Abstract
A relationship between working memory impairment, disordered neuronal oscillations, and abnormal prefrontal GABA function has been hypothesized in schizophrenia; however, in vivo GABA measurements and gamma band neural synchrony have not yet been compared in schizophrenia. This case–control pilot study (N = 24) compared baseline and working memory task-induced neuronal oscillations acquired with high-density electroencephalograms (EEGs) to GABA levels measured in vivo with magnetic resonance spectroscopy. Working memory performance, baseline GABA level in the left dorsolateral prefrontal cortex (DLPFC), and measures of gamma oscillations from EEGs at baseline and during a working memory task were obtained. A major limitation of this study is a relatively small sample size for several analyses due to the integration of diverse methodologies and participant compliance. Working memory performance was significantly lower for patients than for controls. During the working memory task, patients (n = 7) had significantly lower amplitudes in gamma oscillations than controls (n = 9). However, both at rest and across working memory stages, there were significant correlations between gamma oscillation amplitude and left DLPFC GABA level. Peak gamma frequency during the encoding stage of the working memory task (n = 16) significantly correlated with GABA level and working memory performance. Despite gamma band amplitude deficits in patients across working memory stages, both baseline and working memory-induced gamma oscillations showed strong dependence on baseline GABA levels in patients and controls. These findings suggest a critical role for GABA function in gamma band oscillations, even under conditions of system and cognitive impairments as seen in schizophrenia.
Keywords: Schizophrenia, Magnetic resonance spectroscopy, GABA, Gamma oscillation, Dorsolateral prefrontal cortex, Working memory
Highlights
-
•
We compared in vivo GABA measures and gamma band oscillations in schizophrenia.
-
•
Correlations between left DLPFC GABA and gamma amplitude were significant.
-
•
Peak gamma frequency significantly correlated with GABA and performance.
-
•
Patients had significantly lower amplitudes in gamma oscillations than controls.
-
•
Working memory performance was significantly lower for patients than for controls.
1. Introduction
Impairment in working memory is a core deficit in schizophrenia (Elvevag and Goldberg, 2000; Lewis, 2004). Key neurobiological domains that are thought to underlie working memory have shown abnormalities in the illness: prefrontal GABA function (Lewis et al., 2012) and neuronal synchrony (Cho et al., 2006; Uhlhaas and Singer, 2010). In addition to findings of disturbances in all three domains, some studies have examined their relationships. Reports have linked cognitive and GABA dysfunctions (Enomoto et al., 2011), while others related cognitive and neuronal synchrony abnormalities (Cho et al., 2006; Uhlhaas and Singer, 2010) in schizophrenia.
Patients with schizophrenia exhibit clear disturbances in manipulation of transiently stored information (Cannon et al., 2005), including visual working memory (Fleming et al., 1997; Vance et al., 2006). The dorsolateral prefrontal cortex (DLPFC) is part of the integrated cortical network for visuo-spatial working memory (Haxby et al., 2000; Kang et al., 2011), and studies have suggested that working memory deficits in schizophrenia occur in a context of altered activation of DLPFC during task performance (MacDonald et al., 2005; van Veelen et al., 2010). However, the neurochemical disturbances underlying working memory and other cognitive deficits in schizophrenia remain unclear.
A potential physiological substrate for abnormal and inefficient DLPFC function is altered function of GABAergic neurons. Postmortem studies show that fast-spiking, parvalbumin-positive interneurons are affected in schizophrenia, suggesting GABAergic deficits in cortical interneurons (Benes et al., 1991; Benes and Berretta, 2001; Duncan et al., 2010; Lewis and Gonzalez-Burgos, 2008; Lewis and Hashimoto, 2007; Ohnuma et al., 1999; Volk et al., 2002). Recent in vivo MRS studies of prefrontal GABA levels have reported either no difference (Goto et al., 2009; Tayoshi et al., 2010), a trend reduction in older patients (Rowland et al., 2013b), or elevations (Kegeles et al., 2012; Ongur et al., 2010) when compared to healthy controls (see Comment in Kegeles et al., 2012, for discussions on GABA elevations). While the relationship of total tissue GABA levels measured by MRS to synaptic transmission at the fast-spiking interneurons remains unclear, these postmortem and in vivo studies suggest abnormalities in prefrontal GABA function.
Synchrony among large populations of neurons is thought to be essential for healthy cognitive function (Engel et al., 2001; Uhlhaas and Singer, 2006; Von Stein et al., 2000), particularly in fast-spiking parvalbumin-positive interneurons that exhibit abnormalities in postmortem studies. A number of studies have reported aberrant neural oscillations in schizophrenia, particularly in the gamma frequency band (Farzan et al., 2010; Gandal et al., 2012; Uhlhaas and Singer, 2006). Synchronous gamma oscillations (>30 Hz) correlate with several cognitive processes (Fries et al., 2001; Gray et al., 1989; Pesaran et al., 2002; Singer, 1999; Tallon-Baudry and Bertrand, 1999; Varela et al., 2001). During working memory tasks, increasing cognitive load is associated with an increase in gamma oscillations in healthy participants and epilepsy patients (Basar-Eroglu et al., 2007; Howard et al., 2003; Meltzer et al., 2008). Increase in cognitive control also elicits greater modulation of DLPFC gamma neural oscillations in healthy participants, though patients with schizophrenia produce less or no modulation (Barr et al., 2010; Cho et al., 2006). Similarly, gamma-band synchrony during tasks of visual gestalt perception is attenuated in schizophrenia (Spencer et al., 2003), and patients with schizophrenia fail to enhance gamma activity with increasing working memory load (Basar-Eroglu et al., 2007). These previous reports on gamma oscillations during cognitive tasks in patients with schizophrenia might seem to be inconsistent regarding gamma oscillatory amplitudes; such discrepancies might be attributed to differences in tasks and their characteristics in induced and evoked EEG processing. Nonetheless, aberrant modulatory response in gamma frequency band to cognitive challenges is a replicated finding in schizophrenia (Gonzalez-Burgos et al., 2011).
Evidence for a connection between GABA function and cognition has been found using in vivo approaches. A link between GABA dynamics and working memory performances was demonstrated in a prolonged delayed match-to-sample task using healthy participants (Michels et al., 2012). A clinical trial of a novel GABAergic agent showed improvement in some aspects of cognition in schizophrenia (Lewis et al., 2008). In a visual contrast discrimination task, despite lower GABA levels in visual cortex than healthy controls, patients demonstrated the same correlations between GABA levels and task performance (Yoon et al., 2010), further supporting a key role for GABA in cognitive processes in schizophrenia.
