000) but in principle all stages of development can be affected as well, may shift the set-point of the reward system upwards (Koob and Le Moal, 1997, 2005). Therefore, socially accepted types, amounts and/or number of rewards do not have the power to overcome this hurdle any more (Sonuga-Barke, 2011). Chronic VS overstimulation in a highly impulsive, but healthy subject might occur due to impulsive actions such as excessive media consumption or the use of (il)legal stimulants. In combination with other consequences of high impulsivity such as social problems (Kofler et al., 2011), the probability to develop an impulsivity-related psychiatric disorder might increase.
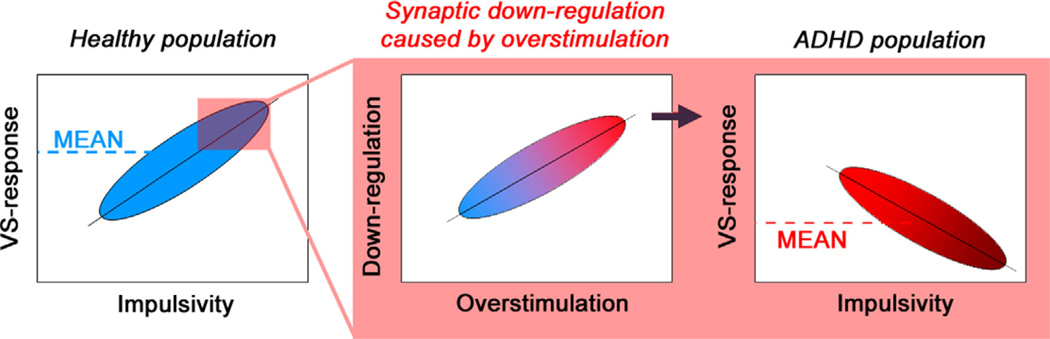
According to this model, the different correlation pattern in the healthy population and in patients with ADHD can be integrated from a developmental perspective (see Fig. 3): An initially hyper-responsive VS-system might increase the probability to behave impulsively in multiple ways (positive correlation). In some individuals, however, putatively those with a genetic risk factor, such repeated VS-overstimulation triggered by impulsive behaviour might lead to a subsequent down-regulation of the reward system (Stice et al., 2013). The degree of down-regulation is presumably positively correlated with the degree of impulsivity and therefore a negative correlation of symptom severity and VS-response results.
Fig. 3.
Left panel shows that an initially hyper-responsive VS-system might increase the probability to behave impulsively in multiple ways (positive correlation in the healthy population). In some individuals, putatively those with additional (genetic) risk factors, such repeated VS-overstimulation triggered by impulsive behaviour might lead to a subsequent down-regulation of the reward system (middle panel). The degree of down-regulation is presumably positively correlated with the degree of impulsivity and therefore a negative correlation of symptom severity and VS-response results (right panel).
This model is a very general approach that might be also useful in understanding VS-hyporesponsiveness in other disorders that share impulsivity as a core symptom (addiction, pathological gambling). According to this model, VS-hyporesponsiveness is related to the concept of “reward deficiency syndrome” (Blum et al., 1995, 2008) and motivational toxicity (Bozarth, 1989).
The dynamical aspect implicated in this model is best supported by a recent study of Stice et al. (2013). They showed that initial VS-hyperresponsivity was a risk factor for developing an impulsivity-related disorder while drug consumption was associated with VS-hyporesponsivity. Comparable studies in the area of ADHD are needed. Prospective longitudinal studies could clarify whether neural plasticity is a candidate mediator. Finally, future studies should clarify whether VS-hyporesponsiveness is indeed correlated with ADHD symptom severity or not. Both a negative correlation and the lack thereof would contradict the findings in the healthy population (higher VS-response in highly impulsive subjects), but VS-hyporesponsiveness without a correlation with ADHD symptom severity would potentially be less disorder specific. At the moment 4 out of 7 relevant studies show a negative correlation between ADHD symptom severity and VS responsiveness (see Table 2).
