Abstract
Schizophrenia is characterized by impaired cognitive functioning, and brain regions involved in cognitive control processes show marked glutamatergic abnormalities. However, it is presently unclear whether aberrant neuronal response is directly related to the observed deficits at the metabolite level in schizophrenia. Here, 17 medicated schizophrenia patients and 17 matched healthy participants underwent functional magnetic resonance imaging (fMRI) when performing an auditory cognitive control task, as well as proton magnetic resonance spectroscopy (1H-MRS) in order to assess resting-state glutamate in the anterior cingulate cortex. The combined fMRI–1H-MRS analysis revealed that glutamate differentially predicted cortical blood-oxygen level-dependent (BOLD) response in patients and controls. While we found a positive correlation between glutamate and BOLD response bilaterally in the inferior parietal lobes in the patients, the corresponding correlation was negative in the healthy control participants. Further, glutamate levels predicted task performance in patients, such that lower glutamate levels were related to impaired cognitive control functioning. This was not seen for the healthy controls. These findings suggest that schizophrenia patients have a glutamate-related dysregulation of the brain network supporting cognitive control functioning. This could be targeted in future research on glutamatergic treatment of cognitive symptoms in schizophrenia.
Keywords: Combined fMRI–MRS, 1H-MRS, Cognitive control, Anterior cingulate cortex, Inferior parietal lobe, Glutamate, BOLD, Connectivity
Highlights
-
•
Neuronal processing of cognitive control is different in schizophrenia patients (SZ).
-
•
Cingulum glutamate levels predict the degree of parietal neuronal response.
-
•
Lower glutamate predicts poorer cognitive control abilities in SZ.
-
•
SZ have a glutamate-related neuronal dysregulation of cognitive control processing.
1. Introduction
Cognitive control dysfunction is one of the core deficits in schizophrenia. It is often manifested as problems in relating daily activities to internal goals and intentions (Miller, 2000), and effective pharmacological treatment for these symptoms is still lacking (Lesh et al., 2011). A fronto-parietal brain network assembling around the dorsal anterior cingulate cortex (ACC) is considered the anatomical substrate of cognitive control processes (Gruber and Goschke, 2004; Ridderinkhof et al., 2004; Vincent et al., 2008). Accordingly, anatomical (Benes et al., 1992; Fornito et al., 2009) as well as functional (Minzenberg et al., 2009) alterations in this brain network have been reported in schizophrenia. These alterations are also reflected on the biochemical level, as revealed in studies using proton magnetic resonance spectroscopy (1H-MRS; Port and Agarwal, 2011). More specifically, studies on ACC and medial prefrontal cortex indicate that schizophrenia patients have alterations in the glutamate system (Benes et al., 1992), often presenting as elevated glutamate levels as measured by 1H-MRS early in the disease, with a decrease or normalization with time and use of medication (Marsman et al., 2011; Poels et al., 2014). Glutamate is the most widely distributed excitatory neurotransmitter in the brain and also acts as an intermediate in cerebral energy metabolism (Rothman et al., 2003). The glutamatergic hypothesis of schizophrenia is currently well recognized, claiming a crucial role of the glutamate system in the genesis of schizophrenia (see Coyle et al., 2012 for a review), although presently the underlying abnormalities are not fully understood. The glutamatergic abnormalities have been linked to a possible excitatory/inhibitory imbalance related to N-methyl-d-aspartate (NMDA) and metabotropic receptors (Coyle et al., 2012; Woo et al., 2008), in addition to a more indirect imbalance through ?-aminobutyric acid (GABA)ergic dysregulation (Lewis et al., 2005).
The combination of functional magnetic-resonance imaging (fMRI) and 1H-MRS constitutes a promising method for probing the relationship between glutamate and neuronal response during tasks and resting-state (Duncan et al., 2011; Enzi et al., 2012; Falkenberg et al., 2012; Horn et al., 2010; Kapogiannis et al., 2013; Schmaal et al., 2012). Using this combined approach, we previously found that resting-state glutamate (rsGlu) levels in the ACC predicted the blood-oxygen level-dependent (BOLD) response in several brain regions when healthy individuals performed a cognitive control task (Falkenberg et al., 2012). Considering schizophrenia patients' problems on tasks assessing cognitive control functions, together with the known glutamatergic pathology, we now suggest that glutamate differentially affects the BOLD response in schizophrenia patients and healthy individuals and that this is a mediating factor behind cognitive impairment in schizophrenia. Such a result would allow us to link glutamatergic mediation of neuronal activity at the metabolite-level to cognitive abnormalities found in schizophrenia. Moreover, we expected that patients with schizophrenia would show impaired performance on a cognitive control task and that the level of task performance would be moderated by glutamate levels.
2. Materials and methods
2.1. Participants
Seventeen patients with schizophrenia (DSM-IV/ICD-10) and 17 healthy participants matched for age, sex, and handedness participated in the study. Exclusion criteria were a history of neurological disorders, traumatic brain injuries, and metallic implants. Patients were assessed with the Positive and Negative Syndrome Scale (PANSS; Table 1; Kay et al., 1987) and obtained a score of four or higher on the P3 Hallucinations-item (moderate to severe hallucinations). The patients were part of a general study on schizophrenia patients with auditory verbal hallucinations, and all were on atypical antipsychotic medication (olanzapine, clozapine, aripiprazole, quetiapine, paliperidone, or amisulpride). Due to the verbal nature of the cognitive control task (see below), hearing threshold was assessed with the Hughson–Westlake audiometric test (Oscilla USB-300, Inmedico, Lystrup, Denmark). Participants with an averaged inter-aural acuity difference of more than 10 dB were excluded. Eleven of the healthy participants were also part of a previous study (Falkenberg et al., 2012) and were included as control subjects for the schizophrenia patients based on the best match according to the above criteria. The study was approved by the Regional Committee for Medical Research Ethics in Western Norway (REK-Vest), and informed consent was obtained from all participants before the study.
Table 1.
Demographic and clinical characteristics.
| Characteristics | SZ (n = 17) | HC (n = 17) |
|---|---|---|
| Age (mean) | 30 ± 10 | 28 ± 4 |
| Sex, no. | ||
| Female | 7 | 7 |
| Male | 10 | 10 |
| Duration of illness, years (mean)a,b | 9 ± 5 | – |
| Handedness, right/left | 14/3 | 14/3 |
| PANSS scores (mean)a | ||
| Positive total | 18 ± 6 | – |
| Negative total | 16 ± 6 | – |
| General total | 30 ± 8 | – |
| PANSS total | 64 ± 17 | – |
± indicates SD.
SZ, schizophrenia patients; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale.
n = 16, data missing for one patient.
Duration = years, from onset of symptoms.