In healthy participants, resting GABA levels have been correlated with peak gamma-band frequency during visual stimulation (Muthukumaraswamy et al., 2009). Animal studies have explored the link between GABA function and gamma band neural synchrony using electrophysiology (Gonzalez-Burgos et al., 2011) and optogenetics (Sohal et al., 2009), showing a crucial role for fast-spiking parvalbumin-positive interneurons in generating synchronous neural oscillations in the gamma frequency band (Hajos et al., 2004; Klausberger et al., 2003; McBain and Fisahn, 2001; Traub et al., 2001). Specific circuit mechanisms of synchronized oscillations via GABAA receptor-mediated inhibition may involve rhythmic interneuron firing with trains of inhibitory postsynaptic currents in their target cells, the pyramidal neurons comprising the major population of the synchronous circuitry (Gonzalez-Burgos et al., 2011). Thus, the need for adequate GABAergic transmission for the generation of synchronous gamma neural oscillations provides the potential basis for aberrant high frequency oscillations in schizophrenia.
Prior studies have generated substantial evidence for cognitive deficits as well as abnormalities in gamma band neuronal synchrony and prefrontal GABA function in schizophrenia. A relationship between working memory impairment, disordered neuronal oscillations, and abnormal prefrontal GABA function has been hypothesized in schizophrenia. Relationships have been reported between cognitive deficits and failure to enhance gamma band synchrony during task performance. Similarly, GABA function has been connected to cognitive performance through a clinical trial (Lewis et al., 2008) and an MRS study (Yoon et al., 2010). Although in-vivo GABA levels of patients with schizophrenia have been showed to associate with oscillatory measures of paired-click paradigm (Rowland et al., 2013a), no studies relating GABA levels to gamma oscillations during a working memory task, or comparing all three domains within the same participants, have been performed in schizophrenia. These comparisons are the subject of the current study: We hypothesize that there are relationships across working memory performance, gamma neural oscillations, and DLPFC GABA levels that indicate a key role for GABA in subserving cognitive functions.
2. Methods
2.1. Participants
The study was approved by the Institutional Review Boards of the New York State Psychiatric Institute (NYSPI) and Columbia University Medical Center. Twelve patients with schizophrenia (three females and nine males, ages 21–49; mean = 31, SD = 10.79), who met the criteria for schizophrenia or schizoaffective disorder as assessed with the Diagnostic Interview for Genetic Studies, were recruited either after voluntary admission to a research unit (Schizophrenia Research Unit, NYSPI) or from the affiliated outpatient research clinic (Lieber Schizophrenia Research Clinic, NYSPI). Inclusion criteria for patients were: (i) no other DSM-IV Axis-I diagnosis, (ii) age 18–60, (iii) no lifetime history of alcohol or substance abuse or dependence, (iv) no concomitant or past severe medical conditions, including head trauma, (v) not pregnant, (vi) no metallic or other material in the body that would preclude safe exposure to MRI, and (vii) ability to provide informed consent. All but three of the patients were taking a fixed, clinically determined dose of a second-generation antipsychotic medication for at least 4 weeks. Those three were stable off medications. A previous study (Minzenberg et al., 2010) had showed that the unmedicated patient group (n = 32) was not different on measures of cognitive control-related gamma deficits compared with the medicated patient group (n = 21).
Twelve controls (six females and six males, ages 24–46; mean = 33.08, SD = 8.23; mean age not different from the patient group, independent-samples t-test, P = 0.600; sex proportion not different from the patient group, chi-squared test, P = 0.206) were recruited from the New York metropolitan area. Inclusion criteria for the control group were absence of past or present Axis-I psychiatric diagnoses, including substance abuse, plus criteria (ii) to (vii) as above for patients. Absence of psychiatric history and/or symptoms in controls was assessed with the SCID non-patient version by a trained rater.
For both patients and controls, assessment of inclusion and exclusion criteria was made by the structured interviews noted, medical/psychiatric history, review of systems, physical and neurological examination, routine blood tests (including pregnancy test), urine toxicology, and EKG. After complete description of the study to the participants, written informed consent was obtained. Patients' capacity to sign informed consent was assessed by a clinician who was not a member of the research team. MRS data for six patients and eight controls were published previously (Kegeles et al., 2012).
2.2. Study procedures
Baseline EEG for all participants was recorded with eyes open for a minimum of 3 min. Subsequently, EEG was recorded while participants performed a modified Sternberg working memory task (Luber et al., 2007) lasting 34 min (see details below and Fig.1 in Luber et al.). MRS data were collected within 3 days of EEG collection.
Fig. 1.
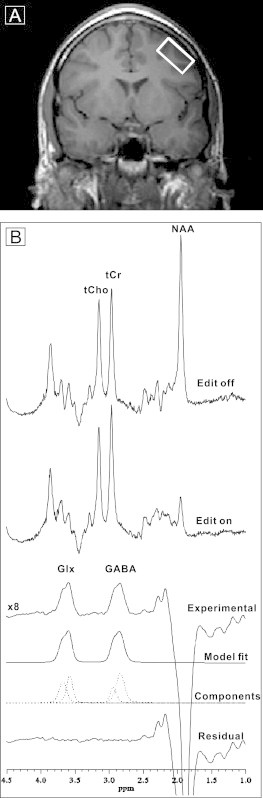
A. Coronal slice through the anterior commissure showing placement of the DLPFC voxel in the left middle frontal gyrus of a patient with schizophrenia. B. An acquisition from this voxel. Six successive curves show spectrum with editing pulse off; editing pulse on; difference spectrum between editing on and off magnified 8-fold showing the resulting Glx and GABA peaks; best fit model spectrum; individual components of the best-fit model; and the residual difference between the measured difference spectrum and the best-fit curve.
2.3. Modified Sternberg working memory task
Each trial lasted 13 s, with the following sequence of three task stages: 1) Encoding stage, an array of one of two possible set sizes (one or six upper case letters) was presented for 3 s. 2) Retention stage, a blank screen was presented for 7 s, during which participants were asked to fixate on the center of the screen and keep the stimulus items in mind. 3) Probe stage, a test stimulus consisting of a single lower case letter appeared for 3 s at the center of the screen. During the probe stage, participants were instructed to indicate by a button press whether the probe letter matched a character in the stimulus array as quickly and as accurately as possible. Choice of set size and positive or negative probe for an individual trial was pseudorandom, with the restriction that there be 16 true positive and 16 true negative probes for each of the two set sizes over a block of 64 trials.