In summary, the “unrelated model” assumes that the association between trait impulsivity and VS-responsiveness in healthy individuals cannot directly be extrapolated to patient populations because of an intermediate factor (e.g. neural plasticity). There is some empirical evidence supporting model III.
5. Limitations
It is important to note that at the present stage the number of existing studies in the healthy population as well as in ADHD groups is relatively low. Therefore, the presented integrative approaches should not be understood as a final answer, but as an attempt to frame future research directions by generating testable hypotheses and giving practical suggestions for future studies.
The multidimensional nature of impulsivity and the heterogeneity of its definition can be a problem. Dawe and Loxton (2004) have argued for subdividing the construct to at least two independent factors which they have labelled as reward sensitivity and rash spontaneous impulsivity. The list of questionnaires in Table 1 shows that the existing studies in the healthy population used assays from both these two facets. Although both facets show positive correlations with VS response in healthy subjects, the question arises as to whether these two separate factors may have separate biological bases.
Furthermore, our focus on task-evoked VS activation is necessarily a limitation of the field of view because of the brains network structure. Here, sophisticated investigation of the complex interactions between VS and other brain areas will definitely complement the picture (Costa Dias et al., 2012; Cubillo et al., 2010, 2012; Hart et al., 2012; Noreika et al., 2012; Rubia et al., 2009). For example, Davis et al. (2012) applied graph theory analyses which have been shown to be reliable fMRI assays (Braun et al., 2012) to characterize the relationship between impulsivity and the functional segregation (“modularity”) of the whole-brain network architecture. They found that in highly impulsive individuals, regulatory structures including medial and lateral regions of the prefrontal cortex were isolated from subcortical structures associated with appetitive drive. Such more complex analyses might help to better understand the apparent differences between patients with ADHD and the healthy population.
6. Conclusions and future directions
The meta-analysis of the existing fMRI studies that focus on reward anticipation in ADHD indicates a medium effect in terms of VS-hyporesponsiveness in ADHD (Cohen’s d = 0.48–0.58). In contrast, the existing fMRI studies in the healthy population suggest that there is a positive correlation between impulsivity-related traits and VS-BOLD response during reward processing including anticipation. We critically discussed how VS-hyporesponsiveness findings in ADHD relate to findings from the healthy population.
The presented three different hypothetical models all explain VS-hyporesponsiveness and, to varying degrees, other empirical evidence in ADHD research such as non-replications, higher variance in the patient group and/or genetic risks as impacting variables. We evaluate both the moderator model and the “unrelated model” to be more closely related to the existing empirical evidence existing so far than the inverted u-shape model.
Clearly, at the moment the number of existing studies in this field of interest is small. With this review, we hope to stimulate attempts to integrate the empirical evidence and to initiate new studies in this field. We close this review paper by making four suggestions for future studies that include task-related issues, impulsivity assessment, sample selection and the developmental perspective:
Task-related issues: Studies comparing ADHD with control groups using the MID task may manipulate probability of winning in order to clarify whether group differences are (partly) driven by uncertainty of reward delivery. Similarly, the relative contribution of (a) the anticipatory delay duration, and (b) learning/remembering the association between cue and outcome to the reported group differences in VS activation need clarification. These questions can be addressed by manipulating delay durations, and by comparing the use of abstract cues with cues that do not require learning an association. Furthermore, although the adaptive RT window of the MID task ensures that important behavioural measures are held constant across subjects and groups (e.g. earned money), other behavioural MID indices (e.g. untimely responses, omission errors, etc.) should also be considered as potentially related to VS-hyporesponsiveness. At a very basal level, and relevant for all three models, registration of eye movements during the functional scans can clarify whether VS-hypoactivation in ADHD is related to the degree of attention towards the stimuli.