2.2. Auditory cognitive control task
The experimental paradigm was a version of the Bergen dichotic listening task, which is an auditory speech perception task with simultaneous pair-wise presentations of consonant–vowel syllables (/ba/, /da/, /ga/, /ka/, /pa/, /ta/; see Hugdahl, 2003 and Falkenberg et al., 2011 for details). A stimulus-driven (bottom-up) and an instruction-driven (top-down) component were included in the paradigm so as to achieve systematic variation in the need for cognitive control. The bottom-up component was implemented by varying the stimulus salience through different levels of inter-aural sound intensity (Hugdahl et al., 2008; Westerhausen and Hugdahl, 2010). Five levels of interaural intensity differences (IIDs) were used: 18 dB in favor of the left ear, 9 dB in favor of the left ear, no intensity difference, 9 dB in favor of the right ear and 18 dB in favor of the right ear. The stimulus was presented at a 70 dB sound pressure level (SPL) at both ears in the condition with no intensity difference. The other four conditions were presented with 70 dB SPL for the louder stimulus, while the weaker was reduced to either 61 or 52 dB SPL. The instruction-driven top-down component was applied by selectively directing the attention of the subject. The subjects were instructed to specifically focus and report from the right- (forced right condition, FR) or the left-ear stimulus (forced left condition, FL; Hugdahl, 2003; Hugdahl and Andersson, 1986). The interaction between the bottom-up and top-down conditions thereby determines the demand for cognitive control, that is when the IID and attention instruction (ATT) are incongruent (e.g., attention focused on the left ear while the right ear is louder) there is higher need for cognitive control mechanisms than when IID and ATT are congruent (Falkenberg et al., 2011; Westerhausen et al., 2010).
The two attentional instructions (FR, FL) combined with the five levels of IID resulted in 10 experimental conditions. Each condition consisted of 18 dichotic presentations, resulting in a total of 180 stimulus presentations pseudo-randomly intermixed with 90 silent null-events. This created a stochastic event-related design for fMRI acquisition (Friston et al., 1999), recorded in a single session. Attention instructions were randomly intermixed, preceded the stimuli by 1.5 s, and were given in writing through goggles mounted on the head coil (NordicNeuroLab Inc., Bergen, Norway). The dichotic syllables were presented at the beginning of the silent gaps of the sparse sampling protocol for the fMRI acquisition (van den Noort et al., 2008) using headphones (NordicNeuroLab Inc.). The participants responded orally immediately after the stimulus by naming the syllable they heard, thereby avoiding movement artifacts during fMRI-acquisition. Stimulus administration and synchronization were performed using E-Prime software (version 2.0, Psychology Software Tools Inc., Pittsburgh, PA, USA).
2.3. Functional magnetic resonance imaging (fMRI) acquisition and analysis
Imaging was performed on a 3.0 T GE Signa HDx scanner, using an eight-channel head coil. A short scout sequence and structural image were acquired first, followed by the fMRI and the 1H-MRS. Structural imaging was performed with a T1-weighted pulse sequence (Fast Spoiled Gradient, FSPGR; TR = 7.9 ms; TE = 3.1 ms; 11° flip angle) measuring 180 sagittal slices of 1 mm thickness (field of view, FOV (mm) = 256 × 256; 256 × 256 scan matrix).
fMRI was performed using an echo-planar imaging (EPI) sequence (TE = 30 ms; 90° flip angle) and was oriented to the structural image. A sparse sampling protocol was used (TR = 3.5 s, TA = 1.5 s) leaving a silent gap of 2.0 s between consecutive scans for task implementation, thereby reducing interference from scanner noise and avoiding movement artifacts during scanning. EPI volumes covered the cerebrum and most of the cerebellum, and contained 25 axial slices of 5 mm thickness (0.5 mm inter-slice gap; FOV 220 × 220 mm, 64 × 64 scan matrix), resulting in a voxel size of 3.44 × 3.44 × 5.0 mm. Image preprocessing and statistical analysis of the data were performed using Statistical Parametrical Mapping (SPM8) analysis software package (Wellcome Department of Cognitive Neurology, London, UK). The EPI images were realigned intra-individually to the first image in each time series and unwarped for correction of head movements and related image distortions. The images were then normalized to standard stereotactic space using the MNI-template and re-sampled to a cubic voxel size of 3 mm as well as smoothed using a 6 mm FWHM Gaussian filter. First-level individual statistical analysis of the fMRI data was set up as a model including a predictor for each of the 10 experimental conditions, and movement parameters were added as regressors. The predictors were convolved with the canonical hemodynamic response function (hrf) and a temporal high pass filter (cutoff at 128 s) was applied. The resulting individual beta-maps were used for the second-level group analysis.
For the fMRI group analysis, only four of the 10 conditions were included in order to investigate the BOLD response during cognitive control. This involved two conditions where the IID and attention were incongruent (FR with 18 dB in favor of the left ear, and FL with 18 dB in favor of the right), and two conditions with congruent IID and attention (FL with 18 dB in favor of the left ear, and FR with 18 dB in favor of the right). These conditions were thought to trigger high and low demands for cognitive control, respectively. The analysis was set up as a full factorial design within the framework of the general linear model, with the predictors attention instruction (ATT; 2 levels), interaural intensity difference (IID; 2 levels), and diagnosis (categorical factor). All analyses were performed using SPM8. We used an intensity threshold of F = 11.34 (equal to voxel-level P < .001) and a cluster threshold of k = 50, which together produced an effective corrected P = 0.5 threshold (cluster-level; Friston et al., 1996; Xiong et al., 1995). This method was applied due to its higher sensitivity to smaller effects, thus reducing the probability of type II errors (Lieberman and Cunningham, 2009). Significant clusters were explored post-hoc using the SPM toolbox MarsBaR (Brett et al., 2002) and MRIcroN software (http://www.cabiatl.com/mricro/mricron). The anatomical location of each cluster was determined using Automated Anatomical Labeling (Tzourio-Mazoyer et al., 2002).
2.4. Magnetic resonance spectroscopy (1H-MRS) acquisition and analysis
An axial T2-weighted image was obtained prior to the 1H-MRS for voxel positioning. In vivo 1H-spectra were obtained from the bilateral dorsal ACC (Fig. 1) using a single voxel point resolved spectroscopy (PRESS) sequence (TR = 1500 ms, TE = 35 ms, voxel size 20 × 20 × 20 mm, 128 averages). The line-width was <8 Hz and water suppression level at least 94%. The spectra were analyzed using LCModel fitting over the spectral range from 0.4 to 4.0 ppm (version 6.2-1A; Provencher, 1993), using a simulated basis-set of 16 metabolites for a TE of 35 ms (l-alanine, aspartate, creatine, phosphocreatine, GABA, glucose, glutamine, glutamate, glycerophosphocholine, phosphocholine, l-lactate, myo-inositol, N-acetyl aspartate, N-acetyl aspartate glutamate, taurine, and glycine). Resting-state glutamate values (rsGlu; relative to creatine) and total creatine concentration (creatine + phosphocreatine) were used from the LCModel output, only including results with a Cramer–Rao lower bound (CRLB) < 20% (Table 2 and Inline Supplementary Fig. S1). A three-way ANOVA with the factors rsGlu, hemisphere, and diagnosis was performed. Effect-size measures were calculated as percentage explained variance (?2), and post-hoc analyses were performed using Fisher's LSD.
Fig. 1.
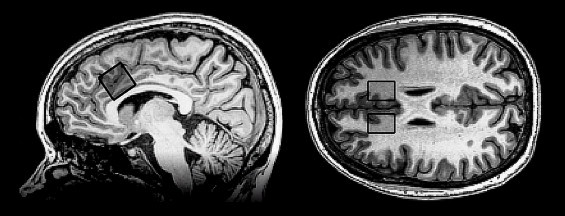
Voxel placements for the 1H-MRS in the bilateral dorsal anterior cingulate cortex.