2.4. EEG recording
We recorded the baseline and working memory EEG using a 66-channel system with direct current BrainAmp MR amplifiers (Brain Products GmbH, Gilching, Germany). Sixty-four EEG channels and two electrooculography (EOG; for the purpose of correcting eye movements, blinks, and micro-saccadic artifacts offline) channels were recorded during rest and working memory task performance. The EEG and EOG signals were referenced to the FCz electrode and an additional forehead electrode, respectively. All signals were hardware-filtered between 0.1 and 1000 Hz and sampled at 1000 Hz with a 60 Hz notch filter (with a bandwidth of 5 Hz, symmetric around 60 Hz with the edge rise of 24 dB/octave). All EEG data were re-referenced to average reference.
2.5. MRS acquisitions
MRS studies were performed using the GE “EXCITE” 3 T magnet (General Electric Medical Systems, Waukesha, Wisconsin). Spectra were recorded using the J-edited spin echo difference technique (Rothman et al., 1993) as modified by Sailasuta et al. (2001), and a receive-only 8-channel phased-array head coil supplied with the instrument (Shungu et al., 2006). The DLPFC voxel was placed in the left middle frontal gyrus (Fig. 1, upper panel) angled parallel to the brain surface with dimensions of 1 cm × 2 cm × 4.8 cm (volume, 9.6 cm3; 26-minute acquisition), with reliable placement attained by using internal landmarks (Kegeles et al., 2006; Kegeles et al., 2012). The scans consisted of a modified standard PRESS sequence with TE = 68 ms and TR = 1500 ms, in which a frequency-selective 180° refocusing radiofrequency pulse was interleaved with a standard non-selective 180° refocusing pulse, such that J-modulation of the GABA C4 H resonance at 3.0 ppm was inhibited and allowed on alternate pulses (Sailasuta et al., 2001). We note that signal from macromolecules co-edits with GABA with this pulse sequence. Using the metabolite nulling method, we have quantified the contribution from mobile macromolecules with this pulse sequence and found it to be about 40% and stable across brain regions (Kegeles et al., 2007).
2.6. Data analysis and statistics
2.6.1. MRS analysis methods
Subtraction of the interleaved PRESS acquisitions with inhibited and allowed J-modulation yielded the edited GABA C4 H resonance at 3.0 ppm (Fig. 1, lower panel). The areas of the spectral peaks, which are proportional to their respective concentrations, were obtained by frequency-domain fitting of each resonance to a Gauss–Lorentz line-shape function using the Levenberg–Marquardt nonlinear least-squares algorithm (Kegeles et al., 2012) written in Interactive Data Language (ITT Exelis Inc., McLean, VA). GABA levels in the edited spectra were then expressed as ratios of the peak area relative to that of the simultaneously acquired unsuppressed water signal from the voxel. To assure goodness of fit, we rejected any cases where shimming resulted in FWHM greater than 20 Hz, which provided good fitting performance. We used chi square statistic from the covariance matrix of the curve fitting parameters normalized to the degrees of freedom (Markwardt, 2009), as we described previously (Simpson et al., 2012). As can be appreciated by examining the sample spectrum presented in Fig. 1, the spectral quality and signal-to-noise ratio (SNR) were consistently very high for successful acquisitions. Therefore, we strictly did not reject any spectra because they were “noisy.” Rather, spectra were rejected because either (a) the shim quality was poor (defined as an FWHM of the water resonance of more than 20 Hz, and/or spectra with unresolved tCr and tCho resonances at 3.03 and 3.22 ppm), or (b) there was excessive head motion during a scan. The criteria for detecting and rejecting motion-degraded spectra were: (1) a very large residual water resonance in the difference or edited spectra due to poor cancelation upon subtracting two subspectra in which the water signal was differentially affected by the head motion; (2) peak phase distortions in all the spectra that could not be automatically adjusted using the phases derived from the unsuppressed water resonance, and (3) degraded SNR in the edited spectra due to incoherent summing of subspectra with motion-induced peak phase and position shifts. Therefore, “noisy spectra” were symptomatic of excessive motion and were rejected as motion-degraded.
2.6.2. Working memory performance
All participants' task performance was assessed by reaction time, overall accuracy, and rates of hit and correct rejection in one and six letter set conditions. Two-tailed independent-samples t-tests were performed.
2.6.3. Baseline gamma amplitude
To evaluate baseline gamma amplitude during rest, EEG data were analyzed as follows: 1) Baseline/resting EEG data were de-artifacted by an amplitude criterion (±500 µV) and then un-filtered EEG data were visually inspected for artifacts. Although participants were not engaged in any task and were relaxed with minimal muscle movement, miniature saccades (Carl et al., 2012; Yuval-Greenberg et al., 2008) and electromyography (EMG) can affect amplitude of the gamma frequency range. Therefore, we visually inspected un-filtered EEG data for saccadic artifacts (Kramer et al., 2008) and monitored the gamma frequency band activity at the outer electrode positions for EMG (Carl et al., 2012). Because the EEG signal is very low compared to other biosignals (e.g., oculomotor activity), we recorded two EOG channels in order to remove oculomotor artifacts off-line. These oculomotor artifacts are inevitable because participants cannot well control spontaneous eye movements or blinks, especially in the patient population with schizophrenia. With signals of vertical and horizontal EOG channels, volume-conducted eye movement or blink artifacts could be effectively removed from the raw EEG by regression-based ocular corrections of first finding ocular-afflicted data stretches in the EOG channels with thresholding techniques, second calculating the regression of the EOG channels with each individual EEG data channel, and last correcting the EEG data (Gratton, 1983). Only clean segments (1000 ms each) without artifacts were analyzed. 2) Fast Fourier transforms were applied and average amplitudes of those baseline segments of the spectrum from 30 to 56 Hz (Spencer, 2012) were computed. This selected gamma frequency band was based on the significant findings of the similar frequency range from a previous study of baseline gamma power in patients and controls (Spencer, 2012). 3) Steps 1 and 2 were repeated for the electrodes from the frontal to central locations of the left (i.e., FP1, AF3, AF7, F1, F3, F5, F7, FC1, FC3, FC5, FT7, C1, C3, C5, and T7) and right (i.e., FP2, AF4, AF8, F2, F4, F6, F8, FC2, FC4, FC6, FT8, C2, C4, C6, and T8) hemispheres. Within each electrode, outliers of the averaged gamma amplitudes were excluded (outside of ±1.5 SDs from the mean).