Impulsivity assessment: Future studies may consider including not only ADHD scales, but also more general measures of trait impulsivity such as the Barratt Impulsiveness Scale, or delay discounting rate. Such an approach would help to clarify whether it is trait impulsivity that is negatively related to VS activation in ADHD or other ADHD-specific facets. Alternatively, researchers could specifically look at the association between the 3 DSM-IV ADHD impulsivity items and VS activation rather than lumping impulsivity and hyperactivity together. Furthermore, emotive processes like delay aversion during reward anticipation might occur at least in some patients with ADHD and should be considered. Such an emotive process might confound the estimate of VS responsiveness towards rewards in ADHD. Therefore, questionnaires on delay aversion (Clare et al., 2010), and/or other measures of subjectively experienced aversion during waiting, could be assessed and taken into account.
Sample-related issues: Researchers may consider including participants covering the full continuum from no ADHD symptoms to a clinical diagnosis of ADHD, especially when they plan to use dimensional analyses. Up till now, the majority of studies select either a healthy group alone, or a clinical group (with at least 6/9 symptoms of inattention and at least 6/9 symptoms of hyperactivity–impulsivity in the case of ADHD-Combined type), and a typical control group that is “symptom-free”. Therefore, there is a lack of studies in which the full continuum, including the “grey area in the middle” is covered (compare Fig. 2A). Including these cases that are in the middle of the continuum may have the important advantage that it will allow for characterization of the actual shape of the relation between impulsivity and VS activation. Finally, based on recent findings (Hahn et al., 2011; Hoogman et al., 2011; Paloyelis et al., 2012) there is a need for linking genetic information, striatal responsivity to rewards and behavioural impulsivity across diagnostic categories.
Developmental aspects: Potentially the most important issue is to perform prospective longitudinal studies that focus on changes in the reward systems’ response. According to the study of Stice et al. (2013), it should be tested whether initial VS-hyperresponsivity is a risk factor for a subsequent downregulation of the reward systems’ response (e.g. due to chronic overstimulation), and therefore a risk factor for impulsivity-related disorders.
Acknowledgements
We thank Antje B.M. Gerdes (University of Mannheim) for proof-reading and her valuable comments on the manuscript. We thank Oliver Grimm (CIMH) for the fruitful discussion. Furthermore, we thank Mingrui Xia from Beijing Normal University for his friendly help on the visualization of the fMRI activation results.
A.S.’s work was supported by grants from the National Institute of Mental Health (NIMH), the Institute for Mental Health Research (IMHR), and the Netherlands Organization for Scientific Research (NWO).
Footnotes
MMP and AS declare having no potential conflict of interest related directly or indirectly to this work.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth ed. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP, Buitelaar J, Banaschewski T, Sonuga-Barke E, Eisenberg J, Manor I, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Faraone SV. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. American Journal of Psychiatry. 2007;164:674–677. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, Arcuri V, Blum SH, Downs BW, Waite RL, Notaro A, Lubar J, Williams L, Prihoda TJ, Palomo T, Oscar-Berman M. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatric Disease and Treatment. 2008;4:893–918. doi: 10.2147/ndt.s2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJH, Comings DE. Dopamine D2 receptor gene variants – association and linkage studies in impulsive–addictive–compulsive behavior. Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. New perspectives on cocaine addiction: recent findings from animal research. Canadian Journal of Physiology and Pharmacology. 1989;67:1158–1167. doi: 10.1139/y89-185. [DOI] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, Sauer C, Haddad L, Grimm O, Mier D, Mohnke S, Heinz A, Erk S, Walter H, Seiferth N, Kirsch P, Meyer-Lindenberg A. Test–retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage. 2012;59:1404–1412. doi: 10.1016/j.neuroimage.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Brown AB, Biederman J, Valera E, Makris N, Doyle A, Whitfield-Gabrieli S, Mick E, Spencer T, Faraone S, Seidman L. Relationship of DAT1 and adult ADHD to task-positive and task-negative working memory networks. Psychiatry Research. 2011;193:7–16. doi: 10.1016/j.pscychresns.