Table 2.
1H-MRS values.
| SZ |
HC |
|||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| rsGlu/Cre | 1.44 ± 0.15a,b | 1.65 ± 0.21b | 1.59 ± 0.18a | 1.66 ± 0.16 |
| FWHM | 0.039 ± 0.006 | 0.045 ± 0.006 | 0.038 ± 0.004 | 0.045 ± 0.008 |
| S/N | 20.24 ± 3.21 | 18.41 ± 2.67 | 22.76 ± 2.08 | 20.06 ± 2.54 |
| CRLB % | 8 ± 1.87a | 7.53 ± 1.12 | 6.65 ± 0.61a | 6.94 ± 0.75 |
| GM | 34.2 ± 6.68 | 32.32 ± 7.1 | 35.14 ± 5.52 | 34.66 ± 5.8 |
| WM | 62.53 ± 7.6 | 64.4 ± 7.65 | 62.46 ± 7.1 | 62.42 ± 6.91 |
± indicates SD.
SZ, schizophrenia patients; HC, healthy controls; rsGlu/Cre, resting-state glutamate relative to creatine; FWHM, full-width-at-half-maximum; S/N, signal-to-noise ratio; CRLB, Cramer–Rao lower bound; GM, gray matter proportion in voxel; WM, white matter proportion in voxel.
P < .05 significant difference between SZ and HC.
P < .05 significant difference between the two hemispheres.
Supplementary Figure S1.
Fig. S1.
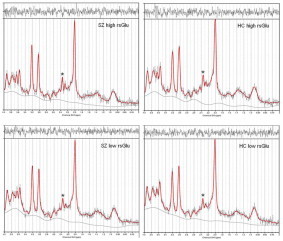
Representative 1H-MRS spectra from the four glutamate-groups. Spectra were acquired by LCModel from four individuals representing the four groups. The absorption peak from which glutamate is measured is marked by an asterisk (*). SZ, schizophrenia patients; HC, healthy controls; rsGlu, resting-state glutamate.
Inline Supplementary Fig. S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2014.03.014.
2.4.1. Control of possible confounding factors
As glutamate levels might depend on the amount of gray and white matter within the measured voxel (Srinivasan et al., 2006), and differences in ACC gray matter have been reported within healthy individuals (Huster et al., 2007) and between healthy controls and schizophrenia patients (Fornito et al., 2009), it was important to test for whether anatomical differences within the voxels could confound the rsGlu values. The proportion of gray and white matter in each region was calculated using scripts developed in-house, leveraging the SPM8 package's combined segmentation and spatial normalization functions. Coordinates and orientation in scanner space were read directly from the raw spectroscopy data files (P*.7, GE P-file format). A structural T2 reference image acquired immediately before the spectroscopy data was coregistered against a higher-resolution T1 image acquired earlier in the session; the resulting rigid body transformation was applied to the spectroscopy voxel coordinates to compensate for any patient movement between the two scans. The structural T1 image was subsequently coregistered to a standard MNI template and segmented into tissue probability maps for three classes of tissue (gray matter, white matter and CSF). These maps were integrated over each transformed spectroscopy voxel to obtain final estimates of region content. However, neither gray matter (r = 0.25, 0.07, 0.02, -0.06, for SZ left, SZ right, HC left, and HC right, respectively), white matter (r = -0.31, 0.00, -0.03, 0.12), nor total brain volume (r = -0.11, -0.01, -0.35, 0.05) correlated significantly (P < .05) with rsGlu levels. Thus, we consider it unlikely that individual differences in ACC structure influenced the reported rsGlu levels in our sample. Additionally, rsGlu levels correlated with neither age (SZ left: r = -0.18, SZ right: r = -0.28, HC left: r = -0.22, HC right: r = -0.1) nor duration of illness (SZ left: r = 0.19, SZ right: r = -0.22).
Creatine is commonly used as an internal reference in 1H-MRS. However, since creatine abnormalities have been found in schizophrenia patients (Öngür et al., 2009) we performed a three-way ANOVA with the factors creatine (Cr + PCr total concentration values, dependent variable), hemisphere (2 levels; right and left) and diagnosis (categorical factor) in order to control for possible creatine differences. Since there were no significant interactions of creatine with either hemisphere (P = .62) or diagnosis (P = .20), we concluded that the use of creatine as an internal standard was valid.
2.5. Combined fMRI–1H-MRS analysis
The contrast images between the incongruent and congruent conditions from the individual first-level fMRI analyses were included as a dependent variable in the combined analysis (full factorial). RsGlu values (continuous, between-subjects factor; z-standardized) and diagnosis (categorical factor), together with the interaction between the two were included as predictors (Cohen et al., 2003). An intensity threshold of t = 3.39 (equivalent to voxel-level P < .001) together with a cluster threshold of k = 50 was used to yield an effective corrected P = 0.05 threshold (Xiong et al., 1995).
2.6. Task performance analysis
The behavioral data from the dichotic listening task (same four conditions as for the fMRI-analysis) were analyzed with a five-way factorial ANOVA with rsGlu (2 levels; high and low) and diagnosis (2 levels; SZ and HC) as categorical factors, together with the repeated-measures factors ATT (2 levels), IID (2 levels), and ear (2 levels; right and left ears). This was done for both right and left hemisphere glutamate, separately. The concept of interest in the present study was the ability of cognitive control, which was defined as the interaction between ATT, IID and ear (see e.g., Falkenberg et al., 2011; Passow et al., 2014; Westerhausen et al., 2010). Hence, the four- and five-way interactions, respectively, of these three factors together with glutamate and diagnosis were the effects of interest in this analysis. For this reason, and based on the omnibus ANOVA (see below), post-hoc three-way ANOVAs with the factors ATT, IID and ear were performed for the four ‘glutamate groups’ in order to determine the effect sizes separately for each group (SZ low rsGlu, SZ high rsGlu, HC low rsGlu, HC high rsGlu). Here, we were interested in the possible difference in how the groups managed to utilize top-down mechanisms and respond to the “correct” ear (represented by the interaction between ATT and ear) and how they were driven by the bottom-up perceptual salience (the interaction of IID and ear). Further post-hoc analyses were performed using Fisher's LSD and effect-size measures were calculated as explained variance (?2).
3. Results
3.1. fMRI
The statistical analysis showed a significant difference in BOLD response between the groups in the right middle occipital gyrus/angular gyrus (BA 19/39), as indicated by a three-way interaction between diagnosis, ATT and IID (Inline Supplementary Fig. S2 and Inline Supplementary Fig. S3). The post-hoc analysis showed that SZ had higher BOLD response in this region during high demand for cognitive control than HC.
Supplementary Figure S2.
Fig. S2.
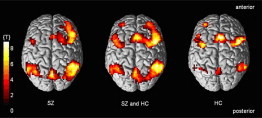
Overall fMRI BOLD response from the auditory cognitive control task. Showing the results for the schizophrenia patients (SZ) and healthy controls (HC) separately, together with the combined BOLD response over the two groups (P < .001, extent threshold k = 50 voxels). See Falkenberg et al. (2011) and Westerhausen et al. (2010) for a more detailed discussion of the above regions and the dichotic listening task in healthy individuals.
Supplementary Figure S3.