2.6.4. Localization of the electrode of interest
Although EEG electrodes of interest were AF3, F3, and FC3 initially because of their anatomic locations over the left DLPFC, baseline/resting data from all frontal to central electrodes were further analyzed to confirm the localization of the electrode of interest (see methodological details of Supplementary Material Fig. 1). Significant results of baseline/rest were found only at three electrode locations, F3 (N = 22, R = 0.53, P = 0.012), F5 (N = 22, R = 0.43, P = 0.046), and FC3 (N = 22, R = 0.45, P = 0.037) with the most prominent results at F3; therefore, we selected the F3 electrode for extensive analysis on three working memory stages (see “Discussion of type I error and using resting-state data” of Supplementary Material Fig. 1).
2.6.5. Working memory task-induced gamma amplitude and relationship with GABA
To investigate gamma amplitude during the three different working memory stages, data from electrode F3 were analyzed extensively as follows: 1) As described above, raw EEG data were de-artifacted (esp., considered saccadic spike potentials, sharp-edged and EMG artifacts). 2) Only correct-response trials of the six letter set were used. In our one letter set condition, there is no performance difference between groups; this difference between patients and controls seemed to be selective to the harder working memory condition, consistent with findings of previous studies (Barr et al., 2010; Barr et al., 2013). 3) For each working memory stage, artifact-free segments were extracted (see a typical example of one single trial in Supplementary Material Fig. 3). 4) Instantaneous amplitudes were extracted by Morlet wavelet decomposition on 98 scales from 0.5 Hz to 100 Hz in MATLAB (The MathWorks, Inc., Natick, MA). Based on studies in working memory and schizophrenia (Barr et al., 2010; Basar-Eroglu et al., 2007; Schmiedt et al., 2005), the gamma frequency range from 30 to 56 Hz (avoiding power line frequency 60 Hz artifacts) was used for computing task-induced gamma amplitude. 5) Time ranges for averaging were selected for the different stages: 500 ms–2500 ms of the encoding stage, the entire duration of the retention stage, and from 500 ms before the correct response until the correct response time in the probe stage. Outliers of the averaged gamma amplitudes were excluded. 6) For each participant, averages across trials were computed for each stage. 7) For each working memory stage, two-tailed Pearson's correlation coefficient between baseline GABA and task-induced gamma amplitude was computed (Fig. 2B). Other cognitive related frequency bands (i.e., theta and beta) were also investigated for assuring the specificity of GABA–oscillation relationships (see Table in Supplementary Materials). 8) For each stage, the difference in task-induced gamma amplitude between groups was also examined (two-tailed independent-samples t-test).
Fig. 2.
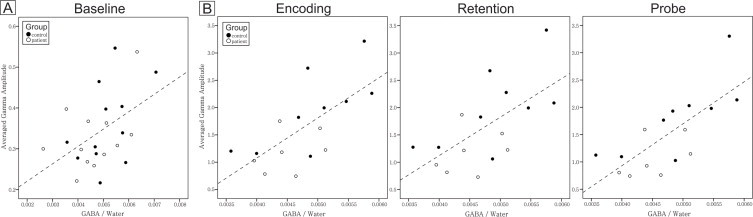
A. Relationship between gamma amplitude and GABA level in the resting state. The filled circles are controls and the open circles are patients. Across groups, the baseline gamma amplitude of the F3 electrode significantly correlated with the baseline GABA level of left DLPFC (two-tailed Pearson's correlation coefficient, N = 24, R = 0.48, P = 0.019). B. Relationships between averaged gamma amplitudes during all three stages (i.e., encoding, retention, and probe) of the working memory task and the baseline GABA level of the left DLPFC. Working memory EEG data consisted of nine controls and seven patients. For each participant, averaged gamma amplitude across trials was computed for each stage. For each working memory stage, two-tailed Pearson's correlation coefficient between baseline GABA and task-induced gamma amplitude was computed. Each stage exhibited a significant correlation between gamma amplitude and GABA level (two-tailed Pearson's correlation coefficients with Bonferroni correction for three stages: corrected alpha level = 0.05/3 = 0.017; n = 16; encoding stage, R = 0.68, P = 0.004; retention stage, R = 0.63, P = 0.009; probe stage, R = 0.73, P = 0.001). Furthermore, within each group and across stages, correlation coefficients are all positive. The correlation coefficients are stronger in healthy controls than in patients.
2.6.6. Peak gamma frequency of the encoding stage and its relationships to GABA and working memory performance
The mode of peak gamma frequencies of single trial wavelets of the encoding stage was calculated for electrode F3 as follows: 1) Using the wavelet decomposition results from above mentioned steps, we found the peak amplitude and frequency for each single trial wavelet within the first 500 ms of the encoding stage. 2) We obtained the mode of peak gamma frequencies of single trial wavelets for each participant. 3) Peak gamma frequency's relationships with GABA (Fig. 3A) and six-letter-set hit rate (Fig. 3B) were examined by two-tailed Pearson's correlation coefficients.
Fig. 3.
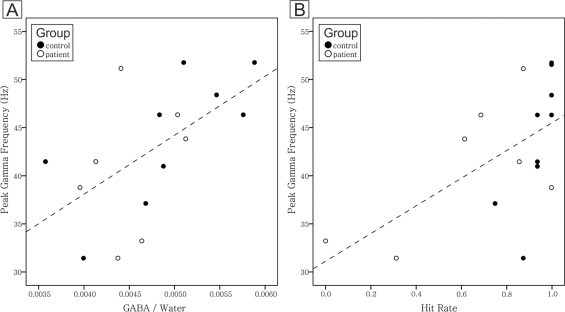
A. Relationship between peak gamma frequency and GABA level. There was a significant correlation between peak gamma frequency and GABA level (two-tailed Pearson's correlation coefficient, n = 16, R = 0.58, P = 0.017). B. Relationship between peak gamma frequency and hit rate. There was also a significant correlation coefficient between peak gamma frequency and hit rate (two-tailed Pearson's correlation coefficient, n = 16, R = 0.59, P = 0.015).
3. Results
Baseline data (N = 24; Fig. 2A) of GABA levels in the left DLPFC (measured by MRS) and gamma frequency band amplitude (recorded by EEG) were collected from 12 controls and 12 patients. Eight controls and six patients completed two blocks of the working memory task (i.e., 128 trials) with good quality EEG recordings and behavioral results. In addition, one control completed three blocks (i.e., 192 trials) and only one patient was able to complete one block (i.e., 64 trials). Therefore, good quality and attentive working memory EEG data consisted of nine controls and seven patients (i.e., 16 out of a total of 24 participants; Fig. 2, Fig. 3). Due to the sample sizes, we may have to consider the results as preliminary.