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos-Quiroga JA, Richarte V, Canals C, Bosch R, Rovira M, Soliva JC, Bulbena A, Tobena A, Casas M, Vilarroya O. Response inhibition and reward anticipation in medication-naive adults with attention-deficit/hyperactivity disorder: a within-subject case-control neuroimaging study. Human Brain Mapping. 2012;33:2350–2361. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. Journal of Comparative Neurology. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Clare S, Helps S, Sonuga-Barke EJ. The quick delay questionnaire: a measure of delay aversion and discounting in adults. Attention Deficit Hyperactivity Disorders. 2010;2:43–48. doi: 10.1007/s12402-010-0020-4. [DOI] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. Journal of Abnormal Child Psychology. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-u-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Seghete KLM, Hudson KA, Nagel BJ. High, low sensation seeking adolescents show distinct patterns of brain activity during reward processing. NeuroImage. 2013;66:184–193. doi: 10.1016/j.neuroimage.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Davis FC, Knodt AR, Sporns O, Lahey BB, Zald DH, Brigidi BD, Hariri AR. Impulsivity and the modular organization of resting-state neural networks. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Reviews. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Downey KK, Stelson FW, Pomerleau OF, Giordani B. Adult attention deficit hyperactivity disorder: psychological test profiles in a clinical population. Journal of Nervous and Mental Disease. 1997;185:32–38. doi: 10.1097/00005053-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Edel MA, Enzi B, Witthaus H, Tegenthoff M, Peters S, Juckel G, Lissek S. Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. Journal of Psychiatric Research. 2013;47:350–356. doi: 10.1016/j.jpsychires.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. Journal of Abnormal Child Psychology. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatric Clinics of North America. 2010;33:159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, Heine M, Jacob CP, Lesch KP, Casas M, Ribases M, Bosch R, Sanchez-Mora C, Gomez-Barros N, Fernandez-Castillo N, Bayes M, Halmoy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJ, Kiemeney LA, Kooij JJ, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities. 2007;40:49–65. doi: 10.1177/00222194070400010401. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis A-C, Plichta MM, Heinzel S, Polak T, Lesch K-P, Breuer F, Jakob PM, Fallgatter AJ. Neural response to reward anticipation is modulated by Gray’s impulsivity. NeuroImage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Hahn T, Heinzel S, Dresler T, Plichta MM, Renner TJ, Markulin F, Jakob PM, Lesch KP, Fallgatter AJ. Association between reward-related activation in the ventral striatum and trait reward sensitivity is moderated by dopamine transporter genotype. Human Brain Mapping. 2011;32:1557–1565. doi: 10.1002/hbm.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Haslam N, Williams B, Prior M, Haslam R, Graetz B, Sawyer M. The latent structure of attention-deficit/hyperactivity disorder: a taxometric analysis. Australian and New Zealand Journal of Psychiatry. 2006;40:639–647. doi: 10.1080/j.1440-1614.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Canals C, Moreno A, Fernandez VR, Picado M, Bosch R, Duno L, Soliva JC, Rovira M, Bulbena A, Tobena A, Casas M, Vilarroya O. Stimulant drugs trigger transient volumetric changes in the human ventral striatum. Brain Structure and Function. 2012 doi: 10.1007/s00429-012-0481-7. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Aarts E, Zwiers M, Slaats-Willemse D, Naber M, Onnink M, Cools R, Kan C, Buitelaar J, Franke B. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. American Journal of Psychiatry. 2011;168:1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Onnink M, Cools R, Aarts E, Kan C, Arias Vasquez A, Buitelaar J, Franke B. The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. European Neuropsychopharmacology. 2013;23:469–478. doi: 10.1016/j.euroneuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16(Suppl. 1):S16L 23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Knutson B, Wrase J, Brune M, Heinz A, Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, Geuze E. Neural correlates of personality: an integrative review. Neuroscience and Biobehavioral Reviews. 2013;37:73–95. doi: 10.1016/j.neubiorev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neuroscience Letters. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:Rc159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. Journal of Abnormal Child Psychology. 2011;39:805–817. doi: 10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Lemiere J, Danckaerts M, Van Hecke W, Mehta MA, Peeters R, Sunaert S, Sonuga-Barke E. Brain activation to cues predicting inescapable delay in adolescent Attention Deficit/Hyperactivity Disorder: an fMRI pilot study. Brain Research. 2012;1450:57–66. doi: 10.1016/j.brainres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action. In: Dagenbach D, Carr RTH, editors. Inhibitory processes in attention, memory and language. San Diego: Academic; 1994. pp. 189–239. [Google Scholar]