Fig. S3.

Group difference in fMRI BOLD response. The red cluster shows the region where schizophrenia patients (SZ) show higher BOLD response during high demands for cognitive control compared to healthy controls (HC; P < .001, extent threshold k = 50 voxels). The blue cluster indicates the region influenced by glutamate levels (see also Fig. 2). LE, left ear; RE, right ear; FR, forced-right attention condition; FL, forced-left condition; “+” indicates the ear with louder (by 18 dB) sound intensity.
Inline Supplementary Figs. S2–S3 can be found online at http://dx.doi.org/10.1016/j.nicl.2014.03.014.
3.2. 1H-MRS
The statistical analysis of the 1H-MRS glutamate data showed a significant interaction between hemisphere and diagnosis (P < .05, F = 4.63, ?2 = .04), with the post-hoc analysis revealing that SZ had lower levels of rsGlu in the left ACC than HC. A significant main effect of hemisphere (P < .001, F = 19.7, ?2 = 0.17) was also driven by lower levels of rsGlu in the left ACC in SZ only. Due to the significant difference in rsGlu between SZ and HC in the left ACC, all further analyses were performed separately for both hemispheres. The main effect for diagnosis was not significant.
3.3. Combined fMRI–1H-MRS
The statistical analysis of the combined fMRI–1H-MRS data revealed a significant modulation by right ACC glutamate, as indicated by a three-way interaction between diagnosis, rsGlu and BOLD response. This interaction was found bilaterally in the inferior parietal lobe (IPL; BA 39/40), with the right-hemispheric cluster located more ventrally (reaching angular gyrus, see Fig. 2). As shown in Fig. 2, right rsGlu moderates the BOLD response differentially in SZ and HC, with BOLD response in the bilateral IPL correlating positively with rsGlu in SZ under high demands for cognitive control, while this relationship is negative in HC. As expected, there was no interaction between rsGlu and ACC BOLD response (see Falkenberg et al., 2012). Moreover, left rsGlu did not show any significant interactions with the BOLD response.
Fig. 2.
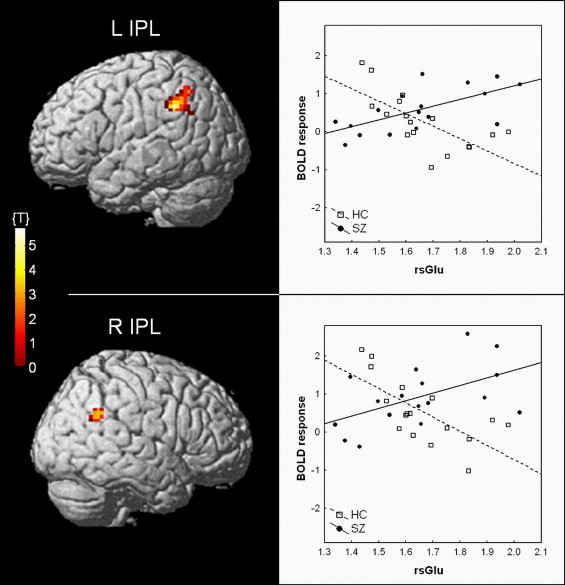
Results from the combined fMRI–1H-MRS analysis, showing the regions where right ACC resting-state glutamate (rsGlu) differentially moderates the BOLD response in the cognitive control task (P < .001, extent threshold k = 50 voxels). Schizophrenia patients (SZ) and healthy controls (HC) display a positive and negative correlation, respectively, between BOLD response and right rsGlu. IPL, inferior parietal lobe; L, left; R, right.
3.4. Task performance
The four-way interactions between ATT, IID, ear, and glutamate or diagnosis were not significant, but the five-way interaction between ATT, IID, ear, rsGlu, and diagnosis showed a clear trend towards significance (P = .07, n2 = .01; Inline Supplementary Table 1). Based on their rsGlu levels, SZ and HC were median-split into low- (n = 8/8; SZ: rsGlu = 1.47 ± 0.11; HC: rsGlu = 1.53 ± 0.07) and high-glutamate (n = 9/9; SZ: rsGlu = 1.81 ± 0.15; HC: rsGlu = 1.77 ± 0.13) groups. Separate ANOVAs performed for each right ACC glutamate group showed that the four groups differed strongly in how much they were influenced by the bottom-up sound intensity manipulation (Fig. 3, Inline Supplementary Table 2). While high rsGlu SZ and both HC groups displayed moderate to large effect sizes for the interaction between IID and ear, the performance of low rsGlu SZ appeared to be mostly driven by the IID during task response. Additionally, the interaction between ATT and ear, showing the ability to follow the attention instruction by using top-down cognitive control, gave moderate to large effect sizes in the HC only. By comparison, the interaction between ATT, IID, and ear did not explain much variance in any of the groups. Other significant main effects and interactions in the five-way ANOVA not related to rsGlu or diagnosis have been discussed in previous analyses of similar data (Falkenberg et al., 2011; Westerhausen et al., 2010). Left hemisphere rsGlu showed no significant main effect or interaction with task performance.
Fig. 3.
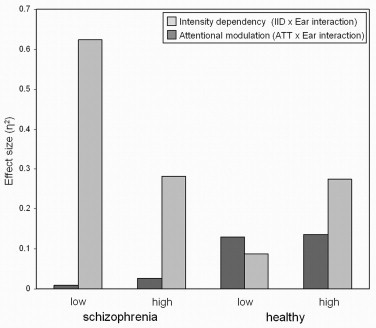
Task performance for the four glutamate-groups. Effect sizes (?2) are shown for the four groups' degree of sound intensity dependency (i.e., the effect size of the IID × ear interaction) and the capability for attentional modulation (i.e., the effect size of the ATT × ear interaction) of the auditory cognitive control task. IID, interaural intensity difference; ATT, attention instruction.
Supplementary Table S1.
Supplementary Table S1.
Task performance. Results of a five-way ANOVA with the factors resting-state glutamate, diagnosis, attention instruction, intensity difference, and ear.