Consistent with a larger and partially overlapping study sample published previously (Kegeles et al., 2012), there were no differences between patients and controls in GABA levels in the left DLPFC (N = 24, t = 1.204, df = 22, P = 0.241). During baseline/rest (Supplementary Material Fig. 1), two electrode locations corresponding to the left DLPFC showed significant correlations between left DLPFC GABA level and gamma frequency band amplitude (significant correlations circled in Supplementary Material Fig. 1; two-tailed Pearson's correlation coefficients, N = 24; F3, R = 0.48, P = 0.019, the solid circle's scatter plot shown in Fig. 2A; F5, R = 0.42, P = 0.042).
We further investigated the relationships between left DLPFC baseline GABA level and averaged gamma amplitude of electrode F3 during three different working memory stages in our participants as a whole (Fig. 2B). These relationships were significant at each stage of the task, i.e., encoding, retention, and probe stages (two-tailed Pearson's correlation coefficients with Bonferroni correction for three stages: corrected alpha level = 0.05/3 = 0.017; n = 16; encoding stage, R = 0.68, P = 0.004; retention stage, R = 0.63, P = 0.009; probe stage, R = 0.73, P = 0.001).
Patients had significantly lower gamma amplitude than controls in every working memory stage (two-tailed independent-samples t-tests, n = 16; encoding stage, t = 2.51, df = 14, P = 0.025; retention stage, t = 2.52, df = 14, P = 0.024; probe stage, t = 2.49, df = 14, P = 0.026), but not during rest (N = 24, t = 0.814, df = 22, P = 0.424).
GABA level was correlated with the mode of peak gamma frequencies of single trial wavelets of the encoding stage (Fig. 3A; two-tailed Pearson's correlation coefficient, n = 16, R = 0.58, P = 0.017). In Fig. 3B, although there is a ceiling effect for controls (i.e., filled dots), higher hit rate was still significantly correlated with higher peak gamma frequency mode (two-tailed Pearson's correlation coefficient, n = 16, R = 0.59, P = 0.015).
Working memory performance is illustrated in Fig. 4. The hit rate mean and standard error of the mean (SEM) in the six letter set were 0.92 (n = 12, SEM = 0.027) and 0.63 (n = 12, SEM = 0.101) for controls and schizophrenia patients, respectively; there was a significant difference between groups (two-tailed independent-samples t-test, equal variances not assumed, N = 24, t = 2.814, df = 12.545, P = 0.015). There was no significant difference between groups on the one letter set (not shown, N = 24, t = 1.351, df = 22, P = 0.190). All other performance measures showed no significant differences between groups; there are no significant correlations between left DLPFC GABA level and all performance measures (two-tailed Pearson's correlation coefficients; N = 24, all P values > 0.05). Furthermore, no significant correlations were found between averaged gamma amplitude of three different working memory stages and all performance measures (two-tailed Pearson's correlation coefficients; n = 16, all P values > 0.05).
Fig. 4.
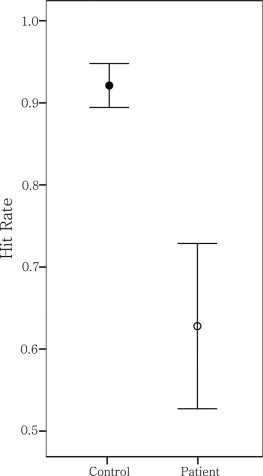
Working memory task hit rate difference between patient and control groups. All participants' task performance was assessed by reaction time, overall accuracy, and rates of hit and correct rejection in one and six letter set conditions. Two-tailed independent-samples t-tests with an alpha level of 0.05 were performed to investigate performance differences between groups. Only the hit rate of the six letter set of the patients was significantly lower than that of the controls (two-tailed independent-samples t-test, equal variances not assumed, n = 24, t = 2.814, df = 12.545, P = 0.015).
4. Discussion
Higher order cognitive processes like working memory depend on synchronization of neural oscillations in gamma frequency bands for optimal function. Since networks of GABAergic neurons are crucial for the generation of gamma oscillations, we hypothesized that DLPFC GABA level and gamma amplitudes during working memory stages (i.e., active states) would be correlated with each other. Our findings showed that, as a whole (patients and controls together), gamma amplitudes during both rest and a working memory task were positively correlated with left DLPFC GABA level (Fig. 2). All three working memory stages showed significant positive correlations between GABA level and gamma amplitude (Fig. 2B). There is no significant correlation coefficient in other cognitive related frequency bands, suggesting the unique and specific relationship between GABA and gamma oscillations (see Table in Supplementary Materials). Due to different scales in Fig. 2A and B, the slopes of Fig. 2A and subplots of Fig. 2B appear very similar; however, for the probe stage, 53% of the variability of the averaged gamma amplitude could be explained by GABA level, followed by 46% and 40% of variability in the encoding and retention stages, respectively. In contrast, only 23% of variability of gamma amplitude during rest was explained by GABA level (Fig. 2A). The higher proportion of gamma amplitude variability during active states explained by GABA level suggests that GABAergic processes are highly responsible for gamma neural oscillations during working memory tasks and more important in cognition than at rest.
Patients' performance and gamma amplitudes during our working memory task were significantly lower than controls'. In our exploratory second-by-second investigations of comparing gamma oscillations across different working memory processes (see Supplementary Material Fig. 2), patients and controls had different modulatory patterns in gamma amplitudes. The gamma-amplitude modulation appeared to be significantly weaker and flatter for patients in terms of the ranges of between- and within-second fluctuations across memory stages. Our data and previous studies have consistently shown working memory deficits and impaired gamma functions in patients with schizophrenia; more importantly, the present study suggests that, due to the robustness of the GABAergic system, it may be able to compensate these impairments and operate in a less optimal capability for achieving minimal daily functions in schizophrenia. Our GABA data of the left DLPFC are consistent with our previous study (Kegeles et al., 2012) that found no alterations in either medicated or unmedicated patient groups compared to the control group; these results are not against the central hypothesis of GABA dysfunction in schizophrenia due to possible compensatory mechanisms that make the GABAergic system robust (see “MPFC GABA Elevations” section under the Comment of Kegeles et al., 2012, for detailed discussion).