- Luman M, Oosterlaan J, Sergeant J. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, van Meel CS, Oosterlaan J, Geurts HM. Reward and punishment sensitivity in children with ADHD: validating the Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children (SPSRQ-C) Journal of Abnormal Child Psychology. 2012;40:145–157. doi: 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, Lucas RE. Trait mechanisms in youth with and without attention-deficit/hyperactivity disorder. Journal of Research in Personality. 2008;42:895–913. doi: 10.1016/j.jrp.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, Thomas AD, Muska C, Hylton JL, Pearlson GD. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mischel W. Processes in delay of gratification. In: Berkowitz L, editor. Advances in Experimental Social Psychology. New York: Academic Press; 1974. pp. 249–292. [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Noreika V, Falter CM, Rubia K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:722–732. e729. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. American Journal of Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, Gerdes AB, Sauer C, Tost H, Esslinger C, Colman P, Wilson F, Kirsch P, Meyer-Lindenberg A. Test–retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage. 2012;60:1746–1758. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Johansen E, Russell V. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shin SH, Chung Y, Jeon SM. Impulsivity and substance use in young adulthood. American Journal on Addictions. 2013;22:39–45. doi: 10.1111/j.1521-0391.2013.00324.x. [DOI] [PubMed] [Google Scholar]
- Sikstrom S, Soderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychological Review. 2007;114:1047–1075. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich HC, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Editorial: ADHD as a reinforcement disorder – moving from general effects to identifying (six) specific models to test. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52:917–918. doi: 10.1111/j.1469-7610.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Wiersema JR, van der Meere JJ, Roeyers H. Context-dependent dynamic processes in attention deficit/hyperactivity disorder: differentiating common and unique effects of state regulation deficits and delay aversion. Neuropsychology Review. 2010;20:86–102. doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ. Psychological heterogeneity in AD/HD – a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ, Taylor E, Heptinstall E. Hyperactivity and delay aversion – II. The effect of self versus externally imposed stimulus presentation periods on memory. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1992a;33:399–409. doi: 10.1111/j.1469-7610.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion – I. The effect of delay on choice. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1992b;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sonuga Barke EJ, Williams E, Hall M, Saxton T. Hyperactivity and delay aversion. III: The effect on cognitive style of imposing delay after errors. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:189–194. doi: 10.1111/j.1469-7610.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Personality and Individual Differences. 2009;47:385–395. [Google Scholar]
- Stark R, Bauer E, Merz CJ, Zimmermann M, Reuter M, Plichta MM, Kirsch P, Lesch KP, Fallgatter AJ, Vaitl D, Herrmann MJ. ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia. 2011;49:426–434. doi: 10.1016/j.neuropsychologia.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Ströhle A. Reward processing in male adults with childhood ADHD – a comparison between drug-naïve and methylphenidate-treated subjects. Psychopharmacology. 2011;215:467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, Juckel G, Knutson B, Lehmkuhl U, Bauer M, Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics. 2010;35:375–375. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of Neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth-Buhler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt-Klein S, Robbins TW, Schumann G, Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wilbertz G, van Elst LT, Delgado MR, Maier S, Feige B, Philipsen A, Blechert J. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage. 2012;60:353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. Journal of Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Biological Bases of Sensation Seeking: Impulsivity, and Anxiety. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1983. [Google Scholar]