| F | dfeffect | dferror | n2 | P value | |
|---|---|---|---|---|---|
| rsGlu | 2.45 | 1 | 38 | .02 | .13 |
| diagn | 1.61 | 1 | 38 | .01 | .21 |
| rsGlu * diagn | 0.01 | 1 | 38 | 0 | .90 |
| ATT | 7.75 | 1 | 38 | 0 | .01 |
| ATT * rsGlu | 3.82 | 1 | 38 | 0 | .06 |
| ATT * diagn | 0.13 | 1 | 38 | 0 | .72 |
| ATT * rsGlu * diagn | 0.51 | 1 | 38 | 0 | .48 |
| IID | 0.19 | 1 | 38 | 0 | .67 |
| IID * rsGlu | 1.51 | 1 | 38 | 0 | .23 |
| IID * diagn | 0.05 | 1 | 38 | 0 | .83 |
| IID * rsGlu * diagn | 0.05 | 1 | 38 | 0 | .83 |
| ear | 7.77 | 1 | 38 | .12 | .01 |
| ear * rsGlu | 0.62 | 1 | 38 | .01 | .43 |
| ear * diagn | 0.87 | 1 | 38 | .01 | .36 |
| ear * rsGlu * diagn | 0.59 | 1 | 38 | .01 | .45 |
| ATT * IID | 4.65 | 1 | 38 | .03 | .04 |
| ATT * IID * rsGlu | 0.17 | 1 | 38 | 0 | .68 |
| ATT * IID * diagn | 4.63 | 1 | 38 | .03 | .04 |
| ATT * IID * rsGlu * diagn | 2.95 | 1 | 38 | .02 | .09 |
| ATT * ear | 12.46 | 1 | 38 | .05 | .00 |
| ATT * ear * rsGlu | 2.37 | 1 | 38 | .01 | .13 |
| ATT * ear * diagn | 3.73 | 1 | 38 | .02 | .06 |
| ATT * ear * rsGlu * diagn | 0.73 | 1 | 38 | 0 | .40 |
| IID * ear | 7.97 | 1 | 38 | .14 | .01 |
| IID * ear * rsGlu | 5.67 | 1 | 38 | .10 | .02 |
| IID * ear * diagn | 6.31 | 1 | 38 | .11 | .02 |
| IID * ear * rsGlu * diagn | 0.40 | 1 | 38 | .01 | .53 |
| ATT * IID * ear | 0.21 | 1 | 38 | 0 | .65 |
| ATT * IID * ear * rsGlu | 1.03 | 1 | 38 | 0 | .32 |
| ATT * IID * ear * diagn | 0.01 | 1 | 38 | 0 | .91 |
| ATT * IID * ear * rsGlu * diagn | 3.48 | 1 | 38 | .01 | .07 |
rsGlu, resting-state glutamate level (high or low, right hemisphere); diagn, diagnosis (schizophrenia patient or healthy control); ATT, attention instruction; IID, interaural intensity difference.
Supplementary Table S2.
Supplementary Table S2.
Effect sizes (?2) from the separate ANOVAs of the task performance for the glutamate groups.
| SZ low | SZ high | HC low | HC high | |
|---|---|---|---|---|
| ATT * ear | 0.008 | 0.025 | 0.131 | 0.136a |
| IID * ear | 0.625 | 0.282a | 0.088 | 0.274a |
| ATT * IID * ear | 0.001 | 0.001 | 0.001 | 0.004 |
SZ, schizophrenia patients; HC, healthy controls; ATT, attention instruction; IID, interaural intensity difference.
significant at P < .05.
Inline Supplementary Tables S1–S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2014.03.014.
4. Discussion
The present results indicate that glutamate measured in the ACC differentially predicts the neuronal response to cognitive control in schizophrenia patients and healthy individuals. Schizophrenia patients with higher levels of rsGlu showed increased BOLD response in the bilateral IPL during high demands for cognitive control, while patients with lower rsGlu did not show a comparable effect. However, the opposite was true for the healthy individuals. Here only individuals with lower levels of rsGlu showed an effect, while healthy individuals with higher rsGlu did not (Fig. 2). In general, the IPL is found to be involved in detection of new events and sustained attention over time (Husain and Nachev, 2007), both in the visual and auditory domains (Corbetta and Shulman, 2002; Salmi et al., 2009; Westerhausen et al., 2010). Moreover, the IPL has been implicated in linguistic functions as part of the phonological–articulatory loop, acting as a feed-forward area between auditory temporal areas and frontal speech production areas (Rauschecker and Scott, 2009). Rauschecker and Scott (2009) propose that the IPL acts as an interface in speech perception through its role in attention modulation and its connections to both frontal and superior temporal regions. It has also been suggested that attentional mechanisms of the IPL are directly modulated by conflict detection and top-down modulation from the ACC (Gruber and Goschke, 2004), an observation which has been strengthened by the detection of high intrinsic functional connectivity of the IPL with other hubs of the frontoparietal control system, including the ACC (Vincent et al., 2008). Finally, coactivation of (Falkenberg et al., 2011; Salmi et al., 2009; Wu et al., 2007) and strong anatomical connections between (Beckmann et al., 2009; Pandya et al., 1981) the IPL and ACC further support the notion of a functional link between these two brain regions during cognitive control processes. Thus, these previous findings corroborate well with the connection found between ACC glutamate and IPL BOLD response.
However, the presence of a link between ACC and IPL in healthy subjects does not provide an explanation as to why the relationship is inverted in schizophrenia. Schizophrenia patients are generally found to engage the ACC and IPL during high demands for cognitive control, as also shown in the present fMRI-results, although often with reduced strength when compared with healthy individuals (Carter et al., 2010; Lesh et al., 2011; Minzenberg et al., 2009). Moreover, anatomical abnormalities have been found in both the ACC (Benes et al., 1992; Fornito et al., 2009) and the IPL (Shenton et al., 2001; Torrey, 2007) in schizophrenia patients, together with altered integrity in white-matter tracts connecting these and other regions considered to take part in cognitive control processes (Repovs et al., 2011; Shergill et al., 2007). Notably, reduced functional connectivity within the frontal–parietal–temporal language network has been found in patients with auditory hallucinations during language processing (Curcic-Blake et al., 2013; Mechelli et al., 2007), and during resting-state fMRI, patients with auditory hallucinations exhibit reduced connectivity between parietal areas and the ACC (Vercammen et al., 2010). Reduced or abnormal connectivity in general, and between frontal and parietal regions in particular, is also a central feature of several theories of auditory hallucinations in schizophrenia (Badcock, 2010; Friston, 1998; Hugdahl et al., 2009; Waters et al., 2012). Related to this, our findings show that only patients with high (right-sided) ACC rsGlu levels engage IPL regions. This could be conceived as a functional disconnection between these regions, which thus is glutamatergically mediated by either direct synaptic activity or neuronal metabolism. Indeed, when it comes to synaptic glutamate, a recent study found that by disrupting glutamatergic neurotransmission through the administration of NMDA antagonist ketamine, long- and short-range circuit communication was impaired both in the fronto-parietal task-positive and in the default-mode network (Anticevic et al., 2012). Ketamine was also found to invoke schizophrenia-like symptoms and to affect cognitive performance (Anticevic et al., 2012), possibly through increased glutamate levels (Fusar-Poli et al., 2011; Stone et al., 2012). Altered glutamate-dependent intrinsic circuitry has also been demonstrated in the ACC in schizophrenia (Benes et al., 1992; Woo et al., 2008), which might contribute to modified connectivity with other regions. Moreover, the task-positive networks are found to undergo critical re-modeling during development (Fair et al., 2007), and as hypothesized by Repovs et al. (2011), it is possible that this re-modeling is disrupted during puberty in individuals that develop schizophrenia, affecting distal connectivity in particular. Since distal corticocortical connections in the brain are mainly glutamatergic, our results could support this view by showing altered glutamate-dependent communication between distal regions in schizophrenia. Thus, the functional connectivity between the ACC and the IPL might be disturbed or desynchronized in schizophrenia patients due to differences in glutamate-dependent modulation of neuronal activity, measured as changes in the BOLD response, also leading to reduced connectivity in more widespread cognitive control and language networks.