In comparison with gamma amplitude as a general index of coordinated neural processing from large numbers of neurons, peak gamma frequencies might reveal that the dominant population response occurs in the frequency range of our gamma amplitude (Brunel and Wang, 2003; Muthukumaraswamy et al., 2009). Controls had a significantly higher hit rate on our working memory task than patients (Fig. 4) and higher peak gamma frequency was significantly correlated with higher hit rate (Fig. 3B). These results are consistent with previous findings that high frequency oscillations are important for working memory function and that deficits in working memory in schizophrenia patients may be due to impaired gamma neural oscillations such that they cannot maintain high frequency oscillations (Light et al., 2006; Spencer et al., 2003). Although synchrony must occur for intact cognitive performance, synchrony has been found at a lower frequency in schizophrenia patients perhaps because of a reduced capability of neuronal networks to support high frequency synchrony (Spencer et al., 2004). Furthermore, the modes of peak gamma frequencies of single trials during the encoding stage were positively correlated with DLPFC GABA levels (Fig. 3A). This is analogous to the previous finding in the medial occipital cortex of healthy participants (Muthukumaraswamy et al., 2009) as peak gamma frequencies during visual processing correlated with resting GABA concentration of the medial occipital cortex (N = 12, R = 0.68, P < .02). The present findings provide new evidence for the role of GABAergic neurons in the left DLPFC during working memory.
4.1. Limitations
The admixture of mobile macromolecule signal in the GABA peak is a study limitation, and it remains unknown whether the macromolecule contribution to the GABA signal is altered in schizophrenia. Another limitation of this study is a relatively small sample size due to the integration of diverse methodologies and participant compliance. Starting with a sample size of 12 healthy participants and 12 patients with schizophrenia, these data consisted of baseline GABA measures of MRS and resting EEGs. This is further reduced to nine and seven, respectively, after we controlled for the qualities of working memory performances and EEGs. It is plausible that the robust results of the study might only be generalized to participants who are capable of finishing intricate, diverse, and lengthy experimental protocols as our empirical experiences clearly indicated that most of participants preferred only to participate in the MRS part of the study without any EEG recording. A future study with a larger sample size and more research resources is needed to settle the issue of generalizability. Another limitation also related to the sample size is that when we performed the confirmatory correlation tests for identifying objectively the electrode of interest (Supplementary Material Fig. 1), multiple comparison corrections were not applied because the sample size is not sufficiently powered to withstand corrections. Hence, we could not rule out the possibility that the localization results could be a capitalization on chance. However, the confirmatory correlation tests showed a cluster of two significant findings with attenuation of other coefficients from the source, its probability of type I error is extremely low (see “Discussion of type I error” of Supplementary Material Fig. 1); furthermore, the arbitrary-planned comparisons of F3 and F5 also indicated that F3 should be the electrode of interest after the alpha level was corrected for two multiple comparisons. Although it is out of the scope of the present study, the relationship between the GABA level and the modulation between the 1 versus 6 letter working memory load is currently being investigated; however, this investigation also inherited the limitations mentioned above and future studies are needed. Lastly, we are aware of the transient-broadband induced gamma-band EEG responses (iGBRtb) that could be caused by miniature saccades from ˜200 to 300 ms following stimulus onsets (Yuval-Greenberg et al., 2008; Yuval-Greenberg et al., 2009). Therefore, we intentionally selected time ranges for averaging gamma amplitude far away from stimulus onsets of the encoding and probe stages, and used EEG data re-referenced to average reference to identify ocular-related artifactual activities that should be maximum around the eyes (Melloni et al., 2009). Our Supplementary Material Fig. 1 showed that there were no significant results for the electrodes near the eyes (e.g., P values of FP1, FP2, AF7, and AF8 =0.423), suggesting our results were not ocular in origin. Furthermore, the iGBRtb is characterized by transient-broadband activities in the gamma frequency range; our wavelet decomposition results of single-trial EEG data of the electrode of interest, F3, did not reveal any transient broadband activity (defined in Yuval-Greenberg et al., 2009: ˜100–150 ms in duration and ˜30–80 Hz in frequency range) not only after stimulus onsets from ˜200 to 300 ms but also during the entire encoding stage (see Supplementary Material Fig. 3). Although our results are unlikely to be ocular in origin and it is out of the scope of this study to investigate the contributions of minisaccade to EEG of the Sternberg working memory task, minisaccades could be modulated by stimulus onsets and cognitive processes such as attention; therefore, we could not rule out all effects of minisaccades as they occur spontaneously throughout the sample period. We have followed the methods of Melloni et al. (2009) and Yuval-Greenberg et al. (2009) to minimize the influence of minisaccades in our data. These methods, which do not use independent component analysis (ICA) and which average gamma amplitude far from stimulus onsets, present potential limitations (Keren et al., 2010). Although there is evidence that removing multiple ICA components associated with saccade activities is a conservative approach to certain data (see the Supplementary Information of Minzenberg et al., 2010), future studies that investigate the role of gamma band neural oscillations in human should consider the significant impact of saccades and compare different detection and correction procedures (Keren et al., 2010). Additional saccadic studies with different applications of ICA (e.g., comparison of different component selections and different ICA algorithms for different experimental paradigms) will be necessary to settle this issue.
5. Conclusion
In patients, both working memory performance and task-induced gamma amplitudes were significantly lower than those in controls. Peak gamma frequency during encoding working memory stage was positively correlated with baseline GABA level of the left DLPFC; additionally, higher peak gamma frequency was significantly correlated with higher hit rate on the working memory task. When grouped together, baseline/resting and task-induced gamma amplitudes were positively correlated with baseline GABA level of the left DLPFC. Despite gamma band amplitude deficits in patients across working memory stages, both baseline and working memory-induced gamma oscillations showed strong dependence on baseline GABA levels in patients and controls. These findings suggest a critical role for GABA function in gamma band oscillations, even under conditions of impairment of these systems and behavioral performances as seen in schizophrenia.
Acknowledgments
This work was supported by the New York State Office of Mental Health, Lieber Center for Schizophrenia Research, Dana Foundation, and Brain and Behavior Research Foundation, K23MH076976 and R01 MH075895. We thank the participants, Ms Jaimie Gowatsky for EEG recordings, and staffs of the Schizophrenia Research Unit and the Division of Translational Imaging at the NYSPI.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2014.03.007.
Appendix. Supplementary Materials
Supplementary Materials for GABA level, gamma oscillation, and working memory performance in schizophrenia.