This assumption can furthermore be supported by the findings of Hutcheson et al. (2012), also applying the combined fMRI–MRS method in schizophrenia patients. The authors report that hippocampal glutamate levels mediate the BOLD response in the inferior frontal gyrus during a memory task in healthy controls. Similar to the present results, schizophrenia patients showed a different pattern of long-range glutamatergic connections during a cognitively demanding task. Previous studies in healthy individuals have found a glutamatergic relationship with regional task-induced BOLD, resting-state BOLD, and with the functional connectivity between regions (Duncan et al., 2011; Duncan et al., 2013; Enzi et al., 2012; Falkenberg et al., 2012; Horn et al., 2010; Kapogiannis et al., 2013; Schmaal et al., 2012). Thus, future studies both in schizophrenia patients and in healthy individuals could benefit from including both task-based and resting state fMRI in order to investigate the glutamatergic influence on the BOLD response in local microcircuits and larger cognitive networks, and maybe its role in the transition between default-mode brain activity and task-related brain responses (see e.g., Fransson, 2006).
Based on the current results, it is however difficult to conclude whether the glutamate-associated BOLD response in the IPL is functionally beneficial or not. Seeing the response as a compensatory mechanism, high glutamate levels in the ACC would be an advantage, possibly through higher rates of energy turnover in this brain region (Mangia et al., 2007; Rothman et al., 2003), and thus IPL engagement might not be necessary. On the other hand, if the IPL is seen as (a) an essential part of cognitive control mechanisms through the fronto-parietal control network (Vincent et al., 2008), and/or (b) important for correct auditory processing through the phonological-articulatory loop (Rauschecker and Scott, 2009), an insufficient recruitment of the IPL would be disadvantageous. Considering the schizophrenia patients' performance on the present task, it appears that low rsGlu levels and a lack of IPL activation are disadvantages, as the patients were exclusively and very strongly influenced by the sound intensity and did not manage to employ control resources to overcome the bottom-up intensity differences (Fig. 3). In SZ with higher levels of rsGlu, the sound intensity influenced task performance to a lesser degree, but they still struggled with applying control. This could perhaps reflect that the latter subgroup put more effort into following the attention instructions, but that the attempt failed (with attention instruction only explaining a small amount of variance). However, neither diagnosis nor glutamate levels alone can explain this difference in task performance (Inline Supplementary Table 1), suggesting that higher levels of glutamate could act as a kind of “buffer” for the schizophrenia patients, which prevents them from showing marked cognitive control deficits. Moreover, the schizophrenia patients showed a generally higher BOLD response during task performance in the right middle occipital gyrus/angular gyrus (BA 19/39) compared to healthy controls, an area adjacent to the glutamate-influenced IPL regions (cf. Inline Supplementary Fig. S3). Together with the engagement of the IPL in patients with higher rsGlu levels, this might prove an additional compensatory neuronal recruitment in these individuals, thereby improving their capability for cognitive control processing. In healthy individuals, however, both glutamate-groups managed to follow instructions and to correctly report the syllable from the to-be attended ear. This indicates that higher levels of glutamate in the ACC may not necessarily be task-relevant or even beneficial in healthy individuals (Falkenberg et al., 2012).
Widespread dysfunction of glutamate neurotransmission is thought to be a part of the pathology behind schizophrenia and is currently under extensive investigation regarding antipsychotic treatment (Moghaddam and Javitt, 2012). Hypofunction of the glutamatergic NMDA receptor seems to be related to schizophrenia symptoms (Coyle et al., 2003), further leading to disinhibition of glutamatergic neurons and desynchronized neural activity through hypostimulation of the GABAergic system (Coyle et al., 2012; Lewis et al., 2005). Moreover, several 1H-MRS schizophrenia studies have found alterations in glutamate levels, although results differ depending on voxel location and disease duration. A general finding has been increased levels of medial frontal glutamate (or combined glutamate and glutamine, glx) in early stages of the disease (Théberge et al., 2002) with levels decreasing or normalizing over time (Marsman et al., 2011; Théberge et al., 2003; see Poels et al., 2014 for a review), possibly due to excitotoxicity or the use of antipsychotic medication (Kegeles et al., 2012). We found lower levels of rsGlu compared to healthy individuals in the left ACC, corroborating previous results in chronic samples. The hemispheric difference in our study was not expected, and is difficult to explain compared with previous results since most 1H-MRS studies place the ACC voxels either across the hemispheres or in one hemisphere only. However, the fact that this difference was found only in the left ACC, and that only right ACC rsGlu interacted with the BOLD response, could indicate a hemispheric imbalance related to glutamatergic regulation in neuronal signaling or local metabolism. Perhaps glutamatergic reduction in the left ACC disturbs cognitive control processing depending on the right ACC, thus contributing to the inverted pattern seen in our results. Generally, this inter-hemispheric difference could be important and should be more thoroughly investigated in future studies.
The current study had several limitations. Firstly, our schizophrenia patients were all on antipsychotic medications, so that medication effects on the results cannot be excluded. Previous clinical as well as animal studies have shown that atypical antipsychotic medication may reduce or normalize glutamate levels in medial frontal regions (Carli et al., 2011; Kegeles et al., 2012). This might be the origin of the reduced rsGlu in left ACC in our results, but would not have any direct impact on the main outcome of the present results, as left-hemisphere rsGlu did not influence the BOLD response. Moreover, none of the currently available antipsychotic medications target the glutamate system directly. Another study has shown that the BOLD response in schizophrenia patients tends to normalize over longer exposure to antipsychotic medications (Abbott et al., 2012). Given the chronic state of the current patient sample, the antipsychotic medications could contribute to the absence of any large between-groups difference in the present fMRI analysis alone. Secondly, at 3 T field strength some of the metabolite peaks in the 1H-MRS spectrum are overlapping. In spite of the high quality of the glutamate output from the LCModel, it is difficult to completely discriminate glutamate from glutamine and GABA signal amplitudes. However, a PRESS sequence with short echo time as we used here is not sensitive to GABA (Henry et al., 2011), and although GABA and glutamate are involved in the same neurotransmitter cycling, they have been found not to correlate within an MRS-voxel (Waddell et al., 2011). Regarding glutamine, future studies using e.g. 2D J-resolved are warranted for full separation (Jensen et al., 2009). Thirdly, due to time limitation, 1H-MRS was only assessed in the ACC. Future studies might benefit from the inclusion of a control measurement region in order to distinguish regional specific effects from possible global effects. In addition, the present study only included schizophrenia patients with moderate to severe auditory verbal hallucinations, thus the results may not generalize to all individuals with schizophrenia.
5. Conclusions
Taken together, the present study indicates glutamate-dependent differences in the engagement of the IPL during cognitive control processing in schizophrenia patients, also showing that cognitive control performance is affected by glutamate levels. The differential glutamate-dependent BOLD response can be seen as an important link between metabolite-level glutamate deficits and cognitive control challenges in schizophrenia, thus implying a more widespread, network-dependent pathology in schizophrenia. Ultimately, these results provide information on glutamate mechanisms that can be of relevance regarding pharmacological development targeting the glutamatergic system.
Acknowledgments
We thank Roger Barndon, Turid Randa, and Eva Øksnes at the Radiology Department at Haukeland University Hospital, Bergen, Norway, for their support in data acquisition and Inga Gerdau, Anne Marie Kinn R?d, and Marina Stibric at the Bergen fMRI Group for their assistance in analysis of the data. This work was funded by an Advanced Grant from the European Research Council (ERC AG # 249516; to K.H.), and a grant from the Research Council of Norway (RCN # 196076; to K.H.).
References
- Abbott C.C., Jaramillo A., Wilco C.E., Hamilton D.A. Antipsychotic drug effects in schizophrenia: a review of longitudinal fMRI investigations and neural interpretations. Current Medicinal Chemistry. 2012;20:428–437. doi: 10.2174/0929867311320030014. 23157635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Gancsos M., Murray J.D., Repovs G., Driesen N.R., Ennis D.J., Niciu M.J., Morgan P.T., Surti T.S., Bloch M.H., Ramani R., Smith M.A., Wang X.J., Krystal J.H., Corlett P.R. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. 23012427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock J.C. The cognitive neuropsychology of auditory hallucinations: a parallel auditory pathways framework. Schizophrenia Bulletin. 2010;36:576–584. doi: 10.1093/schbul/sbn128. 18835839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. 19176826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F.M., Sorensen I., Vincent S.L., Bird E.D., Sathi M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cerebral Cortex (New York, N.Y.: 1991) 1992;2:503–512. doi: 10.1093/cercor/2.6.503. 1282404 [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.J., Valabregue R., Poline J.P. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- Carli M., Calcagno E., Mainolfi P., Mainini E., Invernizzi R.W. Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex. Psychopharmacology. 2011;214:639–652. doi: 10.1007/s00213-010-2065-7. [DOI] [PubMed] [Google Scholar]
- Carter J.D., Bizzell J., Kim C., Bellion C., Carpenter K.L., Dichter G., Belger A. Attention deficits in schizophrenia—preliminary evidence of dissociable transient and sustained deficits. Schizophrenia Research. 2010;122:104–112. doi: 10.1016/j.schres.2010.03.019. 20554160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S.G., Aiken L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2003. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. 11994752 [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Basu A., Benneyworth M., Balu D., Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handbook of Experimental Pharmacology. 2012:267–295. doi: 10.1007/978-3-642-25758-2_10. 23027419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J.T., Tsai G., Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Annals of the New York Academy of Sciences. 2003;1003:318–327. doi: 10.1196/annals.1300.020. 14684455 [DOI] [PubMed] [Google Scholar]
- Curcic-Blake B., Liemburg E., Vercammen A., Swart M., Knegtering H., Bruggeman R., Aleman A. When Broca goes uninformed: reduced information flow to Broca's area in schizophrenia patients with auditory hallucinations. Schizophrenia Bulletin. 2013;39 doi: 10.1093/schbul/sbs107. 10871095 23070537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan N.W., Enzi B., Wiebking C., Northoff G. Involvement of glutamate in rest–stimulus interaction between perigenual and supragenual anterior cingulate cortex: a combined fMRI–MRS study. Human Brain Mapping. 2011;32(12):2172–2182. doi: 10.1002/hbm.21179. 21305662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan N.W., Wiebking C., Tiret B., Marjanska M., Hayes D.J., Lyttleton O., Doyon J., Northoff G. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical–subcortical functional connectivity in humans. PloS One. 2013;8:e60312. doi: 10.1371/journal.pone.0060312. 23573246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi B., Duncan N.W., Kaufmann J., Tempelmann C., Wiebking C., Northoff G. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex—a combined fMRI–MRS study. Neuroscience. 2012;227:102–109. doi: 10.1016/j.neuroscience.2012.09.039. 23022216 [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. 17679691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg L.E., Specht K., Westerhausen R. Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain and Cognition. 2011;76:276–285. doi: 10.1016/j.bandc.2011.02.006. 21398015 [DOI] [PubMed] [Google Scholar]
- Falkenberg L.E., Westerhausen R., Specht K., Hugdahl K. Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5069–5073. doi: 10.1073/pnas.1115628109. 22411802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yucel M., Dean B., Wood S.J., Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophrenia Bulletin. 2009;35:973–993. doi: 10.1093/schbul/sbn025. 18436528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. 16879844 [DOI] [PubMed] [Google Scholar]
- Friston K.J. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/S0920-9964(97)00140-0. 9549774 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A., Poline J.B., Price C.J., Frith C.D. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. 9345513 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Zarahn E., Josephs O., Henson R.N., Dale A.M. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. 10547338 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Stone J.M., Broome M.R., Valli I., Mechelli A., McLean M.A., Lythgoe D.J., O'Gorman R.L., Barker G.J., McGuire P.K. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Archives of General Psychiatry. 2011;68:881–890. doi: 10.1001/archgenpsychiatry.2011.46. 21536967 [DOI] [PubMed] [Google Scholar]
- Gruber O., Goschke T. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychologica. 2004;115:105–121. doi: 10.1016/j.actpsy.2003.12.003. 14962396 [DOI] [PubMed] [Google Scholar]
- Henry M.E., Lauriat T.L., Shanahan M., Renshaw P.F., Jensen J.E. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. Journal of Magnetic Resonance (San Diego, Calif.: 1997) 2011;208:210–218. doi: 10.1016/j.jmr.2010.11.003. 21130670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D.I., Yu C., Steiner J., Buchmann J., Kaufmann J., Osoba A., Eckert U., Zierhut K.C., Schiltz K., He H., Biswal B., Bogerts B., Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00033. 20700385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. In: The Asymmetrical Brain. Hugdahl K., Davidson R.J., editors. MIT Press; Cambridge, MA: 2003. Dichotic listening in the study of auditory laterality; pp. 441–476. [Google Scholar]
- Hugdahl K., Andersson L. The “forced-attention paradigm” in dichotic listening to CV syllables: a comparison between adults and children. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior. 1986;22:417–432. doi: 10.1016/S0010-9452(86)80005-3. 3769494 [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Loberg E.M., Nygard M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Frontiers in Neuroscience. 2009;3:34–45. doi: 10.3389/neuro.01.001.2009. 19753095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Westerhausen R., Alho K., Medvedev S., Hämäläinen H. The effect of stimulus intensity on the right ear advantage in dichotic listening. Neuroscience Letters. 2008;431:90–94. doi: 10.1016/j.neulet.2007.11.046. 18162310 [DOI] [PubMed] [Google Scholar]
- Husain M., Nachev P. Space and the parietal cortex. Trends in Cognitive Sciences. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. 17134935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster R.J., Westerhausen R., Kreuder F., Schweiger E., Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. Neuroimage. 2007;34:888–895. doi: 10.1016/j.neuroimage.2006.10.023. 17161625 [DOI] [PubMed] [Google Scholar]
- Hutcheson N.L., Reid M.A., White D.M., Kraguljac N.V., Avsar K.B., Bolding M.S., Knowlton R.C., den Hollander J.A., Lahti A.C. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophrenia Research. 2012;140:136–142. doi: 10.1016/j.schres.2012.06.039. 22831772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.E., Licata S.C., Ongur D., Friedman S.D., Prescot A.P., Henry M.E., Renshaw P.F. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR in Biomedicine. 2009;22:762–769. doi: 10.1002/nbm.1390. 19388001 [DOI] [PubMed] [Google Scholar]