References
- Barr M.S., Farzan F., Rajji T.K., Voineskos A.N., Blumberger D.M., Arenovich T., Fitzgerald P.B., Daskalakis Z.J. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biological Psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. 23039931 [DOI] [PubMed] [Google Scholar]
- Barr M.S., Farzan F., Tran L.C., Chen R., Fitzgerald P.B., Daskalakis Z.J. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophrenia Research. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. 20598857 [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C., Brand A., Hildebrandt H., Karolina Kedzior K.K., Mathes B., Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. 16962192 [DOI] [PubMed] [Google Scholar]
- Benes F.M., Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. 11377916 [DOI] [PubMed] [Google Scholar]
- Benes F.M., McSparren J., Bird E.D., SanGiovanni J.P., Vincent S.L. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Archives of General Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. 1747023 [DOI] [PubMed] [Google Scholar]
- Brunel N., Wang X.-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation–inhibition balance. Journal of Neurophysiology. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Cannon T.D., Glahn D.C., Kim J., Van Erp T.G.M., Karlsgodt K., Cohen M.S., Nuechterlein K.H., Bava S., Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of General Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. 16203952 [DOI] [PubMed] [Google Scholar]
- Carl C., Açik A., König P., Engel A.K., Hipp J.F. The saccadic spike artifact in MEG. Neuroimage. 2012;59:1657–1667. doi: 10.1016/j.neuroimage.2011.09.020. 21963912 [DOI] [PubMed] [Google Scholar]
- Cho R.Y., Konecky R.O., Carter C.S. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. 17170134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C.E., Webster M.J., Rothmond D.A., Bahn S., Elashoff M., Shannon Weickert C. Prefrontal GABAA receptor a-subunit expression in normal postnatal human development and schizophrenia. Journal of Psychiatric Research. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. 20100621 [DOI] [PubMed] [Google Scholar]
- Elvevåg B., Goldberg T.E. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14:1–21. 11253953 [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Tse M.T., Floresco S.B. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biological Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. 21146155 [DOI] [PubMed] [Google Scholar]
- Farzan F., Barr M.S., Levinson A.J., Chen R., Wong W., Fitzgerald P.B., Daskalakis Z.J. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain: a Journal of Neurology. 2010;133:1505–1514. doi: 10.1093/brain/awq046. 20350936 [DOI] [PubMed] [Google Scholar]
- Fleming K., Goldberg T.E., Binks S., Randolph C., Gold J.M., Weinberger D.R. Visuospatial working memory in patients with schizophrenia. Biological Psychiatry. 1997;41:43–49. doi: 10.1016/S0006-3223(96)00263-6. 8988794 [DOI] [PubMed] [Google Scholar]
- Fries P., Reynolds J.H., Rorie A.E., Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science (New York, N.Y.) 2001;291:1560–1563. doi: 10.1126/science.1055465. 11222864 [DOI] [PubMed] [Google Scholar]
- Gandal M.J., Edgar J.C., Klook K., Siegel S.J. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. 21349276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Fish K.N., Lewis D.A. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plasticity. 2011;2011:723184. doi: 10.1155/2011/723184. 21904685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N., Yoshimura R., Moriya J., Kakeda S., Ueda N., Ikenouchi-Sugita A., Umene-Nakano W., Hayashi K., Oonari N., Korogi Y., Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3 T proton MRS study. Schizophrenia Research. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. 19464152 [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. 6187540 [DOI] [PubMed] [Google Scholar]
- Gray C.M., König P., Engel A.K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–339. doi: 10.1038/338334a0. 2922061 [DOI] [PubMed] [Google Scholar]
- Hájos N., Pálhalmi J., Mann E.O., Németh B., Paulsen O., Freund T.F. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. 15483131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Petit L., Ungerleider L.G., Courtney S.M. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–391. doi: 10.1006/nimg.2000.0592. 10806025 [DOI] [PubMed] [Google Scholar]
- Howard M.W., Rizzuto D.S., Caplan J.B., Madsen J.R., Lisman J.E., Aschenbrenner-Scheibe R., Schulze-Bonhage A., Kahana M.J. Gamma oscillations correlate with working memory load in humans. Cerebral Cortex (New York, N.Y.: 1991) 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. 14615302 [DOI] [PubMed] [Google Scholar]
- Kang S.S., Sponheim S.R., Chafee M.V., MacDonald A.W., III Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia. 2011;49:2836–2847. doi: 10.1016/j.neuropsychologia.2011.06.009. 21703287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles L.S., Mao X., Dyke J., Gonsalez R., Soones T.N., Shungu D.C. Test–retest reliability of dorsolateral prefrontal cortical GABA measurement using an 8-channel phased-array head coil with the J-editing technique at 3 T. Proceedings of the International Society for Magnetic Resonance in Medicine. 2006;14:489. [Google Scholar]
- Kegeles L.S., Mao X., Gonsalez R., Shungu D.C. Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3.0 T. Proceedings of the International Society for Magnetic Resonance in Medicine. 2007;15:1391. [Google Scholar]
- Kegeles L.S., Mao X., Stanford A.D., Girgis R., Ojeil N., Xu X., Gil R., Slifstein M., Abi-Dargham A., Lisanby S.H., Shungu D.C. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate–glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Archives of General Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. 22213769 [DOI] [PubMed] [Google Scholar]
- Keren A.S., Yuval-Greenberg S., Deouell L.Y. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. NeuroImage. 2010;49:2248–2263. doi: 10.1016/j.neuroimage.2009.10.057. 19874901 [DOI] [PubMed] [Google Scholar]
- Klausberger T., Magill P.J., Márton L.F., Roberts J.D.B., Cobden P.M., Buzsáki G., Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. 12594513 [DOI] [PubMed] [Google Scholar]
- Kramer M.A., Tort A.B.L., Kopell N.J. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. Journal of Neuroscience Methods. 2008;170:352–357. doi: 10.1016/j.jneumeth.2008.01.020. 18328571 [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Cho R.Y., Carter C.S., Eklund K., Forster S., Kelly M.A., Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. American Journal of Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. 18923067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. 22154068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. 17805309 [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Hashimoto T. In: International Review of Neurobiology. Lewis D.A., Hashimoto T., editors. Academic Press; 2007. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons; pp. 109–131. [DOI] [PubMed] [Google Scholar]
- Lewis R. Should cognitive deficit be a diagnostic criterion for schizophrenia? Journal of Psychiatry & Neuroscience: JPN. 2004;29:102–113. 15069464 [PMC free article] [PubMed] [Google Scholar]
- Light G.A., Hsu J.L., Hsieh M.H., Meyer-Gomes K., Sprock J., Swerdlow N.R., Braff D.L. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biological Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. 16893524 [DOI] [PubMed] [Google Scholar]