- Kapogiannis D., Reiter D.A., Willette A.A., Mattson M.P. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. 23000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Kegeles L.S., Mao X., Stanford A.D., Girgis R., Ojeil N., Xu X., Gil R., Slifstein M., Abi-Dargham A., Lisanby S.H., Shungu D.C. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate–glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Archives of General Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. 22213769 [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. 20844478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat. Reviews in the Neurosciences. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. 20035017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S., Tkac I., Gruetter R., Van de Moortele P.F., Maraviglia B., Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. 17033694 [DOI] [PubMed] [Google Scholar]
- Marsman A., van den Heuvel M.P., Klomp D.W., Kahn R.S., Luijten P.R., Hulshoff Pol H.E. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophrenia Bulletin. 2011;39(1):120–129. doi: 10.1093/schbul/sbr069. 21746807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Allen P., Amaro E., Jr., Fu C.H., Williams S.C., Brammer M.J., Johns L.C., McGuire P.K. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Human Brain Mapping. 2007;28:1213–1222. doi: 10.1002/hbm.20341. 17266108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K. The prefrontal cortex and cognitive control. Nature. Reviews in the Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. 19652121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. 21956446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D., Prescot A.P., Jensen J.E., Cohen B.M., Renshaw P.F. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. 19239984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D.N., Van Hoesen G.W., Mesulam M.M. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research. 1981;42:319–330. doi: 10.1007/BF00237497. 6165607 [DOI] [PubMed] [Google Scholar]
- Passow S., Westerhausen R., Hugdahl K., Wartenburger I., Heekeren H.R., Lindenberger U., Li S.C. Electrophysiological correlates of adult age differences in attentional control of auditory processing. Cerebral Cortex (New York, N.Y.: 1991) 2014;24:249–260. doi: 10.1093/cercor/bhs306. 23042734 [DOI] [PubMed] [Google Scholar]
- Port J.D., Agarwal N. MR spectroscopy in schizophrenia. Journal of Magnetic Resonance imaging: JMRI. 2011;34:1251–1261. doi: 10.1002/jmri.22787. 22102558 [DOI] [PubMed] [Google Scholar]
- Poels E.M., Kegeles L.S., Kantrowitz J.T., Javitt D.C., Lieberman J.A., Abi-Dargham A., Girgis R.R. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophrenia Research. 2014;152:325–332. doi: 10.1016/j.schres.2013.12.013. 24418122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. 8139448 [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Scott S.K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature Neuroscience. 2009;12:718–724. doi: 10.1038/nn.2331. 19471271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biological Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. 21193174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science (New York, N.Y.) 2004;306:443–447. doi: 10.1126/science.1100301. 15486290 [DOI] [PubMed] [Google Scholar]
- Rothman D.L., Behar K.L., Hyder F., Shulman R.G. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annual Review of Physiology. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. 12524459 [DOI] [PubMed] [Google Scholar]
- Salmi J., Rinne T., Koistinen S., Salonen O., Alho K. Brain networks of bottom-up triggered and top-down controlled shifting of auditory attention. Brain Research. 2009;1286:155–164. doi: 10.1016/j.brainres.2009.06.083. 19577551 [DOI] [PubMed] [Google Scholar]
- Schmaal L., Goudriaan A.E., van der Meer J., van den Brink W., Veltman D.J. The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain and Behavior. 2012;2:553–562. doi: 10.1002/brb3.74. 23139901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M.E., Dickey C.C., Frumin M., McCarley R.W. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/S0920-9964(01)00159-110.1016/S0920-9964(01)00163-3. 11343862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill S.S., Kanaan R.A., Chitnis X.A., O'Daly O., Jones D.K., Frangou S., Williams S.C., Howard R.J., Barker G.J., Murray R.M., McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. American Journal of Psychiatry. 2007;164:467–473. doi: 10.1176/appi.ajp.164.3.467. 17329472 [DOI] [PubMed] [Google Scholar]
- Srinivasan R., Cunningham C., Chen A., Vigneron D., Hurd R., Nelson S., Pelletier D. TE-averaged two-dimensional proton spectroscopic imaging of glutamate at 3 T. Neuroimage. 2006;30:1171–1178. doi: 10.1016/j.neuroimage.2005.10.048. 16431138 [DOI] [PubMed] [Google Scholar]
- Stone J.M., Dietrich C., Edden R., Mehta M.A., De Simoni S., Reed L.J., Krystal J.H., Nutt D., Barker G.J. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Molecular Psychiatry. 2012;17:664–665. doi: 10.1038/mp.2011.171. 22212598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge J., Al-Semaan Y., Williamson P.C., Menon R.S., Neufeld R.W., Rajakumar N., Schaefer B., Densmore M., Drost D.J. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. American Journal of Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. 14638596 [DOI] [PubMed] [Google Scholar]
- Théberge J., Bartha R., Drost D.J., Menon R.S., Malla A., Takhar J., Neufeld R.W., Rogers J., Pavlosky W., Schaefer B., Densmore M., Al-Semaan Y., Williamson P.C. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. American Journal of Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. 12411236 [DOI] [PubMed] [Google Scholar]
- Torrey E.F. Schizophrenia and the inferior parietal lobule. Schizophrenia Research. 2007;97:215–225. doi: 10.1016/j.schres.2007.08.023. 17851044 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- van den Noort M., Specht K., Rimol L.M., Ersland L., Hugdahl K. A new verbal reports fMRI dichotic listening paradigm for studies of hemispheric asymmetry. Neuroimage. 2008;40:902–911. doi: 10.1016/j.neuroimage.2007.11.051. 18234509 [DOI] [PubMed] [Google Scholar]
- Vercammen A., Knegtering H., den Boer J.A., Liemburg E.J., Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biological Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. 20060103 [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. 18799601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell K.W., Zanjanipour P., Pradhan S., Xu L., Welch E.B., Joers J.M., Martin P.R., Avison M.J., Gore J.C. Anterior cingulate and cerebellar GABA and Glu correlations measured by 1 H J-difference spectroscopy. Magnetic Resonance imaging. 2011;29:19–24. doi: 10.1016/j.mri.2010.07.005. 20884148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F., Allen P., Aleman A., Fernyhough C., Woodward T.S., Badcock J.C., Barkus E., Johns L., Varese F., Menon M., Vercammen A., Laroi F. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophrenia Bulletin. 2012;38:683–693. doi: 10.1093/schbul/sbs045. 22446568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R., Hugdahl K. In: The Two Halves of the Brain. Hugdahl K., Westerhausen R., editors. MIT Press; Cambridge, MA: 2010. Cognitive control of auditory laterality; pp. 469–497. [Google Scholar]
- Westerhausen R., Moosmann M., Alho K., Belsby S.O., Hamalainen H., Medvedev S., Specht K., Hugdahl K. Identification of attention and cognitive control networks in a parametric auditory fMRI study. Neuropsychologia. 2010;48:2075–2081. doi: 10.1016/j.neuropsychologia.2010.03.028. 20363236 [DOI] [PubMed] [Google Scholar]
- Woo T.U., Shrestha K., Lamb D., Minns M.M., Benes F.M. N-methyl-d-aspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biological Psychiatry. 2008;64:803–809. doi: 10.1016/j.biopsych.2008.04.034. 18585682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.T., Weissman D.H., Roberts K.C., Woldorff M.G. The neural circuitry underlying the executive control of auditory spatial attention. Brain Research. 2007;1134:187–198. doi: 10.1016/j.brainres.2006.11.088. 17204249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Gao J.-H., Lancaster J.L., Fox P.T. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. doi: 10.1002/hbm.460030404. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