- Luber B., Kinnunen L.H., Rakitin B.C., Ellsasser R., Stern Y., Lisanby S.H. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency- and time-dependent effects. Brain Research. 2007;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. 17113573 [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., III, Carter C.S., Kerns J.G., Ursu S., Barch D.M., Holmes A.J., Stenger V.A., Cohen J.D. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. 15741464 [DOI] [PubMed] [Google Scholar]
- Markwardt C. Non-linear least squares fitting in IDL with MPFIT. Proceedings of Astronomical Data Analysis Software and Systems. 2009;XVIII(411):251–254. [Google Scholar]
- McBain C.J., Fisahn A. Interneurons unbound. Nature Reviews. Neuroscience. 2001;2:11–23. doi: 10.1038/3504904710.1038/35047544. 11253355 [DOI] [PubMed] [Google Scholar]
- Melloni L., Schwiedrzik C.M., Wibral M., Rodriguez E., Singer W. Response to: Yuval-Greenberg et al., “Transient induced gamma-band response in EEG as a manifestation of miniature saccades”. Neuron 58, 429–441. Neuron. 2009;62:8–10. doi: 10.1016/j.neuron.2009.04.002. 19376062 [DOI] [PubMed] [Google Scholar]
- Meltzer J.A., Zaveri H.P., Goncharova I.I., Distasio M.M., Papademetris X., Spencer S.S., Spencer D.D., Constable R.T. Effects of working memory load on oscillatory power in human intracranial EEG. Cerebral Cortex (New York, N.Y.: 1991) 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. 18056698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L., Martin E., Klaver P., Edden R., Zelaya F., Lythgoe D.J., Lüchinger R., Brandeis D., O'Gorman R.L. Frontal GABA levels change during working memory. PLOS ONE. 2012;7:e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J., Firl A.J., Yoon J.H., Gomes G.C., Reinking C., Carter C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. 20827271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Edden R.A.E., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. 19416820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T., Augood S.J., Arai H., McKenna P.J., Emson P.C. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA A receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neurosciences. 1999;93:441–448. doi: 10.1016/S0306-4522(99)00189-X. [DOI] [PubMed] [Google Scholar]
- Ongür D., Prescot A.P., McCarthy J., Cohen B.M., Renshaw P.F. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. 20598290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B., Pezaris J.S., Sahani M., Mitra P.P., Andersen R.A. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nature Neuroscience. 2002;5:805–811. doi: 10.1038/nn890. 12134152 [DOI] [PubMed] [Google Scholar]
- Rothman D.L., Petroff O.A.C., Behar K.L., Mattson R.H. Localized 1H NMR measurements of ?-aminobutyric acid in human brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. 8516315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L.M., Edden R.A.E., Kontson K., Zhu H., Barker P.B., Hong L.E. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2013;25:83–87. doi: 10.1176/appi.neuropsych.11120368. 23487198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L.M., Kontson K., West J., Edden R.A., Zhu H., Wijtenburg S.A., Holcomb H.H., Barker P.B. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophrenia Bulletin. 2013;39:1096–1104. doi: 10.1093/schbul/sbs092. 23081992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N., LeRoux P., Hurd R., Wang P., Sachs N., Ketter T. Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proceedings of the International Society for Magnetic Resonance in Medicine. 2001;9:1011. [Google Scholar]
- Schmiedt C., Brand A., Hildebrandt H., Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Research. Cognitive Brain Research. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. 16289526 [DOI] [PubMed] [Google Scholar]
- Shungu D.C., Mao X., Kegeles L.S. Evaluation of GABA detection sensitivity gains achieved with an 8-channel phased-array head coil at 3.0 T in the human dorsolateral prefrontal cortex using the J-editing technique. Proceedings of the International Society for Magnetic Resonance in Medicine. 2006;14:488. [Google Scholar]
- Simpson H.B., Shungu D.C., Bender J., Jr., Mao X., Xu X., Slifstein M., Kegeles L.S. Investigation of cortical glutamate–glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37:2684–2692. doi: 10.1038/npp.2012.132. 22850733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neurobiology. Striving for coherence. Nature. 1999;397:391–393. doi: 10.1038/17021. 9989402 [DOI] [PubMed] [Google Scholar]
- Sohal V.S., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. 19396159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K.M. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Frontiers in Human Neuroscience. 2012;5 doi: 10.3389/fnhum.2011.00190. 22319485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K.M., Nestor P.G., Niznikiewicz M.A., Salisbury D.F., Shenton M.E., McCarley R.W. Abnormal neural synchrony in schizophrenia. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. 12917376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K.M., Nestor P.G., Perlmutter R., Niznikiewicz M.A., Klump M.C., Frumin M., Shenton M.E., McCarley R.W. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. 15546988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/S1364-6613(99)01299-1. 10322469 [DOI] [PubMed] [Google Scholar]
- Tayoshi S., Nakataki M., Sumitani S., Taniguchi K., Shibuya-Tayoshi S., Numata S., Iga J., Ueno S., Harada M., Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophrenia Research. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. 20022731 [DOI] [PubMed] [Google Scholar]
- Traub R.D., Kopell N., Bibbig A., Buhl E.H., LeBeau F.E.N., Whittington M.A. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. 11717382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. 17015233 [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. 20087360 [DOI] [PubMed] [Google Scholar]
- van Veelen N.M.J., Vink M., Ramsey N.F., Kahn R.S. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophrenia Research. 2010;123:22–29. doi: 10.1016/j.schres.2010.07.004. 20724113 [DOI] [PubMed] [Google Scholar]
- Vance A., Hall N., Bellgrove M.A., Casey M., Karsz F., Maruff P. Visuospatial working memory deficits in adolescent onset schizophrenia. Schizophrenia Research. 2006;87:223–227. doi: 10.1016/j.schres.2006.04.025. 16793240 [DOI] [PubMed] [Google Scholar]
- Varela F., Lachaux J.P., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews. Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. 11283746 [DOI] [PubMed] [Google Scholar]
- Volk D.W., Pierri J.N., Fritschy J.M., Auh S., Sampson A.R., Lewis D.A. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cerebral Cortex (New York, N.Y.: 1991) 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. 12217970 [DOI] [PubMed] [Google Scholar]
- Von Stein A., Chiang C., König P. Top-down processing mediated by interareal synchronization. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. 11121074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Maddock R.J., Rokem A., Silver M.A., Minzenberg M.J., Ragland J.D., Carter C.S. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. 20220012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S., Keren A.S., Tomer O., Nelken I., Deouell L.Y. Response to Letter: Melloni et al., “Transient induced gamma-band response in EEG as a manifestation of miniature saccades.” Neuron 58, 429–441. Neuron. 2009;62:10–12. doi: 10.1016/j.neuron.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S., Tomer O., Keren A.S., Nelken I., Deouell L.Y. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. 18466752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials for GABA level, gamma oscillation, and working memory performance in schizophrenia.


